ABSTRACT
Introduction of primary COVID-19 vaccination has helped reduce severe disease and death caused by SARS-CoV-2 infection. Understanding the protection conferred by heterologous booster regimens informs alternative vaccination strategies that enable programmatic resilience and can catalyze vaccine confidence and coverage. Inactivated SARS-CoV-2 vaccines are among the most widely used vaccines worldwide. This review synthesizes the available evidence identified as of May 26, 2022, on the safety, immunogenicity, and effectiveness of a heterologous BNT162b2 (Pfizer-BioNTech) mRNA vaccine booster dose after an inactivated SARS-CoV-2 vaccine primary series, to help protect against COVID-19. Evidence showed that the heterologous BNT16b2 mRNA vaccine booster enhances immunogenicity and improves vaccine effectiveness against COVID-19, and no new safety concerns were identified with heterologous inactivated primary series with mRNA booster combinations.
Introduction
More than 12.9 billion coronavirus disease 2019 (COVID-19) vaccine doses have been administered globally, with 5.4 billion individuals fully vaccinated and approximately 2.3 billion boosted to reduce severe disease and death caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).Citation1,Citation2 Inactivated virus vaccines account for about half of the COVID-19 vaccine doses delivered globally and have been administered in primarily low- and middle-income countries.Citation3,Citation4 Three inactivated vaccines have received the World Health Organization (WHO) Emergency Use Listing (EUL): CoronaVac (Sinovac Life Sciences Co., Ltd; used in 60 countries), BBIBP-Cor-V (Beijing Institute of Biological Products Co., Ltd./Sinopharm; used in 93 countries), and Covaxin (Bharat Biotech International; used in 27 countries).Citation5–9 In coming years, as the various vaccines are mixed and matched, understanding the safety, effectiveness, and durability of protection of heterologous primary and booster regimens will be important to help support alternative vaccination strategies and programmatic flexibility amid supply delays, increase population confidence, and impact vaccination uptake.Citation10 Strategies for maximizing protection against SARS-CoV-2 should be considered; in that regard, emerging evidence indicates that heterologous booster vaccination is associated with an improved immune response and effectiveness compared with homologous vaccination, at least with respect to certain combinations.Citation11,Citation12 Additionally, current recommendations regarding the available vaccines in low- and middle-income countries,Citation13,Citation14 together with real-world evidence (RWE) from these countries, reinforce the important role of a heterologous booster in some regions. A detailed assessment of all existing evidence on which vaccine is best as a booster dose after primary vaccination with inactivated vaccines is not currently available.
Messenger RNA (mRNA) vaccines are emerging as the preferred heterologous booster in recommendations from vaccine technical committees, such as the WHO-Strategic Advisory Group of Experts on Immunization (SAGE)Citation15 and the US Centers for Disease Control and Prevention (CDC),Citation16 based on the accumulated evidence of safety, high vaccine effectiveness (VE), and breadth of protection against emerging variants. Given the widespread use of inactivated SARS-CoV-2 vaccines, particularly in low- and middle-income countries, and that BNT162b2 is the most widely used mRNA COVID-19 vaccine globally, we reviewed available evidence on the safety, immunogenicity, and effectiveness of a heterologous BNT162b2 booster dose after complete primary vaccination with an inactivated SARS-CoV-2 vaccine. It is hoped that this evidence will help regional and country vaccine technical committees make decisions regarding vaccination strategies to overcome the COVID-19 pandemic.
Search methods
For the evidence summary, a targeted search was conducted of PubMed and EMBASE databases for published articles, as well as bioRxiv for preprint articles, to identify studies evaluating the safety, immunogenicity, or VE of BNT162b2 booster vaccination after primary vaccination with an inactivated SARS-CoV-2 vaccine, published up to May 26, 2022. English language articles identified through a search for heterologous booster, COVID-19 vaccine booster, heterologous vaccination, mixed vaccination, inactivated SARS-CoV2 vaccine, inactivated COVID-19 vaccine, mRNA vaccine, and mRNA COVID-19 vaccine were reviewed. Those reporting RWE or clinical studies of homologous or heterologous COVID-19 vaccination (inactivated vaccine primary and booster dose vs inactivated vaccine primary series with BNT162b2 booster dose) were included. Case reports, reviews, editorials, and letters to editor that did not have the original data on homologous or heterologous COVID-19 vaccination were not included. To avoid missing relevant studies, we checked the bibliographies of the published meta-analyses and systematic reviews. For the pooled analysis, data preparation for analysis and cleaning was done using Microsoft Office 365, and analysis was performed by R Version 4.0.5 software. Estimates of VE were extracted from different publications. Point estimates of ln(1–VE/100) and 95% CIs were calculated from VE results. Random effects models were used to estimate pooled effects.
Results of evidence synthesis
Thirty-three studies were identified with inactivated-mRNA or inactivated-viral vector combinations,Citation17–49 with the majority of studies (28/33) involving the CoronaVac inactivated vaccine primary series (). Among the identified studies, three randomized clinical trials,Citation23,Citation33,Citation36 and five nonrandomized, uncontrolled clinical studiesCitation19,Citation30,Citation32,Citation41,Citation49 contributed safety and immunogenicity data. Six real-world studies contributed VE data,Citation21,Citation27,Citation37,Citation38,Citation40,Citation45 and 19 studies contributed immunogenicity (including B- and T-cell responses) data alone.Citation11,Citation17,Citation18,Citation20,Citation24–26,Citation28–29,Citation31–34,Citation35–39,Citation42–44,Citation46–48 outlines the study designs and methodological details of the included studies. describes the dosing intervals utilized between the last dose of the primary immunization series and the booster dose for included studies.
Figure 1. Summary of dosing interval between the last dose of the primary immunization series and the booster dose for each study. NR, not reported.
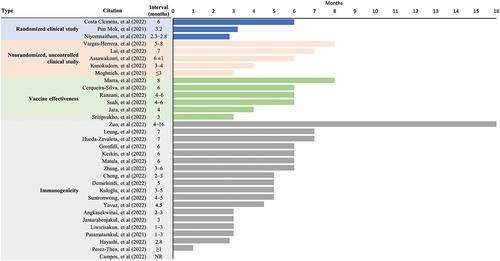
Table 1. Details of all included studiesa.
Randomized controlled trials evaluating immunogenicity and safety of a heterologous BNT162b2 booster dose after primary vaccination with two doses of inactivated vaccine
The safety and immunogenicity of the BNT162b2 booster vaccination after primary vaccination with an inactivated virus COVID-19 vaccine (heterologous booster dose) is primarily inferred from three independent randomized clinical studies conducted in Brazil, Thailand, and Hong Kong.Citation23,Citation33,Citation36 Two of these studies evaluated a heterologous booster dose of BNT162b2 after two doses of CoronaVac compared with a homologous booster dose,Citation23,Citation36 and the third study examined a heterologous booster dose of BNT162b2 after one dose of ChAdOx1 and one dose of CoronaVac in any sequence ().Citation33
A phase 4, participant-blind, randomized clinical trial conducted by the Ministry of Health in Brazil evaluated a heterologous BNT162b2 booster (third) dose between August 16 and September 1, 2021, in adults ≥18 y of age who had completed a 2-dose primary vaccination with CoronaVac 6 months earlier and who reported no history of SARS-CoV-2 infection.Citation23 The primary analysis (N = 1205) compared participants randomized to receive a heterologous booster (third) dose of BNT162b2 (n = 333), Ad26.COV2.S (n = 295), or ChAdOx1 nCoV-19 (n = 296) with participants who received a homologous third dose of CoronaVac (n = 281). Noninferiority of anti-spike IgG levels 28 d after the booster dose in heterologous versus homologous booster regimens (primary outcome) was determined using 0.67 as margin for the geometric mean ratio (GMR). Secondary outcomes included Day 28 pseudovirus neutralizing antibody titers measured by a lentivirus expressing the D614 SARS-CoV-2 spike protein and by live virus neutralization using SARS-CoV-2 variants (Delta [B.1.617.2] and Omicron [B.1.1.529]), local and systemic reactogenicity profiles, adverse events (AEs), and serious AEs. At 6 months after the primary vaccination with two doses of CoronaVac, concentrations of antibodies were low and neutralizing antibodies were largely undetectable. All heterologous regimens were superior to the homologous regimen, both with regard to anti-spike IgG antibodies and neutralizing antibody titers (all p < .001). Heterologous BNT162b2 booster elicited the highest magnitude immune response; the GMR of anti-spike IgG (heterologous vs homologous) was greatest with the BNT162b2 booster (13.4; 95% CI: 11.6, 15.3) followed by ChAdOx1 nCoV-19 (7.0, 95% CI: 6.1, 8.1) and Ad26.COV2.S (6.7; 95% CI: 5.8, 7.7). Similarly, GMR for pseudovirus neutralizing antibodies (heterologous vs homologous) was highest with the BNT162b2 booster (21.5; 95% CI: 14.5, 31.9), followed by ChAdOx1 nCoV-19 (10.6; 95% CI 7.2, 15.6) and Ad26.COV2.S (8.7; 95% CI: 5.9, 12.9); a third vaccine dose also boosted in vitro live virus neutralization of Delta and Omicron variants. AEs were assessed through 28 d after the booster dose. Increased reactogenicity observed with the heterologous versus homologous booster dose was consistent with previous studies, such as the Com-CoV Boost study from the United Kingdom.Citation50 Three of five serious AEs (including one in the BNT162b2 group and two in the Ad26.COV2.S group) were deemed possibly related to the booster vaccine; however, all participants recovered and returned home.
