ABSTRACT
Pneumococcal conjugate vaccine ten valent (PCV 10) was introduced into Nigeria in three phases. Phase 3 introduction started in August 2016. However, its impact on pneumonia admissions and mortality among vaccinated Nigerian children has not been determined. Data in the period before PCV-10 introduction (3 August 2013–2 August 2016), and after (3 August 2017–2 August 2020) were retrospectively extracted from the medical charts of eligible patients aged 3–24 months with hospitalized radiological pneumonia at the University College Hospital (UCH), Ibadan; National Hospital (NH), Abuja; and Federal Teaching Hospital (FTH), Gombe, allowing for an intervening period of 1 year. Proportions of the patients with hospitalized pneumonia and case fatality rates were determined during both periods. The results were compared using z-test, multiple logistic regression analysis and p < .05 was considered significant. Adjusted pneumonia hospitalization rates between the two periods increased at the NH Abuja (10.7% vs 14.6%); decreased at the UCH, Ibadan (8.7% vs 6.9%); and decreased at the FTH, Gombe (28.5% vs 18.9%). Case fatality rates decreased across all the sites during the post-PCV introduction period: NH Abuja, from 6.6% to 4.4% (p = .106); FTH, Gombe, 11.7% to 7.7% (p = .477); and UCH, Ibadan, 2.0% to 0% (p = .045); but only significant at Ibadan. Overall, proportion of hospitalized pneumonia cases decreased after 3 years of PCV 10 introduction into the National Immunization Programme in Nigeria. The case fatality rate during post-PCV 10 introduction decreased at all the three sites, but this difference was significant at the UCH, Ibadan.
Plain Language Summary
Pneumonia is the commonest killer of Nigerian children aged less than 5 years. Pneumonia vaccine (PCV 10) was introduced into Nigeria Vaccination Program between 2014 and 2016, but up till now the value has not been confirmed. We conducted a retrospective study in which data before and after PCV 10 introduction were compared. The study sites were the University College Hospital (UCH), Ibadan; National Hospital (NH), Abuja; and Federal Teaching Hospital (FTH), Gombe. The data were extracted from the medical charts of eligible patients aged 3–24 months who were admitted for severe pneumonia with evidences on lung radiographs. We found that the proportion of hospitalized pneumonia cases decreased after 3 years of PCV 10 introduction into the National Immunization Program in Nigeria. The death rate during post-PCV 10 introduction decreased at all the three sites, but was only significantly decreased at the UCH, Ibadan.
Introduction
Background
Streptococcus pneumoniae causes severe illnesses such as pneumonia, meningitis, and septicemia in children aged 0 up to 5 years.Citation1 S. pneumoniae is the commonest cause of bacterial pneumonia mortality worldwide, killing about 800,000 under-5s annually.Citation2 In Nigeria, pneumonia is the commonest cause of under-5 deaths.Citation3 Most of these infections are preventable by the use of pneumococcal conjugate vaccines (PCVs) that are already licensed and in routine use in most developed and many developing countries.Citation4
In an hospital-based study of invasive pneumococcal diseases in Nigeria, 100% of the pneumococcal serotypes identified (4, 5, and 19F) are contained in the 10- and 13-valent PCVs currently in use.Citation5
The most accurate method of monitoring trends in disease incidence over time is population-based active surveillance for laboratory-confirmed cases of invasive bacterial diseases (IBD).Citation6 There are five methods that can be used to measure vaccine effectiveness and efficacy of PCV in the field. Demonstrating vaccine efficacy through double-blinded, randomized, placebo-controlled trials is necessary before licensure of PCV but may not be ethical or necessary for current PCV, and, therefore, there is a role for observational methods of post-licensure field impact studies. A case-control study with invasive bacterial disease as the outcome is probably the most feasible method to measure accurately PCV effectiveness in most settings. Cluster randomized trials including stepped-wedge studies, which are expensive, are required if also it is intended to capture the part of vaccination impact that is driven by suboptimal coverage and indirect protection. However, in Nigeria, a simple method of monitoring pneumonia hospitalization and deaths will be more feasible than the enumerated methods.
