ABSTRACT
At the beginning of each flu season, the Italian Ministry of Health defines the categories at higher risk of influenza complications, for which vaccination is actively and freely offered. The vaccine coverage (VC) of the influenza vaccine in subjects from 6 months to 64 years of age suffering from diseases that increase the risk of complications from influenza during the 2020–2021 season was evaluated. Our study wants to evaluate the VCs of the influenza vaccine in these subjects during the 2020/2021 season in Apulia. The digital archives relative to the Apulian population were used. A retrospective cohort study design was performed. 484,636 Apulian residents aged between 6 months and 64 years suffered from at least one chronic disease; 139,222 of 484,636 subjects received the influenza vaccine (VC: 28.7%) from October 2020 to January 2021. Considering the single comorbidities, the greatest values are found for pathologies for which major surgical interventions are planned and chronic renal failure/adrenal insufficiency patients, while the worst for chronic liver diseases and pathologies for which major surgical interventions are planned. In any case, it would seem that better VC is achieved in subjects with more than one chronic condition. Influenza vaccination must be promoted as a central public health measure, also because by reducing the burden on hospitals, it can greatly benefit the management of COVID-19 patients. Greater efforts by public health institutions must be implemented in order to achieve better VC in the target categories, including chronic patients.
Introduction
Influenza viruses typically circulate each year in the northern hemisphere, most commonly from the late fall through the early spring. Most persons who become ill after influenza virus infection recover without serious complications or sequelae. However, influenza can be associated with serious illnesses, hospitalizations, and deaths, particularly among older adults, very young children, pregnant women, and persons of all ages with certain chronic medical conditions.Citation1 Routine annual influenza vaccination provides important protection from influenza illness and its potential complications; vaccination for all persons aged ≥6 months who do not have contraindications has been recommended by the Center for Disease Control and Prevention (CDC) and the Advisory Committee on Immunization Practices (ACIP) since 2010.Citation1
At the beginning of each flu season, the Italian Ministry of Health defines the categories at higher risk of influenza complications, according to international Public Health institution recommendations,Citation2 for which vaccination is actively and freely offered. For 2020/2021 influenza season, the target categories were subjects with age ≥65 years, pregnant women, subjects employed in public services of primary collective interest (including healthcare workers), workers who are in contact with animals that could be a source of non-human influenza virus infection, blood donors, and subjects from 6 months to 64 years of age suffering from diseases that increase the risk of complications from influenza.Citation3 Furthermore, the objectives of vaccine coverage (VC) are defined as follows: 75% as the minimum achievable goal and 95% as an optimal goal.Citation3
Influenza cases among people suffering from chronic diseases are a major issue from a Public Health point of view. A 2019 New Zealand studyCitation4 estimated that from 2012 to 2015 2435 influenza-associated severe acute respiratory illness hospitalizations occurred; hospitalization rates were significantly higher in those with chronic medical conditions compared with healthy one. The largest effects occurred with congestive heart failure, end-stage renal disease, and chronic obstructive pulmonary disease. CDC estimated that during 2019/20 influenza season in US among 6,399 flu-associated hospitalized adults, 92.6% had at least one reported underlying medical condition that placed them at high risk for influenza-associated complications.Citation5
Thus, in this study, we evaluated the VCs of the influenza vaccine in subjects from 6 months to 64 years of age suffering from diseases that increase the risk of complications from influenza during the 2020/2021 season. This topic is poor investigated in the literature; the Regional Office for Europe of the World Health Organization reported a VC of 33.8% in Italian residents with chronic diseases.Citation6 A 2019 Italian studyCitation7 investigated the knowledge and attitudes concerning influenza vaccination in a sample of 700 adults with chronic conditions; less than half of the sample (42.1%) received influenza vaccine in the last season, and 46.9% declared the will receive influenza vaccination in the next season.
Our study was carried out in Apulia (South Italy, around 4,000,000 inhabitants). In Apulia, before the 2020–2021 influenza season, 974,643 residents were vaccinated (VC: 26.0%); 415,583 doses were administered to 6 months-64 years old subjects (VC: 13.6%), and 559,060 to ≥65 years old residents (VC: 62.7%).
Material and methods
This is a retrospective cohort study.