A randomized clinical trial conducted between August and October 2021 in Hong Kong evaluated a heterologous BNT162b2 booster (third) dose following two doses of CoronaVac.Citation36 Adults 34–73 y of age who had completed primary vaccination with the CoronaVac 2-dose series about 3.2 months earlier (n = 80) were randomized to receive a third, heterologous dose of BNT162b2 (n = 40) or CoronaVac (n = 40). Primary outcomes measured at 1 month after heterologous versus homologous booster dose were surrogate virus neutralization tests (sVNTs), 50% plaque reduction neutralization tests, and anti–N-terminal domain-specific Ig antibodies. Secondary outcomes included AEs at 7 d and at 1 month after the booster. The heterologous BNT162b2 booster elicited a significantly higher immune response than the homologous booster based on percentage inhibition in the sVNT assay, both overall (96.8% vs 57.8%; p < .001) and against the Beta (92.3% vs 38.8%), Gamma (92.5% vs 32.2%), and Delta (95.3% vs 48.9%; all p < .001) variants. No new safety concerns were identified. Although significantly more participants who received the heterologous versus a homologous booster dose reported fatigue and muscle pain, they were considered acceptable.
Results of an open-label, randomized clinical trial in Thailand (conducted January–June 2021) that evaluated a heterologous triple platform vaccination support a mix-and-match approach using a heterologous primary series (CoronaVac/ChAdOx1) and BNT162b2 as the final (third) dose.Citation33 Adults 18–60 y of age with no known history of SARS-CoV-2 infection received a heterologous 2-dose primary ChAdOx1-CoronaVac (n = 30; n = 29 analyzed) or CoronaVac-ChAdOx1 (n = 30) series, followed by a heterologous booster (third) dose of BNT162b2. Results reported herein are within the context of a larger trial (N = 210) in which participants were randomized to one of seven groups that received 2-dose homologous BNT162b2 or heterologous combinations of CoronaVac, ChAdOx1, and BNT162b2 administered 4 weeks apart. Neutralizing antibodies against SARS-CoV-2 variants were measured. A heterologous BNT162b2 booster (third dose) given to participants who received heterologous CoronaVac-ChAdOx1 or ChAdOx1-CoronaVac primary series, in either sequence, induced higher levels of neutralizing antibodies against the Delta and Omicron variants. Notably, higher neutralizing antibodies were observed against Omicron with ChAdOx1-CoronaVac-BNT162b2 (1151-fold increase vs after dose 2) versus CoronaVac-ChAdOx1-BNT162b2 regimens (146-fold increase vs after dose 2). AEs reported after the BNT162b2 booster (third dose) were similar to those reported after the BNT162b2 second dose.
Safety results were generally consistent across the three studies, with no new safety concerns identified.Citation23,Citation33,Citation36 Increased reactogenicity was observed with heterologous versus homologous booster dose,Citation23,Citation36 which was consistent with previous studies, such as the Com-CoV Boost study from the United KingdomCitation50 ().
Table 2. Safety overview of data from included studiesa.
Real-world vaccine effectiveness of a BNT162b2 booster dose after primary vaccination with two doses of inactivated vaccine
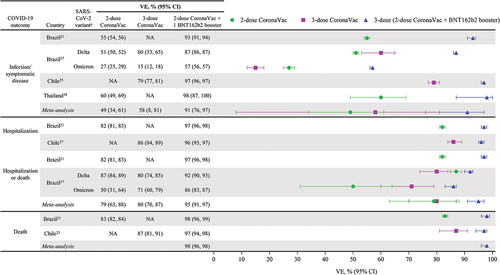
Six real-world effectiveness studies from four countries (Brazil, Chile, Malaysia, and Thailand) measured VE after a heterologous booster with BNT162b2 that followed inactivated virus primary vaccination.Citation21,Citation27,Citation37,Citation38,Citation40,Citation45 A test-negative case–control study evaluated the VE of a heterologous BNT162b2 booster dose after CoronaVac primary vaccination against severe COVID-19 outcomes, including hospitalization and death, among adults ≥18 y of age in Brazil during January to November 2021, when Gamma and Delta variants were predominant.Citation21 National vaccine administration databases were analyzed using additive logistic regression to evaluate the VE of the 2-dose schedule of CoronaVac and a single booster dose of BNT162b2 on reverse transcription-polymerase chain reaction (RT-PCR)–confirmed SARS-CoV-2 infection and COVID-19 hospitalization or death. From approximately 7.3 million eligible individuals, test-positive cases were generally matched to test-negative controls based on clinical and sociodemographic characteristics. Among 913,052 individuals who were vaccinated with CoronaVac, 7863 received a booster dose of BNT162b2, of whom >90% were tested within 30 d after receiving the booster. Over the 6 months after primary vaccination, CoronaVac VE waned from 55.0% (95% CI: 54.3%, 55.7%) to 34.7% (95% CI: 33.1%, 36.2%) against infection and from 82.1% (95% CI: 81.4%, 82.8%) to 72.5% (95% CI: 70.9%, 74.0%) against severe outcomes. A heterologous booster dose of BNT162b2 improved VE to 92.7% (95% CI: 91.0%, 94.0%) against infection and 97.3% (95% CI: 96.1%, 98.1%) against severe outcomes at 14–30 d, values greater than initially achieved with the 2-dose CoronaVac regimen (). Waning of CoronaVac VE was higher among ≥80-year-olds versus younger adults, but the BNT162b2 booster dose increased levels of protection across the age groups assessed (). BNT162b2 is used primarily as a booster in Brazil’s national immunization program, and a longer follow-up time is needed to assess duration of protection after the booster dose.
Figure 2. Heterologous BNT162b2 booster dose restores waning VE against COVID-19 outcomes, with Delta and Omicron variants based on VE (95% CI) over time reported in real-world effectiveness studies. NA, not available; VE, vaccine effectiveness.
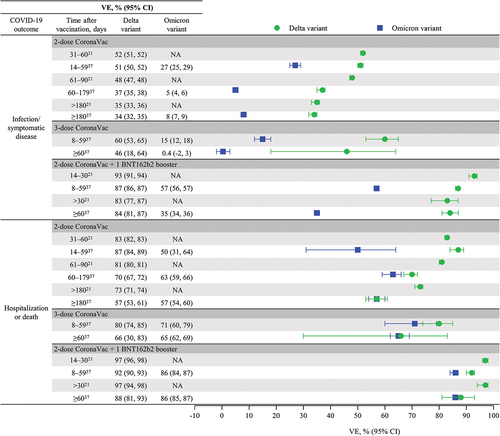
Figure 3. Heterologous BNT162b2 booster dose improves VE against COVID-19 hospitalization and death in the elderly (≥60 y of age) from real-world effectiveness studies. NA, not available; NE, not evaluable; VE, vaccine effectiveness.