Justification for the study
The first phase of PCV-10 introduction into the Nigeria immunization programme was on 22 December 2014;Citation7 the second phase was in November 2015; and the third phase vaccine introduction into 18 states including Federal Capital Territory, Oyo ,and Gombe states was in August 2016. This present study took place in three sites which are located in Oyo and Gombe states, as well as FCT.
Pneumococcal conjugate vaccine impact with co-introduction of other vaccines can be measured. In Nigeria, Haemophilus influenzae type B (Hib) conjugate vaccine was introduced into the routine childhood immunization in two phases in 2012 and 2013Citation8 with an estimated average national coverage of 97%; and rotavirus vaccine is being taken by some infants from private health facilities. These other vaccines may have a more measurable impact on mortality in under-5s than each vaccine separately.
Despite pneumonia being the most common clinical manifestation of pneumococcal infection, measuring the impact of PCV on pneumonia can be difficult because the case definition of pneumonia does not specifically identify pneumococcus as the causative organism.
Using chest radiographs that are interpreted based on WHO criteria to determine lobar pneumonia can improve specificity. Studies that aim to measure the effect on clinically defined pneumonia will almost certainly fail to see an effect because of the nonspecific outcome measure. This may lead to inaccurate conclusions that the vaccine is not effective against pneumonia when, in fact, it is working against Hib and/or pneumococcal pneumonia, but these are only a fraction of the cases identified as clinical pneumonia.
In Nigeria, there is no robust baseline data on pneumococcal disease burden and serotype distributions; and the sources and scope of available baseline dataCitation5,Citation9 cannot be generalized for the whole country. Given this setting, there is a need for the best way to generate baseline data for pneumococcal disease, to compare with post-implementation PCV impact data following the introduction of PCV-10 on 22 December 2014 into the National Program on Immunization.
Statement for the hypothesis
We hypothesized that the introduction of PCV-10 into Nigeria Immunization Program reduces pneumonia hospitalization and case fatality rates in children aged 3 to 24 months in Nigeria.
Patients and methods
An observational retrospective medical chart analysis study was performed in three tertiary hospitals in Nigeria (NH Abuja, FTH Gombe, UCH Ibadan) to assess PCV-10 impact on pneumonia hospitalization and mortality in children aged 3 to 24 months.
Case-definitions and endpoints
Pneumonia was diagnosed with chest radiography, and interpretation was accepted as written in the medical charts of patients, reporting either lobar pneumonia or bronchopneumonia, and empyema with or without pneumothorax. These reports are aligned to the WHO Standard for reporting chest radiographs in which there is presence of a dense or fluffy opacity that occupies a portion or whole of a lobe or of the entire lung, presence of fluid in the lateral pleural space between the lung and chest wall, or both.Citation10,Citation11
The endpoints were number of pneumonia hospitalizations and deaths among children 3–24-month-old. The proportion of pneumonia hospitalizations was calculated before and after vaccination periods. The case-fatality rates were calculated from the deaths among the pneumonia admissions.
Data collection before vaccine introduction
Total pediatric wards’ admissions for the period from 3 August 2013 to 2 August 2016 (3 years before start of PCV-10 vaccination) were determined from the registers, using the patients’ hospital numbers and sorting done with a dedicated computer. Pneumonia admissions and deaths in the target age group were extracted. The medical charts of these patients were retrieved from the hospital Medical Record Departments with the assistance of the Record Officers. Those that satisfied the inclusion criteria after reviewing of the charts were sorted out and data such as patient’s age, gender, clinical parameters, and vaccination record were extracted from these records and entered into the case report form by the study physician. Vaccination record is typically recorded in patients’ charts as up-to-date without specifying the names of the vaccines nor indicating the particular dates.
Data collection after vaccine introduction
For three years (3 August 2017 to 2 August 2020), after allowing one year for rise in vaccine coverage and vaccine impact, all children aged 3 to 24 months who were hospitalized because of radiologically confirmed pneumonia (as defined in the endpoints) were identified using the same method as for the pre-PCV 10 introduction period. Specifically, first-year post-vaccination data extraction started on 3 August 2018; second year on 3 August 2019; and third year on 3 August 2020. This quasi-retrospective method was employed to create a situation as close as possible to collection of data before vaccine introduction and thereby reduce information bias.