Information on chronic diseases was checked using the Edotto platform of the Apulian Health Information System. Edotto, set up in 2012, allows the integration of various branches of the Italian healthcare system (Public Health Department, Regional Health Agency, healthcare companies, general practitioners, pharmacies, hospital physicians, etc.). From Edotto’s regional archive, the list of Apulian inhabitants aged from 6 months to 64 years was extracted; the reference period was from 1 October 2020 to January 31, 2021. High-risk patients were defined according to the findings of a 2018 Italian paper by Martinelli et al.Citation8 Those authors reviewed published recommendations on medical conditions for which vaccination against influenza was indicated and then they associated these medical conditions with the Italian user-fee exemption codes.Citation8 Finally, the dataset was decoded using the risk categories defined by Martinelli D et al., in order to identify patients with at least one chronic disease. Furthermore, 11 risk categories were identified (according to the Italian Ministry of HealthCitation3): chronic lung diseases, cardiopathies, diabetes mellitus, and other metabolic diseases, chronic renal failure/adrenal insufficiency, hematopathies and hemoglobinopathies, tumors, HIV and immunodepression, chronic inflammatory diseases and bowel malabsorption syndromes, pathologies for which major surgical interventions are planned, pathologies associated with an increased risk of aspiration of respiratory secretions, chronic liver diseases.
The overall vaccination status was assessed using the Regional Immunization Database (GIAVA). GIAVA is a computerized vaccination registry containing information on the vaccination history of every Apulian inhabitant;Citation9 it can also be used to generate an immunization schedule. Records of influenza vaccination between October 2020 and January 2021 were extracted and matched with the data obtained from Edotto using the patients’ unique PINs. The vaccine recommended during 2020/21 influenza season in Apulia were the quadrivalent inactivated influenza vaccine (Vaxigrip Tetra®, Sanofi Italia, Italy, Mian (MI), 20158) from 6 months to 74 years old, the quadrivalent inactivated cell-based influenza vaccine (Flucelvax® Tetra, Seqirus, Italy, Monteriggioni (SI), 53035) for healthcare workers ages <75 years old and the trivalent inactivated adjuvanted influenza vaccine (Fluad™, Seqirus, Italy, Monteriggioni (SI), 53035) for ≥75 years old subjects and for 60–74 years old frail patients, at the discretion of the medical opinion.Citation10
The final dataset was created as an Excel spreadsheet that included information on sex, age, influenza vaccine (YES/NO), vaccines formula, chronic disease (YES/NO) and risk categories. An anonymized data analysis was performed using the STATA MP17 software.
Continuous variables are presented as the median and interquartile range (IQR) and categorical variables as proportions.
The determinants of vaccination (YES/NO) were assessed using multivariate logistic regression model, considering sex (male vs. female), age (years), and number of comorbidities as determinants. The adjusted odds ratio (aOR) was calculated together with the 95%CI.
For all the tests, a two-sided p-value <.05 was considered to indicate statistical significance.
Results
From Edotto’s regional archive, 484,636 on 3,061,463 (15.8%) Apulian residents aged between 6 months and 64 years reported an exemption for at least one chronic disease. Of these, 271,025 (55.9%) were females and the median age was of 53 years (range IQR: 42–59). 392,650 (81.0%) subjects reported one comorbidity, 79,915 (16.5%) reported two comorbidities, 10,719 (2.2%) reported three comorbidities and 1,352 (0.3%) reported more than three comorbidities. describes the number of Apulian inhabitants aged between 6 months and 64 years and suffering from at least one chronic disease, per comorbidity.
Table 1. Frequency of Apulian inhabitants aged between 6 months and 64 years and suffering from at least one chronic disease, per comorbidity.
139,222 of 484,636 subjects received the influenza vaccine (VC: 28.7%); the quadrivalent inactivated influenza vaccine (qIIV) was administered to 118,202 (84.8%) subjects, the quadrivalent inactivated cell-based influenza vaccine (qIcbV) was administered to 10,925 (7.9%) subjects, and the trivalent inactivated adjuvanted influenza vaccine (tIaIV) was administer to 10,095 (7.3%) subjects.
VC increases as the number of chronic diseases increases, with 26.0% (n = 101,939) in subjects with one comorbidity, 39.4% (n = 31,463) in subjects with two comorbidities, 47.8% (n = 5,128) in subjects with three comorbidities and 51.2% (n = 692) in subjects with more than three comorbidities. The VCs reached per chronic disease are described in , and the characteristics of the vaccinated subjects according to their specific chronic condition are described in .
