ABSTRACT
Despite widespread mass rollout programs, the rapid spread of the SARS-CoV-2 Omicron variant called into question the effectiveness of the existing vaccines against infection, hospitalization, severity, and mortality compared to previous variants. This systematic review summarizes and compares the effectiveness of the COVID-19 vaccines, with respect to the above outcomes in adults, children, and adolescents. A comprehensive literature search was undertaken on several databases. Only 51 studies met our inclusion criteria, revealing that the protection from primary vaccination against Omicron infection is inferior to protection against Delta and Alpha infections and wanes faster over time. However, mRNA vaccine boosters were reported to reestablish effectiveness, although to a lower extent against Omicron. Nonetheless, primary vaccination was shown to preserve strong protection against Omicron-associated hospitalization, severity, and death, even months after last dose. However, boosters provide more robust and longer-lasting protection against hospitalizations due to Omicron as compared to only primary series.
Introduction
The Coronavirus Disease 2019 (COVID-19) is known to be caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) belonging to the Coronaviridae family and the genus Betacoronavirus.Citation1 The SARS-CoV-2-induced COVID-19 has signaled the most challenging coronavirus outbreak since its outbreak in Wuhan, China, in December 2019. By February 2021, it had already spread globally, making it one of the most contagious viruses in history.Citation1 As of writing, five variants of concern (VOC) have been identified by the World Health Organization (WHO): Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2 and AY lineages), and Omicron (B.1.1.529, then reclassified into BA sub lineages, notably, BA.1 and BA.2). Delta is one of the special interest and concern as it may be responsible for more severe illness.Citation2 The original Omicron variant (BA.1) was first reported from Botswana and South Africa in November 2021. Thereafter, the cases began to appear in other countries and multiply rapidly. By December 2021, Omicron was responsible for over a million daily cases in the United States (US).
Although the infectiousness of Omicron is determined mainly by the binding affinity to ACE2 and RBD complex on the S protein, the furin cleavage site is also found to play a crucial role in its infectivity and ability to evade natural immunity from previous infections. Omicron harbors around 30 amino acid mutations in its spike (S) protein, about 15 proteins in its receptor binding domain (RBD), and three mutations on the furin cleavage site that make it very contagious compared to other strains of SARS-CoV-2.Citation3 These changes help the virus escape antibody protection from previous infections.Citation4 Further, Omicron reportedly had lower and higher viral copy numbers in lung epithelial cells in the nasal airway epithelial cells of infected individuals, respectively. These observations explain the reduced disease severity and increased transmissibility of Omicron, respectively. Studies have also shown that Omicron has a reduced ability to induce syncytia in tissues, which has been shown to increase disease severity. Syncytia formation requires viral infection through membrane fusion involving the membrane protein transmembrane protease, serine 2 (TMPRSS2). The low rate of syncytia formation indicates Omicron infection through endosomal fusion. This switch in Omicron’s infection mechanism increases the number of cell types that Omicron affects.Citation5
Vaccination has shown to be the most effective means for COVID-19 prevention and control. The current COVID-19 vaccines target S protein. Mohammed et al.Citation6 reported that the COVID-19 vaccines have successfully reduced the rates of infections, severity, hospitalization, and death caused by the pre-Omicron variants of SARS-CoV-2. The highest reported vaccine effectiveness (VE) values of Pfizer in reducing infection were 99.5% overall,Citation7 94% against Alpha,Citation8 and 75% against Beta.Citation9 Against hospitalization, severity, and mortality, 100% VE was reported against the Alpha and/or the Beta variants.Citation9,Citation10 Moderna was found to be 93.3%, 86%, and 100% effective against infection, severity, and hospitalization, respectively.Citation10 The highest reported VE values for Janssen were 66.9% against infection, 100% against hospitalization, and 83.5% against severity.Citation11 The highest VE of Sinovac was 73.8% against infection.Citation12 At the time, two doses of the Pfizer or Moderna vaccines were effective in preventing infection, severe disease, hospitalization, and mortality (Pfizer only).
Despite of the relatively high reported effectiveness of the COVID-19 vaccines against the pre-Omicron variants, it was observed that many of the Omicron-infected patients were fully vaccinated or boosted which raised concerns regarding the effectiveness of the COVID-19 vaccines against Omicron. This large comprehensive review is among the first to compile data regarding the effectiveness of different COVID-19 vaccines against Omicron-associated infection, severity, hospitalization, and mortality, compared to other VOC, with further attention to dose number, time since vaccination, and vaccinee age group. diagrammatically illustrates the different sections of the review to facilitate the comprehensive understanding of its structure.
Methods
The preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement were used to develop the protocol of this systematic review.Citation13
Information sources and search strategy
A comprehensive search was conducted to target any studies about the new variant of SARS-CoV-2 using the following two keywords: Omicron and B.1.1.529. The following databases were searched in March 2022: PubMed, Medline, Embase, Scopus, Web of Science, Science Direct, MedRxiv, and Lens.org. All searches were limited by year to 2020 through 2022.
Eligibility criteria
We conducted a comprehensive literature search of medical studies that reported any data related to the effectiveness of the COVID-19 vaccines against Omicron. No restrictions were made based on country, age, or gender. Any articles that did not have primary data, such as review articles, were excluded from the study after removing the duplicates. Furthermore, studies that were not in English were excluded. During the full-text screening, any studies that reported the effectiveness of the COVID-19 vaccines against Omicron were included. Any studies that reported populations with positive SARS-CoV-2 infection without stratifying the data based on the variant were excluded.
Study selection and data collection
Title and abstract screening, full-text screening, and data extraction were conducted by two independent reviewers for each study using Covidence. Disagreements were resolved by consensus.
Data items
VE values as well as hazard ratios (HR), odd ratios (OR) or relative risk ratios (RR) related to the manifestations of the Omicron infection in vaccinated populations as compared to the other variants.
Risk of bias and quality assessment
Quality assessment (QA) of each included study was performed by two independent reviewers using the Newcastle-Ottawa QA Scale (NOS).Citation14
Results
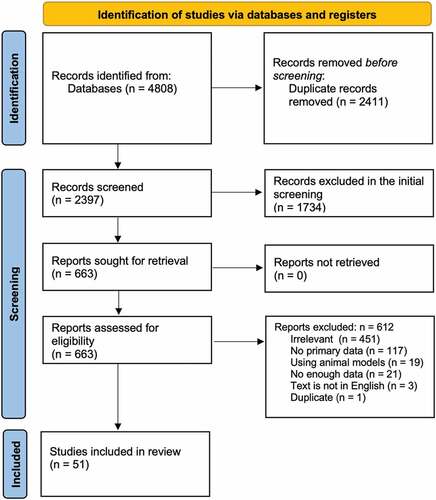
shows the flow diagram of our protocol. After removing duplicates, the titles and abstracts of 2397 studies were screened, of which 663 were selected for full-text screening. Only 51 studies met our inclusion criteria. Of the 612 excluded studies, 451 were irrelevant, 21 did not have enough data, 117 had no primary data, 3 were not in English, 19 used animal models, and 1 was a duplicate of another study. Supplementary Tables S1–S5 summarize the types of studies and the VE against Omicron infection, hospitalization, severity, and death, respectively.Citation15–65 Furthermore, the supplementary tables summarize any reported values related to the HR, OR, or RR related to the manifestations of the Omicron infection in vaccinated populations as compared to the other variants. compare the lowest and highest COVID-19 VE values against Omicron versus other variants in terms of infection, hospitalization, severity, and death, respectively.
Table 1. Highest and lowest reported vaccine effectiveness (VE, with 95% confidence intervals) values of the COVID-19 vaccines against the Omicron variant in reducing infection as compared to the Delta variant.
Table 2. Highest and lowest reported vaccine effectiveness (VE, with 95% confidence intervals) values of the COVID-19 vaccines against the Omicron variant in reducing hospitalization as compared to the Delta and Alpha variants.
Table 3. Highest and lowest reported vaccine effectiveness (VE, with 95% confidence intervals) values of the COVID-19 vaccines against the Omicron variant in reducing the severity of infection as compared to the Delta variant.
Table 4. Highest and lowest reported vaccine effectiveness (VE with 95% confidence intervals) values of the COVID-19 vaccines against the Omicron variant in reducing mortality as compared to the Delta variant.
