?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Limited data are available on the effectiveness of COVID-19 vaccines used in China in real-world outbreaks – especially against Omicron variants in vaccinated individuals. Two outbreaks of SARS-CoV-2 Omicron variants – the first involving the sub-lineage BA.2 and the second the BA.1 variant – occurred in Quzhou. Infected people and their close contacts were divided according to vaccination status: unvaccinated, partially vaccinated, fully vaccinated, and boosted. The Cox proportional-hazard regression model was used to estimate the evolving hazard for vaccinated individuals after their first immunization. 138 people had been infected with the SARS-CoV-2 Omicron BA.2 variant and 13 with the BA.1 variant. Of the 151 infections, 99.34% (150/151) were mild or asymptomatic and 90.07% (136/151) were vaccine breakthrough cases. The total vaccine effectiveness (VE) of partial, full, and booster vaccinations during the two outbreaks was 47.4% (95%CI: 0–93.1%), 28.9% (95%CI: 0–60.2%), and 27.5% (95%CI: 0–58.3%). The VE of booster vaccination against the Omicron BA.1 variant was higher than that for the BA.2 variant. The cumulative hazard began to increase 220 days after the first immunization. The transmissibility of the Omicron BA.2 variant as for BA.1 did not increase in vaccinated individuals; booster vaccination after a primary course substantially increased protection. Our study found that the SARS-CoV-2 Omicron variant caused less severe illness and that the VE of boosters against the Omicron variant was less than 30%. Timely administration of the booster dose was important, especially for individuals aged over 80 years old.
Introduction
Since the onset of the 2019 novel coronavirus disease (COVID-19) pandemic, variants of concern (VOCs) have emerged repeatedly. The fifth VOC was designated Omicron by the WHO.Citation1 From October 2021 to the beginning of 2022, two genetically divergent subvariants of Omicron, BA.1 and BA.2, emerged and rapidly became the dominant subvariants in several countries, including China.Citation2 BA.2 differs from BA.1 in its genetic sequence – resulting in amino acid differences in the spike protein and other proteins. Studies have shown that BA.2 has a growth advantage over BA.1 and appears inherently more transmissible than BA.1.Citation3 According to the Chinese Government’s COVID-19 prevention and control policy of zero tolerance for local transmission, all infections are identified in a timely manner and reported by local health departments for contact tracing, isolated treatment, quarantine of close contacts, and testing for acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA. Additional COVID-19 prevention requirements are stringent physical distancing measures, immunization with a COVID-19 vaccine, and the use of personal protective measures.
In China, 3.44 billion doses of COVID-19 vaccines have been administered as of 12 October 2022, approximate 90% of which are inactivated COVID-19 vaccines.Citation4 COVID-19 vaccination started on 1 October 2020 in Quzhou and went through four stages. The first stage was emergency vaccination for medical personnel, which took place from October 1 to 15 December 2020. The second stage was extended vaccination for public service personnel, which occurred from 15 December 2020 to 14 March 2021. The third stage started on 15 March 2021 for residents over 18 years old and on 1 August 2021 for children of 3 to 17 years old. The fourth stage, from October 2021, was booster vaccination for residents over 18 years old. Children aged 3 to 17 years are not eligible for booster immunization. Three types of COVID-19 vaccine were used in China: an inactivated COVID-19 vaccine (vero cell); a recombinant subunit COVID-19 vaccine (CHO cell); and an adenovirus vector COVID-19 vaccine (Ad5). There is no approved COVID-19 vaccine for children under 3 years of age. As of October 2022, the coverage of COVID-19 vaccination among the population aged over 3 years old in Quzhou is 98.6% and the coverage of booster vaccination among residents over 18 years old is 96.1%. The three vaccines were confirmed to be effective against the COVID-19 Delta variant in the short term following the vaccination campaign and most effective at preventing serious illness.Citation5
Protection against variants and the continued protection by COVID-19 vaccines need to be constantly evaluated using real-world studies. Under high vaccination coverage and strict control measures, Quzhou maintained a low circulation of COVID-19 until March 2022. As a result of a major community epidemic of SARS-CoV-2 Omicron BA.2 in neighboring provinces, from March 1 to 30 April 2022, two COVID-19 outbreaks of variants BA.2 and BA.1, respectively, occurred in Quzhou. These outbreaks presented a valuable opportunity to measure the effectiveness of vaccines used in China in a real-world, high-risk environment.
