?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
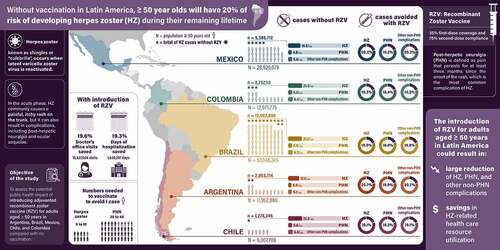
This study aimed to: (1) estimate the disease burden of herpes zoster (HZ) and (2) assess the potential public health impact of introducing adjuvanted recombinant zoster vaccine (RZV) compared with no vaccination in adults aged ≥50 years in Argentina, Brazil, Mexico, Chile, and Colombia using the ZOster ecoNomic Analysis (ZONA) static multicohort Markov model. The model followed individuals aged ≥50 years from administration of RZV over their remaining lifetime. Inputs were based, most often, on local data. First dose coverage was assumed to be 35%, with 75% second dose compliance. It was predicted that without RZV, there would be 23,558,675 HZ cases, 6,115,981 post-herpetic neuralgia (PHN) cases, and 7,058,779 non-PHN complications in the five countries, but introducing RZV under assumed coverage could avoid 4,583,787 (19%) HZ cases, 1,130,751 (18%) PHN cases, and 1,373,419 (19%) non-PHN complications. Also, 10427,504 (20%) doctor’s office visits and 1,630,201 (19%) days of hospitalization could be averted in the three countries (Argentina, Brazil, and Mexico) with available input data. The numbers needed to be vaccinated to avoid one case of HZ were 9–10 across countries, and to avoid one case of PHN, 35–40. One-way sensitivity analyses showed that the input parameters with the largest impact on the estimated number of HZ cases avoided were first dose coverage, initial HZ incidence, and vaccine efficacy waning. In conclusion, the introduction of RZV for older adults in Latin America could greatly reduce the public health burden of HZ and reduce the related doctor visits and hospitalization days.
Plain Language Summary
Why was the study done?
Herpes zoster (HZ), commonly known as shingles or “culebrilla,” typically causes a painful, itchy rash on the trunk in older adults, and can result in long-term complications. It is difficult to study the lifetime burden of HZ due to follow-up time constraints. We therefore wanted to predict how many people could develop HZ as they age and how many cases of HZ could be avoided by introducing adjuvanted recombinant zoster vaccine (RZV) in people aged 50 years and older in five Latin American countries (Argentina, Brazil, Mexico, Chile, and Colombia).
What did the researchers do and find?
Using a mathematical model, we predicted that nearly 5 million of an estimated 24 million cases of HZ could be avoided by vaccinating 35% of older adults with RZV in the five countries. This vaccination approach would also avert various complications of HZ, including post-herpetic neuralgia (long-lasting pain at the rash site) and save doctor’s office visits and hospitalizations for HZ.
What do the results mean?
Introducing RZV for older adults in Latin America – as is already the case in various other countries – could prevent a substantial proportion of HZ cases, leading to improved public health and less health care resource utilization.
What is the objective influence on the wider field?
In the absence of real-world data on the potential impact of RZV on HZ in Latin America, these predictions could help policymakers to assess the potential value of introducing RZV for older adults in Latin America.
Introduction
Herpes zoster (HZ) (commonly known as shingles or “culebrilla”) occurs when latent varicella zoster virus (which causes chickenpox) is reactivated.Citation1 In the acute phase, HZ commonly causes a painful, itchy rash on the trunk, but it can also result in complications, including post-herpetic neuralgia (PHN) (i.e. long-lasting pain at the rash site) and ocular sequelae (including loss of vision).Citation1 The yearly incidence of HZ increases with age due to natural waning of immune function,Citation2 from approximately 5 per 1,000 people aged 50–54 years to around 11 per 1,000 people aged ≥85 years.Citation3 The incidence of HZ also increases considerably in people with comorbiditiesCitation4 (e.g. diabetes,Citation5 chronic obstructive pulmonary disease, and asthma) and those who are immunocompromised due to disease or therapy.Citation6 Studies in Latin America have indicated an incidence density of 6–37 cases per 1,000 person-years among higher-risk patients.Citation7 In the United States (US), approximately 1/3 of people will develop HZ at some point during their lifetime,Citation1 with around 15–30% of those who do also developing PHN, the risk of which increases with age.Citation8
A pooled analysis of studies from Argentina, Brazil, and Mexico found that 79% of patients with HZ visited a doctor’s office, 49% the emergency room, 38% a specialist, and 6% were hospitalized, resulting in a direct cost of $763 per case (2015 US$).Citation9 When work days and productivity losses were taken into account, the total cost increased to $1,465 per case.Citation9 Health care resource utilization (HCRU) and costs were considerably higher among patients who also developed PHN (e.g. direct costs $1,228 vs $422; total costs $2,001 vs $868).Citation9
There are two vaccines for the prevention of HZ, namely adjuvanted recombinant zoster vaccine (RZV; Shingrix, GSK) and zoster vaccine live (ZVL; Zostavax, Merck Sharp & Dohme Corp.). Placebo-controlled randomized controlled trials (RCTs) have shown RZV to have a vaccine efficacy >90% against HZ during the first 3 years after vaccination of older adults (≥50 or ≥70 years).Citation10 Another placebo-controlled RCT has reported that ZVL had a vaccine efficacy of 61% among older adults (≥60 years) over a similar time frame.Citation11 Of note, ZVL is contraindicated for immunocompromised patients.Citation12 Also, following a period of preference for RZV over ZVL,Citation13 the Advisory Committee on Immunization Practices in the US now only recommends a two-dose series of RZV for all adults aged ≥50 years, including those previously vaccinated with ZVL.Citation14 Multiple studies – in North America,Citation15–19 Europe,Citation20–25 and AsiaCitation26–29 – have shown that the introduction of routine RZV vaccination for older adults could reduce the public health burden of HZ. Two recent meta-analyses have shown that routine RZV vaccination for older adults has the potential to be cost-effectiveCitation30 and consistently dominant over routine ZVL vaccination.Citation31
Currently, RZV has only been implemented in Brazil in the private sector and is recommended by the Brazilian Society of Immunization for adults aged ≥50 years and those aged ≥18 who are immunocompromised.Citation32 The objective of the current study was, therefore, to estimate the burden of HZ in five Latin American countries (Argentina, Brazil, Mexico, Chile, and Colombia) and to assess the potential public health impact of introducing RZV for adults aged ≥50 years in each country, compared with no vaccination. The public health impact was defined as the numbers of cases of HZ, PHN, and non-PHN complications avoided, which is reflective of the natural history of HZ infection. When country-specific data were available, the numbers of doctor’s office visits and days of hospitalization avoided were estimated to represent the downstream impact of clinical cases of HZ.