Study characteristics and demographic data
Supplementary Table S1 summarizes the types of studies, their countries, and the number of subjects in each study.Citation15–65 Among the 51 included studies, 17 were from the US, 7 from South Africa, 2 from France, 1 from Italy, 2 from Norway, 7 from the UK, 3 from Denmark, 2 from Canada, 2 from the Netherlands, 3 from Qatar, 2 from Israel, and 1 each from Faroe Island, Portugal, and the Czech Republic. The studies included 14 test-negative case-control studies, 12 retrospective observational studies, 1 study used data linkages, 2 case only approach/case studies, 9 cohort studies, 3 registry-based studies, 1 survey data analysis, 3 prospective observational studies, 1 longitudinal cohort study, 2 database-based cohort studies, 1 population/cohort-based surveillance, 2 clinical outcome analysis studies. The 51 studies collectively included at least 10,581,027 subjects. Furthermore, Supplementary Table S1 illustrates the NOS QA score for each study, which ranged from 4 to 10.
Vaccine effectiveness against infection, hospitalization, severity, and death in Omicron infected patients as compared to the other variants
The VE for reducing rates of infections, hospitalization, severity, and death with respect to Omicron and other SARS-CoV-2 VOC is summarized in Supplementary Tables S2–S5, respectively. summarize the lowest and highest reported VE values mainly against Omicron and the other VOC based on each outcome above. Below, we summarize the main findings with a focus on the VE (%) against particular outcomes, of different vaccination status, and for the individual vaccine types. Wherever data for individual vaccines are not available, data reported for mixed or heterogeneous vaccine regimens are summarized.
Vaccine effectiveness against infection
Supplementary Table S2 summarizes the VE of different COVID-19 vaccines against infection, showing that, consistently across different vaccine types and doses, lower VE were reported against Omicron versus Delta. Comparison of VE against Omicron infection stratified based on recipient age groups and/or time interval following vaccination also yielded similar results. In addition, higher HR and OR were reported for Omicron infections versus other VOCs at different doses of vaccination. summarizes the lowest and highest reported VE against infection for each vaccine type.
Effectiveness of partial vaccination
The effectiveness of partial vaccination (“partial VE”) with Pfizer against Omicron infection ranged from 9.5 (95% CI: −39.8–41.3) to 51.4 (95% CI: 42.7–58.8) vs 30.9 (95% CI: 25.4–36) to 75.9 (95% CI: 74.3–77.3) against Delta. The lowest VE for both Omicron and Delta was reported at 0–13 days and >105 days, respectively, after vaccination, and the highest were reported at 14–20 days for both Omicron and Delta within 16–17 years old age group. The partial VE with Moderna against Omicron ranged from −16.8 (95% CI: −137.8–42.6, >14 days after vaccination) to 47.9 (95% CI: 43.1–52.3, 1–3 weeks after vaccination) vs 56.7 (95% CI: 40.7–68.4) to 60.1 (95% CI: 51.8–66.9, 1–3 weeks after vaccination) against Delta. The partial VE with AstraZeneca against Omicron was reported as 17.7 (95% CI: 14.3–21, 4 weeks after vaccination) vs 42.9 (95% CI: 39.8–45.9, 25 weeks after vaccination) against Delta.
Effectiveness of full vaccination
The effectiveness of full vaccination (“full VE”) with Pfizer against Omicron ranged from −3 (95% CI: −30–18, >150 days after vaccination) to 83.1 (95% CI: 78.2–86.9, 7–13 days after vaccination) vs 53.8 (95% CI: 52.9–54.6, 91–150 days after vaccination) to 93.2 (95% CI: 81.5–97.5, 7–13 days after vaccination in 12–15 years old children) against Delta. The full VE with Moderna against Omicron ranged from −39.3 (95% CI: −61.6- −20, 91–150 days after vaccination) to 75.1 (95% CI: 70.8–78.7, 2–4 weeks after vaccination) vs 61.3 (95% CI: 55–66.7, >270 days after vaccination) to 94.5 (95% CI: 90.5–96.9), 2–4 weeks after vaccination) against Delta. The full VE with AstraZeneca against Omicron ranged from −2.7 (95% CI: −4.2- −1.2, >25 weeks after vaccination) to 48.9 (39.2–57.1, 2–4 weeks after vaccination) vs 43.5 (95% CI: 42.4–44.5, >25 weeks after vaccination) to 82.8 (95% CI: 74.5–88.4, 2–4 weeks after vaccination) against Delta.
Effectiveness of receiving the booster
The effectiveness of the booster vaccination (“booster VE”) with Pfizer against Omicron ranged from 3.6 (95% CI: 0.6–6.5, 4 months after vaccination compared to 5-month post-vaccination) to 81 (95% CI: 59–91 > 7 days after vaccination in 16–17 years old) vs 81.2 (95% CI: 79.2–82.9, 1–30 days after vaccination) to 97 (96–98, >7 days after vaccination) against Delta. The Moderna booster VE against Omicron ranged from 34.9 (95% CI: 14.6–50.4, >6 weeks after vaccination) to 71.6 (95% CI: 69.7–43.4, <60 days after vaccination) vs 86 (78.1–91.1, >60 days after vaccination) to 97 (95% CI: 95–98, >7 days after vaccination) against Delta. The AstraZeneca booster VE against Omicron was 47 (95% CI: 2–70). Vaccination using heterogeneous regimens was reported using different combination of vaccine types (). The highest reported VE against Omicron was 74 (95% CI: 73.1–74.9, 1 week after vaccination) using Moderna for full vaccination and Pfizer as booster.
Vaccine effectiveness against hospitalization
Supplementary Table S3 summarizes the VE of different COVID-19 vaccines against hospitalization; consistently lower VE was reported against Omicron versus Delta after full vaccination with different vaccine types (without the booster). However, some studies reported comparable VE against Omicron and Delta-related hospitalization after the booster dose. In addition, the HR and/or OR were reported for the Omicron infections and/or the other variants at different doses of vaccination; however, no consistent pattern was observed. summarizes the lowest and highest reported VE against hospitalization for each vaccine type.
Effectiveness of full vaccination
The full Pfizer VE ranged from 46 (95% CI: −15–77) to 100 (95% CI: −189–100) against Omicron vs 93 (95% CI: 90–94) against Delta. The full Moderna VE was 84.5 (95% CI: 23–96.9) against Omicron vs 99 (95% CI: 93.9–99) against Delta.
Effectiveness of receiving the booster
A noticeable increase in the reported VE against hospitalization due to Omicron infection was observed after receiving the booster dose. The Moderna booster VE was reported as 99.2 (95% CI: 76.3–100) against Omicron vs 99.7 (95% CI: 96.5–100) against Delta. The Pfizer or Moderna booster VE ranged from 78 (95% CI: 67–85 > 4 months after vaccination) to 91 (95% CI: 88–93 < 2 months after vaccination) against Omicron vs 76 (95% CI: 14–93 > 4 months after vaccination) to 97 (95% CI: 95–98) against Delta. The Janssen booster VE against Omicron was 85 (95% CI: 54–95, 27–87 days after vaccination).
Vaccine effectiveness against severity
The Supplementary Table S4 summarizes the VE of different COVID-19 vaccines against severe disease. Similar to VE against hospitalization measures, although protection against Omicron severe disease was lower compared to Delta after full vaccination, they were comparable post-booster. Further, no consistent pattern regarding HR and/or OR against severity of disease between VOC were observed. summarizes the lowest and highest reported VE against severe disease for each vaccine type.
Effectiveness of full vaccination
The effectiveness of full vaccination with Pfizer, Moderna, AstraZeneca, or Janssen vaccines against severe Omicron disease ranged from 32 (95% CI: 20–43, >2 months after vaccination) to 57 (95% CI: 32–72, <2 months after vaccination) for intensive care unit (ICU) admission and 37 (95% CI: 12–55 > 2 months after vaccination) to 58 (95% CI: 3–82, <2 months after vaccination) for the progression to required use of invasive mechanical ventilation (IMV) and death, vs 82 (95% CI: 76–87 < 2 months after vaccination) to 82 (95% CI: 80–83, >2 months after vaccination) for ICU admission and 84 (95% CI: 72–91 < 2 months after vaccination) to 86 (95% CI: 83–88, >2 months after) for the progression to required use of IMV and death for Delta-associated severe disease, respectively.