The VE of a heterologous BNT162b2 booster dose after CoronaVac primary vaccination against symptomatic and severe COVID-19 was assessed in Brazil among adults ≥18 y of age using data from two national databases during periods when the Delta variant (September 6–December 14, 2021) and the Omicron variant (December 25, 2021–March 10, 2022) predominated.Citation37 This test-negative case–control study used conditional logistic regression analyses to estimate the VE of the 2-dose schedule of CoronaVac and a single booster dose of BNT162b2 (administered 4‒6 months after primary series completion) on RT-PCR‒ or antigen-test‒confirmed SARS-CoV-2 symptomatic infection and COVID-19 hospitalization or death. Overall, 1,339,986 cases were matched to 1,339,986 test-negative controls across Delta and Omicron predominance periods based on sex, age group, geography, calendar week of PCR test, and adjusted for chronic comorbidities, self-reported race, and any reported previous symptomatic event. During the Delta period, VE against symptomatic infection waned to 33.5% (95% CI: 31.7%, 35.3%) over 6 months after primary vaccination with 2-dose CoronaVac, increased to 59.8% (95% CI: 53.3, 65.3%) within 59 d after a homologous CoronaVac booster, and then decreased to 45.5% (95% CI: 18.1%, 63.7%) by >60 d after the booster. In contrast, a heterologous BNT162b2 booster increased VE substantially to 86.6% (95% CI: 85.9%, 87.3%) and showed little waning at 60 d and beyond (VE, 84.3%; 95% CI: 80.9, 87.2%; ). Although VE against symptomatic infection waned to a greater degree after primary vaccination and with both homologous and heterologous boosters, a similar pattern was observed during the Omicron period ().
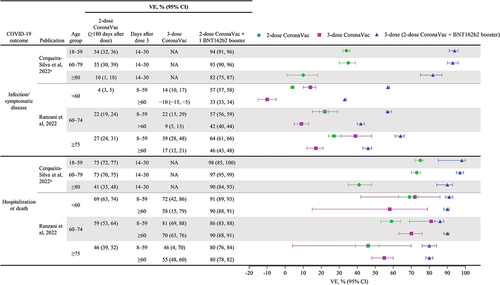
Jara and colleaguesCitation27 reported the VE of a heterologous BNT162b2 booster dose after CoronaVac primary vaccination against infection, symptomatic disease, hospitalization, and intensive care unit (ICU) admission among individuals ≥16 y of age between February 2 and November 10, 2021, when the Delta variant was predominant in Chile. This prospective cohort study analyzed a national administrative dataset representing 80% of the Chilean population to estimate hazard ratios (HRs) from adjusted Cox models between treated individuals who received homologous CoronaVac and heterologous BNT162b2 or ChAdOx1 vaccine boosters administered 4 months after completion of a 2-dose CoronaVac primary series and unvaccinated individuals. Vaccine effectiveness was calculated as 1 minus the corresponding HR and expressed as a percentage. Analysis of data from 11.2 million eligible individuals was adjusted for individual characteristics associated with the probability of treatment (i.e., vaccination) such as age, sex, number of comorbidities, and nationality. A total of 7,016,865 individuals (74.5%) were vaccinated with CoronaVac, of whom 186,946 received a third dose of CoronaVac, 2,019,260 received a BNT162b2 booster dose, and 1,921,340 received the ChAdOx1 booster dose. Compared with a homologous booster, a heterologous booster postprimary vaccination with CoronaVac showed higher VE for all outcomes. VE against symptomatic COVID-19 was higher after a heterologous booster (third) dose with BNT162b2 (96.5%; 95% CI: 96.2%, 96.7%) or ChAdOx1 (93.2%; 95% CI: 92.9%, 93.6%) than with a 3-dose CoronaVac regimen (78.8%; 95% CI: 76.8%, 80.6%). Similarly, VE against hospitalization, ICU admission, and COVID-19–related deaths were higher after a heterologous booster (third) dose with BNT162b2 (96.1%, 96.2%, and 96.8%, respectively) or ChAdOx1 (97.7%, 98.9%, and 98.1%, respectively) than after a 3-dose CoronaVac regimen (86.3%, 92.2%, and 86.7%, respectively).
A prospective test-negative case-control study (N = 3353) used logistic regression analyses to evaluate the VE of a heterologous booster dose (BNT162b2 or ChAdOx1) administered 3 months after CoronaVac primary vaccination for preventing COVID-19 among adults ≥18 y of age in Thailand between July 25 and October 23, 2021, when the Delta variant accounted for 95–100% of cases.Citation38 Test-positive cases were matched 1:2 to test-negative controls adjusted for sex, age group, number of comorbidities, and being a health-care worker (HCW). The adjusted VE against COVID-19 was highest with the 2-dose CoronaVac primary series plus a third dose of BNT162b2 (98%; 95% CI: 87%, 100%), followed by a third dose of ChAdOx1 (86%; 95% CI: 74%, 93%); both heterologous booster regimens had greater VE than the 2-dose CoronaVac regimen (60%; 95% CI: 49%, 69%).
Vaccine effectiveness against hospitalization or death during the Delta period waned over 6 months to 57.3% (95% CI: 53.4%, 60.9%) after primary vaccination with CoronaVac, increased to 80% (95% CI: 74%, 85%) within 59 d after homologous booster, and waned to 66% (95% CI: 30%, 83%) thereafter, whereas a heterologous BNT162b2 booster increased VE substantially to 92% (95% CI: 90%, 93%) within 59 d and showed little waning thereafter, with 88% (95% CI: 81%, 93%) VE (). Similarly, during the Omicron period, VE against hospitalization or death waned to 57% (95% CI: 54%, 60%) over 6 months after primary vaccination, increased to 71% (95% CI: 60%, 79%) within 59 d after a homologous (CoronaVac) booster, and then decreased slightly to 65% (95% CI: 62%, 69%) thereafter. In contrast, a heterologous BNT162b2 booster increased VE to 86% (95% CI: 84%, 87%) within 59 d, and at 60 d and later was unchanged at 86% (95% CI: 85%, 87%). Although VE of the primary CoronaVac series, the CoronaVac booster, and the BNT162b2 against hospitalization or death were lower among individuals ≥75 y of age than those aged <60 and 60–74 y, VE against hospitalization or death was significantly higher among those ≥75 y of age who received a BNT162b2 booster versus a CoronaVac booster (). Overall, these results indicate that a heterologous BNT162b2 booster restored high effectiveness against severe COVID-19 outcomes, while a homologous CoronaVac booster was unable to augment protection.
A report by Suah and colleagues evaluated VE of a heterologous BNT162b2 booster dose after CoronaVac primary vaccination in preventing COVID-19 in Malaysia by analyzing national administrative data, including a COVID-19 vaccine recipients registry, an RT-PCR and antigen tests registry, a listing of confirmed cases, and a listing of confirmed contacts from the national automated contact tracing system.Citation40 This test-negative case–control study used multivariable logistic regression analyses to evaluate the VE of a heterologous BNT162b2 or ChAdOx1 booster dose received 4–6 months (between October 27, 2021, and February 4, 2022) after completion of a 2-dose CoronaVac primary series (between July 1 and September 30, 2021) for preventing COVID-19 among adults ≥18 y of age, during periods when Delta and Omicron variants were predominant. Of the approximately 12.6 million eligible individuals, the study enrolled approximately 1.9 million participants over the Delta-predominant period and approximately 1.0 million participants over Omicron-predominant period; test-positive cases were matched 1:5 for Delta and 1:2 for Omicron to test-negative controls. Analyses were adjusted for sex, age group, comorbidities, ethnicity, geography, being an HCW, vaccine receipt from a private source or national program, month of primary vaccination, number of SARS-CoV-2 tests taken, and number of times flagged as a contact in the contact-tracing system. Notably, this study used individuals who received a 2-dose BNT162b2 regimen as a reference group (instead of unvaccinated individuals) to measure the marginal effectiveness of 3-dose regimens with homologous and heterologous booster vaccines. A heterologous regimen consisting of 2-dose CoronaVac followed by a BNT162b2 booster demonstrated an improved marginal VE compared with a homologous 3-dose CoronaVac regimen of 48% (95% CI: 47%, 48%) versus 33% (95% CI: 32%, 35%) during the Omicron period and 85% (95% CI: 85%, 85%) versus 82% (95% CI: 82%, 83%) during the Delta period, respectively.