Figure 1. Vaccine coverage (%) of Apulian inhabitants aged between 6 months and 64 years and suffering from at least one chronic disease, per comorbidity.
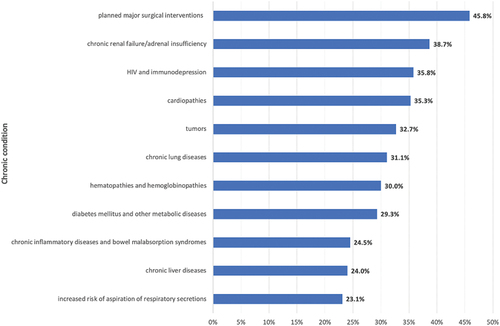
Table 2. Characteristic of vaccinated Apulian inhabitants aged between 6 months and 64 years, per comorbidity.
The results of the multivariate analysis are described in .
Table 3. The determinants of vaccination according to multivariate logistic regression model.
Discussion
Our study shows a vaccination coverage value of 29% in the study population, ranging from 23% to 46% depending on the underlying disease; these values are very far from the minimum achievable goal (75%), as defined by the Italian Ministry of Health.Citation3 According to the PASSI survey, the VC in n 18–64 years old Italian subjects with at least one chronic disease was 23.3% in the 2019/2020 influenza season.Citation11 Considering the individual comorbidities, the lack of vaccination in Italian hepatic subjects was investigated in a 2021 Italian study,Citation12 that interviewed 818 patients reporting a VC of 26.9% in subjects aged <65 years, confirming the value found in our study (28.2%). The authors concluded that more efforts are need to reach higher VC in this subgroup population. It must be considered that the progression of liver disease is associated with immune dysregulation and complications from common acute infections such as influenza cause significant morbidity and mortalityCitation13 and so vaccination in these subjects is a priority. Surprisingly, diabetes, cardiopathies, HIV and immunodepression, chronic inflammatory diseases and bowel malabsorption syndromes and chronic renal failure/adrenal insufficiency did not reach high values of VC. As reported by CDC, people with diabetes are at higher risk of developing serious influenza complications, which can result in hospitalization and death; in this subgroup population, influenza vaccination has been shown to reduce the risk of getting sick as well as reduce the risk of having a serious influenza outcome (hospitalization or intensive care unit).Citation14 Regarding cardiovascular diseases, a 2021 reviewCitation15 showed that influenza vaccine was associated with a lower risk of all-cause mortality (RR = 0.75; 95%CI = 0.60–0.93), cardiovascular mortality (RR = 0.82; 95%CI = 0.80–0.84) and major adverse cardiovascular events (RR = 0.87; 95%CI = 0.80–0.94). Epidemiological data have confirmed that immunocompromised patients are at high risk of influenza-associated complications. Even if the immunogenicity of the influenza vaccine is overall reduced in these subjects, the efficacy and safety of the vaccination in immunocompromised subjects is comparable to that of healthy subjects.Citation16 Even for chronic inflammatory diseases and bowel malabsorption syndromes, the vaccine is an indispensable tool to protect the patient from the complications of the influenza.Citation17 Finally, it must be considered that infections are the second most common cause of death in the chronic kidney disease population.Citation18
From the analyzed data it would seem that there is greater attention in vaccinating the subjects with more than one comorbidity, as also confirmed by the multivariate regression model. Moreover, the multivariate model also highlights how vaccination compliance increases with age, and male subjects seem to be associated with vaccination uptake.
In summary, the VC achieved in our sample is very far from the minimum achievable goal (75%); considering the single comorbidities, the greatest values are found for pathologies for which major surgical interventions are planned and chronic renal failure/adrenal insufficiency patients, while the worst for chronic liver diseases and pathologies for which major surgical interventions are planned. In any case, it would seem that better vaccination coverage is achieved in subjects with more than one chronic condition. Several reasons could explain the low value of VC; firstly, a main role is assigned to physicians, especially the General Practitioner and the branch specialists. Indeed, patients, especially chronic ones, identify these figures as a major source of trust; therefore, it is a duty to recommend the vaccine to patients in order to protect them from any serious complications of the flu. To ensure this, physicians should be adequately trained and up-to-date on seasonal flu vaccine recommendations, so that they can recommend it with good knowledge and conscience. Secondly, the lack of a culture of prevention is deeply rooted in Italy, and this leads to unconsidered choices by the patients themselves. Thirdly, the difficulty of government and public health institutions in proposing effective and non-confusing vaccine communication and promotion campaigns is an important factor in vaccine hesitancy.Citation19 Finally, the fear of adverse events following immunization, and the unjustified fear of a worsening of clinical conditions after the vaccine could be an explanation for bad attitude.