Materials and methods
Analysis plan
The ZOster ecoNomic Analysis (ZONA) is a static multicohort Markov model that was developed in Microsoft Excel (Figure S1).Citation16,Citation18−Citation20,Citation22−Citation29 This was adapted – to Argentina, Brazil, Mexico, Chile, and Colombia – and used to assess the potential public health impact of the introduction of RZV for older adults. These five Latin American countries were selected as a previously published systematic review on the epidemiology of HZ in Latin AmericaCitation7 included multiple studies from these countries. Further, Argentina, Brazil, Mexico, and Colombia are the top four most populous countries in Latin America. Two strategies were compared in each country: no RZV and vaccination with RZV.
Study population
The population was stratified into five age cohorts: 50–59, 60–64, 65–69, 70–79, and ≥80 years. The model followed individuals aged ≥50 years within each cohort from their year of receipt of RZV over their remaining lifetime with annual cycle length. The population could transition between different health states (no HZ, HZ, PHN, HZ-related non-PHN complications, recurrent HZ, HZ-related death, and death due to other causes) as shown in Figure S1.Citation22 For the overall cohort, the model assumed that individuals in each age cohort were vaccinated at 50, 60, 65, 70, or 80 years of age.
Inputs
Demographic inputs included national data from population projection pyramids and life tables and were collected from official websites.Citation33–42 Natural mortality rates from the life tables were used to calculate the size of the susceptible population by age in each country. As the life tables in Brazil and Chile ended at an open interval of ≥80 years old, an exponential extrapolation of death rates into older age cohorts was applied, which provided relatively more conservative estimates of annual death rates and probabilities compared with the original estimates for all individuals ≥80 years old from the life tables and compared with their neighboring countries (Argentina, Mexico, and Colombia). To close the life table and match with the model time horizon, no people were assumed to live beyond 100 years old. Country-specific population sizes and mortality rates are shown in Tables S1 and S2, respectively.Citation33–42
Key base case inputs are listed in .Citation3,Citation8,Citation23,Citation43−Citation46 Due to a paucity of robust local data, age-specific annual HZ incidence was taken from a worldwide meta-regression.Citation3 Local incidence data from three small studies were tested in one-way sensitivity analyses (OWSAs).Citation47–49 The recurrence rate of HZ episodes was assumed to be the same as the first occurrence rate.Citation44 The percentages of HZ cases that resulted in PHN and non-PHN complications (ocular, neurological, cutaneous, and other complications) were based on data from a tertiary care hospital in Brazil.Citation43 This study reported overall rates, but as age is a risk factor for PHN, the age distribution of PHN from Kawai et al.Citation8 was used to calculate age-specific PHN percentages. The recurrent HZ cases were assumed to be associated with the same risk of developing PHN. In the absence of credible sources for case fatality rates of HZ, this was conservatively assumed to be 0%.
Figure 1. Epidemiological inputs.
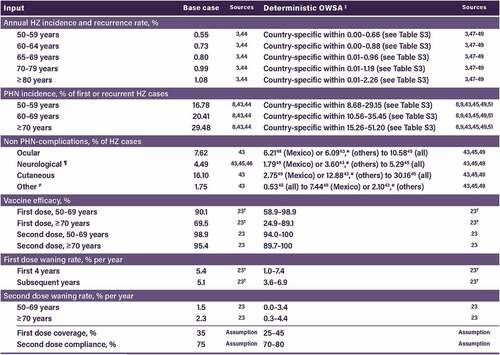
Vaccine efficacy and waning rates were taken from an 8-year long-term follow-up trial (ZOE-LTFU),Citation23 which was conducted in Germany (see for age-specific values). First dose coverage was conservatively assumed to be 35%, with a second dose compliance of 75%.
The mean numbers of doctor’s office visits (3.4) and days of hospitalization (0.30) per patient with HZ for Argentina and Mexico were taken from a study that pooled patients from Argentina, Brazil, and Mexico.Citation9 Data for Brazil (2.3 doctor’s office visits and 0.52 days of hospitalization) were taken from a study performed in São Paulo.Citation50 Due to the absence of suitable HCRU data in Chile and Colombia and the limitation of generalizability of the HCRU data from other countries, the estimation of HCRU was not conducted in Chile and Colombia.
Outcomes
Key outcomes were the numbers of HZ cases, PHN cases, and HZ-related non-PHN complications (neurological, ocular, cutaneous, and other) and the number of HZ-related doctor’s office visits and days of hospitalization. As the model followed all subjects within each age cohort (50–59, 60–64, 65–69, 70–79, and ≥80 years) from the year of vaccination over their remaining lifetime (see Table S2 for mortality rates), all subjects remained in their initial cohort and all numbers of events (for no RZV, along with the numbers saved with RZV) are reported in that cohort. We also report grand totals for all cohorts combined over their remaining lifetimes.
Numbers needed to vaccinate (NNVs) to avoid one HZ case and one PHN case were calculated using the following equation:
Deterministic one-way sensitivity analysis
To test the impact of parameter uncertainty on HZ cases avoided, deterministic OWSAs for each of the five countries were performed. The low and high values used are shown in , with further details in Table S3.Citation3,Citation8,Citation9,Citation23,Citation43,Citation45,Citation47–49,Citation51 For HZ incidence, a small study of 340 patients with HZ in a community hospital in Buenos Aires during 2013–2014 was used for Argentina.Citation47 A Brazilian study that reported monthly numbers of hospitalizations due to varicella and HZ during 2008–2013 provided values for Brazil.Citation48 For Mexico, the numbers of inpatient and emergency department HZ cases from the Mexico Health Secretariat and General Health Information OfficeCitation49 was used. For inputs for which low and high values were not available, the base case was varied by ±20%.
The results of the OWSAs – obtained by modifying the value of one base case parameter at a time – are summarized in tornado diagrams that present the five inputs that had the largest impact on predicted HZ cases avoided in each country.
Probabilistic sensitivity analysis
Probabilistic sensitivity analyses (PSAs) for each country were conducted to account for the full uncertainty in model inputs and explore the impact on the number of HZ cases avoided. A total of 1,000 Monte Carlo simulations were run, in which input values were simultaneously sampled from probability distributions. All parameters were sampled across beta distributions. Age-specific incidence parameters that varied across age cohorts were assumed to be correlated using a correlation of 0.5.