Methods
Study setting
Quzhou is a prefecture-level city located in the Zhejiang province in eastern China and at the edge of Fujian, Jiangxi, and Anhui provinces, which means it enjoys convenient transportation and plays an important role in the rapid spread of infectious diseases. It covers an area of 8,844 km2 and the residents number about 2.28 million. In order to control COVID-19 earlier, Zhejiang province developed an electronic information system in January 2022, the Precision Intelligent Control System (PICS), which demanded that all local COVID-19 infections and close contacts, and close contacts of close contacts, should be registered in the system. Other relevant information, including nucleic acid sampling detection, vaccination information, transport isolation, and so on, needed to be fed into this system.
Data source
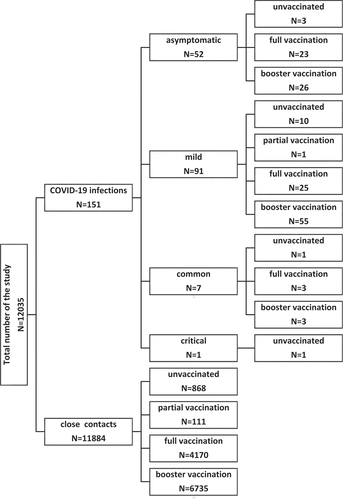
There were two COVID-19 outbreaks in Quzhou between March 1 and 30 April 2022.Citation6 One occurred between March 6 and March 24 and infected people with the SARS-CoV-2 Omicron BA.2 variant. The other outbreak occurred between April 25 and April 30 and infected people with the SARS-CoV-2 Omicron BA.1 variant. Infection in exposed people was detected through real time polymerase chain reaction (RT-PCR) tests using nasopharyngeal swab samples.Citation7 Those who had tests that were positive for nucleic acid had to be transferred to a designated hospital. Those who had negative test results had to isolate for medical observation for 14 days from last contact.Citation8 According to the Diagnosis and Treatment Protocol for COVID-19 (Trial Version 9), doctors in each designated hospital divided positive infections into five types:Citation9 (1) Asymptomatic: only the result of RT-PCR test was positive and no clinical symptoms. (2) Mild: the clinical symptoms were mild, and the imaging findings were not pneumonia. (3) Common: With clinical symptoms, and the imaging findings can be seen pneumonia manifestations. (4) Severe: for adult cases, severe illness must meet any of the following criteria: (a) respiratory distress (respiration rate [RR] ≥ 30 breaths per minute (BPM)), (b) oxygen saturation ≤93% at rest, (c) arterial partial pressure of oxygen/fraction of inspired oxygen ≤300 mmHg, and (d) chest imaging shows obvious lesion progression within 24–48 h > 50%. For child cases, severe illness must meet any of the following criteria: (a) persistent high fever over 3 days; (b) tachypnea (RR ≥ 60 BPM for infants aged below 2 months; RR ≥ 50 BPM for infants aged 2–12 months; RR ≥ 40 BPM for children aged 1–5 years, and RR ≥ 30 BPM for children >5 years), independent of fever and crying; (c)oxygen saturation ≤93% on finger pulse oximeter taken at rest; (d) labored breathing (moaning, nasal fluttering, and infrasternal, supraclavicular, and intercostal retraction), cyanosis, and intermittent apnea; (e)lethargy and convulsion; and (f) difficulty feeding and signs of dehydration. (5)critical: critical illness must meet any of the following criteria: (a) respiratory failure and requiring mechanical ventilation, (b) shock, and (c) other organ failures that require intensive care unit (ICU) care. Our study analyzed infected people and their close contacts in the two outbreaks. Data on the two outbreaks were extracted from the PICS on 30 June 2022. The Zhejiang Provincial Immunization Information System (ZJIIS) recorded all information about COVID-19 vaccination, including vaccination date, vaccine type, vaccine manufacturer, vaccination dose, vaccination site, and immunization clinic. This system was linked to the PICS; vaccination information, matched cases, and close contacts information were extracted on 30 June 2022 ().