Effectiveness of receiving the booster
A noticeable increase in the reported VE against the severity of Omicron was observed after receiving the booster dose. The Pfizer post-booster VE ranged from 95 (95% CI: 87–98 > 7 days after vaccination) to 100 (95% CI: 71.4–100, >7 days after vaccination) against Omicron vs 90.1 (95% CI: 80.6–95 > 7 weeks after vaccination) to 99 (95% CI: 98–99 > 7 days after vaccination) against Delta. The Moderna or Pfizer VE post-booster was reported as 95 (95% CI: 87–98 > 7 days after vaccination) against Omicron vs 99 (95% CI: 98–99 > 7 days after vaccination) against Delta. The AstraZeneca post-booster VE was reported as 93 (95% CI: 74–98 > 7 days after vaccination) against Omicron vs 100 (95% CI: 98–100 > 7 days after vaccination) against Delta. The post-booster VE of Pfizer, Moderna, AstraZeneca, or Janssen vaccines against Omicron ranged from 85 (95% CI: 80–88 > 2 months after vaccination) to 90 (95% CI: 87–92 < 2 months after vaccination) for ICU admission and 60 (95% CI: 37–74 > 2 months after vaccination) to 83 (95% CI: 75–89 < 2 months after vaccination) for the progression to the use of IMV and death vs 97 (95% CI: 95–98 > 2 months after vaccination) to 98 (95% CI: 98–98 < 2 months after vaccination) for ICU admission and 97 (95% CI: 92–99 > 2 months after vaccination) to 98 (95% CI: 97–99 > 2 months after) for the progression to required use of IMV and death against Delta.
Vaccine effectiveness against death
Limited data were available about the effectiveness of the different COVID-19 vaccines against Omicron and/or other VOC associated mortality (Supplementary Table S5). However, post-booster, the effectiveness noticeably increased to a comparable level for both Omicron and Delta. shows that the full vaccination with Pfizer or Moderna had a VE of 75 (95% CI: 52–87 > 14 days after vaccination) for Omicron vs 93 (95% CI: 85–97, >14 days after vaccination) for Delta. The VE increases after receiving the booster dose to 94 (95% CI: 85–98, >14 days after vaccination) for Omicron vs 96 (95% CI: 88–99, >14 days after vaccination) for Delta.
Discussion
Our systematic review included 51 studies with at least 10,581,027 subjects, marking one of the largest reviews on the subject till date. Since the emergence of Omicron, it was not clear how effective the COVID-19 vaccines are against this variant compared to previous VOC. Earlier, Chenchula et al.Citation66 rapidly reviewed 27 studies to examine the effect of booster vaccination against Omicron; evidence at the time supported rapid roll-out of booster-doses to protect the most vulnerable; however, data to compare VE versus other VOC or of partial/full regimens as well as detailed analysis was not present in this study. Similarly, Du et al.Citation67 reviewed that serum antibodies from booster vaccine recipients tested via pseudovirus live virus neutralization tests were significantly more effective against Omicron compared to two-dose regimen; however, patient-level data was not considered in this study. Zeng et al.Citation68 provided detailed in-depth and broad analysis concerning various vaccines with respect to various VOC; however, data concerning VE against hospitalization and mortality were not considered. In this comprehensive review, we compiled all reported data about the effectiveness of the COVID-19 vaccines against the Omicron-related infections, hospitalization, severity, or death, in comparison to other VOC, and with attention to time since vaccination, vaccinee age group, and dose number.
How were variants and subvariants identified?
Variants were confirmed as Omicron using whole-genome sequencing or a novel variant-specific PCR test and assumed Delta otherwise. Other studies implemented S-gene target failure (SGTF), identified by genotyping by detecting the del69/70 mutation in the S-gene, as a proxy for Omicron (BA.1) variant. A minority of studies designated variant by solely matching dates of PCR-test and national variant-trends. In countries such as Qatar, viral genome sequencing and multiplex RT-PCR variant screening of random positive clinical samples are the basis of variant screening; this is also complemented by deep sequencing of wastewater samples. Well into surge of Omicron, non-SGTF genotyped cases were proxied for the Omicron BA.2 subvariant.
COVID-19 vaccine effectiveness against Omicron and other variants in reducing infection only
Pfizer
The Pfizer vaccine has been the most widely investigated vaccine across the world given its status as one of the first-approved and most efficacious vaccines against the SARS-CoV-2 wildtype. Its waning effectiveness against Delta was earlier called into question,Citation6 although recent studies revealed that its protection against Omicron infection was much more scrutinized. Across studies that investigated this relationship, the conclusion pointed toward the booster being a necessity in preventing further Omicron spread given the lower vaccine effectiveness just months following primary course completion; naturally, partial vaccination has been shown to be even less effective. In their early investigation of the Danish national database between November 20 and December 12, 2021, Hansen et al.Citation44 reported a VE of 55.2% against Omicron infection in the 1st month after primary vaccination, which waned substantially to −76.5% during the 4th and 5th months. While the negative VE value may quantitatively indicate a higher risk of infection in the vaccinated group as compared to the control group, the wide 95% CI range (−95.3 to −59.5) can be only explained as waning of the vaccine effectiveness. Although VE against Delta also decreased, it was not as substantial as against Omicron. Booster vaccination reestablished VE to 54.6% against Omicron and to 81.2% against Delta during a 1-month follow-up, providing an early hint for the need of boosters to counter Omicron. Similar inter-variant conclusions were reached by Šmíd et al.Citation37 which reaffirmed two observations: protection against Omicron infection from partial and full courses are both, less effective, and shorter lasting, against Omicron compared to Delta. Findings from the UK also revealed that protection against symptomatic Delta disease is both initially stronger and lasts longer compared to Omicron, with respect to partial, full and booster Pfizer vaccination.Citation43,Citation63,Citation69 Large-scale studies across the US have confirmed and reproduced the results described in the abovementioned studies across Europe.Citation20 Furthermore, Abu-Raddad et al.Citation19 conducted a large, matched retrospective cohort study during a period of Omicron predominance between December 19, 2021, and January 26, 2022. The study reaffirms and quantifies findings regarding the marginal effectiveness of boosters over only primary series, against the Omicron and Delta variants. In a parallel but substantially extended Qatar-based study by Chemaitelly et al.Citation30 results revealed that the effectiveness of full vaccination against symptomatic Omicron BA.1 and BA.2 infection peaked in the first 3 months after receiving the 2nd dose and waned significantly over 7 months for both subvariants. Although booster doses increased effectiveness discriminately against the two subvariants, after 3rd dose, it waned quickly against both subvariants after ≥1 month.
In conclusion, full course Pfizer vaccination provides moderate protection against Delta-, but not Omicron-associated infections; this reduction is further accelerated by time since last dose. Although boosters significantly reestablish protection against both variants, it is less robust and again subject to accelerated waning with time against Omicron.