Inclusion criteria
Patients were enrolled into the study if aged 3–24 months; and admitted with a diagnosis of radiologically confirmed acute pneumonia.
Exclusion criteria
Patients were excluded from the study if: (a) aged below 3 months or above 24 months; (b) duration of symptoms was 14 days and more; (c) wheezing illness documented; (d) confirmed to have comorbidities related to cardiac diseases, central nervous system diseases, birth defects affecting upper and lower respiratory tract systems such as cleft lip and craniofacial abnormalities, gastroesophageal reflux, or aspiration pneumonitis; (e) premature infants (gestational age <37 completed weeks); and (f) the pneumonia was not confirmed radiologically.
Sample size
It is expected that 50% of the severe pneumonia admissions will be radiologically confirmed with a margin of error ranging between 3% and 5%. Since the total population in the catchment area was not exactly known, we used the population parameter as unknown. The confidence limit was 95%. The calculated sample size was 900 from all the three sites (see details in the Supplemental Material). The following formula was used to calculate the number of medical charts required for both vaccination periods.
SS = Z2*p*(1-p)/C2
SS = Sample size
Z = Z value (1.96 for 95% CI)
P = expected proportion of the disease (pneumonia) to be picked up
C = Margin of error
Data management and analyses
For both the retrospective and the prospective (which was quasi-retrospective) components of the study, the study clinician ascertained from the admission register the total number of admissions and those children with diagnosis of pneumonia admitted into the pediatric wards during the study period. A case record form was completed. All filled case record forms were cross-checked for consistency. Dual data entry was done by two different data entry clerks using Epi-Data version 3.1 to allow data verification and cleaning. After cleaning, the data was transferred to IBM® SPSS version 21 and Stata for analyses.
The number of admitted children aged 3–24 months was the denominator of the study while the number of children in the same age group and with radiologically confirmed pneumonia admissions constituted the numerator to calculate the proportion.
Proportions of pneumonia admissions before and after vaccine introduction, and case fatality rates, were compared using z-test and a p < .05 was considered significant. Multiple logistic regression analysis was employed to examine for confounding factors.
Ethical consideration
Ethical approval was obtained from the University/Teaching Hospital Institutional Review Board of each study site.
Results
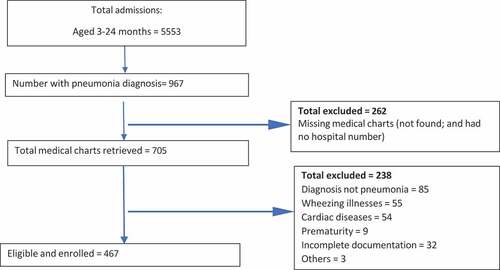
For the pre-PCV 10 introduction period, there were 5553 children aged 3–24 months identified from admission registers of the three teaching hospitals (). Out of 967 pneumonia admissions, only 705 medical charts were retrieved because 262 were either not found or the hospital number known. Only 467 fulfilled the inclusion criteria ().
Figure 1. Flow diagram for enrolling 3–24-month-olds with radiological pneumonia admitted into the three sites (Pre-PCV 10 vaccine introduction period).
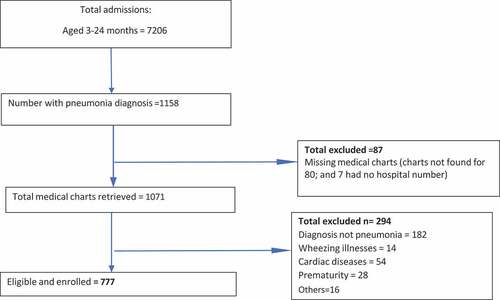
After PCV 10 introduction, 7206 children aged 3–24 months were identified from the admission registers (). There were 1158 pneumonia admissions. Because 87 medical charts were either not found or the hospital number known, 1071 medical charts were retrieved. Finally, 777 fulfilled the inclusion criteria ().
Figure 2. Flow diagram for enrolling 3–24-month-olds with radiological pneumonia admitted into the three sites (post-PCV 10 introduction period).
Baseline characteristics
Total admissions of the 3–24-month-olds in the pre-PCV 10 introduction period was 5553 and is less than 7206 in the post-PCV 10 introduction period (). During these latter periods, increase in admissions was seen in other departments of the three hospitals such as Medical, Surgical, Obstetric & Gynecological, and Accident & Emergency (data not shown).