Vaccination status
According to the Technical Vaccination Recommendations for COVID-19 Vaccines in China (First Edition), vaccination status was divided into four types:Citation10 The first type is unvaccinated with no history of COVID-19 vaccination before the last SARS-CoV-2 exposure date. The second type is partial vaccinated where have one dose of adenovirus vector vaccine administered but less than 14 days before the last SARS-CoV-2 exposure date; only received one dose of inactivated vaccine, or two doses of inactivated vaccine administered but less than 14 days before the last SARS-CoV-2 exposure date; received two doses of recombinant protein (CHO cell) vaccine or three doses of recombinant protein (CHO cell) vaccine but less than 14 days before the last SARS-CoV-2 exposure date. The third type is fully vaccinated with one dose of adenovirus vector vaccine or two doses of inactivated vaccine or three doses of recombinant protein (CHO cell) vaccine completed more than 14 days before the last SARS-CoV-2 exposure date, but not boosted; two doses of adenovirus vector vaccine administered less than 7 days before the last SARS-CoV-2 exposure date; two doses of inactivated vaccine administered and boosted with one dose of inactivated vaccine or adenovirus vector vaccine or recombinant protein (CHO cell) vaccine less than 7 days before the last SARS-CoV-2 exposure date. The fourth type is booster vaccinated with having received two doses of adenovirus vector vaccine more than 7 days before the last SARS-CoV-2 exposure date; had two doses of inactivated vaccine and was boosted with one dose of inactivated vaccine or adenovirus vector vaccine or recombinant protein (CHO cell) vaccine more than 7 days before the last SARS-CoV-2 exposure date (). In our study, inactivated vaccine included the COVID-19 vaccines produced by Sinovac, Sinopharm CNBG Beijing and Wuhan; recombinant protein (CHO cell) vaccine referred to the COVID-19 vaccine produced by Anhui Zhifei Longcom Biopharmaceutical Institute of Microbiology; and adenovirus vector vaccine referred to Cansino Ad5-nCoV-S COVID-19 vaccine.
Table 1. The definition of vaccination status according to the technical vaccination recommendations for COVID-19 vaccines in China (First Edition).
Statistical analysis
Exposed persons in the outbreak included people infected and close contacts. The attack rate (AR) was equal to the number of infections divided by the number of exposed persons.Citation11 The relative risk (RR) was equal to the AR of the analysis group divided by the AR of the reference group. A histogram and a forest plot were used to summarize the infections’ distribution and RR with 95% confidence intervals (CI) in COVID-19 outbreaks of SARS-CoV-2 Omicron variant, respectively.
When estimating the effectiveness of partial vaccination, people who had received full vaccination or a booster vaccination were excluded from the population vaccinated. Similarly, people who had received a booster vaccination were excluded from calculations that estimated the effectiveness of full vaccination.Citation12 Days after immunization included the first injection date to the diagnosis date for infections and the first injection date to the confirmation date for close contacts. Hazard ratios (HR) with 95% CI and cumulative hazard curves were estimated using Cox proportional-hazard regression analysis. Analyses were performed with statistics software IBM SPSS 16.0 (SPSS Inc., Chicago, Illinois, USA) and GraphPad Prism 5 statistical packages at a significance level of 0.05.