Moderna
Most of the studies discussed above also investigated the effectiveness of partial, full, and/or booster Moderna vaccination given their similar distribution owing to comparable efficacies and legislative approval time frames. Although most studies showed that the protection provided by the two mRNA vaccines is comparable, a few have reported contrasting findings. For instance, Hansen et al.Citation44 reported non-significant VEs of 36.7% against Omicron in just the 1st month after primary vaccination, which further waned considerably to −39.3% during the 4th and 5th months. Although VE of the primary course also warned against Delta over time, the decline was less substantial (88.2% during 1st month to 65.0% during the 4th–5th months). Booster vaccination reestablished VE against Delta to 82.8% reaffirming its significance against Delta-associated infection; however, insufficient number of Omicron cases disallowed Moderna booster VE estimation against Omicron. However, a comparison can be made using the results by Šmíd et al.Citation37 who reported the effectiveness of Moderna vaccines using a large national database in the Czech Republic. Their observations reaffirmed previous inter-variant findings, in addition to hinting toward slightly better protection compared to Pfizer. Additionally, the results of Moderna VE obtained by Andrews et al.Citation63 in England greatly align with those seen from use of Pfizer, showing that although protection is limited and shorter lasting against Omicron compared to Delta, outcomes fare much better when compared to those unvaccinated. Studies conducted in the US further add to the increasing literature investigating the Moderna VE against both variants. Both Accorsi et al.Citation20 and Tseng et al.Citation15 reported comparable findings where boosters revamped protection but also waned relatively quickly after vaccination. The studies from Qatar further contribute to this discussion given the widespread distribution of both mRNA vaccines across the city-state. In a study focused on investigating the marginal effectiveness of dose 3 compared to dose 2, Abu-Raddad et al.Citation19 showed that Moderna boosters were 47.3% effective against symptomatic Omicron infection compared to primary series only. This aligns with the comparison of the Pfizer booster, compared to its primary course. Further, Chemaitelly et al.Citation30 showed that partial Moderna vaccination was not reliable in their protection against both BA.1 and BA.2 symptomatic infection. Full vaccination against Omicron BA.1 was 71% effective in the first 3 months following 2nd dose compared to unvaccinated, but this protection rapidly waned by ≥7 months. Against BA.2 subvariant, full course of Moderna was not effective at any point from 1 to ≥7 months after 2nd dose. This finding is in contrast to what the same authors reported for Pfizer vaccination, hence signaling lower protection of Moderna primary series against this subvariant. However, booster doses greatly increased effectiveness to 51.5% (BA.1) and 39.4% (BA.2) within a month after 3rd dose, once again revealing a slightly greater predisposition of BA.2 to escape Moderna-induced immune response, although a similar pattern was also observed with Pfizer-induced protection between BA.1 and BA.2. In conclusion, Moderna parallels Pfizer vaccines with respect to protection against Omicron and Delta VOC infection and the effect of time on it; further, while most studies show comparable VE of the two mRNA agents, few studies show conflicting results, although not very significantly.
Janssen
Although many studies have reported the use of Janssen along with other vaccines in their populations, very few have reported on Janssen efficacies independent of other manufacturers, making it difficult to comment on its efficacy alone. However, owing to their large national database, Šmíd et al.Citation37 show modest and long-lasting protection of Janssen against Delta. However, their results highlighted that the viral vector vaccine is also susceptible to waning VE with time and Omicron-specific immune evasion. Notably, Janssen appears to be comparable in effectiveness to full vaccination with either mRNA vaccine against Omicron, but not Delta, where mRNA vaccines provide greater protection.
AstraZeneca
Given the lack of legislative approval in the US, only studies across Europe have reported on the protection provided by the AstraZeneca vaccine independent of others. Andrews et al.Citation63 showed that partial AstraZeneca vaccination protected against symptomatic Delta infection less robustly compared to both mRNA vaccines, with mean VEs of 42.9% and 17.7% against symptomatic Delta and Omicron variants at ≥4 weeks, respectively. Completion of primary series increased the VE against both variants. However, by the third-dose eligibility period, at ≥25 weeks, effectiveness of 2-doses against both variants had dropped, to 43.5% against Delta and −2.7% against Omicron. These trends have also been reproduced from the large Czech study by Šmíd et al.Citation37 and Spensley et al.Citation43 However, Andrews et al.Citation63 further revealed that an AstraZeneca booster dose reestablished VE to 77.1% against Delta and 57.7% against Omicron; however, these were the lowest VEs among all primary course plus booster combinations (combinations with mRNA vaccines, either as primary course or boosters, have been discussed in the following section). Hence, a novel indication of these findings is that both primary series and booster protection with purely AstraZeneca vaccines are less effective at preventing symptomatic Delta and Omicron infection compared to a combination with mRNA or Janssen vaccines; their effect against Delta was more comparable, although still less protective.
Heterogenous or mixed vaccine regimens
Regimens composed of purely one type of vaccine have been discussed in their respective sections above; here we discuss heterogenous combinations.
Pfizer, Moderna, and/or AstraZeneca
Andrews et al.Citation63 reported that the vast majority of individuals who had completed primary series with either Pfizer, Moderna, or AstraZeneca were administered mRNA boosters, as per national policy. Against Delta variant, both mRNA-boosters resulted in mean VE > 95% by 2nd – 4th week of inoculation, regardless of primary course with either of the three vaccines and remained high (>88%) over the longest follow-up period of ≥10 weeks. However, against Omicron, the results were more concerning. Among AstraZeneca primary recipients, Pfizer boosters increased VE to 62.4% at 2nd − 4th week, dropping to 39.6% by ≥10 weeks, whereas Moderna boosters after AstraZeneca primary series increased VE to 70.1% at 2nd − 4th weeks, dropping to 60.9% at 5th − 9th weeks. Among BioNTech and Moderna primary course recipients, corresponding 2nd − 4th week VEs were 73.9% and 64.9 in those receiving Moderna and Pfizer boosters, respectively, which were similar to VEs observed from homogenous mRNA-vaccine boosting.
Pfizer and/or AstraZeneca
In addition to homogenous full courses of Pfizer and AstraZeneca, as well as 3-dose course with Pfizer, Spensley et al.Citation43 also reported that a Pfizer booster dose following a primary series of AstraZeneca was protective in preventing Omicron infection in hemodialysis patients in the UK with a VE of 47%, whereas primary series using either of the two were not effective. VE was also reestablished in the cohort which received Pfizer as both primary and booster vaccination, further adding to the growing evidence that mRNA vaccine-boosting plays a significant part in preventing infection, irrespective of primary course.
Pfizer, Moderna, and/or Janssen
Lewnard et al.Citation26 conducted a prospective cohort study in South California, US, to report characteristic and clinical outcomes among Delta and Omicron BA.1/BA.2 cases detected in an outpatient setting between November 1, 2021 and March 17, 2022. They show that when vaccinated with Janssen primary series and further mRNA vaccine-boosted, the (adjusted) odds of Omicron infection was about 2.32 times more likely than Delta infection compared to when unvaccinated. The findings revealed Omicron’s increased tendency to escape infection fighting immune protection compared to Delta. However, there was no difference in the odds of Omicron BA.2 vs BA.1 infection compared to unvaccinated.
COVID-19 vaccine effectiveness against Omicron and other variants in reducing hospitalization only
Pfizer
In one of the earliest reports investigating the effectiveness of primary Pfizer vaccination against Omicron-associated hospitalization in South Africa, where the variant was first detected, Collie et al.Citation47 conducted a retrospective test-negative cohort study in comparison to a Delta-predominated period. Compared to a VE of 93% in Delta periods, the VE of the primary Pfizer series against Omicron-period hospitalization was 70%. These early findings showed that although protection ameliorated due to the novel variant, vaccination still plays an important role against COVID-19 related hospitalization. Šmíd et al.Citation37 reported that similar patterns as seen against infection, but with consistently higher protection, were seen against hospitalization due to vaccination. Hence, although time-sensitive waning of effectiveness is also observed against Delta-induced hospitalization, it is dramatically accelerated against Omicron-induced hospitalization. Finally, in their observational and prospective study at 21 hospitals in the US, the report by Lauring et al.Citation22 revealed that the Pfizer primary series was equally effective (82%) against both Alpha and Delta-variants associated hospitalization. Other heterogeneous combinations have been discussed below.
Moderna
Similar to the protection conferred by the Pfizer mRNA vaccine, Šmíd et al.Citation37 reported that protection from partial and full Moderna vaccinations was both reasonably high and durable against Delta-associated hospitalization, but was much less reliable against Omicron-associated hospitalization. However, Tseng et al.Citation15 reported that partial vaccination did not offer reliable protection against Delta-associated hospitalization, with a VE of 71.2%. In contrast, two-dose and three-dose regimens were shown to successfully produce effectiveness figures of 99.0% and 99.7% against Delta-variant hospitalization, respectively. Although primary series VE against Omicron-associated hospitalization was lower at 84.5%, a booster dose successfully increased protection to 99.2%, rivaling protection against Delta.
AstraZeneca
Šmíd et al.Citation37 showed that although protection against Delta-associated hospitalizations form AstraZeneca full courses are effective up to 3 months from latest dose, the same cannot be said for protection against Omicron-associated hospitalization, where effectiveness is statistically non-significant, even at 2 months from primary series.