Table 1. Total admissions of 3–24-month-olds pre-PCV 10 and post-PCV 10 introduction periods.
Pneumonia admissions during the post-PCV introduction period was 777 () and is more than 467 in the pre-PCV introduction period (). There was a higher proportion of missing medical charts during pre-vaccination period (262/967, 27.1%; ) compared to post-vaccination period (87/1158, 7.5%; ).
Hospitalization proportions and case fatality rates
Hospitalization proportions per hospital by year of observation is as shown in . Because per year hospitalization in the pre- and post-period are not balanced in each hospital, there was high variability in the number of hospitalizations across years and hospitals. Therefore, overall proportions of hospitalization due to community-acquired pneumonia (CAP) per hospital in the pre- and post-period was adjustedCitation12 and compared. summarizes the differences in crude rates and adjusted rates of pneumonia admissions at the three tertiary centers for pre- and post-PCV 10 introduction periods. Adjusted pneumonia hospitalization rates between the two periods increased at the NH Abuja (10.7% vs 14.6%); decreased at the UCH, Ibadan (8.7% vs 6.9%); and decreased at the FTH, Gombe (28.5% vs 18.9%). This translates to a calculated weighted adjusted difference pre- vs post-vaccination per hospital of: NH Abuja (increase of 37%); FTH, Gombe (decrease of 34%); and UCH, Ibadan (decrease of 21%).
Table 2. Proportions of hospitalized children aged 3–24 months with pneumonia per hospital by year of observation.
Table 3. Crude and adjusted proportions of pneumonia hospitalization at the three hospitals during pre- and post-PCV-10 introduction periods.
describes the pneumonia admissions stratified into two age groups at the three sites during the pre-PCV 10 and post-PCV 10 introduction periods. Categorizing all hospitalizations per age group could not be done because we did not have the total admissions stratified into “3-11” and 12-24” months, which are what we required for the denominator to calculate the fractions. This problem is inherent in the study because the case record form (CRF) was not designed to capture the ages of the non-pneumonia admissions, except that of pneumonia admissions. Nevertheless, this table generally reflects the findings in .
Table 4. Pneumonia admissions stratified into two age groups at the three sites during the pre-PCV 10 and post-PCV 10 introduction periods.
shows the vaccination history and outcome of pneumonia admissions. A child who had received three doses of PCV 10 at the age of 14 weeks is said to be fully immunized against pneumococcal disease. Using this criterion, the PCV-10 vaccine uptake was 94.5% at NH Abuja; 92.3% at FTH, Gombe; and 88.9% at the UCH, Ibadan. This information was mostly collected from verbal reports of the caregivers/guardians/mothers. Only in a few instances could this information be confirmed through sighting of the vaccination cards. In Nigeria, mothers rarely bring the health cards containing vaccination records to health facilities. Although they were encouraged to bring the vaccination cards for sighting before discharge, only a few complied. In , the case fatality rates decreased across all the sites during the post-PCV 10 introduction period but only significantly at one site: NH Abuja, from 6.6% to 4.4% (p = .106); FTH, Gombe, 11.7% to 7.7% (0.477); and UCH, Ibadan, 2.0% to 0% (p = .045).
Table 5. Vaccination history, pneumonia admissions and outcome in each of the three teaching hospitals during the pre-PCV 10 and post-PCV 10 introduction periods.
Table 6. Case fatality rates among 3–24-month-olds admitted with pneumonia in each of the three teaching hospitals during the pre- and post-PCV 10 introduction periods.
Discussion
There were low outlier values during the pre-PCV 10 period 2013–2014 of 77 total admissions of 3–24-month-olds at the UCH, Ibadan giving atypically high 15.6% pneumonia admissions compared with the other two sites at the same observation pre-PCV 10 period. Similarly, low outlier values for total admissions for the 3-year pre-PCV 10 period (2013–2016) at FTH, Gombe was 354. These findings were probably due to the effects of industrial strikes in the health sectors at the corresponding times.Citation13 Furthermore, total admissions for the 3–24-month-olds during post-PCV-10 period (2019–2020) at the NH Abuja was 331. Because of this low value, the proportion of pneumonia admissions for this observation year was apparently higher than other two sites at 20.2% (67/331) – . Coronavirus disease 2019 (COVID-19) lockdown in Abuja, Nigeria, was associated with reduction in the overall acute trauma volume in the emergency room among acute trauma patients.Citation14 It is not clear if this effect of COVID-19 pandemic was responsible for the lower admission of pneumonia at the children emergency ward of NH Abuja than the other two sites.