Scenario analyses
As RZV is given as a two-dose series and the number of doses received affects the efficacy and waning of the vaccine, different proportions of the population receiving one dose and two doses were explored in two scenario analyses. One was performed to explore how many HZ cases could be saved with different first dose coverage rates (0–100%), while a second compared different second dose compliance rates (55% and 95%).
Results
Impact of recombinant zoster vaccine on cases
Without RZV vaccination, the model predicted 23,558,675 cases of HZ (ranging from 1,278,246 in Chile to 12,082,890 in Brazil) in the remaining lifetime of subjects aged ≥50 years (i.e. 20.2 HZ cases per 100 population of that age) in the five Latin America countries, with more cases predicted per 100 population among younger people due to their longer remaining lifetimes ( and Table S4). Vaccinating 35% of the ≥50-year-old population with RZV was predicted to avoid 4,583,787 (ranging from 244,610 in Chile to 2,292,296 in Brazil) HZ cases (i.e. 19.5% of cases) in the five Latin America countries, with higher percentages of cases avoided among older people due to less vaccine efficacy waning ( and Table S4).
Figure 2. Numbers of cases of HZ, PHN, and non-PHN complications without RZV and avoided with RZV, by country, overall, and by age group.
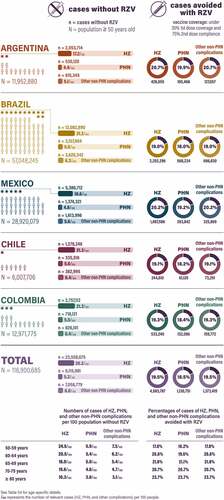
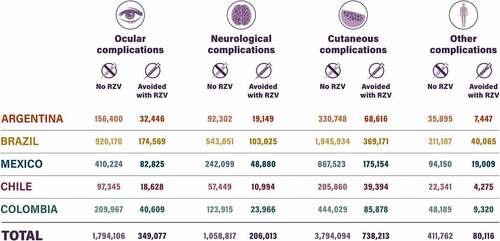
Without RZV vaccination, the model predicted 6,115,981 (ranging from 335,516 in Chile to 3,157,904 in Brazil) cases of PHN and 7,058,779 (ranging from 382,995 in Chile to 3,620,342 in Brazil) non-PHN complications in the remaining lifetime of individuals aged ≥50 years in the five Latin America countries ( and Table S4). With RZV vaccination for 35% of the ≥50-year-old population, it was predicted that 1,130,751 (ranging from 61,120 in Chile to 568,234 in Brazil) PHN cases and 1,373,419 (ranging from 73,291 in Chile to 686,830 in Brazil) non-PHN complications would be avoided. As for HZ, more PHN cases were predicted among younger people without RZV vaccination, but a higher percentage of PHN cases were predicted to be avoided among older people with RZV vaccination ( and Table S4).
Details on the numbers of non-PHN complications (i.e. ocular, neurological, and other) predicted without RZV and saved with RZV are shown in (and detailed in Table S5).
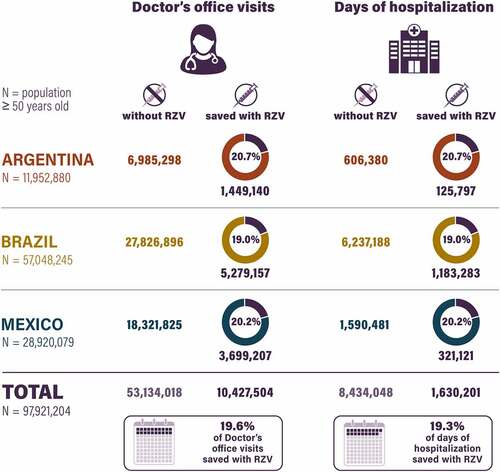
Impact of recombinant zoster vaccine on health care resource utilization
Without RZV, the model predicted 53,134,018 (6,985,298 in Argentina, 18321,825 in Mexico, and 27,826,896 in Brazil) doctor’s office visits and 8,434,048 (606,380 in Argentina, 1,590,481 in Mexico, and 6,237,188 in Brazil) days of hospitalization due to HZ in the remaining lifetime of ≥50-year-old population in the three Latin America countries ( and Table S6). With RZV, it was predicted that 10,427,504 (1,449,140 in Argentina, 3,699,207 in Mexico, and 5,279,157 in Brazil) doctor’s office visits and 1,630,201 (125,797 in Argentina, 321,121 in Mexico, and 1,183,283 in Brazil) hospitalization days could be saved.
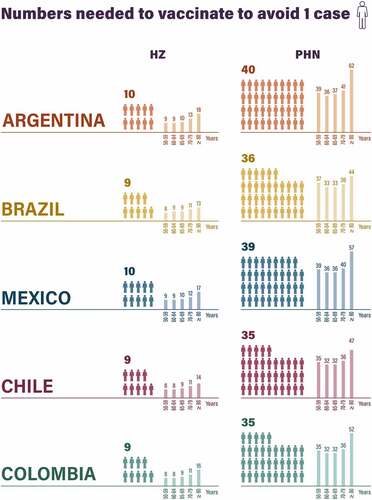
Numbers needed to vaccinate
NNVs to avoid one case of HZ were 8 or 9 (depending on the country) for people aged 50–59 years, increasing to 13–18 for those aged ≥80 years (). Similarly, NNVs to avoid one case of PHN were 35–39 in the youngest cohort to 44–62 in the oldest cohort.
One-way sensitivity analysis
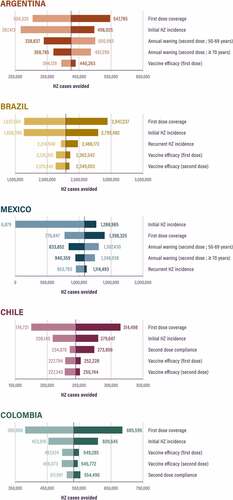
The top five parameters that had the largest impact on predicted HZ cases avoided in each of the five countries are shown in . The most influential parameter was first dose coverage (in Argentina, Brazil, Chile, and Colombia) and initial HZ incidence (in Mexico), due to the very low incidence of inpatient/emergency HZ cases reported in the passive surveillance system [see Table S3]). Other influential parameters included vaccine efficacy, annual waning, recurrent HZ incidence, and second dose compliance.
Figure 6. Deterministic OWSA results (top five inputs per country) for HZ cases avoided for Argentina, Brazil, Mexico, Chile, and Colombia. Light color indicates low values; dark color indicates high values. For input values used, please see .
Probabilistic sensitivity analysis
The probability distributions in the five countries are shown in Figure S2.
Scenario analyses
When the first dose coverage was varied from 0% to 100%, the predicted numbers of cases of HZ avoided increased linearly from 0 up to 6,549,416 (Brazil), 3,107,389 (Mexico), 1,584,258 (Colombia), 1,217,299 (Argentina), or 726,691 (Chile) (Figure S3).