Results
Outbreak description
Two COVID-19 outbreaks occurred in Quzhou between March 1 and 30 April 2022. A total of 151 infections occurred with 138 infections with the SARS-CoV-2 Omicron BA.2 variant were reported between March 6 and March 24, and 13 infections with the SARS-CoV-2 Omicron BA.1 variant were reported between April 25 and April 30. These 151 infections were: 52 asymptomatic; 91 mild; 7 common; and 1 critical. Of all the infections, 9.9% (15/151) were unvaccinated. The level of partial, full, and booster vaccination was 0.7% (1/151), 33.8% (51/151), and 55.6% (84/151), respectively. There were 12,035 exposed persons, resulting in a total attack rate (AR) for the two outbreaks of 1.25%. There was no statistically difference in relative risk (RR) in terms of gender (p = .27) and virus type (p = .49). Compared with the unvaccinated group, there was no statistically significant difference between partial, full, and booster vaccinations when it came to the RR, but intergroup differences need further analysis. There were significant differences in the AR among age groups. There was no difference in the RR in other age groups when compared with under-3 group ().
Table 2. Vaccine effectiveness for partial, full, and booster immunization with COVID-19 vaccines by age group and virus type during two outbreaks in Quzhou, China.
Vaccine effectiveness
As shown in , the total VE of partial, full, and booster vaccination during the two outbreaks was 47.4% (95%CI: 0–93.1%), 28.9% (95%CI: 0–60.2%), and 27.5% (95%CI: 0–58.3%). Compared with the unvaccinated group, the VE of partial vaccination among all age groups, full and booster vaccination in the under-40 group, and full vaccination in the over-80 group were not significant in the two outbreaks. The VE of full vaccination in the groups aged 40 to 59 years and 60 to 79 years was 73.7% (95%CI: 20.9%−91.2%) and 45.1% (95%CI: 0–89.1%). The VE of booster vaccination in the groups aged 40 to 59 years, 60 to 79 years, and over 80 was 47.2% (95%CI: 0–77.5%), 59.3% (95%CI: 0–88.7%) and 48.4% (95%CI: 0–96.9%). The VE of booster vaccination against the SARS-CoV-2 Omicron BA.1 variant was 60.0% (95%CI: 0–92.4%), which was higher than that of the SARS-CoV-2 Omicron BA.2 variant value, but the increase in the value was no statistically significant (p > .05). With regard to the SARS-CoV-2 Omicron BA.2 variant, there was no significant difference in VE among partial vaccination, full vaccination and booster vaccination. According to the COVID clinical patterns, 94.23% (49/52) of the asymptomatic infections, 87.91% (80/91) of the mild cases and 85.71 (6/7) of the common cases were full or booster vaccination, critical case was only one and had no history of COVID-19 vaccination ().
Cumulative hazard
shows the results of Cox proportional-hazard regression analysis adjusted for the covariates, which suggests that vaccination status and age group had a significant effect on infection with the SARS-CoV-2 Omicron variant after first immunization, even after adjusting for potential confounding effects in the study. The virus types, BA.1 and BA.2 had no statistical significance (p = .07). The cumulative hazard of infection with the SARS-CoV-2 Omicron variant was the same 220 days after for either vaccination status or age group immunization (). The risk of catching the disease was highest when a person was only partially vaccinated, followed by full vaccination and then booster vaccination, which had the lowest risk. Among age groups, the risk for those between 3 and 17 years was highest, followed by those over 80 years. The levels of risk were slightly different for the other three age groups ().
Figure 2. Cox proportional-hazard regression curves among immune population in COVID-19 outbreaks of SARS-CoV-2 Omicron variant in Quzhou, China.
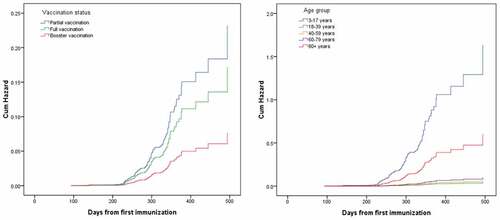
Table 3. The relative risk and results of Cox proportional-hazard regression model among immune population in COVID-19 outbreaks of SARS-CoV-2 Omicron variant in Quzhou, China.