In a retrospective cohort study, Marra and colleagues analyzed data from symptom-based testing of adult HCWs at a large hospital in São Paulo, Brazil, to evaluate the VE of a heterologous BNT162b2 booster dose after CoronaVac primary vaccination against laboratory-confirmed COVID-19.Citation45 Vaccine effectiveness (defined as the incidence rate ratio) was determined using Poisson models of the occurrence of laboratory-confirmed COVID-19 infection, adjusting for age, sex, and job type. The study compared VE of the 2-dose schedule of CoronaVac followed a median of 8 months later by a single booster dose of BNT162b2 with that of two doses of CoronaVac with no booster among adult HCWs ≥18 y of age during January 1 to December 30, 2021, when Gamma and Delta variants were circulating in Brazil. Overall, 11,427 HCWs met inclusion criteria (71% female, median age 36 y): 1157 (10%) received the 2-dose CoronaVac vaccine, and 4472 (39%) received the 2-dose CoronaVac vaccine followed by a single BNT162b2 booster dose. Compared with the individuals who received primary vaccination only, the group that received the booster vaccine was significantly older, had greater proportions of HCWs with patient contact, and had higher rates of comorbidities. Cases of COVID-19 occurred in 0.9% of those who received the 2-dose CoronaVac plus BNT162b2 booster regimen versus 31.5% of those who received the 2-dose CoronaVac vaccine only, corresponding to an adjusted VE of 92% (95% CI: 89%, 94%) for the 2-dose CoronaVac vaccine plus BNT162b2 booster regimen. Genomic sequencing of COVID-19 case isolates revealed that 22% were Gamma and 78% were Delta; furthermore, Gamma isolates were predominant in July through August, whereas Delta was predominant in September through December 2021. Taken together, these results indicate that a BNT162b2 heterologous booster given after the CoronaVac primary series confers substantial protection.
Pooled analysis to estimate vaccine effectiveness of heterologous booster regimen
Four of the six studies calculated vaccine effectiveness by comparing vaccinated versus unvaccinated individuals as reference and were included in a pooled analysis ().Citation21,Citation27,Citation37,Citation38 Two studies did not use unvaccinated individuals as reference and were excluded from the pooled analysis: Suah et al. used individuals vaccinated with the 2-dose BNT162b2 primary series as a reference and compared VE in this group with other vaccinated groups in the study;Citation40 Marra et al. used individuals vaccinated with the 2-dose CoronaVac primary series as a reference and compared VE in this group with other vaccinated groups in the study.Citation45 Of the seven variables estimated via the pooled analysis, the heterogeneity tests indicated high heterogeneity (>75%) in six tests except dose 2 to 3 for the death outcome test. The pooled analysis showed that at 7 or 14 d after vaccination, the VE of a 2-dose CoronaVac regimen was 49% (95% CI: 34%, 61%) against infection/symptomatic disease and 79% (95% CI: 63%, 88%) against hospitalization/death (). The VE of a homologous booster (three doses of CoronaVac) was similar or only slightly higher than the 2-dose CoronaVac regimen against both infection/symptomatic disease (58%; 95% CI: 8%, 81%) and hospitalization/death (80%; 95% CI: 70%, 87%). In contrast, heterologous boosting with BNT162b2 after two doses of CoronaVac achieved considerably greater VE against infection/symptomatic disease (91%; 95% CI: 76%, 97%), hospitalization (95%; 95% CI: 91%, 97%), and death (98%; 95% CI: 96%, 98%).
Figure 4. Heterologous BNT162b2 booster dose after inactivated virus vaccine improves VE across COVID-19 outcomes, including Delta and Omicron variants from real-world effectiveness studies. aVE is attributable to variants in circulation during the study period. BNT, BNT162b2; NA, not available; VE, vaccine effectiveness.
Nonrandomized uncontrolled studies of a BNT162b2 booster dose after primary vaccination with two doses of inactivated vaccine
In addition to the randomized and real-world studies described above, we identified three nonrandomized uncontrolled clinical studies evaluating heterologous BNT162b2 booster after the primary series with CoronaVac and BBIBP-CorV.Citation19,Citation32,Citation49 Assawakosri and colleagues compared the booster effect of BNT162b2 in healthy Thai adults (n = 54) who had completed the 2-dose CoronaVac primary series 6 ± 1 month earlier with similar-sized cohorts who received heterologous boosters of BBIBP-CorV, ChAdOx1, and mRNA-1273.Citation19 Kanokudom and colleagues compared the effect of a BNT162b2 booster dose (n = 60) with that of a BBIBP-CorV booster (n = 60) or a ChAdOx1 booster (n = 57) in healthy adults from Thailand, who had completed the 2-dose CoronaVac primary series 3 ± 1 month earlier.Citation49 Moghnieh and colleagues evaluated the booster effect of BNT162b2 in healthy adults from Lebanon (n = 50) who had completed a 2-dose BBIBP-CorV primary series up to 3 months earlier compared with two doses of the BNT162b2 primary series (n = 50).Citation32 In general, results showed that heterologous BNT162b2 booster significantly improved spike-RBD binding Ig levels, increased the neutralizing activity against SARS-CoV-2 variants Alpha, Beta, and Delta, and increased interferon-γ–secreting T-cell response where tested versus the comparator vaccines.Citation19,Citation32,Citation49 No serious AEs were observed with the heterologous BNT162b2 booster dose.
Finally, we identified 19 observational studies that measured humoral and cellular responses in nonrandomized cohorts of between 31 and 1587 individuals who received the 2-dose CoronaVac or BBIBP-CorV primary series followed at various dosing intervals (1‒16 months) by a heterologous booster dose of BNT16b2 or other viral vector or mRNA vaccine; some studies included multiple comparisons.Citation11,Citation17,Citation18,Citation20,Citation24–26,Citation28–29,Citation31–34,Citation35–39,Citation42–44,Citation46–48 Findings with heterologous booster varied by combinations examined, order of vaccine administration, and dosing interval used, and must be interpreted cautiously in the absence of a consensus immune correlate for short- and long-term protection. A heterologous BNT162b2 booster (third) dose after the 2-dose CoronaVac primary series enhanced humoral and cellular responses.Citation25,Citation28,Citation42,Citation43 Broad neutralization of SARS-CoV-2 variants was higher with a heterologous BNT162b2 booster (third) dose after a 2-dose CoronaVac primary series compared with a homologous CoronaVac booster,Citation29 including against DeltaCitation34 and OmicronCitation11 variants. Similar results were observed among studies that pooled data from patients receiving either of the two authorized mRNA vaccines (BNT162b2 and mRNA-1273) as a booster after primary vaccination with an inactivated vaccine.Citation47,Citation48 Additionally, neutralization of SARS-CoV-2 variants, including Alpha, Beta, Delta, Gamma, and Omicron (with exception of one studyCitation44), was significantly greater with a heterologous BNT162b2 booster versus a heterologous ChAdOx1 booster after a 2-dose CoronaVac primary series.Citation17,Citation43,Citation44 A heterologous BNT162b2 booster after mixed primary regimen of 1-dose CoronaVac and 1-dose ChAdOx1 showed a greater degree of enhancement of humoral and cellular responses and neutralization of Delta and Omicron variants than with a ChAdOx1 booster.Citation46
Studies of a heterologous BNT162b2 booster after primary vaccination with a viral vector vaccine
Findings of the current analysis are consistent with the results of studies examining a heterologous BNT162b2 booster after primary vaccination with a viral vector vaccine. An open-label, nonrandomized clinical trial performed in the United States demonstrated robust binding antibody, neutralizing antibody, and T-cell responses after primary vaccination with two doses of Ad26.COV2.S or mRNA-1273 followed ≥3 months later by a BNT162b2 booster.Citation51 In a phase 2, multicenter, randomized, controlled clinical trial conducted in the United Kingdom (the COV-BOOST trial), heterologous boosting with a single-dose of BNT162b2 after a 2-dose primary vaccination series with ChAdOx1 induced substantial anti-spike IgG and cellular responses that were comparable in participants <70 y of age and in those 70 y and older.Citation52 These humoral (B-cell) and T-cell responses persisted over 3 months.Citation53 In the two randomized clinical trials, heterologous BNT162b2 boosting was generally safe and well tolerated, with no new safety concerns identified.Citation51,Citation52 Among 22 adults primed with a 2-dose ChAdOx1 primary vaccination series in a study conducted in Italy, a heterologous BNT162b2 booster dose elicited broadly reactive neutralizing antibodies against variants of concern, including Beta, Delta, and Omicron.Citation54 Additionally, RWE from the United Kingdom demonstrated that after waning of VE was observed after a primary series with ChAdOx1, a heterologous BNT162b2 booster improved VE against symptomatic COVID-19 and COVID-19 hospitalization caused by Delta and Omicron variants to above the highest VE levels initially achieved by primary vaccination with ChAdOx1.Citation55 Recent findings from the COV-BOOST trial showed that a heterologous fourth dose of a COVID-19 mRNA booster vaccine (BNT162b2 or mRNA-1273) after 2-dose primary vaccination with ChAdOx1 and a single booster with BNT162b was well tolerated and boosted humoral and cellular immunity.Citation56 Peak responses after the fourth vaccine dose were comparable with, and possibly greater than, peak responses after the third vaccine dose (i.e., the first dose of BNT162b2).