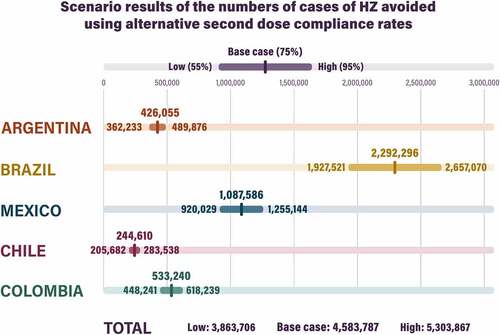
When the second dose compliance was reduced to 55% or increased to 95%, the predicted numbers of cases of HZ avoided decreased or increased accordingly, as detailed in .
Discussion
Key study findings are summarized in the associated Plain Language Summary and Graphical Abstract. Overall, this study indicates that the public health burden of HZ is likely to be substantial during the remaining lifetime of adults aged ≥50 years in the five included Latin America countries. Further, that the introduction of RZV among these populations – even with only 35% first-dose coverage – could avoid approximately 20% of cases of HZ, PHN, and non-PHN complications; and HZ-related doctor’s office visits and days of hospitalization at proportional magnitudes. Obviously, the public health impact of RZV would increase as coverage rates increase over time. Although it is difficult to compare absolute case number reductions with those estimated in other studies due to the different population sizes, multiple studies from North America,Citation15–19 Europe,Citation20–25 and AsiaCitation26–29 have also estimated that the introduction of routine RZV vaccination for older adults would proportionally reduce the burden of HZ.
Impact on cases by age group
By age, the current model predicted that, over the course of their remaining lifetimes, the youngest vs oldest cohorts (50–59 vs ≥80 years) would: (1) have the most cases of HZ (12 million vs 1 million) and PHN (3 million vs 0.3 million) without HZ vaccination and (2) avoid the most cases of HZ (2 million vs 0.2 million) and PHN (0.5 million vs 0.1 million) with HZ vaccination. This is mainly because the youngest cohort included the most people (50 million vs 1 million), but also because of their longer life expectancy. After accounting for the different cohort sizes, the model still predicted that the youngest vs oldest cohorts would have the most cases of HZ (24.5 vs 10.3 per 100 people) and PHN (5.9 vs 3.0 per 100 people) without vaccination. However, the highest percentages of HZ and PHN cases avoided with vaccination were predicted to be in the oldest vs youngest cohorts (HZ: 23.7% vs 17.8%; PHN: 23.7% vs 16.2%) because there was less time for vaccine efficacy to wane.
Our prediction that the greatest lifetime burden of HZ without vaccination would be among the youngest cohort (24.5, 20.5, 18.0, 15.6, and 10.3 per 100 people aged 50–59, 60–64, 65–69, 70–79, and ≥80 years, respectively) is aligned with findings from the US.Citation17,Citation19 Prosser et al.Citation19 estimated that, without vaccination, there would be 26.5, 20.4, 13.8, 8.1, and 4.2 cases of HZ per 100 people aged 50–59, 60–69, 70–79, 80–89, and 90–99 years, respectively. Similarly, Carpenter et al. estimated that 31.3%, 26.2%, and 19.7% of 50-, 60-, and 70-year-olds, respectively, would develop HZ during their remaining lifetime.Citation17
Our prediction that the youngest cohort would avoid the greatest number of HZ cases with vaccination is aligned with three studies from GermanyCitation21–23 and one from China.Citation26 Our predictions that the percentages of HZ and PHN cases avoided would increase with age (HZ: 17.8% to 23.7%; PHN: 16.2% to 23.7%) aligns with data from US, which predicted that 21% to 41% of HZ cases and 8% to 41% of PHN cases would be prevented among people vaccinated at age 50 to 70 years, respectively.Citation17
Overall, the estimated optimal age for vaccination depends on the model and its input parameters, but our results and various other studies tend to support vaccination at age 50 or 60 years, rather than at 70 or 80 years. However, one should note that people vaccinated at age 50 years would likely benefit from a booster HZ vaccine dose in later life.
Impact on health care resource utilization
In the current study, it was estimated that 1.4, 3.7, and 5.3 million doctor’s office visits and 0.1, 0.3, and 1.2 million days of hospitalization could be saved in Argentina, Mexico, and Brazil, respectively. Most of this difference is due to the different population sizes, but some is due to the lower rate of doctor’s office visits (2.3 vs 3.4 visits per HZ case) and longer hospitalization (0.52 vs 0.30 days per HZ case) assumed for Brazil vs Argentina and Mexico.Citation9,Citation50 To put this into context, 21% (Argentina), 20% (Mexico), and 19% (Brazil) of doctor’s office visits and days of hospitalization were predicted to be saved among the population aged ≥50 years. Of note, country-specific data are essential to run HCRU analyses because each country’s healthcare practice varies. Brazilian local inputs of HCRUCitation50 were identified, while for Argentina and Mexico, a pooled analysisCitation9 of HZ patients from Argentina (n = 96), Brazil (n = 145), and Mexico (n = 142) was used due to the paucity of better local data. In Chile and Colombia, no HCRU data were identified.
Numbers needed to vaccinate
As HZ is a relatively common afflictionCitation1 and recurrence is possible,Citation44 the current model predicted that only 8–9 people aged 50–59 years up to 13–18 people aged ≥80 years would need to be vaccinated with RZV to prevent one case of HZ in the various countries studied due to the long-term protection afforded by this vaccine. These results are well aligned with results from Canada,Citation52 Germany,Citation21–23 the UK,Citation20 China,Citation26,Citation53 and Japan.Citation27
Regarding PHN, the current model predicted that 35–39 people aged 50–59 years would need to be vaccinated to prevent one case, decreasing slightly to 32–37 among those aged 60–69 years, then increasing to 36–41 and 44–62 among those aged 70–79 and ≥80 years, respectively. This J-curve pattern has also been reported in studies from Canada,Citation52 the UK,Citation20 and Germany,Citation22,Citation23 while one from Japan reported similar NNVs for each age cohortCitation27 and one from China reported NNVs that tended to decrease with age.Citation26 In terms of actual NNVs, our results were generally in line with various studies,Citation22,Citation23,Citation27,Citation52 but somewhat lower than in other studies.Citation20,Citation26
One way sensitivity analysis
In the deterministic OWSAs, factors with a beneficial impact on estimated HZ cases avoided with RZV included higher first dose coverage, higher initial HZ incidence, higher vaccine efficacy, slower waning of vaccine efficacy, higher second dose compliance, and higher recurrent HZ incidence. Not surprisingly, a similar study from ChinaCitation26 also found these factors to be influential. Increasing the first dose coverage and the second dose compliance beyond the levels tested in the OWSAs increased the estimated cases avoided even further, as shown in the scenario analyses. Of note, when very low HZ incidence rates based on passive surveillance were tested in the OWSA for Mexico (0.00–0.01Citation49 vs 0.55–1.08Citation3,Citation44 across age cohorts), the predicted number of HZ cases avoided dropped to 6,879. However, this is likely a gross underestimation, as has been shown with data of other pathologies from passive surveillance in Mexico.Citation54 This is because these data were based only on inpatient and emergency cases that were notified to the Mexico Health Secretariat and General Health Information Office, HZ is not a mandatorily notifiable disease, and HZ is often not captured as a primary diagnosis in the surveillance system.