Discussion
The two COVID-19 outbreaks in our study were detected as being of the SARS-CoV-2 Omicron variant. There were indications that these variants were more transmissible, because their missense mutations made them better at evading neutralizing antibodies and innate immunity than the wild-type virus.Citation13 Quzhou had no COVID-19 outbreaks before 1 March 2022. After detecting the COVID-19 outbreaks, several rounds of SARS-CoV-2 tests, each involving more than 2.28 million people and isolation measures were conducted, the outbreaks were quickly stopped, only 151 infections were reported. Most of the infections were mild or asymptomatic, only one critical COVID-19 case was reported. But in January 2020, 26% of the 138 hospitalized patients with confirmed COVID-19 were transferred to the intensive care unit (ICU) because of complications, and mortality was 4.3% in Wuhan, China.Citation14 Evidence suggested that Omicron caused less severe disease,Citation15 this was probably associated with lower virulence, infection-acquired immunity, and higher vaccination coverage.Citation16 Because of high population-wide COVID-19 vaccination coverage during the outbreaks, 90% of the cases were breakthrough cases, which has aroused concerns about the effectiveness of current vaccines against the SARS-CoV-2 Omicron variant.Citation17
There was little difference in the AR in terms of gender between the two outbreaks, which was similar to the finding of a Shanghai, China study.Citation18 BA.1 and BA.2 was seen for the Quzhou outbreaks, consistent with other studies.Citation19 Our study found that COVID-19 vaccines provided at least some protection against Omicron variants. The VE against the Omicron variant for the fully vaccinated was similar to that for those who had the booster. Both had a VE of less than 30%, but the VE for the BA.1 variant was higher than that for BA.2. The study in Shanghai showed that booster vaccination with inactivated vaccines achieved 16.3% (95% CI: 15.4%−17.2%) effective against SARS-CoV-2 Omicron BA.2 variant infection.Citation18 A Danish study suggested that Omicron BA.2 had greater infectivity than BA.1, which caused it to rapidly replace BA.1 as the dominant subvariant in several countries, and accelerated immune evasiveness, reducing the effect of vaccination.Citation20 The VE of full vaccination in the 40–59 age group was 73.7% (95%CI: 20.9%−91.2%), which was higher than that in 60–79 age group. Both groups had higher VE than the results from a two-dose schedule, 28 days apart, of Sinovac’s inactivated COVID-19 vaccine administered in children of 3–5 years of age in Chile during the Omicron outbreak, which was 38.2% (95% CI: 36.5%−39.9%).Citation21 Conversely, the VE of booster vaccination in the 60–79 age group was higher than that in the VE value of the 40–59 age group, and also higher than that of full vaccination in the same age group. Two doses of inactivated COVID-19 vaccine with 28 days between doses in Chile showed that the adjusted vaccine effectiveness in the subgroup of fully immunized persons 60 years of age or older was 66.6% (95% CI: 65.4%−67.8%).Citation22 The VE of partial vaccination was 47.4% (95%CI: 0–93.1%), which was apparently higher than that of full and booster vaccination, it may be related to the fact that there were too few people in the study who had been partially vaccinated.