Janssen
Šmíd et al.Citation37 also reported that Janssen full vaccination was also effective at preventing Delta variant-associated hospitalizations. However, unlike other vaccines, it is interesting to note that the protection from Janssen appears to increase with time, reaching highest protection at 3 months from vaccination; however, the 1-month protection was non-significant. This promising feature of Janssen has been reproduced in another study. Lewnard et al.Citation26 showed that compared to unvaccinated, primary Janssen vaccination was effective at preventing both Delta and Omicron-associated symptomatic hospital admissions that were tested in an outpatient setting, although protection against Omicron was lower versus Delta. This was reflected in the comparison of symptomatic hospital admission association between Omicron vs Delta, which reflects that Omicron was less severe compared to Delta in those vaccinated with Janssen.
Heterogenous or mixed vaccine regimens
Lewnard et al.Citation26 highlight that full Janssen vaccination followed by an mRNA booster was effective at preventing both Delta and Omicron associated symptomatic hospitalizations tested in outpatient settings compared to only the primary series, although protection against Delta was greater than that against Omicron. This was evident from the direct comparison of Omicron vs Delta symptomatic hospitalization revealing that occurrence of symptomatic hospitalization was less likely with Janssen plus mRNA booster regimen.
COVID-19 vaccine effectiveness against Omicron and other variants in reducing infection, hospitalization, severity, and/or death
Abu-Raddad et al.Citation19 investigated the effects of Pfizer boosters on COVID-19 related severe, critical, or fatal disease, defined as acute-care hospitalizations, ICU admissions, and deaths, respectively (WHO definitionCitation70,Citation71). Earlier, we have described how both mRNA-boosters were approximately 50% more effective in preventing symptomatic Omicron infections compared to the primary series. However, Pfizer booster was also shown to prevent COVID-19 related severe or fatal disease with greater VE (76.5%) compared to full vaccination, which adds to the observation that despite the great incidence of infection among the individuals who received the booster, there were very few severe cases and no critical or fatal cases. Additionally, such severe cases were low in number in both booster and non-booster cohorts despite the large number of infections, reaffirming that primary series are still durable against severe disease months after receipt of second dose. Findings from Chemaitelly et al.Citation30 further show that partial vaccination provided no protection (40.9%), whereas effectiveness of full vaccination was 70.4% at 1–6 months after 2nd dose and 77.5% by ≥7 months, whereas VE of booster was 90.0% at 1–6 weeks after 3rd dose and 90.1% by ≥7 weeks, without immediate signs of waning. Patalon et al.Citation29 noted that the odds of Omicron-associated hospitalization or death were not significantly affected by time from the 3rd dose, highlighting the relatively long-lasting protection against severe disease compared to protection against infection only. Finally, compared to primary series vaccinees, the authors showed significant marginal VE of boosters against hospitalization and death, although it also waned slightly over time, from 72.2% at 3 months after booster to 54.5% 5 months after booster, revealing the clinically significant superiority of Pfizer boosters to prevent severe Omicron-induced disease compared to primary course alone. With respect to Moderna, remarkably, Chemaitelly et al.Citation30 reported that partial Moderna vaccination could successfully prevent (100.0%) all Omicron-associated severe, critical, or fatal disease (WHO definitionsCitation70,Citation71); however, this was likely a result of insufficient cases in the cohort. Full vaccination provided good protection (87.1%); however, this quickly decreased to 68.4% by ≥7 months after 2nd dose. Protection from Moderna boosters was also difficult to deduce due to low number of severe cases.
COVID-19 vaccine effectiveness against Omicron and other variants in reducing infection, hospitalization, severity, and/or death in children and adolescents
Based on clinical trial data, FDA authorizations and US CDC recommendations, compared to children aged 12–17 years who are administered the same dose of Pfizer as adults (30 µg), those aged 5–11 years are administered a smaller dose of 10 µg; those aged 6 months–4 years are given 3 µg per dose.Citation72 For Moderna, similar limitations apply, with doses of 100 µg, 50 µg, and 25 µg, respectively. As of writing, other vaccines have not been approved for children.
Klein et al.Citation65 investigated 39,217 ED/UC encounters and 1,699 hospitalizations of children aged 5–17 years with COVID-19–like illness from April 9, 2021, to January 29, 2022, thus involving periods of both Delta and Omicron predominance. During periods of Delta predominance, the initial Pfizer VE of primary series in children aged 12–15 years was 92% but waned quickly over time to 79% by ≥150 days. In the 16-17-year group, this was 85% and 77%, respectively. Booster dose effectiveness could not be calculated owing to small number of cases. However, against Omicron-infection, VE was 51%, 45%, and 34% recently after receiving the 2nd dose in the 5–11, 12–15, and 16–17-year age groups, respectively. The primary series offered no protection against Omicron infection by ≥150 days in both the 12–15 and 16–17-year-old cohorts. Boosters reestablished efficacy back to 81% against Omicron ≥7 days after vaccination. Hence, the effectiveness of the vaccine seemed to wane over time even in children, and additionally, the effectiveness of the vaccine was significantly lower amongst children aged 5–11 years compared to adolescents aged 12–17 years. However, it must be noted that most of the infections in the 5–11-year-old age group occurred during Omicron period where the effectiveness of the vaccine was lower in all age groups. Finally, the authors also reported that primary Pfizer vaccination provided robust, long-lasting, and effective protection against ‘Omicron or Delta’ hospitalization in all age groups, except 5–11 years, which could be attributed to low cases and thus statistical bias, but is nonetheless, a concerning statistic.
Another study by Dorabawila et al.Citation45 also probed into the effectiveness of the Pfizer vaccine against infection and hospitalization in children aged 5–17 between December 13, 2021, to January 30, 2022, in New York, US, in a large cohort of 852,384 children aged 12–17 and another 365,502 aged 5–11, all of whom had been fully vaccinated. Since the authors did not utilize laboratory testing for population-level cases to determine the variant, it was difficult to link the effectiveness to particular strains. Nevertheless, using data published by the New York State GISAIDCitation73 and the CDC,Citation74 it was clear that a vast majority (>80%) of SARS-COV-2 cases sequenced by 1st January 2022 were of Omicron variant. The study showed that the VE of the primary series against infection amongst the 12–17 years old children was 85% during Delta variant predominance which quickly declined to 51% during a period of Omicron predominance. For those aged 5–11 years, VE against infection waned from 68% at a period when Omicron constituted 1/5th of all cases, to merely 12% during Omicron predominance. Although reduced from a peak during Delta predominance, VE against hospitalization remained relatively high during Omicron predominance in the 12–17-year-old group, with a protection rate of 73%. However, the protection against Omicron-induced hospitalization in the 5–11-year-old group was non-significant. The study also showed that VE waned significantly with time since vaccination among both age groups. In fact, VE against infection was consistently higher in the older cohort. Further, by 35–41 days from 2nd dose, the primary series no longer provided any protection in those aged 5–11 years. These concerning findings among the 5–11-year-old group were likely due to the lower vaccine dose administered in this population, thus calling for a study of the optimal numbers of doses, amount per dose, dose timing, and/or antigens targeted for children in this age group regarding Pfizer vaccination.
Similarly, Powell et al.Citation39 conducted a test-negative case–control study across the UK aiming to estimate VE of partial and full Pfizer vaccination against symptomatic Omicron and Delta variant infection and hospitalization in adolescents. Among 12-15-year olds, one-dose protection was highest at days 14–20 against both variants, although VE against Delta was much higher (74.5%) compared to Omicron (49.6%). Protection waned drastically over time, reaching lows of 45.9% and 16.1% against Delta and Omicron, respectively, both at days 70–83. Full vaccination reestablished protection against both variants, at days 7–13 after 2nd dose. Similar results were obtained for the 16-17-year-old group. Although protection against Omicron could not be assessed due to insufficient follow-up, the authors reported that partial Pfizer vaccination protected against Delta-associated hospitalization following infection only at days 28+ in the 12–15-year-old cohort and at day 0–27 and 28+ in the 16–17-year-old cohort, respectively. The authors conclude that the adolescent immunization program at the time would not sustain protection as a standalone intervention in the face of newer strains without regular boosters. The findings from our review align with those of Sabu et al.Citation75 hinting at waning immunity over time and calling for further research for the role of boosters in this age group.
How long does the immunity last after vaccination?