Probabilistic sensitivity analyses
Following standard statistical methods, beta distributions were applied to match for binomial data in the PSA. Due to the limited information on the parameters, a conservative approach was adopted with an appropriately broad range of ±20% of the base case estimates. The results were robust under PSA, with >90% of simulations showing reductions of ≥240,000, ≥1,600,000, ≥600,000, ≥180,000, and ≥400,000 HZ cases with respect to no vaccination in Argentina, Brazil, Mexico, Chile, and Colombia, respectively. The choice of parameter distribution was deemed appropriate and correlation of parameter was considered.
Scenario analyses
The scenario analysis results indicate that, not surprisingly, improving first dose coverage and second dose compliance would result in higher numbers of HZ cases avoided. To put this into context, first dose coverage rates of 35% (base case), 60%, and 80% could be expected to avoid approximately 19% (base case), 34%, and 45% of HZ cases, respectively. Similarly, second dose compliance rates of 75% (base case) and 95% could be expected to avoid approximately 19% (base case) and 23% of HZ cases, respectively. If both parameters improved over time, the proportions of HZ cases avoided could be even higher.
Strengths and limitations
This is the first public health impact study to assess the potential impact of RZV in Latin America. Modeling the lifetime risk of HZ and its complications with a multiple-cohort approach allows us to make projections for outcomes that have not been reported in real-world evidence. The model incorporated age-specific vaccine efficacy and waning rates based on long-term RCT data. A static cohort model was used as HZ is caused by the reactivation of varicella zoster virus in an individual rather than its transmission in the population. Static cohort models of HZ have been widely used for the economic evaluation of HZ,Citation55 which has a constant risk of infection. Although our models are a simplification of the reactivation of varicella zoster virus among people aged ≥50 years, we believe that they are useful for highlighting the burden and public health impact of HZ.
It has been hypothesized that decreased virus circulation following varicella vaccination implementation and the resultant diminished exogenous boosting could potentially impact on the epidemiology of HZ.Citation56–59 However, there is no consistent evidence showing an extensive surge in HZ incidence after varicella vaccination implementation,Citation60–63 the recent first-dose coverage rates of which are 66% (Brazil, 2021),Citation64 72% (Argentina, 2020),Citation65 79% (Chile, 2022),Citation66 and 93% (Colombia, 2019),Citation67 while varicella vaccination is not included in the universal mass vaccination program in Mexico. Instead, the key contributing factor to HZ is immune senescence in older adults.Citation68,Citation69 Any impact of reduced exogenous boosting following varicella vaccination implementation will, therefore, not be expected to impact our results.
However, there are some limitations to note. Firstly, while we aimed to use the most suitable inputs, country- and age-specific data were not always available. For example, we used worldwide meta-regression data for HZ incidence,Citation3 due to a lack of robust local data, which were only available from an Argentinian hospital study (age ≥60 years),Citation47 a Brazilian study that only reported hospitalizations,Citation48 or from passive surveillance of inpatient/emergency cases in Mexico.Citation49 However, the global meta-regressionCitation3 data showed no significant difference in HZ incidence across continents and the local data were tested in the OWSAs. Secondly, while our vaccine efficacy rates were based on RCTs, so should be robust, waning rates beyond 8 years are, as yet, unknown.Citation10,Citation23 Thirdly, we only estimated the impact of RZV on doctor’s office visits and hospitalizations, but it is likely that other types of HCRU could also be avoided. Further studies could be performed to estimate the costs prevented with RZV vaccination. In terms of generalizability, the absolute numbers of cases and HCRU avoided are country specific, but the relative percentages may be pertinent to other countries in Latin America. Lastly, this analysis employed a static multiple-cohort Markov model, which allowed the inclusion of the long-term benefits of RZV. However, as we did not account for the potential effect of the RZV vaccine on the severity of HZ and PHN,Citation70 we may have underestimated the overall benefits of RZV.
Conclusions
The current public health impact study predicted that there could be approximately 24 million cases of HZ during the remaining lifetimes of those aged ≥50 years in Argentina, Brazil, Mexico, Chile, and Colombia combined. Further, nearly 5 million of these cases could be avoided if RZV vaccination was introduced with an assumed first-dose coverage rate of 35% for older adults in these five Latin American countries. There would also be large reductions in PHN and non-PHN complications, along with savings in HZ-related doctor’s office visits and days of hospitalization. These results could help policymakers to assess the potential value of introducing RZV for people aged ≥50 years in Latin America.
Trademark
Shingrix is a trademark owned by or licensed to GSK. Zostavax is a trademark of Merck Sharp & Dohme Corp.
Supplemental Material
Download PDF (730.6 KB)Acknowledgments
The authors would like to thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Jenny Lloyd (Compass Healthcare Communications Ltd., on behalf of GSK) provided medical writing support.
Disclosure statement
RH, JAG, BdV, TP, AG-H, JN, and DAMvO are employees of GSK. AG-H, JN, and DAMvO hold shares in GSK. All authors declare no other financial or non-financial relationships and activities and no other conflicts of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2164144.
Additional information
Funding
References
- Centers for Disease Control and Prevention (CDC). Shingles (herpes zoster); [accessed 2022 Jun 23]. https://www.cdc.gov/shingles/hcp/clinical-overview.html.
- Oxman MN. Herpes zoster pathogenesis and cell-mediated immunity and immunosenescence. J Am Osteopath Assoc. 2009;109:1–14.
- Curran D, Callegaro A, Fahrbach K, Neupane B, Vroling H, van Oorschot D, Yawn BP. Meta-regression of herpes zoster incidence worldwide. Infect Dis Ther. 2022;11:389–403. doi:10.1007/s40121-021-00567-8.
- Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin Proc. 2017;92:1806–21. doi:10.1016/j.mayocp.2017.10.009.