Since 90% of all infections were breakthrough infections with the SARS-CoV-2 Omicron variant in the two outbreaks, it is necessary to research the factors influencing breakthrough infections. Our results, using Cox proportional-hazard regression analysis, suggested that the cumulative hazard of infection with the SARS-CoV-2 Omicron variant began to increase 220 days after first immunization. This observation is consistent with the results of the clinical trials, which demonstrated that neutralizing antibody titers decline substantially 6 months after two doses of inactivated COVID-19 vaccines (vero cell).Citation23 A study from the United Kingdom also showed that 25 weeks or more after two doses, VE was 14.8% (95% CI: 12.9%−16.7%) against BA.1 and 27.8% (95% CI: 25.9%−29.7%) against BA.2.Citation24 After adjusting for potential confounding effects that the interaction among gender, vaccination status, virus type and age group in the outbreaks, the transmissibility of either the Omicron BA.1 or BA.2 variant in vaccinated individuals was found not to increase. The risk was lowest in the booster vaccination group. The risk in the 3–17 year-old group was the highest, likely because there was no booster immunization for children aged 3 to 17 years. Receiving a third dose in a timely fashion resulted in a remarkable increase in the concentration of antibodies.Citation25 Primary immunization with two doses of ChAdOx1 nCoV-19 (Vaxzevria [AstraZeneca]) or the BNT162b2 (Comirnaty, [Pfizer-BioNTech]) vaccine provided limited protection against symptomatic disease caused by the Omicron variant.Citation16 The VE of booster immunization after the primary course increased to 70%, but the protection waned over time.Citation26 A study in Israel showed that a third dose for adults aged over 60 years who had received two doses of the BNT162b2 messenger RNA vaccine (Pfizer-BioNTech) at least 5 months earlier, the rate of confirmed COVID-19 infection and severe illness were substantially lower by a factor of 11.3% (95%CI: 10.4%−12.3%) and 19.5% (95%CI: 12.9%−29.5%).Citation27 Our study also showed that vaccinated individuals aged over 80 years were at high risk, the hazard ratio was 0.37 times than that of the 3–17 year-old group and was much higher than the other three groups. A study in Hong Kong found vaccine effectiveness among two-dose inactivated COVID-19 vaccine recipients waned over time, particularly in those aged 80 years or over.Citation28 The waning of immunity in the elderly may be related to the aging of the body. Increasing the uptake of a third vaccine doses will be important, particularly in older adults, to achieve the VE of 48.4% (95% CI: 0–96.9%). Therefore, the booster dose of COVID-19 vaccines should be employed as soon as possible, especially for individuals aged over 80 years old, to reduce the risk of infection with COVID-19.
There were several limitations in our study. First, due to the timely detection of the BA.1 outbreak, the number of infections was very small, making the sample size too small to evaluate VE among subgroups, such as partial and full vaccination. Second, although a variety of COVID-19 vaccines were widely available in Quzhou, the vast majority were inactivated vaccines, making it impossible to compare the VE of different types of vaccines used in China. Third, one of the criteria for determining close contacts in China was for field investigators to assess other individuals who met the criteria for determining close contacts, and the number of exposed persons in the outbreaks may be either underestimated or overestimated.
In conclusion, our study found that the SARS-CoV-2 Omicron variants caused less severe illness compare to the Delta variant of Wuhan, China. The VE of booster vaccination against the Omicron variant was less than 30%, but it was higher for variant BA.1 than for BA.2. Infection risk began to increase 220 days after first immunization. Timely administration of the booster dose was important, especially for individuals aged over 80 years old.
Authors’ contributions
Zhiying Yin designed and wrote the study. Quanjun Fang, Tingcui Wen, Canjie Zheng, and Canya Fu organized and analyzed the data. Shuangqing Wang, Junji Li, and Xiaoying Gong contributed to obtaining the data.
Ethical considerations
Personal data were anonymized by deleting personal identifiers (such as name, address, and telephone number) and determined as exempt from ethical review by the ethics committee of the Quzhou Center for Disease Control and Prevention (QZCDC).
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author on reasonable request .
Additional information
Funding
References
- Viana R, Moyo S, Amoako DG, Tegally H, Scheepers C, Althaus CL, Anyaneji UJ, Bester PA, Boni MF, Chand M, et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022;603:(7902):679–8. doi:10.1038/s41586-022-04411-y. Epub 2022 Jan 7.
- Tian D, Nie W, Sun Y, Ye Q. The epidemiological features of the SARS-CoV-2 Omicron subvariant BA.5 and its evasion of the neutralizing activity of vaccination and prior infection. Vaccines. 2022;10(10):1699. doi:10.3390/vaccines10101699.