An early report by Hansen et al.Citation44 shows that VE for Pfizer against Omicron wanes as quickly as 31–60 days after primary vaccination. The authors suggest that this decreased VE against Omicron is likely the result of rapid spread via super-spreading events causing various infections among young and vaccinated individuals, however, this is also the fastest waning estimate among all studies, likely attributable to statistical bias stemming from low number of cases due to its early timeframe. Consensus among other large studies shows that immunity from the Pfizer primary series lasts for a period of between 3 and 6 months against Omicron BA.1 subvariant infection, and about ≥7 months against Omicron BA.2 subvariant, based on findings from Accorsi et al.Citation20 and Chemaitelly et al.Citation30 Šmíd et al.Citation37 report that protection from Omicron-induced hospitalization waned by the first month and against Delta-associated hospitalization by the 2nd month, with respect to partial Pfizer vaccination. Based on findings by Accorsi et al.Citation20 and Chemaitelly et al.Citation30 protection catered by the 2-dose primary Moderna series against Omicron BA.1 infection is no longer significant after 6–7 months, but this protection does not last even 1–3 months against Omicron BA.2. Andrews et al.Citation63 report that primary AstraZeneca vaccination is no longer protective by 25+ weeks, while Šmíd et al.Citation37 report that for protection against Omicron-induced hospitalization swayed by 2 months for the same 2-dose series. Šmíd et al.Citation37 report that for protection from Omicron-associated hospitalization, full Janssen vaccination effectiveness does not last the first month. More data and details related to the above sections, including mixed data for different vaccine types, are summarized in Appendix A.
Conclusion and recommendations
The rapid spread of Omicron is attributed to various factors, notably its increased infectiousness, transmissibility, or immune evasion compared to previous variants, including Delta. Our results show that although primary vaccination course protects against Omicron infection more effectively than partial vaccination or unvaccinated, this effect is greatly reduced compared to the protection observed against Delta variant infection. Further, primary series protection has been shown to wane significantly over time against both variants; however, more so against Omicron than Delta. In fact, 2-dose mRNA vaccination has been shown to be ineffective by 3–5 months since second dose across multiple studies. Subsequently, mRNA boosters have been shown, regardless of primary vaccination series and consistently across multiple studies, to reestablish effectiveness against infection with Delta, and to a lower margin, against Omicron, often to levels equal or greater than that conferred by primary series alone. Further, one study has shown that 3-dose mRNA series to be significantly more effective than a 2-dose series against Omicron-associated infection. Despite these benefits, the effectiveness of a booster dose against Omicron infection also begins to wane modestly over the course of a few months. In contrast to these findings against infection, primary vaccination courses have been shown to still provide consistent and strong protection against Omicron-associated hospitalization, severity, and death, months after last dose; although to a lower degree than against Delta-associated morbidity and mortality. However, boosters provide robust and longer-lasting protection against hospitalizations against Omicron, often on par to protection conferred against Delta. There was no evidence of any difference between the immune evasion of, and vaccine effectiveness against, the two different Omicron subvariants, BA.1 and BA.2. Further, while the two mRNA vaccines were both equally the most promising against Omicron-associated infection and complications, AstraZeneca was shown to cater the lowest protection; however, mRNA boosters reestablished effectiveness to on par to 3-dose mRNA courses, irrespective of primary course, aligning with existing literature.Citation76 Investigation of vaccine effectiveness in children revealed greatly lower protection conferred by the Pfizer vaccine against those aged 5–11 years old, calling for revision of dosage and dose-intervals in this age group. Consensus recommendation included promoting the administration of boosters to protect populations and healthcare systems against rapid resurgence of severe COVID-19 infection by reestablishing waning protection in the short- and middle-term. However, there is no hiding that there is an urgent need for renewed efforts to develop, manufacture, and administer en masse novel, broad-acting, next-generation vaccines to curb the ongoing and future surges of the COVID-19 pandemic, potentially fueled by newer and more virulent variants, instead of cowering behind a policy of repeatedly administering boosters of current vaccines. In the interim, increased research and funding should be refocused on developing more effective public health policy, development of antivirals and non-pharmacological interventions, and production of more streamlined national and regional channels for vaccine rollout.Citation77
Limitations
This study has a few limitations, mostly due to heterogeneous methodologies and target populations between the analyzed studies, making it difficult to compare reported results from different age groups, follow-up periods, and vaccine types. This disallows assessment of vaccine effectiveness due to widely varying follow-up times or non-specification of the vaccines utilized. Additionally, dose number and corresponding vaccination status were not clarified in a few studies, which adds to the complexity of assessing effectiveness in studies reporting on a population receiving multiple types of vaccines. The definition of severity levels varied between studies, thus further complicating analysis of VE, whereas in other studies, effectiveness against severity, hospitalization, and death was pooled into one entity owing to low number of outcomes. Moreover, many studies often utilized SGTF or community-based strain predominance as proxies to assess VE against particular variants or subvariants, allowing for gap in confidence for protection against such subtypes.
Data sharing
The data that supports the findings of this study are available in the supplementary material of this article.
Supplemental Material
Download PDF (1,017.2 KB)Acknowledgments
We would like to thank Mr. Sa’ad Laws for his help during the early phases of this project including developing the search strategy and importing papers. We would like also to thank Weill Cornell Medicine-Qatar for the continuous support. Open Access funding provided by the Qatar National Library.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2167410
Additional information
Funding
References
- Khan NA, Al-Thani H, El-Menyar A. The emergence of new SARS-CoV-2 variant (Omicron) and increasing calls for COVID-19 vaccine boosters-the debate continues. Travel Med Infect Dis. 2022;45:102246. doi:10.1016/j.tmaid.2021.102246.
- Le Page M. Vaccines vs variants. New Sci. 2021;250(3336):8–23. doi:10.1016/S0262-4079(21)00895-2.
- Christoph J, Dorota K, Lennart K, Zech F, Jacob T, Sparrer KMJ, Kirchhoff F. Omicron: what makes the latest SARS-CoV-2 variant of concern so concerning? J Virol. 2022;96(6): e02077-21. doi:10.1128/jvi.02077-21.
- Ou X, Yang Z, Zhu D, Mao S, Wang M, Jia R, Chen S, Liu M, Yang Q, Wu Y, et al. Microbial GWAS studies revealing combinations of Omicron RBD mutations existed and may contribute to antibody evasion and ACE2 binding. medRxiv. Published online Jan 1. doi:10.1101/2022.01.19.22269510.
- Pia L, Rowland-Jones S. Omicron entry route. Nat Rev Immunol. 2022;22(3):144. doi:10.1038/s41577-022-00681-9.
- Mohammed I, Nauman A, Paul P, Ganesan S, Chen K-H, Jalil SMS, Jaouni SH, Kawas H, Khan WA, Vattoth AL, et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: a systematic review. Hum Vaccin Immunother. 2022;18(1):1–20. doi:10.1080/21645515.2022.2027160.
- Yelin I, Katz R, Herzel E, Berman-Zilberstein T, Ben-Tov A, Kuint J, Gazit S, Patalon T, Chodick G, Kishony R, et al. Associations of the BNT162b2 COVID-19 vaccine effectiveness with patient age and comorbidities. medRxiv. Published online Jan 1. doi:10.1101/2021.03.16.21253686.
- Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD. Bnt162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–23. doi:10.1056/NEJMoa2101765.
- Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med. 2021;385(2):187–89. doi:10.1056/NEJMc2104974.
- Pawlowski C, Lenehan P, Puranik A, Agarwal V, Venkatakrishnan AJ, Niesen MJM, O’Horo JC, Virk A, Swift MD, Badley AD, et al. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med. 2021;2(8):979–92.e8. doi:10.1016/j.medj.2021.06.007.
- Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–201. doi:10.1056/NEJMoa2101544.
- de Faria E, Guedes AR, Oliveira MS, de Godoy Moreira MV, Maia FL, dos Santos Barboza A, Leme MD, Letaif LSH, Miethke-Morais A, Bonfá E, et al. Performance of vaccination with CoronaVac in a cohort of healthcare workers (HCW) - preliminary report. medRxiv. Published online Jan 1. doi:10.1101/2021.04.12.21255308.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. doi:10.1016/j.ijsu.2010.02.007.
- Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital Research Institute. Published 2021. [Accessed 2022 Nov 14]. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Tseng HF, Ackerson BK, Luo Y, Sy LS, Talarico CA, Tian Y, Bruxvoort KJ, Tubert JE, Florea A, Ku JH, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med. 2022; PG-. doi:10.1038/s41591-022-01753-y.