To our knowledge, no other studies are reported in the literature that investigate the topic in question with such a large sample and for such a wide range of diseases. An above cited 2021 studyCitation12 reported a VC of 26.9% in subjects aged <65 years affected by chronic hepatic diseases in 2018/19 influenza season. A 2020 Italian studyCitation20 investigated the coverage rate in four consecutive influenza seasons (from 2011/2012 to 2014/2015) in subjects affected by chronic heart disease; the levels of influenza vaccine uptake ranged from the 28.3% in 2011/2012 season and to 22.3% in the following year in the 15–64 years old age group. Finally, a 2021 reviewCitation21 estimated a VC of 53% in splenectomized patients, worldwide.
The strength of our study is to investigate a topic not much studied in the literature in a very large sample. Moreover, the study’s design allowed us to evaluate the cause–effect relationship between the vaccine uptake and many determinants. However, it should also be noted that the referenced archives were established for administrative and non-epidemiological purposes such that, at least theoretically, there was a risk of related bias. Moreover, considering that only subjects that obtained an active exemption were enrolled, the possibility that other individuals with chronic condition but without exemption were not included cannot be ignored. The sources of our data do not allow to perform further analyses, as the evaluation of vaccine effectiveness or the surveillance of adverse events after immunization in this population. Another limitation of the study was that the vaccination campaign of 2020/21 influenza season was developed in the context of the COVID pandemic; compared to previous flu seasons, higher coverage values were achieved, presumably due to the pandemic. Therefore, the results of our study must also be read in this light. Future studies should similarly evaluate the role of other vaccines (e.g. anti-pneumococcal vaccine) and should record the VC values during multiple influenza seasons.
The results of this study highlight that important immunization strategies in this subset of patients need to be implemented to achieve better vaccination coverage. First of all, it must be specified that in Italy the immunization of the population enrolled is entrusted to General Practitioners; a proposal could be to support them with specialists from various medical branches, as well as vaccinologists. A more important role should be guaranteed by the Department of Public Health, which could provide facilities and health personnel to immunize some target categories; for example, it could organize open days inviting the population at risk, or evaluate on-site vaccination teams in target facilities (e.g. school, commercial facilities); indeed, on-site vaccination has proved to be an effective strategy to immunize some population subgroups.Citation22 Nosocomial structures should also be involved in immunization strategies; in fact, as already experimented with the vaccination campaign against COVID19, hospital wards could call their chronic patients for ad hoc vaccination sessions. Also in this case, the active offer of vaccination to chronic patients has proved to be a winning strategy.Citation23
Influenza vaccination must be promoted as an important public health measure during the coronavirus pandemic, given that, as noted by Paget J and Caini S,Citation24 by preventing influenza infections it can both simplify the differential diagnosis of COVID-19 and free up healthcare facilities to deal with these patients. Indeed, one of the major issues of the COVID-19 pandemic has been the overwhelming of healthcare systems;Citation25 it may also contain the health effects and social consequences of the COVID-19 pandemic.Citation26,Citation27
In conclusion, greater efforts by public health institutions must be implemented in order to achieve better vaccination coverage in the target categories, including chronic patients. The organization implemented during the COVID-19 vaccination campaign could be taken as an example to ensure quick access to the influenza vaccine for a large part of the population. Influenza vaccine safety and efficacy dataCitation28–30 require greater efforts to immunize a category of patients at high risk for influenza complications.
Informed consent statement
As this study constituted public health surveillance, ethical approval from the institutional review board was not required. All data were provided and analyzed anonymously.