- World Health Organization. Statement on Omicron sublineage BA.2 [accessed 2022 Oct 12]. https://www.who.int/news/item/22-02-2022-statement-on-omicron-sublineage-ba.2.
- China Food and Drug Administration. COVID-19 vaccination status (as of October 12) [accessed 2022 Oct 13]. http://www.gov.cn/xinwen/2022-10/13/content_5718121.htm.
- Ma C, Sun W, Tang T, Jia M, Liu Y, Wan Y, Han J, Rodewald L, Li J, Song Y, et al. Effectiveness of adenovirus type 5 vectored and inactivated COVID-19 vaccines against symptomatic COVID-19, COVID-19 pneumonia, and severe COVID-19 caused by the B.1.617.2 (Delta) variant: evidence from an outbreak in Yunnan, China, 2021. Vaccine. 2022;40:(20):2869–74. doi:10.1016/j.vaccine.2022.03.067.
- Huang Y, Zheng ZW, Chen C, Li K, Chen SY, Chen YY, Jing QL, Ma Y, Luo L, Yang ZC, et al. Epidemiological characteristics of two local COVID-19 outbreaks caused by 2019-nCov Omicron variant in Guangzhou, China. Zhonghua Liu Xing Bing Xue Za Zhi. 2022;43:1705–10. doi:10.3760/cma.j.cn112338-20220523-00450.
- Song Y, Chen C, Wang Y, Zhang J, Chen M, Gao G, Wang S, Yang D, Song R, Wang L, et al. Early and consecutive RT-PCR tests with both oropharyngeal swabs and sputum could improve testing yield for patients with COVID-19: an observation cohort study in China. Int J Infect Dis. 2021;107:242–46. doi:10.1016/j.ijid.2021.04.076.
- Liu F, Zheng C, Wang L, Geng M, Chen H, Zhou S, Ran L, Li Z, Zhang Y, Feng Z, et al. Interpretation of the Protocol for Prevention and Control of COVID-19 in China (Edition 8). China CDC Wkly. 2021;3(25):527–30. doi:10.46234/ccdcw2021.138.
- National Health Commission, State Administration of Traditional Chinese Medicine. Diagnosis and treatment protocol for COVID-19 (trial version 9) [accessed 2022 Oct 12]. http://www.gov.cn/zhengce/zhengceku/2022-03/15/5679257/files/49854a49c7004f4ea9e622f3f2c568d8.pdf.
- Covid-Vaccine Technical Working Group. Technical vaccination recommendations for COVID-19 vaccines in China (First edition). China CDC Wkly. 2021;3:459–61.
- Covid-Vaccine Technical Working Group. Technical guidelines for evaluation of epidemiological protection effect of COVID-19 vaccine in outbreak sites (trial version). Chin J Vacc Immuniz 2022; 28:257–62.
- Yin Z, Wen T, Fang Q, Zheng C, Gong X, Li J, Wang S, Xiang Z. Assessment of mumps-containing vaccine effectiveness by dose during 2006 to 2020 in Quzhou, China. Hum Vacc Immunotherap. 2022;18(5):2086774. doi:10.1080/21645515.2022.2086774.
- Chen J, Wang R, Gilby NB, Wei GW Omicron variant (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model 2022; 62:412–22. doi:10.1021/acs.jcim.1c01451
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–69. doi:10.1001/jama.2020.1585.
- Madhi SA, Kwatra G, Myers JE, Jassat W, Dhar N, Mukendi CK, Nana AJ, Blumberg L, Welch R, Ngorima-Mabhena N, et al. Population immunity and Covid-19 severity with Omicron variant in South Africa. N Engl J Med. 2022;386:(14):1314–26. doi:10.1056/NEJMoa2119658.