Discussion
We reviewed the available evidence on the safety, immunogenicity, and effectiveness of a heterologous BNT162b2 booster dose after complete primary vaccination with an inactivated SARS-CoV-2 vaccine to help protect against COVID-19. Thirty-three studies reported use of mRNA and inactivated-viral vector vaccines, no studies included protein-based vaccines, and the majority of reports involved primary vaccination with CoronaVac inactivated vaccine. Heterologous boosting with BNT162b2 after inactivated virus primary vaccination afforded higher VE than a homologous booster with an inactivated virus vaccine for all COVID-19 outcomes but especially for infection/symptomatic disease (). Higher VE was observed with the heterologous BNT162b2 booster versus the inactivated virus vaccine booster against COVID-19 hospitalization and death in the elderly (). By restoring waning VE against COVID-19 outcomes after primary vaccination (), a heterologous BNT162b2 booster extended long-term protection. Additionally, higher VE was observed with the heterologous BNT162b2 booster compared with the inactivated virus vaccine booster against Delta and Omicron variants (). The studies reviewed here measured VE of a heterologous BNT162b2 booster during circulation of various SARS-CoV-2 variants, Alpha, Beta, Gamma, Delta, Omicron (), and the overall VE data are undoubtedly encouraging. Calculating cross-reactive responses across variants, however, requires additional specific analyses with larger study populations.
Vaccine-mediated protection against COVID-19 wanes over time and is especially challenged by divergent, emerging SARS-CoV-2 variants, such as Delta and Omicron,Citation57–59 and has created the need for booster doses. For coverage of Omicron and its descendants, booster vaccination may be necessary to help protect against Omicron-related infection and disease.Citation60 Added benefits of primary and booster vaccination include higher VE in older age groups in terms of protection against COVID19‒related mortality, as well as potential protection from post-acute sequelae of COVID-19 (also called “long COVID”) and Multisystem Inflammatory Syndrome in children (MIS-C).Citation61–64 Immunocompromised sub-populations are heterogeneous, consisting of patients with diverse immune-mediated inflammatory diseases, hemato-oncological malignancies, solid-organ transplantation, and patients undergoing hemodialysis, nevertheless, there is accumulating evidence showing safety and effectiveness of additional doses of COVID-19 vaccines.Citation65 However, there are very limited data directly comparing immunogenicity and safety of homologous versus heterologous booster vaccination among immunocompromised or immunosuppressed individuals, and additional studies with larger patient cohorts are required. Finally, maintaining protective community immunity through high booster vaccination coverage provides a broader societal benefit by reducing health-care resource utilization, restoring population mobility, and resuming economic activity.Citation66 Therefore, public awareness efforts and education about the need for boosters will be critical to ensure adherence with and confidence in vaccination.
During the COVID-19 pandemic, concerns about access to sufficient and predictable supply of mRNA vaccines have contributed to utilization of a heterologous vaccination strategy by many health authorities worldwide. The WHO-SAGE supports a flexible approach to vaccination strategies based on product availability and recommends that countries using WHO EUL inactivated vaccines for primary vaccination as part of a heterologous schedule consider WHO EUL mRNA or viral vector vaccines for subsequent doses.Citation15 Aligned with this flexible WHO recommendation, and in view of the overall lower efficacy of inactivated vaccines like CoronaVac and their limited efficacy data against the Omicron variant, Singapore recommends that only those individuals unable to complete the full two doses of mRNA vaccine due to medical reasons receive the 3-dose CoronaVac vaccine.Citation67,Citation68 In the United States, mRNA vaccines are preferentially recommended for use as a heterologous booster.Citation16 Furthermore, due to the risk of thrombosis with thrombocytopenia syndrome, the US Food and Drug Administration has limited authorized use of Ad26.COV2.S to adults for whom other COVID-19 vaccines are not available or are not clinically appropriate and to those who choose this vaccine because they would not otherwise receive a COVID-19 vaccine.Citation69 The European Medicines Agency recommends consideration of an mRNA vaccine dose after a single dose of a viral vector vaccine based on evidence of tolerability of this sequence and an enhanced immune response relative to the homologous viral vector vaccine regimen.Citation10
As countries transition from the pandemic to an endemic-like co-existence with SARS-CoV-2, heterologous booster regimens will likely become a prominent consideration in their national immunization program strategy and plans. For integrating COVID-19 vaccines into routine immunization programs, it will be necessary to measure real-world effectiveness of the third dose of inactivated virus vaccines in order to make recommendations, especially in the context of variants of concern. It will also be important to understand the cost-effectiveness of mRNA vaccines, such as BNT162b2, versus inactivated vaccines. Ideally, the evaluation would take into account the higher VE of mRNA vaccines on reduction of health-care resource use and other costs associated with COVID-19. Vaccine supply is now less of an issue than in the early days of COVID-19 vaccine rollout, although equitable distribution remains an unresolved challenge. Therefore, countries may consider rationalizing their expanded portfolio of four to six different COVID-19 vaccines down to perhaps one to two vaccines based on the clinical evidence and balancing programmatic feasibility, and access. Recommendations from global public health agencies like the WHOCitation70,Citation71 will be very helpful in this regard.
The current review has strengths and limitations. We attempted to collate the rapidly emerging evidence on heterologous booster regimens used in COVID-19 vaccination programs globally, by surveying both peer-reviewed and pre-print publications and by analyzing data from a diverse set of randomized, non-randomized, and real-world studies. One limitation is that we may not have captured all available studies, which will likely emerge from future systematic literature reviews. However, we believe that we covered a substantial number of relevant publications that will improve the scientific understanding of heterologous regimens and hopefully sustain future discussions regarding the optimal use of these regimens in national immunization programs during the COVID-19 pandemic and beyond. We conducted a pooled analysis to calculate a comprehensive VE estimate for the regimens involving a heterologous BNT162b2 mRNA vaccine booster dose after an inactivated vaccine primary series. Our estimate is based on four of six studies identified in this review and it is possible that including additional studies might yield an improved VE estimate that is closer to the ground truth. Finally, we were unable to gather data on the durability and breath of coverage across variants of concern for these heterologous booster regimens due to paucity of emerging data because of the longer follow-up times needed after implementation of the booster dose.
Conclusion
The accumulated evidence summarized in this review indicates that heterologous boosting with the BNT162b2 mRNA vaccine after an inactivated vaccine primary series enhances immunogenicity and improves vaccine effectiveness against COVID-19. Higher VE was observed with the heterologous BNT162b2 booster compared with the inactivated virus vaccine booster against Delta and Omicron variants. In addition, no new safety concerns have been identified with heterologous inactivated-mRNA booster combinations. Based on our collective experience with COVID-19 vaccination during the pandemic, heterologous boosting is a promising strategy to attain longer duration and greater breadth of protection against emerging SARS-CoV-2 variants. Furthermore, heterologous boosting may provide flexibility to help balance programmatic implementation across a diverse portfolio of COVID-19 vaccines used in many countries.