- Suaya JA, Chen SY, Li Q, Burstin SJ, Levin MJ. Incidence of herpes zoster and persistent post-zoster pain in adults with or without diabetes in the United States. Open Forum Infect Dis. 2014;1:ofu049. doi:10.1093/ofid/ofu049.
- Yanni EA, Ferreira G, Guennec M, El Hahi Y, El Ghachi A, Haguinet F, Espie E, Bianco V. Burden of herpes zoster in 16 selected immunocompromised populations in England: a cohort study in the clinical practice research datalink 2000-2012. BMJ Open. 2018;8:e020528. doi:10.1136/bmjopen-2017-020528.
- Bardach AE, Palermo C, Alconada T, Sandoval M, Balan DJ, Nieto Guevara J, Gomez J, Ciapponi A, Andrei G. Herpes zoster epidemiology in Latin America: a systematic review and meta-analysis. PLoS One. 2021;16(8):e0255877. doi:10.1371/journal.pone.0255877.
- Kawai K, Rampakakis E, Tsai TF, Cheong HJ, Dhitavat J, Covarrubias AO, Yang L, Cashat-Cruz M, Monsanto H, Johnson K, et al. Predictors of postherpetic neuralgia in patients with herpes zoster: a pooled analysis of prospective cohort studies from North and Latin America and Asia. Int J Infect Dis. 2015;34:126–31. doi:10.1016/j.ijid.2015.03.022.
- Rampakakis E, Pollock C, Vujacich C, Toniolo Neto J, Ortiz Covarrubias A, Monsanto H, Johnson KD. Economic burden of herpes zoster (“culebrilla”) in Latin America. Int J Infect Dis. 2017;58:22–26. doi:10.1016/j.ijid.2017.02.021.
- Boutry C, Hastie A, Diez-Domingo J, Tinoco JC, Yu CJ, Andrews C, Beytout J, Caso C, Cheng HS, Cheong HJ, et al. The adjuvanted recombinant zoster vaccine confers long-term protection against herpes zoster: interim results of an extension study of the pivotal phase 3 clinical trials ZOE-50 and ZOE-70. Clin Infect Dis. 2022;74:1459–67. doi:10.1093/cid/ciab629.
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. doi:10.1056/NEJMoa051016.
- Merck & Co, Inc. ZOSTAVAX® (Zoster Vaccine Live); [accessed 2022 Aug 5]. https://www.fda.gov/media/82524/download.
- Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, Harpaz R. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103–08. doi:10.15585/mmwr.mm6703a5.
- Centers for Disease Control and Prevention (CDC). Shingrix Recommendations; [accessed 2022 Aug 12]. https://www.cdc.gov/vaccines/vpd/shingles/hcp/shingrix/recommendations.html#:~:text=CDC%20recommends%20Shingrix%20/recombinant%20zoster,prior%20episode%20of%20herpes%20zoster.
- Patterson BJ, Buck PO, Curran D, Van Oorschot D, Carrico J, Herring WL, Zhang Y, Stoddard JJ. Estimated public health impact of the recombinant zoster vaccine. Mayo Clin Proc Innov Qual Outcomes. 2021;5:596–604. doi:10.1016/j.mayocpiqo.2021.03.006.
- Curran D, Patterson B, Varghese L, Van Oorschot D, Buck P, Carrico J, Hicks K, Lee B, Yawn B. Cost-effectiveness of an adjuvanted recombinant zoster vaccine in older adults in the United States. Vaccine. 2018;36:5037–45. doi:10.1016/j.vaccine.2018.07.005.
- Carpenter CF, Aljassem A, Stassinopoulos J, Pisacreta G, Hutton D. A cost-effectiveness analysis of an adjuvanted subunit vaccine for the prevention of Herpes Zoster and post-herpetic neuralgia. Open Forum Infect Dis. 2019;6:ofz219. doi:10.1093/ofid/ofz219.
- McGirr A, Van Oorschot D, Widenmaier R, Stokes M, Ganz ML, Jung H, Varghese L, Curran D. Public health impact and cost-effectiveness of non-live adjuvanted recombinant zoster vaccine in Canadian adults. Appl Health Econ Health Policy. 2019;17:723–32. doi:10.1007/s40258-019-00491-6.
- Prosser LA, Harpaz R, Rose AM, Gebremariam A, Guo A, Ortega-Sanchez IR, Zhou F, Dooling K. A cost-effectiveness analysis of vaccination for prevention of herpes zoster and related complications: input for national recommendations. Ann Intern Med. 2019;170:380–88. doi:10.7326/M18-2347.
- van Oorschot DAM, Hunjan M, Bracke B, Lorenc S, Curran D, Starkie-Camejo H. Public health impact model estimating the impact of introducing an adjuvanted recombinant zoster vaccine into the UK universal mass vaccination programme. BMJ Open. 2019;9:e025553. doi:10.1136/bmjopen-2018-025553.
- Van Oorschot D, Anastassopoulou A, Poulsen Nautrup B, Varghese L, von Krempelhuber A, Neine M, Lorenc S, Curran D. Cost-effectiveness of the recombinant zoster vaccine in the German population aged ≥60 years old. Hum Vaccin Immunother. 2019;15:34–44. doi:10.1080/21645515.2018.1509645.
- Curran D, Van Oorschot D, Varghese L, Oostvogels L, Mrkvan T, Colindres R, von Krempelhuber A, Anastassopoulou A. Assessment of the potential public health impact of herpes zoster vaccination in Germany. Hum Vaccin Immunother. 2017;13:2213–21. doi:10.1080/21645515.2017.1345399.
- Curran D, Van Oorschot D, Matthews S, Hain J, Salem AE, Schwarz M. Long-term efficacy data for the recombinant zoster vaccine: impact on public health and cost effectiveness in Germany. Hum Vaccin Immunother. 2021;17:5296–303. doi:10.1080/21645515.2021.2002085.
- Volpi A, Boccalini S, Dari S, Clarke C, Curran D, Loiacono I, Pitrelli A, Puggina A, Tosatto R, Van Oorschot D, et al. The potential public health impact of herpes zoster vaccination in the 65 years of age cohort in Italy. Hum Vaccin Immunother. 2020;16:327–34. doi:10.1080/21645515.2019.1657753.
- de Boer PT, van Lier A, de Melker H, van Wijck AJM, Wilschut JC, van Hoek AJ, Postma MJ. Cost-effectiveness of vaccination of immunocompetent older adults against herpes zoster in the Netherlands: a comparison between the adjuvanted subunit and live-attenuated vaccines. BMC Med. 2018;16:228. doi:10.1186/s12916-018-1213-5.