There were differences in the proportions of community-acquired pneumonia (CAP) hospitalization across the three hospitals during the pre- and post-PCV 10 introduction periods: highest at the FTH, Gombe and lowest at the UCH, Ibadan, and NH Abuja taking a second position (). However, the absolute number of CAP hospitalizations was highest at the NH Abuja, which might reflect the economic potentials and ability of the parents of children in Abuja to afford care at tertiary level compared to Gombe and Ibadan. Catastrophic out-of-pocket expenditure has been shown to impact negatively on Nigerians,Citation15 thereby determining health care-seeking behaviors, in which poor households seek low-quality care, avoid seeking healthcare at all, or resort to self-medication due to inability to pay for healthcare services.Citation16 The wealth quintiles in FCT, Abuja was greatest (lowest, 0.7% to highest, 55.1%); modest in Oyo State (11.1% to 25.2%); and least in Gombe State (36.7% to 8.6%).Citation17 Consequently, the apparent highest CAP hospitalization proportion at Gombe site might be due to incomplete data retrieval.
Adjusted pneumonia hospitalization rates between the two periods increased at the NH Abuja (10.7% vs 14.6%); decreased at the UCH, Ibadan (8.7% vs 6.9%); and decreased at the FTH, Gombe (28.5% vs 18.9%). This translates to a calculated weighted adjusted difference pre- vs post-vaccination per hospital of: NH Abuja (increase of 36.4%); FTH, Gombe (decrease of 34%); and UCH, Ibadan (decrease of 21%).
Trying to explain this finding, we looked at the pneumonia admissions stratified into two age groups at the three sites during the pre-PCV 10 and post-PCV 10 introduction periods (). But we could not categorize all hospitalizations per age group because the total admissions stratified into 3–11 and 12–24 months were not available to calculate the fractions.
We employed a similar strategy to that of Iceland to evaluate the impact of PCV 10 in reducing pneumonia admissions. There was significant reduction of pneumonia, which was noted very early after initiation of the vaccination.Citation18 The contrast noted in our study might be due to inadequate retrieval of data which is inherent in a system without electronic medical record.
The WHO-Federal Government National invasive bacterial disease longitudinal surveillance in Nigeria from 2010 to 2016 showed pneumococcus as the commonest causative organism of under-five bacterial meningitis and is also likely the commonest cause of under-five pneumonia (since the meningitis surveillance is a proxy for pneumonia). Nearly a half of the pneumococcal meningitis cases successfully serotyped (46.4%: 13/28) were caused by serotypes that are included in the 10-valent pneumococcal conjugate vaccine.Citation19
The vaccination history was obtained mostly from verbal reports of the caregivers/guardians/mothers. Only in a few instances could this information be confirmed through sighting of the vaccination cards. In Nigeria, mothers rarely bring the health cards containing vaccination records to health facilities. Using this method, the PCV-10 vaccine uptake across the 3 sites was good, ranging from 88.9% at the UCH, Ibadan, through 92.3% at the FTH, Gombe to 94.5% at the NH, Abuja. However, the WHO/UNICEF estimates of third-dose pneumococcal conjugate vaccine (PCV3) immunization coverage in Nigeria from 2016 to 2020 were in the range of 49% in 2016 to 57% in 2020.Citation20 The reasons for this discrepancy include: the WHO/UNICEF estimates for Nigeria rely mostly on population-based surveys whereas in our study, we used facility-based data which were not primarily obtained to assess vaccine uptake. In addition, our data relied mostly on caregivers’ recall and could have resulted in overestimation of PCV-10 vaccine uptake. Variation in agreement between vaccine card and caregiver’s recall has been previously documented.Citation21
Case fatality rates decreased across all the sites during the post-PCV 10 introduction period but only significant at Ibadan. The higher case fatality rate observed in FTH, Gombe compared with the other study centers might be attributed to sociodemographic differences among the study sites. Gombe which is located in the northeast geopolitical region is characterized by extreme poverty, large family size, poor female education and empowerment, and recently insurgency.Citation22
Limitations of the study
Our endpoint was not exactly as recommended by the WHO Standard reporting for chest radiographs due to logistic reasons. The reporting physicians of the radiographs, before and after PCV-10 introduction periods, did not use WHO definitions; instead, the radiographs were read as lobar pneumonia and bronchopneumonia.