Acknowledgments
Medical writing support was provided by Andrea Bothwell, BSc, and Adrienne Drinkwater, PhD (ICON, Blue Bell, PA) and was funded by Pfizer Inc.
Disclosure statement
MK and JS are employees of Pfizer Inc and may hold stock or stock options. AS was a Pfizer employee during manuscript development and may hold stock options; he is currently employed by Orbital Therapeutics. LZ is an employee of Boehringer Ingelheim. HO declares no financial conflicts of interest and is involved in two ongoing studies of COVID-19 vaccine immunogenicity in health-care workers in Singapore.
Additional information
Funding
References
- World Health Organization. WHO Coronavirus disease (COVID-19) dashboard. [accessed 2022 Nov 4]. https://covid19.who.int.
- Mallapaty S, Callaway E, Kozlov M, Ledford H, Pickrell J, Van Noorden R. How COVID vaccines shaped 2021 in eight powerful charts. Nature. 2021;600(7890):580–16. doi:10.1038/d41586-021-03686-x.
- Dolgin E. Omicron thwarts some of the world’s most-used COVID vaccines. Nature. 2022;601(7893):311. doi:10.1038/d41586-022-00079-6.
- J. H. Tracking coronavirus vaccinations around the world. The New York Times. [accessed 2022 Jul 12]. https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html.
- World Health Organization. WHO - prequalification of medical products (IVDs, medicines, vaccines and immunization devices, vector control). COVID-19 vaccines with WHO Emergency Use Listing. [accessed 2022 Jun 16]. https://extranet.who.int/pqweb/vaccines/vaccinescovid-19-vaccine-eul-issued.
- Shrotri M, Swinnen T, Kampmann B, Parker EPK. An interactive website tracking COVID-19 vaccine development. Lancet Glob Health. 2021;9(5):e590–92. doi:10.1016/s2214-109x(21)00043-7.
- COVID-19 Vaccine Tracker. Equity of vaccine roll-out. [accessed 2022 Jun 16]. https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/.
- World Health Organization. WHO recommendation COVID-19 vaccine Beijing Institute of Biological Products Co., Ltd. (BIBP)/Sinopharm. [accessed 2022 Jul 12]. https://extranet.who.int/pqweb/vaccines/who-recommendation-covid-19-vaccine-bibp.
- World Health Organization. COVID-19 vaccines with WHO emergency use listing. [accessed 2022 Jul 12]. https://extranet.who.int/pqweb/vaccines/vaccinescovid-19-vaccine-eul-issued/.
- European Medicines Agency. Heterologous primary and booster COVID-19 vaccination: evidence based regulatory considerations. [accessed 2022 Jul 6]. https://www.ema.europa.eu/en/documents/report/heterologous-primary-booster-covid-19-vaccination-evidence-based-regulatory-considerations_en.pdf.
- Cheng H, Peng Z, Si S, Alifu X, Zhou H, Chi P, Zhuang Y, Mo M, Yu Y. Immunogenicity and safety of homologous and heterologous prime–boost immunization with COVID-19 vaccine: systematic review and meta-analysis. Vaccines (Basel). 2022;10(5):798. doi:10.3390/vaccines10050798.
- Au WY, Cheung PP. Effectiveness of heterologous and homologous COVID-19 vaccine regimens: living systematic review with network meta-analysis. BMJ. 2022;377:e069989. doi:10.1136/bmj-2022-069989.
- Ministério da Saúde. Secretaria Extraordinária de Enfrentamento à COVID-19 Gabinete. NOTA TÉCNICA Nº 11/2022-SECOVID/GAB/SECOVID/MS. Nota Técnica que tem por objetivo de consolidar as Notas Técnicas referentes a vacinação da população maior de 12 anos. [accessed 2022 Jun 14]. https://www.gov.br/saude/pt-br/coronavirus/vacinas/plano-nacional-de-operacionalizacao-da-vacina-contra-a-covid-19/notas-tecnicas/2022/nota-tecnica-no11.pdf/view.
- Subsecretaría de Salud Pública División de Prevención y Control de Enfermedades Departamento de Inmunizaciones. Tercera dosis de vacuna contra SARS-COV-2 EN pacientes pediátricos inmunocomprometidos (3-11 años). [accessed 2022 Jun 14]. https://www.minsal.cl/wp-content/uploads/2022/02/Tercera-dosis-de-vacuna-contra-SARS-CoV-2-en-pacientes-pedi%C3%A1tricos-inmunocomprometidos-3-11-a%C3%B1os.pdf.
- World Health Organization. Interim recommendations for heterologous COVID-19 vaccine schedules. [accessed 2022 Jul 6]. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-vaccines-SAGE-recommendation-heterologous-schedules.
- Centers for Disease Control and Prevention. Summary document for interim clinical considerations for use of COVID-19 vaccines currently authorized or approved in the United States. [accessed 2022 Jul 12]. https://www.cdc.gov/vaccines/covid-19/downloads/summary-interim-clinical-considerations.pdf.
- Angkasekwinai N, Niyomnaitham S, Sewatanon J, Phumiamorn S, Sukapirom K, Senawong S, Toh ZQ, Umrod P, Somporn T, Chumpol S, et al. The immunogenicity and reactogenicity of four COVID-19 booster vaccinations against SARS-CoV-2 variants of concerns (Delta, Beta, and Omicron) following CoronaVac or ChAdox1 nCov-19 primary series. medRxiv. 2022. doi:10.1101/2021.11.29.21266947.
- Leung NHL, Cheng SMS, Martín-Sánchez M, Au NYM, Ng YY, Luk LLH, Chan KCK, JKC L, Leung YWY, Tsang LCH, et al. Immunogenicity of a third dose of BNT162b2 to ancestral SARS-CoV-2 & Omicron variant in adults who received two doses of inactivated vaccine. Clin Infect Dis. 2022. doi:10.1093/cid/ciac458.
- Assawakosri S, Kanokudom S, Suntronwong N, Auphimai C, Nilyanimit P, Vichaiwattana P, Thongmee T, Duangchinda T, Chantima W, Pakchotanon P, et al. Neutralizing activities against the Omicron variant after a heterologous booster in healthy adults receiving two doses of CoronaVac vaccination. J Infect Dis. 2022;226(8):1372–81. Epub ahead of print. doi:10.1093/infdis/jiac092.
- Campos GRF, Almeida NBF, Filgueiras PS, Corsini CA, Gomes SVC, de Miranda DAP, de Assis JV, de Souza Silva TB, Alves PA, da Rocha Fernandes G, et al. Booster dose of BNT162b2 after two doses of CoronaVac improves neutralization of SARS-CoV-2 Omicron variant. Commun Med. 2022;2:(1):76. doi:10.1038/s43856-022-00141-4.
- Cerqueira-Silva T, Katikireddi SV, de Araujo Oliveira V, Flores-Ortiz R, Júnior JB, Paixão ES, Robertson C, Penna GO, Werneck GL, Barreto ML, et al. Vaccine effectiveness of heterologous CoronaVac plus BNT162b2 in Brazil. Nat Med. 2022;28:(4):838–43. doi:10.1038/s41591-022-01701-w.
- Cheng SMS, Mok CKP, Leung YWY, Ng SS, Chan KCK, Ko FW, Chen C, Yiu K, Lam BHS, Lau EHY, et al. Neutralizing antibodies against the SARS-CoV-2 Omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat Med. 2022;28:(3):486–89. doi:10.1038/s41591-022-01704-7.
- Costa Clemens SA, Weckx L, Clemens R, Almeida Mendes AV, Ramos Souza A, Silveira MBV, da Guarda SNF, de Nobrega MM, de Moraes Pinto MI, Gonzalez IGS, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399:(10324):521–29. doi:10.1016/s0140-6736(22)00094-0.
- Grenfell RFQ, Almeida NB, Filgueiras PS, Corsini CA, Gomes SV, de Miranda DA, Lourenço AJ, Martins Filho OA, de Oliveira JG, Teixeira-Carvalho A. Immunogenicity, effectiveness, and safety of inactivated virus (CoronaVac) vaccine in a two-dose primary protocol and BNT162b2 heterologous booster in Brazil (Immunita-001): a one year period follow up phase 4 study. Front Immunol. 2022;13:918896. doi:10.2139/ssrn.4070408.