- Lee C, Jiang N, Tang H, Ye C, Yuan Y, Curran D. Potential public health impact of the adjuvanted recombinant zoster vaccine among people aged 50 years and older in Beijing. Hum Vaccin Immunother. 2021;17:3735–46. doi:10.1080/21645515.2021.1932216.
- Watanabe D, Mizukami A, Holl K, Curran D, Van Oorschot D, Varghese L, Shiragami M. The potential public health impact of herpes zoster vaccination of people aged ≥ 50 years in Japan: results of a Markov model analysis. Dermatol Ther (Heidelb). 2018;8:269–84. doi:10.1007/s13555-018-0236-3.
- Teng L, Mizukami A, Ng C, Giannelos N, Curran D, Sato T, Lee C, Matsuki T. Cost-effectiveness analysis update of the adjuvanted recombinant zoster vaccine in Japanese older adults. Dermatol Ther (Heidelb). 2022;12:1447–67. doi:10.1007/s13555-022-00744-8.
- Shiragami M, Mizukami A, Kaise T, Curran D, Van Oorschot D, Bracke B, Watanabe D. Cost-effectiveness of the adjuvant recombinant zoster vaccine in Japanese adults aged 65 years and older. Dermatol Ther (Heidelb). 2019;9:281–97. doi:10.1007/s13555-019-0291-4.
- Udayachalerm S, Renouard MG, Anothaisintawee T, Thakkinstian A, Veettil SK, Chaiyakunapruk N. Incremental net monetary benefit of herpes zoster vaccination: a systematic review and meta-analysis of cost-effectiveness evidence. J Med Econ. 2022;25:26–37. doi:10.1080/13696998.2021.2008195.
- Meredith NR, Armstrong EP. Cost-effectiveness of herpes zoster vaccines in the U.S.: a systematic review. Prev Med Rep. 2022;29:101923. doi:10.1016/j.pmedr.2022.101923.
- Sociedade Brasileira de Imunizações (SBIm). Nota Técnica SBIm: vacina herpes-zóster inativada recombinante (Shingrix®; [accessed 2022 Aug 12]. https://sbim.org.br/informes-e-notas-tecnicas/sbim/1692-nota-tecnica-sbim-vacina-herpes-zoster-inativada-recombinante-shingrix-220608.
- Instituto Nacional de Estadìstica y Censos (INDEC). Estimaciones y proyecciones de población 2010-2040. Total del paìs; [accessed 2022 Jun 23]. https://www.indec.gob.ar/ftp/cuadros/publicaciones/proyeccionesyestimaciones_nac_2010_2040.pdf.
- Instituto Brasileiro de Geografia e Estatística (IBGE). Projeções da População (Projeções da população por sexo e idades); [accessed 2022 Jun 23]. https://www.ibge.gov.br/estatisticas/sociais/populacao/9109-projecao-da-populacao.html?=&t=resultados.
- Consejo Nacional de Población (CONAPO). Proyecciones de la Población de México y de las Entidades Federativas, 2016-2050. Proyecciones de la Población de los Municipios de México, 2015-2030 (base 1); [accessed 2021 Nov 30]. https://datos.gob.mx/busca/dataset/proyecciones-de-la-poblacion-de-mexico-y-de-las-entidades-federativas-2016-2050.
- Instituto Nacional de Estadística (INE). Chile. Proyecciones de población. CUADROS ESTADÍSTICOS. Proyección base 2017. Estimaciones y proyecciones 2002-2035, comuna y área urbana y rural; [accessed 2021 Nov 30]. https://www.ine.cl/estadisticas/sociales/demografia-y-vitales/proyecciones-de-poblacion.
- DANE. Proyecciones de población. Serie nacional de población por área, sexo y edad para el periodo 2018 – 2070 [accessed 2021 Nov 30]. https://www.dane.gov.co/index.php/estadisticas-por-tema/demografia-y-poblacion/proyecciones-de-poblacion.
- Instituto Nacional de Estadìstica y Censos (INDEC). TABLA DE MORTALIDAD 2008-2010. TOTAL DEL PAÍS. AMBOS SEXOS; [accessed 2022 Aug 7]. https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwww.indec.gob.ar%2Fftp%2Fcuadros%2Fpoblacion%2Ftm_0810_totalpais.xls&wdOrigin=BROWSELINK.
- Instituto Brasileiro de Geografia e Estatística (IBGE). Tábuas Completas de Mortalidade (Ambos os Sexos) [accessed 2021 Nov 30]. https://www.ibge.gov.br/estatisticas/sociais/populacao/9126-tabuas-completas-de-mortalidade.html?=&t=resultados.
- Consejo Nacional de Población (CONAPO). Proyecciones de la Población de México y de las Entidades Federativas, 2016-2050. Dufunciones 1950 - 2050; [accessed 2021 Nov 30]. https://datos.gob.mx/busca/dataset/proyecciones-de-la-poblacion-de-mexico-y-de-las-entidades-federativas-2016-2050/.
- Departamento de Estadísticas e Información de Salud (DEIS). Estadísticas de Mortalidad. Defunciones según grupo de edad, región, gran grupo de causas de muerte y sexo. Chile; 2014 [accessed 2021 Nov 30]. https://deis.minsal.cl/#estadisticas.
- DANE. Defunciones no fetales 2019. Cuadro 1. Defunciones, por área donde ocurrió la defunción y sexo, según grupos de edad. Total nacional; [accessed 2021 Nov 30]. https://www.dane.gov.co/index.php/estadisticas-por-tema/salud/nacimientos-y-defunciones/defunciones-no-fetales/defunciones-no-fetales-2019.
- Antoniolli L, Rodrigues C, Borges R, Goldani LZ. Epidemiology and clinical characteristics of herpes zoster in a tertiary care hospital in Brazil. Braz J Infect Dis. 2019;23:143–45. doi:10.1016/j.bjid.2019.03.001.
- Yawn BP, Wollan PC, Kurland MJ, St Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86:88–93. doi:10.4065/mcp.2010.0618.
- Abreu AJL, Venys A, Jacob W, Silva T, Henrique A, Gopala K, Barros ENC. Burden of herpes zoster among Brazilian adults – a hospital-based study. Geriatr Gerontol Aging. 2021;15:e0210035. doi:10.53886/gga.e0210035.
- Bollea-Garlatti ML, Bollea-Garlatti LA, Vacas AS, Torre AC, Kowalczuk AM, Galimberti RL, Ferreyro BL. Clinical characteristics and outcomes in a population with disseminated herpes zoster: a retrospective cohort study. Actas Dermosifiliogr. 2017;108:145–52. doi:10.1016/j.ad.2016.10.009.