- Young-Xu Y, Zwain GM, Izurieta HS, Korves C, Powell EI, Smith J, Balajee A, Holodniy M, Beenhouwer DO, Rodriguez-Barradas MC, et al. Effectiveness of mRNA COVID-19 vaccines against Omicron and Delta variants in a matched test-negative case–control study among US veterans. BMJ Open. 2022;12(8):e063935. doi:10.1136/bmjopen-2022-063935.
- Eggink D, Andeweg SP, Vennema H, van Maarseveen N, Vermaas K, Vlaemynck B, Schepers R, van Gageldonk-Lafeber AB, van den Hof S, Reusken CB, et al. Increased risk of infection with SARS-CoV-2 Omicron BA.1 compared with Delta in vaccinated and previously infected individuals, the Netherlands, 22 November 2021 to 19 January 2022. Euro Surveill. 2022;27(4). doi:10.2807/1560-7917.Es.2022.27.4.2101196.
- Allen H, Tessier E, Turner C, Anderson C, Blomquist P, Simons D, Løchen A, Jarvis CI, Groves N, Capelastegui F, et al. Comparative transmission of SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) variants and the impact of vaccination: national cohort study, England. medRxiv. 2022; PG-. doi:10.1101/2022.02.15.22271001.
- Abu-Raddad LJ, Chemaitelly H, Ayoub HH, AlMukdad S, Yassine HM, Al-Khatib HA, Smatti MK, Tang P, Hasan MR, Coyle P, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386(19):1804–16. doi:10.1056/NEJMoa2200797.
- Accorsi EK, Britton A, Fleming-Dutra KE, Smith ZR, Shang N, Derado G, Miller J, Schrag SJ, Verani JR. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. JAMA. 2022;327(7):639–51. doi:10.1001/jama.2022.0470.
- Jalali N, Brustad HK, Frigessi A, MacDonald EA, Meijerink H, Feruglio SL, Nygård KM, Rø G, Madslien EH, de Blasio BF. Increased household transmission and immune escape of the SARS-CoV-2 Omicron compared to Delta variants. Nat Commun. 2022;13(1):5706. doi:10.1038/s41467-022-33233-9.
- Lauring AS, Tenforde MW, Chappell JD, Gaglani M, Ginde AA, McNeal T, Ghamande S, Douin DJ, Talbot HK, Casey JD, et al. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376:e069761. doi:10.1136/bmj-2021-069761.
- Lyngse FP, Mortensen LH, Denwood MJ, Christiansen LE, Møller CH, Skov RL, Spiess K, Fomsgaard A, Lassaunière R, Rasmussen M, et al. Household transmission of the SARS-CoV-2 Omicron variant in Denmark. Nat Commun. 2022;13(1):5573. doi:10.1038/s41467-022-33328-3.
- Paredes MI, Lunn SM, Famulare M, Frisbie LA, Painter I, Burstein R, Roychoudhury P, Xie H, Mohamed Bakhash SA, Perez R, et al. Associations between severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) variants and risk of coronavirus disease 2019 (COVID-19) hospitalization among confirmed cases in Washington state: a retrospective cohort study. Clin Infect Dis. 2022;75(1):e536–44. doi:10.1093/cid/ciac279.
- Lee M, Quinn R, Pradhan K, Fedorov K, Levitz D, Fromowitz A, Thakkar A, Shapiro LC, Kabarriti R, Ruiz RE, et al. Impact of COVID-19 on case fatality rate of patients with cancer during the Omicron wave. Cancer Cell. 2022;40(4):343–45. doi:10.1016/j.ccell.2022.02.012.
- Lewnard JA, Hong PM VX, Kahn R, Lipsitch M, Tartof SY. Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in southern California. Nat Med. Published online 2022 Jun;28(9):1933–43. doi:10.1038/s41591-022-01887-z.
- Veneti L, Bøås H, Bråthen Kristoffersen A, Stålcrantz J, Bragstad K, Hungnes O, Storm ML, Aasand N, Rø G, Starrfelt J, et al. Reduced risk of hospitalisation among reported COVID-19 cases infected with the SARS-CoV-2 Omicron BA.1 variant compared with the Delta variant, Norway, December 2021 to January 2022. Euro Surveill. 2022;27(4): PG- doi: 10.2807/1560-7917.Es.2022.27.4.2200077.
- Nunes MC, Mbotwe-Sibanda S, Baillie VL, Kwatra G, Aguas R, Madhi SA. SARS-CoV-2 Omicron symptomatic infections in previously infected or vaccinated South African healthcare workers. Vaccines. 2022;10(3):459. doi:10.3390/vaccines10030459.
- Patalon T, Saciuk Y, Peretz A, Perez G, Lurie Y, Maor Y, Gazit S. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat Commun. 2022;13(1):3203. doi:10.1038/s41467-022-30884-6.
- Chemaitelly H, Ayoub HH, AlMukdad S, Coyle P, Tang P, Yassine HM, Al-Khatib HA, Smatti MK, Hasan MR, Al-Kanaani Z, et al. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun. 2022;13(1):3082. doi:10.1038/s41467-022-30895-3.
- Helmsdal G, Hansen OK, Møller LF, Christiansen DH, Petersen MS, Kristiansen MF. Omicron outbreak at a private gathering in the Faroe Islands, infecting 21 of 33 triple-vaccinated healthcare workers. Clin Infect Dis. 2022;75(5):893–96; PG-. doi:10.1093/cid/ciac089.
- Andeweg SP, de Gier B, Eggink D, van den Ende C, van Maarseveen N, Ali L, Vlaemynck B, Schepers R, Hahné SJM, Reusken CBEM, et al. Protection of COVID-19 vaccination and previous infection against Omicron BA.1, BA.2 and Delta SARS-CoV-2 infections. Nat Commun. 2022;13(1):4738. doi:10.1038/s41467-022-31838-8.
- Davies M-A, Kassanjee R, Rousseau P, Morden E, Johnson L, Solomon W, Hsiao N-Y, Hussey H, Meintjes G, Paleker M, et al. Outcomes of laboratory-confirmed SARS-CoV -2 infection in the Omicron-driven fourth wave compared with previous waves in the Western Cape Province, South Africa. Trop Med Int Heal. 2022;27(6):564–73. doi:10.1111/tmi.13752.
- Ferdinands JM, Rao S, Dixon BE, Mitchell PK, DeSilva MB, Irving SA, Lewis N, Natarajan K, Stenehjem E, Grannis SJ, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance — VISION network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):255–63. doi:10.15585/mmwr.mm7107e2.
- Robinson ML, Morris CP, Betz J, Zhang Y, Bollinger R, Wang N, Thiemann DR, Fall A, Eldesouki RE, Norton JM, et al. Impact of SARS-CoV-2 variants on inpatient clinical outcome. medRxiv. 2022; PG-. doi:10.1101/2022.02.02.22270337.
- Rufino J, Baquero C, Frey D, Glorioso CA, Ortega A, Reščič N, Roberts JC, Lillo RE, Menezes R, Champati JP, et al. Using survey data to estimate the impact of the Omicron variant on vaccine efficacy against COVID-19 infection. medRxiv. 2022; PG-. doi:10.1101/2022.01.21.22269636.
- Šmíd M, Berec L, Přibylová L, Májek O, Pavlík T, Jarkovský J, Weiner J, Barusová T, Trnka J. Protection by vaccines and previous infection against the Omicron variant of severe acute respiratory syndrome Coronavirus 2. J Infect Dis. Published online 2022 Apr 28;226(8):jiac161. doi:10.1093/infdis/jiac161.
- Ulloa AC, Buchan SA, Daneman N, Brown KA. Estimates of SARS-CoV-2 Omicron variant severity in Ontario, Canada. JAMA. 2022;327(13):1286. doi:10.1001/jama.2022.2274.
- Powell AA, Kirsebom F, Stowe J, McOwat K, Saliba V, Ramsay ME, Lopez-Bernal J, Andrews N, Ladhani SN. Effectiveness of BNT162b2 against COVID-19 in adolescents. Lancet Infect Dis. 2022;22(5):581–83. doi:10.1016/S1473-3099(22)00177-3.
- Tai CG, Maragakis LL, Connolly S, DiFiori J, Anderson DJ, Grad YH, Mack CD. Association between COVID-19 booster vaccination and Omicron infection in a highly vaccinated cohort of players and staff in the National Basketball Association. JAMA. 2022;328(2):209–11. doi:10.1001/jama.2022.9479.