Abbreviations
| WHO | = | World Health Organization |
| CDC | = | Center for Disease Control and Prevention |
| GIAVA | = | Regional Immunization Database |
| Edotto | = | Apulian Health Information System |
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Grohskopf LA, Alyanak E, Broder KR, Blanton LH, Fry AM, Jernigan DB, Atmar RL. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2020-21 influenza season. MMWR Recomm Rep. 2020 Aug 21;69(8):1–6. doi:10.15585/mmwr.rr6908a1.
- CDC. People at higher risk of flu complications. [accessed 2021 Oct 13]. https://www.cdc.gov/flu/highrisk/index.htm.
- Italian Ministry of Health. Influenza prevention and control: recommendations for the 2020-2021 season. [accessed 2021 Oct 20]. https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2020&codLeg=74451&parte=1%20&serie=null.
- Walker TA, Waite B, Thompson MG, McArthur C, Wong C, Baker MG, Wood T, Haubrock J, Roberts S, Gross DK, et al. Risk of severe influenza among adults with chronic medical conditions. J Infect Dis. 2020 Jan 2;221(2):183–90. doi:10.1093/infdis/jiz570.
- Xu X, Blanton L, Elal AIA, Alabi N, Barnes J, Biggerstaff M, Brammer L, Budd AP, Burns E, Cummings CN, et al. Update: influenza activity in the United States during the 2018-19 season and composition of the 2019-20 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2019 Jun 21;68(24):544–51. doi:10.15585/mmwr.mm6824a3.
- WHO regional office for Europe. Influenza vaccination coverage, chronic diseases. [accessed 2021 Oct 21]. https://gateway.euro.who.int/en/indicators/infl_14-influenza-vaccination-coverage-chronic-diseases/.
- Bertoldo G, Pesce A, Pepe A, Pelullo CP, Di Giuseppe G, Manzoli L, Collaborative Working Group. Seasonal influenza: knowledge, attitude and vaccine uptake among adults with chronic conditions in Italy. PLoS One. 2019 May 1;14(5):e0215978. doi:10.1371/journal.pone.0215978.
- Martinelli D, Fortunato F, Iannazzo S, Cappelli MG, Prato R. Using routine data sources to feed an immunization information system for high-risk patients-a pilot study. Front Public Health. 2018 Feb 16;6:37. doi:10.3389/fpubh.2018.00037.
- Pedote PD, Termite S, Gigliobianco A, Lopalco PL, Bianchi FP. Influenza vaccination and health outcomes in COVID-19 patients: a retrospective cohort study. Vaccin Basel. 2021 Apr 8;9(4):358. doi:10.3390/vaccines9040358.
- Apulia Region. Influenza vaccination campaign in the apulia region for the 2020-2021 season. [accessed 2022 Dec 2]. https://vaccinarsinpuglia.org/assets/uploads/files/22/2020-08-20-circolare-campagna-vaccinazione-antinfluenzal.pdf.
- Epicentro. Influenza vaccine, Italian data. [accessed 2021 Oct 23]. https://www.epicentro.iss.it/passi/dati/VaccinazioneAntinfluenzale.
- Stroffolini T, Lombardi A, Ciancio A, Niro GA, Colloredo G, Marignani M, Vinci M, Morisco F, Babudieri S, Ferrigno L, et al. Low influenza vaccination coverage in subjects with liver cirrhosis. An alert waiting for winter season 2020-2021 during the COVID-19 pandemic. J Med Virol. 2021 Apr;93(4):2446–52. doi:10.1002/jmv.26763.
- Härmälä S, Parisinos CA, Shallcross L, O’Brien A, Hayward A. Effectiveness of influenza vaccines in adults with chronic liver disease: a systematic review and meta-analysis. BMJ Open. 2019 Sep 6;9(9):e031070. doi:10.1136/bmjopen-2019-031070.
- CDC. Flu & people with diabetes. [accessed 2021 Oct 22]. https://www.cdc.gov/flu/highrisk/diabetes.htm.
- Yedlapati SH, Khan SU, Talluri S, Lone AN, Khan MZ, Khan MS, Navar AM, Gulati M, Johnson H, Baum S, et al. Effects of influenza vaccine on mortality and cardiovascular outcomes in patients with cardiovascular disease: a systematic review and meta-analysis. J Am Heart Assoc. 2021;10(6):e019636. doi:10.1161/JAHA.120.019636.