- Rozenek M, Romani A, Aronson S, Ramilo MD, Abellan V, Perez MA, Camera L. Herpes zoster in elderly adults in a community hospital in Buenos Aires. June 2013-May 2014. Medicina (B Aires). 2017;77:24–30.
- de Martino Mota A, Carvalho-Costa FA. Varicella zoster virus related deaths and hospitalizations before the introduction of universal vaccination with the tetraviral vaccine. J Pediatr (Rio J). 2016;92:361–66. doi:10.1016/j.jped.2015.10.003.
- DGIS. Egresos Hospitalarios, Urgencias y Defunciones. Cubos dinámicos. Secretaría de Salud DGIS Mexico; [accessed 2020 Aug 16]. http://www.dgis.salud.gob.mx/contenidos/basesdedatos/bdc_egresoshosp_gobmx.html.
- Toniolo-Neto J, Psaradellis E, Karellis A, Rampakakis E, Rockett TY, Sampalis JS, Johnson KD, Monsanto HA, Acosta CJ. Measuring herpes zoster disease burden in Sao Paulo, Brazil: a clinico-epidemiological single-center study. Clinics (Sao Paulo). 2018;73:e243. doi:10.6061/clinics/2018/e243.
- Vujacich C, de Wouters L, Margari A, Gordóvil M, Kawai K, Lemos E, Rampakakis E, Pollock C, White R, Acosta CJ, et al. Assessment of burden of illness due to herpes zoster in Argentina: a prospective observational study. Value Health. 2013;16:A668. doi:10.1016/j.jval.2013.08.1925.
- Drolet M, Brisson M, Schmader K, Levin M, Johnson R, Oxman M, Patrick D, Camden S, Mansi JA. Predictors of postherpetic neuralgia among patients with herpes zoster: a prospective study. J Pain. 2010;11:1211–21. doi:10.1016/j.jpain.2010.02.020.
- You JHS, Ming WK, Tsang OT, Chan PK. Optimal gender-specific age for cost-effective vaccination with adjuvanted herpes zoster subunit vaccine in Chinese adults. PLoS One. 2019;14:e0210005. doi:10.1371/journal.pone.0210005.
- Nieto Guevara J, Guzman-Holst A. Laboratory-based surveillance in Latin America: attributes and limitations in evaluation of pneumococcal vaccine impact. Hum Vaccin Immunother. 2021;17:4667–72. doi:10.1080/21645515.2021.1972709.
- Damm O, Ultsch B, Horn J, Mikolajczyk RT, Greiner W, Wichmann O. Systematic review of models assessing the economic value of routine varicella and herpes zoster vaccination in high-income countries. BMC Public Health. 2015;15:533. doi:10.1186/s12889-015-1861-8.
- Garnett GP, Grenfell BT. The epidemiology of varicella-zoster virus infections: the influence of varicella on the prevalence of herpes zoster. Epidemiol Infect. 1992;108:513–28. doi:10.1017/s0950268800050019.
- Karhunen M, Leino T, Salo H, Davidkin I, Kilpi T, Auranen K. Modelling the impact of varicella vaccination on varicella and zoster. Epidemiol Infect. 2010;138:469–81. doi:10.1017/S0950268809990768.
- Guzzetta G, Poletti P, Merler S, Manfredi P. The epidemiology of herpes zoster after varicella immunization under different biological hypotheses: perspectives from mathematical modeling. Am J Epidemiol. 2016;183:765–73. doi:10.1093/aje/kwv240.
- Brisson M, Gay NJ, Edmunds WJ, Andrews NJ. Exposure to varicella boosts immunity to herpes-zoster: implications for mass vaccination against chickenpox. Vaccine. 2002;20:2500–07. doi:10.1016/s0264-410x(02)00180-9.
- Leung J, Dooling K, Marin M, Anderson TC, Harpaz R. The impact of universal varicella vaccination on herpes zoster incidence in the United States: comparison of birth cohorts preceding and following varicella vaccination program launch. J Infect Dis. 2022;226:S470–77. doi:10.1093/infdis/jiac255.
- Harder T, Siedler A. Systematic review and meta-analysis of chickenpox vaccination and risk of herpes zoster: a quantitative view on the “exogenous boosting hypothesis”. Clin Infect Dis. 2019;69:1329–38. doi:10.1093/cid/ciy1099.
- Harpaz R. Do varicella vaccination programs change the epidemiology of herpes zoster? A comprehensive review, with focus on the United States. Expert Rev Vaccines. 2019;18:793–811. doi:10.1080/14760584.2019.1646129.
- Talbird SE, La EM, Mauskopf J, Altland A, Daniels V, Wolfson LJ. Understanding the role of exogenous boosting in modeling varicella vaccination. Expert Rev Vaccines. 2018;17:1021–35. doi:10.1080/14760584.2018.1538801.
- Brazil Ministry of Health, SUS Informatics Department (DATASUS). Tabnet. National immunization program information system. Immunizations since 1994. Coverages (“Coberturas”); [accessed 2022 Nov 30]. http://tabnet.datasus.gov.br/cgi/webtabx.exe?bd_pni/cpnibr.def.
- de Salud Argentina M. Coberturas de Vacunación por Jurisdicción. Calendario Nacional de Vacunación 2009-2020; [accessed 2022 Dec 5]. https://bancos.salud.gob.ar/sites/default/files/2021-12/coberturas-de-vacunacion-por-jurisdiccion-cnv-2009-2020.pdf.
- de Salud M. Informe de Cobertura Nacional de Inmunizaciones año; 2022 [accessed 2022 Dec 5]. https://vacunas.minsal.cl/wp-content/uploads/2022/08/Informe-de-Coberturas_2022_enero_junio.pdf.
- Pawaskar M, Gil-Rojas Y, Irene Parellada C, Rey-Velasco A, Beltran C, Prieto E, Lasalvia P. The impact of universal varicella vaccination on the clinical burden of varicella in Colombia: a national database analysis, 2008-2019. Vaccine. 2022;40:5095–102. doi:10.1016/j.vaccine.2022.07.012.
- Levin MJ. Immune senescence and vaccines to prevent herpes zoster in older persons. Curr Opin Immunol. 2012;24:494–500. doi:10.1016/j.coi.2012.06.002.
- Laemmle L, Goldstein RS, Kinchington PR. Modeling varicella zoster virus persistence and reactivation - closer to resolving a perplexing persistent state. Front Microbiol. 2019;10:1634. doi:10.3389/fmicb.2019.01634.
- Syed YY. Recombinant zoster vaccine (Shingrix®): a review in herpes zoster. Drugs Aging. 2018;35:1031–40. doi:10.1007/s40266-018-0603-x.