Immunization history for both the retrospective data, and even the quasi-retrospective data, was not card-verified.
The causative bacterial pathogens of the pneumonia were not isolated; and there was no way to exclude viral etiologies of some, or possibly many of the admission pneumonia cases. Although we excluded all the wheezing illnesses, there are reports of viral pneumonias without wheezes.
Finally, we could not categorize all hospitalizations per age group because the total admissions were not stratified into “3-11” and 12-24” months, which are what we required for the denominator to calculate the fractions. This problem is inherent in the study because the case record form (CRF) was not designed to capture the ages of the non-pneumonia admissions, except that of pneumonia admissions.
Conclusions
The introduction of the pneumococcal conjugate vaccine ten valent (PHiD-CV-10) into the National Immunization Program in August 2016 in Oyo and Gombe states, and the Federal Capital Territory, Abuja was associated with a reduction in pneumonia hospitalizations and mortality in children aged 3 to 24 months in Nigeria population studied. However, the vaccination coverages as well as magnitude of decline in hospitalization and deaths varied across study sites. Efforts should be made to improve and sustain the national vaccine coverage in Nigeria. Introduction of electronic clinical record system into the major hospitals in Nigeria should facilitate better data capture for future impact studies.
Authors contributions
The following contributions were made: conceptualization AGF, JI, DRS, BNT, WNO; data collection AIA, AOF, AR, SN, IWE, RM-N, PA, AAB; formal analysis BOY, OBO; funding acquisition AGF; investigation AGF, JI, DRS, BNT, WNO; methodology AGF, JI, DRS, BNT, WNO; writing – original draft AGF; writing – review/editing AGF, JI, DRS, BNT, WNO, BOY, AOF, AR, SN, IWE, RM-N, PA, OBO, AAB, BOY, OOA.
Supplemental Material
Download PDF (95 KB)Acknowledgments
GSK was provided the opportunity to review a preliminary version of this manuscript for factual accuracy, but the authors are solely responsible for final content and interpretation. We are grateful to the parents of all our participants in the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2162289.
Additional information
Funding
References
- Greenwood B, Anderson RM. The epidemiology of pneumococcal infections in children in the developing world. Phil Trans R Soc Lond B Biol Sci. 1999;354:777–9. doi:10.1098/rstb.1999.0430.
- O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009 Sep 12;374(9693):893–902. doi:10.1016/S0140-6736(09)61204-6. PMID: 19748398.
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, et al. Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012 Jun 9;379(9832):2151–61. doi:10.1016/S0140-6736(12)60560-1. Epub 2012 May 11. Erratum in: Lancet. 2012 Oct 13;380(9850):1308. PMID: 22579125.
- The state of the world’s children 2010: special Edition. [ accessed 2015 Nov 20]. https://www.unicef.org/reports/state-worlds-children-2010.
- Falade AG, Lagunju IA, Bakare RA, Odekanmi AA, Adegbola RA. Invasive pneumococcal disease in children aged < 5 years admitted to 3 urban hospitals in Ibadan, Nigeria. Clin Infect Dis. 2009;48(Supplement 2):S190–6. doi:10.1086/596500.
- WHO/IVB/12.08: measuring impact of Streptococcus pneumoniae and Haemophilus influenzae type b conjugate vaccination. 2012 Sep [accessed 2015 Nov 20]. http://apps.who.int/iris/bitstream/10665/75835/1/WHO_IVB_12.08_eng.pdf.
- FG introduces pneumococcal conjugate vaccine. [accessed 2015 Dec 14]. http://www.nursingworldnigeria.com/2014/12/fg-introduces-pneumococcal-conjugate-vaccine.