- Zbinden D, Manuel O. Influenza vaccination in immunocompromised patients: efficacy and safety. Immunotherapy. 2014;6(2):131–39. doi:10.2217/imt.13.171.
- Chan W, Salazar E, Lim TG, Ong WC, Shim HH. Vaccinations and inflammatory bowel disease - a systematic review. Dig Liver Dis. 2021 Sep;53(9):1079–88. doi:10.1016/j.dld.2021.04.015.
- Reddy S, Chitturi C, Yee J. Vaccination in chronic kidney disease. Adv Chronic Kidney Dis. 2019 Jan;26(1):72–78. doi:10.1053/j.ackd.2018.10.002.
- Levi M, Sinisgalli E, Lorini C, Santomauro F, Chellini M, Bonanni P. The “fluad case” in Italy: could it have been dealt differently? Hum Vaccin Immunother. 2017 Feb;13(2):379–84. doi:10.1080/21645515.2017.1264738.
- Stefanati A, Lupi S, Campo G, Cocchio S, Furlan P, Baldo V, Gabutti G. Influenza coverage rates in subjects with chronic heart diseases: results obtained in four consecutive immunisation seasons in the local health unit of ferrara (North Italy). Arch Public Health. 2020 Oct 16;78:103.
- Bianchi FP, Stefanizzi P, Spinelli G, Mascipinto S, Tafuri S. Immunization coverage among asplenic patients and strategies to increase vaccination compliance: a systematic review and meta-analysis. Expert Rev Vaccin. 2021 Mar;20(3):297–308. doi:10.1080/14760584.2021.1886085.
- Bianchi FP, Tafuri S, Spinelli G, Carlucci M, Migliore G, Calabrese G, Daleno A, Melpignano L, Vimercati L, Stefanizzi P. Two years of on-site influenza vaccination strategy in an Italian university hospital: main results and lessons learned. Hum Vaccin Immunother. 2021 Nov;18(1):1–6. doi:10.1080/21645515.2021.1993039.
- Bianchi FP, Rizzo LA, De Nitto S, Stefanizzi P, Tafuri S. Influenza vaccination coverage among splenectomized patients: an Italian study on the role of active recall in the vaccination compliance. Hum Vaccin Immunother. 2019;15(11):2644–49. doi:10.1080/21645515.2019.1599678.
- Paget J, Caini S, Cowling B, Esposito S, Falsey AR, Gentile A, Kyncl J, MacIntyre C, Pitman R, Lina B. The impact of influenza vaccination on the COVID-19 pandemic? evidence and lessons for public health policies. Vaccin. 2020 Sep 29;38(42):6485–86. doi:10.1016/j.vaccine.2020.08.024.
- Armocida B, Formenti B, Ussai S, Palestra F, Missoni E. The Italian health system and the COVID-19 challenge. Lancet Public Health. 2020 May;5(5):e253. doi:10.1016/S2468-2667(20)30074-8.
- Cocco P, Meloni F, Coratza A, Schirru D, Campagna M, De Matteis S. Vaccination against seasonal influenza and socio-economic and environmental factors as determinants of the geographic variation of COVID-19 incidence and mortality in the Italian elderly. Prev Med. 2021 Feb;143:106351. doi:10.1016/j.ypmed.2020.106351.
- Boersma C, Postma MJ. Health economics of vaccines: from current practice to future perspectives. Value Health. 2021 Jan;24(1):1–2. doi:10.1016/j.jval.2020.11.006.
- Stefanizzi P, De Nitto S, Spinelli G, Lattanzio S, Stella P, Ancona D, Dell’Aera M, Padovano M, Soldano S, Tafuri S, et al. Post-marketing active surveillance of adverse reactions following influenza cell-based quadrivalent vaccine: an italian prospective observational study. Vaccin Basel. 2021 May 4;9(5):456. doi:10.3390/vaccines9050456.
- Dhakal S, Klein SL, Coyne CB. Host factors impact vaccine efficacy: implications for seasonal and universal influenza vaccine programs. J Virol. 2019 Oct 15;93(21):e00797–19. doi:10.1128/JVI.00797-19.
- Trombetta CM, Gianchecchi E, Montomoli E. Influenza vaccines: evaluation of the safety profile. Hum Vaccin Immunother. 2018 Mar 4;14(3):657–70. doi:10.1080/21645515.2017.1423153.