- Hayashi JY, Simizo A, Miyamoto JG, Costa LVS, Souza OF, Chiarelli T, Bacarov NBS, Hidalgo R, Garcia LD, Soane MM, et al. Humoral and cellular responses to vaccination with homologous CoronaVac or ChAdox1 and heterologous third dose with BNT162b2. J Infect. 2022;84:(6):834–72. doi:10.1016/j.jinf.2022.02.026.
- Hueda-Zavaleta M, Gómez de la Torre JC, Cáceres-Del Aguila JA, Muro-Rojo C, De La Cruz-Escurra N, Arenas Siles D, Minchón-Vizconde D, Copaja-Corzo C, Bardales-Silva F, Benites-Zapata VA, et al. Evaluation of the humoral immune response of a heterologous vaccination between BBIBP-CorV and BNT162b2 with a temporal separation of 7 months, in Peruvian healthcare workers with and without a history of SARS-CoV-2 infection. Vaccines (Basel). 2022;10:(4):502. doi:10.3390/vaccines10040502.
- Jara A, Undurraga EA, Zubizarreta JR, Gonzalez C, Pizarro A, Acevedo J, Leo K, Paredes F, Bralic T, Vergara V. Effectiveness of homologous and heterologous booster shots for an inactivated SARS-CoV-2 vaccine: a large-scale observational study. 2022. Epub ahead of print. doi:10.2139/ssrn.4005130
- Keskin AU, Bolukcu S, Ciragil P, Topkaya AE. SARS-CoV-2 specific antibody responses after third CoronaVac or BNT162b2 vaccine following two-dose CoronaVac vaccine regimen. J Med Virol. 2022;94(1):39–41. doi:10.1002/jmv.27350.
- Kuloglu ZE, El R, Guney-Esken G, Tok Y, Talay ZG, Barlas T, Kuskucu MA, Albayrak O, Dogan O, Yavuz SS, et al. Effect of BTN162b2 and CoronaVac boosters on humoral and cellular immunity of individuals previously fully vaccinated with CoronaVac against SARS-CoV-2: a longitudinal study. Allergy. 2022;77(8):2459–67. Epub ahead of print. doi:10.1111/all.15316.
- Lai KT, Wan Loong EY L, Fung TL, Luk LW, Lau CC, Zee JS, Ma ES, Tang BS. Safety and immunogenicity of a booster vaccination by CoronaVac or BNT162b2 in previously two-dose inactivated virus vaccinated individuals with negative neutralizing antibody. Vaccines (Basel). 2022;10(4):556. doi:10.3390/vaccines10040556.
- Matula Z, Gonczi M, Beko G, Kadar B, Ajzner E, Uher F, Valyi-Nagy I. Antibody and T cell responses against SARS-CoV-2 elicited by the third dose of BBIBP-CorV (Sinopharm) and BNT162b2 (Pfizer-BioNtech) vaccines using a homologous or heterologous booster vaccination strategy. Vaccines (Basel). 2022;10(4):539. doi:10.3390/vaccines10040539.
- Moghnieh R, Mekdashi R, El-Hassan S, Abdallah D, Jisr T, Bader M, Jizi I, Sayegh MH, Rahman Bizri A. Immunogenicity and reactogenicity of BNT162b2 booster in BBIBP-CorV-vaccinated individuals compared with homologous BNT162b2 vaccination: results of a pilot prospective cohort study from Lebanon. Vaccine. 2021;39(46):6713–19. doi:10.1016/j.vaccine.2021.10.007.
- Niyomnaitham S, Quan Toh Z, Wongprompitak P, Jansarikit L, Srisutthisamphan K, Sapsutthipas S, Jantraphakorn Y, Mingngamsup N, Licciardi PV, Chokephaibulkit K. Immunogenicity and reactogenicity against the SARS-CoV-2 variants following heterologous primary series involving CoronaVac, ChAdox1 nCov-19 and BNT162b2 plus BNT162b2 booster vaccination: an open-label randomized study in healthy Thai adults. Hum Vaccin Immunother. 2022;18(6):2091865. doi:10.1080/21645515.2022.2091865.
- Patamatamkul S, Thammawat S, Buranrat B. Induction of robust neutralizing antibodies against the COVID-19 Delta variant with ChAdox1 nCov-19 or BNT162b2 as a booster following a primary vaccination series with CoronaVac. medRxiv. 2021. doi:10.1101/2021.09.25.21264099.
- Pérez-Then E, Lucas C, Monteiro VS, Miric M, Brache V, Cochon L, Vogels CBF, Malik AA, De la Cruz E, Jorge A, et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med. 2022;28:(3):481–85. doi:10.1038/s41591-022-01705-6.
- Mok CKP, Chen C, Yiu K, Chan TO, Lai KC, Ling KC, Sun Y, Hui DS, Cheng SMS, Peiris M. A randomized clinical trial using CoronaVac or BNT162b2 vaccine as a third dose in adults vaccinated with two doses of CoronaVac. Am J Respir Crit Care Med. 2022;205(7):844–47. doi:10.1164/rccm.202111-2655LE.
- Ranzani OT, Hitchings MD, de Melo RL, de França GV, de Fátima RF C, Lind ML, Torres MSS, Tsuha DH, David LC, Said RF. Effectiveness of an inactivated Covid-19 vaccine with homologous and heterologous boosters against the Omicron (B. 1.1. 529) variant. medRxiv. 2022. doi:10.1101/2022.03.30.22273193.
- Sritipsukho P, Khawcharoenporn T, Siribumrungwong B, Damronglerd P, Suwantarat N, Satdhabudha A, Chaiyakulsil C, Sinlapamongkolkul P, Tangsathapornpong A, Bunjoungmanee P, et al. Comparing real-life effectiveness of various COVID-19 vaccine regimens during the delta variant-dominant pandemic: a test-negative case-control study. Emerg Microbes Infect. 2022;11:(1):585–92. doi:10.1080/22221751.2022.2037398.
- Yavuz E, Gunal O, Basbulut E, Sen A. SARS-CoV-2 specific antibody responses in healthcare workers after a third booster dose of CoronaVac or BNT162b2 vaccine. J Med Virol. 2022;94(8):3768–75. doi:10.1002/jmv.27794.
- Suah JLB, Tng BHP, Keng Tok PM, Husin MM, Thevananthan TB, Peariasamy KMM, Sivasampu SM. Real-world effectiveness of homologous and heterologous BNT162b2, CoronaVac, and AZD1222 booster vaccination against Delta and Omicron SARS-CoV-2 infection. Emerg Microbes Infect. 2022;11(1):1–17. doi:10.1080/22221751.2022.2072773.
- Vargas-Herrera N, Fernández-Navarro M, Cabezudo NE, Soto-Becerra P, Solís-Sánchez G, Escobar-Ágreda S, Silva-Valencia J, Pampa-Espinoza L, Bado-Pérez R, Solari L, et al. Immunogenicity and reactogenicity of a third dose of BNT162b2 vaccine for COVID-19 after a primary regimen with BBIBP-CorV or BNT162b2 vaccines in Lima, Peru. medRxiv. 2022. doi:10.1101/2022.05.01.22274548.
- Demirhindi H, Mete B, Tanir F, Kara E, Kibar F, Cetiner S, Candevir A, Akti SE. Effect of heterologous vaccination strategy on humoral response against COVID-19 with CoronaVac plus BNT162b2: a prospective cohort study. Vaccines (Basel). 2022;10(5):687. doi:10.3390/vaccines10050687.
- Jantarabenjakul W, Sodsai P, Chantasrisawad N, Jitsatja A, Ninwattana S, Thippamom N, Ruenjaiman V, Tan CW, Pradit R, Sophonphan J, et al. Dynamics of neutralizing antibody and T-cell responses to SARS-CoV-2 and variants of concern after primary immunization with CoronaVac and booster with BNT162b2 or ChAdox1 in health care workers. Vaccines (Basel). 2022;10:(5):639. doi:10.3390/vaccines10050639.