- The World Health Organization. Nigeria launches penta vaccine. [accessed 2016 Mar 12]. http://www.afro.who.int/en/nigeria/press-materials/item/4735-nigeria-launches-penta-vaccine.html.
- Obaro S, Lawson L, Essen U, Ibrahim K, Brooks K, Otuneye A, Shetima D, Ahmed P, Ajose T, Olugbile M, et al. Community-acquired bacteremia in young children from central Nigeria–a pilot study. BMC Infect Dis. 2019;11(1):137. doi:10.1186/1471-2334-11-137.
- World Health Organization Pneumonia Vaccine Trial Investigators Group. Standardisation of chest radiographs for the diagnosis of pneumonia in children. Geneva: World Health Organization; 2001.
- Cherian T, Mulholland EK, Carlin JB, Ostensen H, Amin R, de Campo M, Greenberg D, Lagos R, Lucero M, Madhi SA, et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ. 2005;83:353–59.
- Kaufman JS. Statistics, adjusted statistics, and maladjusted statistics. Am J Law Med. 2017 May;43(2–3):193–208. doi:10.1177/0098858817723659. PMID: 29254468.
- Oleribe OO, Udofia D, Oladipo O, Ishola TA, Taylor-Robinson SD. Healthcare workers’ industrial action in Nigeria: a cross-sectional survey of Nigerian physicians. Hum Resour Health. 2018;16:54. doi:10.1186/s12960-018-0322-8.
- Okoye OG, Olaomi OO, Gwaram UA, Apollo KD. The impact of COVID-19 lockdown on acute trauma patients seen at the National Hospital Trauma Centre Abuja, Nigeria. Pan Afr Med J. 2021;38(414). doi:10.11604/pamj.2021.38.414.28431.
- Aregbeshola BS, Khan SM. Out-of-Pocket payments, catastrophic health expenditure and poverty among households in Nigeria 2010. Int J Health Policy Manag. 2018 Sep 1;7(9):798–806. doi:10.15171/ijhpm.2018.19. PMID: 30316228; PMCID: PMC6186489.
- World Health Organization. The African health monitor: health financing in the African region. Congo, Brazzaville: World Health Organization Regional Office for Africa; 2013.
- Fagbamigbe AF, Bamgboye EA, Yusuf BO, Akinyemi JO, Issa BK, Ngige E, Amida P, Bashorun A, Abatta E. The Nigeria wealth distribution and health seeking behaviour: evidence from the 2012 national HIV/AIDS and reproductive health survey. Health Econ Rev. 2015 Feb 11;5:5. doi:10.1186/s13561-015-0043-9. PMID: 25853003; PMCID: PMC4384915.
- Sigurdsson S, Kristinsson KG, Erlendsdóttir H, Hrafnkelsson B, Haraldsson Á. Decreased incidence of respiratory infections in children after vaccination with ten-valent pneumococcal vaccine. Pediatr Infect Dis J. 2015;34:1385–90. doi:10.1097/INF.0000000000000899.
- Tagbo BN, Bancroft RE, Fajolu I, Abdulkadir MB, Bashir MF, Okunola P, Isiaka AH, Lawal NM, Edelu BO, Onyejiaka N, et al. Paediatric bacterial meningitis surveillance in Nigeria from 2010-2016; prior to and during the phased introduction of the 10-valent pneumococcal conjugate vaccine. Clin Infect Dis. 2019;69(Suppl. 2):S81–88. doi:10.1093/cid/ciz474.
- Immunization coverage estimates data visualization. Jul 2021 [accessed 2022 Mar 12]. https://unicef.shinyapps.io/wuenic-analytics-2021/.
- Modi RN, King C, Bar-Zeev N, Colbourn T. Caregiver recall in childhood vaccination surveys: systematic review of recall quality and use in low- and middle-income settings. Vaccine. 2018;36(29):4161–70. doi:10.1016/j.vaccine.2018.05.089.
- Imasuen E. Insurgency and humanitarian crises in Northern Nigeria: the case of Boko Haram AJPSIR. Afr J Polit Sci Int Relat. 2015;9(7):284–96. doi:10.5897/AJPSIR2015.0789.