- Altarawneh HN, Chemaitelly H, Hasan MR, Ayoub HH, Qassim S, AlMukdad S, Coyle P, Yassine HM, Al-Khatib HA, Benslimane FM, et al. Protection against the Omicron variant from previous SARS-CoV-2 infection. N Engl J Med. 2022;386:1288–90. doi:10.1056/NEJMc2200133.
- Luliano AD, Brunkard JM, Boehmer TK, Peterson E, Adjei S, Binder AM, Cobb S, Graff P, Hidalgo P, Panaggio MJ, et al. Trends in Disease Severity and Health Care Utilization During the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146–52. doi:10.1558/5mmwr.mm7104e4.
- Huang Z, Xu S, Liu J, Wu L, Qiu J, Wang N, Ren J, Li Z, Guo X, Tao F, et al. Effectiveness of inactivated and Ad5-nCov COVID-19 vaccines against SARS-CoV-2 Omicron BA. 2 variant infection, severe illness, and death. BMC Med. 2022;20:400. doi:10.1186/s12916-022-02606-8.
- Esper FP, Adhikari TM, Tu ZJ, Cheng YW, El-Haddad K, Farkas DH, Bosler D, Rhoads D, Procop GW, Ko JS, et al. Alpha to Omicron: disease severity and clinical outcomes of major SARS-CoV-2 variants. J Infect Dis 2022. doi:10.1093/infdis/jiac411
- Lyngse FP, Kirkeby CT, Denwood M, Christiansen LE, Mølbak K, Møller CH, Skov RL, Krause TG, Rasmussen M, Sieber RN, et al. Transmission of SARS-CoV-2 Omicron VOC subvariants BA.1 and BA.2: evidence from Danish households. MedRxiv. 2022 Jun 28;22270044. Preprint. doi:10.1101/2022.01.28.22270044.
- Jara A, Undurraga EA, Zubizarreta JR, Gonzalez C, Acevedo J, Pizarro A, Vergara V, Soto-Marchant M, Gilabert R, Flores JC, et al. Effectiveness of CoronaVac in children 3–5 years of age during the SARS-CoV-2 Omicron outbreak in Chile. Nat Med. 2022;28:1377–80. doi:10.1038/s41591-022-01874-4.
- Jara A, Undurraga EA, Gonzalez C, Paredes F, Fontecilla T, Jara G, Pizarro A, Acevedo J, Leo K, Leon F, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385:875–84. doi:10.1056/NEJMoa2107715.
- Zeng G, Wu Q, Pan H, Li M, Yang J, Wang L, Wu Z, Jiang D, Deng X, Chu K, et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis. 2022;22:483–95. doi:10.1016/S1473-3099(21)00681-2.
- Kirsebom FCM, Andrews N, Stowe J, Toffa S, Sachdeva R, Gallagher E, Groves N, O’Connell A-M, Chand M, Ramsay M, et al. COVID-19 vaccine effectiveness against the omicron (BA.2) variant in England. Lancet Infect Dis. 2022;22:931–33. doi:10.1016/S1473-3099(22)00309-7.
- Madhi SA, Kwatra G, Richardson SI, Koen AL, Baillie V, Cutland CL, Fairlie L, Padayachee SD, Dheda K, Barnabas SL, et al. Durability of ChAdox1 nCov-19 (AZD1222) vaccine and hybrid humoral immunity against variants including omicron BA.1 and BA.4 6 months after vaccination (COV005): a post-hoc analysis of a randomised, phase 1b–2a trial. Lancet Infect Dis 2022. doi:10.1016/S1473-3099(22)00596-5
- Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O’Connell A-M, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–46. doi:10.1056/NEJMoa2119451.
- Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, Mizrahi B, Alroy-Preis S, Ash N, Milo R, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393–400. doi:10.1056/NEJMoa2114255.
- McMenamin ME, Nealon J, Lin Y, Wong JY, Cheung JK, Lau EHY, Wu P, Leung GM, Cowling BJ Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis 2022; 22:1435–43. doi:10.1016/S1473-3099(22)00345-0