- Liwsrisakun C, Pata S, Laopajon W, Takheaw N, Chaiwong W, Inchai J, Pothirat C, Bumroongkit C, Deesomchok A, Theerakittikul T, et al. Neutralizing antibody and T cell responses against SARS-CoV-2 variants of concern following ChAdox-1 or BNT162b2 boosting in the elderly previously immunized with CoronaVac vaccine. Immun Ageing. 2022;19:(1):24. doi:10.1186/s12979-022-00279-8.
- Marra AR, Miraglia JL, Malheiros DT, Guozhang Y, Teich VD, da Silva Victor E, Pinho JRR, Cypriano A, Vieira LW, Polonio M, et al. Effectiveness of heterologous coronavirus disease 2019 (COVID-19) vaccine Booster Dosing in Brazilian healthcare workers, 2021. Clin Infect Dis. 2022. Epub ahead of print. doi:10.1093/cid/ciac430.
- Suntronwong N, Kanokudom S, Auphimai C, Assawakosri S, Thongmee T, Vichaiwattana P, Duangchinda T, Chantima W, Pakchotanon P, Chansaenroj J, et al. Effects of boosted mRNA and adenoviral-vectored vaccines on immune responses to omicron BA.1 and BA.2 following the heterologous CoronaVac/AZD1222 vaccination. medRxiv. 2022. doi:10.1101/2022.04.25.22274294.
- Zhang B, Huo J, Huang Y, Teo SY, Li YF, Toh LK, Lam K-P, Xu S. mRNA booster vaccination enhances antibody responses against SARS-CoV2 Omicron variant in individuals primed with mRNA or inactivated virus vaccines. Res Square. 2022. Preprint. doi:10.21203/rs.3.rs-1577475/v1.
- Zuo F, Abolhassani H, Du L, Piralla A, Bertoglio F, de Campos-Mata L, Wan H, Schubert M, Cassaniti I, Wang Y, et al. Heterologous immunization with inactivated vaccine followed by mRNA-booster elicits strong immunity against SARS-CoV-2 Omicron variant. Nat Commun. 2022;13:(1):2670. doi:10.1038/s41467-022-30340-5.
- Kanokudom S, Assawakosri S, Suntronwong N, Auphimai C, Nilyanimit P, Vichaiwattana P, Thongmee T, Yorsaeng R, Srimuan D, Thatsanatorn T, et al. Safety and immunogenicity of the third booster dose with inactivated, viral vector, and mRNA COVID-19 vaccines in fully immunized healthy adults with inactivated vaccine. Vaccines (Basel). 2022;10:(1):86. doi:10.3390/vaccines10010086.
- Shaw RH, Stuart A, Greenland M, Liu X, Van-Tam JS N, Snape MD. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397(10289):2043–46. doi:10.1016/s0140-6736(21)01115-6.
- Atmar RL, Lyke KE, Deming ME, Jackson LA, Branche AR, El Sahly HM, Rostad CA, Martin JM, Johnston C, Rupp RE, et al. Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:(11):1046–57. doi:10.1056/NEJMoa2116414.
- Munro APS, Janani L, Cornelius V, Aley PK, Babbage G, Baxter D, Bula M, Cathie K, Chatterjee K, Dodd K, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdox1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398(10318):2258–76. doi:10.1016/s0140-6736(21)02717-3.
- Liu X, Munro APS, Feng S, Janani L, Aley PK, Babbage G, Baxter D, Bula M, Cathie K, Chatterjee K, et al. Persistence of immunogenicity after seven COVID-19 vaccines given as third dose boosters following two doses of ChAdox1 nCov-19 or BNT162b2 in the UK: three month analyses of the COV-BOOST trial. J Infect. 2022;84:(6):795–813. doi:10.1016/j.jinf.2022.04.018.
- Pagliari M, Mazzetto E, Gastaldelli M, Bortolami A, Donà D, Padoan A, Di Chiara C, Pezzani MD, Cosma C, Napolitan A, et al. Omicron neutralization and the inference of correlates of protection based on anti-SARS-CoV-2 S-RBD IgG levels in boosted individuals. SSRN Electron J. 2022. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4016530.
- UK Health Security Agency. COVID-19 vaccine surveillance report: week 9. Department of Health and Social Care. [accessed 2022 Jul 8]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1058464/Vaccine-surveillance-report-week-9.pdf.
- Munro APS, Feng S, Janani L, Cornelius V, Aley PK, Babbage G, Baxter D, Bula M, Cathie K, Chatterjee K, et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdox1 nCov-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): a multicentre, blinded, phase 2, randomised trial. Lancet Infect Dis. 2022;22:(8):1131–41. doi:10.1016/s1473-3099(22)00271-7.
- Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O’Connell AM, et al. COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386:(16):1532–46. doi:10.1056/NEJMoa2119451.
- Chemaitelly H, Abu-Raddad LJ. Waning effectiveness of COVID-19 vaccines. Lancet. 2022;399(10327):771–73. doi:10.1016/s0140-6736(22)00277-x.
- Ferdinands JM, Rao S, Dixon BE, Mitchell PK, DeSilva MB, Irving SA, Lewis N, Natarajan K, Stenehjem E, Grannis SJ, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance - VISION network, 10 states, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:(7):255–63. doi:10.15585/mmwr.mm7107e2.
- Feikin DR, Abu-Raddad LJ, Andrews N, Davies MA, Higdon MM, Orenstein WA, Patel MK. Assessing vaccine effectiveness against severe COVID-19 disease caused by omicron variant. Report from a meeting of the World Health Organization. Vaccine. 2022;40(26):3516–27. doi:10.1016/j.vaccine.2022.04.069.
- Arbel R, Hammerman A, Sergienko R, Friger M, Peretz A, Netzer D, Yaron S. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385(26):2413–20. doi:10.1056/NEJMoa2115624.
- Ayoubkhani D, Bermingham C, Pouwels KB, Glickman M, Nafilyan V, Zaccardi F, Khunti K, Alwan NA, Walker AS. Trajectory of long covid symptoms after COVID-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676.
- Azzolini E, Levi R, Sarti R, Pozzi C, Mollura M, Mantovani A, Rescigno M. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA. 2022;328(7):676. doi:10.1001/jama.2022.11691.
- Holm M, Espenhain L, Glenthøj J, Schmidt LS, Nordly SB, Hartling UB, Nygaard U. Risk and phenotype of multisystem inflammatory syndrome in vaccinated and unvaccinated Danish children before and during the Omicron wave. JAMA Pediatr. 2022;176(8):821. doi:10.1001/jamapediatrics.2022.2206.
- Napuri NI, Curcio D, Swerdlow DL, Srivastava A. Immune response to COVID-19 and mRNA vaccination in immunocompromised individuals: a narrative review. Infect Dis Ther. 2022;11(4):1391–414. doi:10.1007/s40121-022-00648-2.
- Kirson N, Swallow E, Lu J, Mesa-Frias M, Bookhart B, Maynard J, Shivdasani Y, Lefebvre P. The societal economic value of COVID-19 vaccines in the United States. J Med Econ. 2022;25(1):119–28. doi:10.1080/13696998.2022.2026118.
- Premikha M, Chiew CJ, Wei WE, Leo YS, Ong B, Lye DC, Lee VJ, Tan KB. Comparative effectiveness of mRNA and inactivated whole virus vaccines against COVID-19 infection and severe disease in Singapore. Clin Infect Dis. 2022;75(8):1442–45. doi:10.1093/cid/ciac288.
- Singapore Ministry of Health. FAQS - safety and efficacy of the COVID-19 vaccine. [accessed 2022 Aug 15]. https://www.moh.gov.sg/covid-19/vaccination/faqs—safety-and-efficacy-of-the-covid-19-vaccine.
- US Food & Drug Administration. Coronavirus (COVID-19) update: FDA limits use of Janssen COVID-19 vaccine to certain individuals. [accessed 2022 Jun 16]. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-janssen-covid-19-vaccine-certain-individuals.
- UNICEF. COVAX: ensuring global equitable access to COVID-19 vaccines. [accessed 2022 Jul 19]. https://www.unicef.org/supply/covax-ensuring-global-equitable-access-covid-19-vaccines.
- UNICEF. COVID-19 vaccine market dashboard. [accessed 2022 Jul 19]. https://www.unicef.org/supply/covid-19-vaccine-market-dashboard.