- Nguyen VG, Yavlinsky A, Beale S, Hoskins S, Lampos V, Braithwaite I, Byrne TE, Erica Fong WL, Fragaszy E, Geismar C, et al. Comparative effectiveness of different primary vaccination courses on mRNA based booster vaccines against SARs-COV-2 infections: a time-varying cohort analysis using trial emulation in the Virus Watch community cohort. medRxiv. 2022; PG-. doi:10.1101/2022.02.04.22270479.
- Shrestha NK, Burke PC, Nowacki AS, Terpeluk P, Gordon SM. Necessity of Coronavirus disease 2019 (COVID-19) vaccination in persons who have already had COVID-19. Clin Infect Dis. 2022;75(1):e662–71; PG-. doi:10.1093/cid/ciac022.
- Spensley KJ, Gleeson S, Martin P, Thomson T, Clarke CL, Pickard G, Thomas D, McAdoo SP, Randell P, Kelleher P, et al. Comparison of vaccine effectiveness against the Omicron (B.1.1.529) variant in hemodialysis patients. Kidney Int Rep. 2022;7(6):1406–09. doi:10.1016/j.ekir.2022.04.005.
- Hansen CH, Schelde AB, Moustsen-Helm IR, Emborg H-D, Krause TG, Mølbak K, Valentiner-Branth P, on behalf of the Infectious Disease Preparedness Group at Statens Serum Institut. et al. Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: a danish cohort study. medRxiv. 2021; PG-. doi:10.1101/2021.12.20.21267966.
- Dorabawila V, Hoefer D, Bauer UE, Bassett MT, Lutterloh E, Rosenberg ES. Effectiveness of the BNT162b2 vaccine among children 5-11 and 12-17 years in New York after the emergence of the Omicron variant. medRxiv. 2022; PG-. doi:10.1101/2022.02.25.22271454.
- Buchan SA, Chung H, Brown KA, Austin PC, Fell DB, Gubbay JB, Nasreen S, Schwartz KL, Sundaram ME, Tadrous M, et al. Estimated effectiveness of COVID-19 vaccines against Omicron or Delta symptomatic infection and severe outcomes. JAMA Netw Open. 2022;5(9):e2232760. doi:10.1001/jamanetworkopen.2022.32760.
- Collie S, Champion J, Moultrie H, Bekker L-G, Gray G. Effectiveness of BNT162b2 vaccine against Omicron variant in South Africa. N Engl J Med. 2022;386(5 PG–494–496):494–96. doi:10.1056/NEJMc2119270.
- Krutikov M, Stirrup O, Nacer-Laidi H, Azmi B, Fuller C, Tut G, Palmer T, Shrotri M, Irwin-Singer A, Baynton V, et al. Outcomes of SARS-CoV-2 omicron infection in residents of long-term care facilities in England (VIVALDI): a prospective, cohort study. Lancet Heal Longev. 2022;3(5):e347–55. doi:10.1016/S2666-7568(22)00093-9.
- Wolter N, Jassat W, von Gottberg A, Cohen C. Clinical severity of omicron lineage BA.2 infection compared with BA.1 infection in South Africa. Lancet (London, England). 2022;400(10346):93–96. doi:10.1016/S0140-6736(22)00981-3.
- Ward IL, Bermingham C, Ayoubkhani D, Gethings OJ, Pouwels KB, Yates T, Khunti K, Hippisley-Cox J, Banerjee A, Walker AS, et al. Risk of COVID-19 related deaths for SARS-CoV-2 omicron (B.1.1.529) compared with delta (B.1.617.2): retrospective cohort study. BMJ. 2022;378:e070695. doi:10.1136/bmj-2022-070695.
- Wolter N, Jassat W, Walaza S, Welch R, Moultrie H, Groome M, Amoako DG, Everatt J, Bhiman JN, Scheepers C, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet (London, England). 2022;399(10323 PG–437–446):437–46. doi:10.1016/S0140-6736(22)00017-4.
- Marks KJ, Whitaker M, Anglin O, Milucky J, Patel K, Pham H, Chai SJ, Kirley PD, Armistead I, McLafferty S, et al. Hospitalizations of Children and adolescents with laboratory-confirmed COVID-19 — COVID-NET, 14 States, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):271–78. doi:10.15585/mmwr.mm7107e4.
- Chaguza C, Coppi A, Earnest R, Ferguson D, Kerantzas N, Warner F, Young HP, Breban MI, Billig K, Koch RT, et al. Rapid emergence of SARS-CoV-2 Omicron variant is associated with an infection advantage over Delta in vaccinated persons. Med. 2022;3(5):325–34.e4. doi:10.1016/j.medj.2022.03.010.
- Chemaitelly H, Ayoub HH, Coyle P, Tang P, Yassine HM, Al-Khatib HA, Smatti MK, Hasan MR, Al-Kanaani Z, Al-Kuwari E, et al. Protection of Omicron sub-lineage infection against reinfection with another Omicron sub-lineage. Nat Commun. 2022;13(1):4675. doi:10.1038/s41467-022-32363-4.
- Boscolo-Rizzo P, Tirelli G, Meloni P, Hopkins C, Madeddu G, De Vito A, Gardenal N, Valentinotti R, Tofanelli M, Borsetto D, et al. Coronavirus disease 2019 (COVID-19)–related smell and taste impairment with widespread diffusion of severe acute respiratory syndrome–coronavirus-2 (SARS-CoV-2) Omicron variant. Int Forum Allergy Rhinol. 2022;12(10):1273–81. doi:10.1002/alr.22995.
- Auvigne V, Vaux S, Le SY, Schaeffer J, Fournier L, Tamandjou C, Montagnat C, Coignard B, Levy-Bruhl D, Parent du Châtelet I. Severe hospital events following symptomatic infection with Sars-CoV-2 Omicron and Delta variants in France, December 2021–January 2022: a retrospective, population-based, matched cohort study. eClinicalMedicine. 2022;48:48. doi:10.1016/j.eclinm.2022.101455.
- Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Amir O, Freedman L, Alroy-Preis S, Ash N, Huppert A, Milo R. Protection by a fourth dose of BNT162b2 against Omicron in Israel. N Engl J Med. 2022;386(18):1712–20. doi:10.1056/NEJMoa2201570.
- Boucau J, Marino C, Regan J, Uddin R, Choudhary MC, Flynn JP, Chen G, Stuckwisch AM, Mathews J, Liew MY, et al. Duration of viable virus shedding in SARS-CoV-2 omicron variant infection. medRxiv. 2022; PG-. doi:10.1101/2022.03.01.22271582.
- Peralta-Santos A, Rodrigues EF, Moreno J, Ricoca V, Casaca P, Fernandes E, Gomes JP, Ferreira R, Isidro J, Pinto M, et al. Omicron (BA.1) SARS-CoV-2 variant is associated with reduced risk of hospitalization and length of stay compared with Delta (B.1.617.2). medRxiv. 2022; PG-. doi:10.1101/2022.01.20.22269406.
- Thompson MG, Natarajan K, Irving SA, Rowley EA, Griggs EP, Gaglani M, Klein NP, Grannis SJ, DeSilva MB, Stenehjem E, et al. Effectiveness of a third dose of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance — VISION network, 10 States, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):139–45. doi:10.15585/mmwr.mm7104e3.
- Lyngse FP, Kirkeby CT, Denwood M, Christiansen LE, Mølbak K, Møller CH, Skov RL, Krause TG, Rasmussen M, Sieber RN, et al. Household transmission of SARS-CoV-2 Omicron variant of concern subvariants BA.1 and BA.2 in Denmark. Nat Commun. 2022;13(1):5760. doi:10.1038/s41467-022-33498-0.
- Vieillard-Baron A, Flicoteaux R, Salmona M, Annane D, Ayed S, Azoulay E, Bellaiche R, Beloucif S, Berti E, Bertier A, et al. Epidemiological characteristics and severity of omicron variant cases in the aphp critical care units. medRxiv. 2022; PG-. doi:10.1101/2022.01.25.22269839.
- Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O’Connell A-M, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–46. doi:10.1056/NEJMoa2119451.
- Gray GE, Collie S, Garrett N, Goga A, Champion J, Zylstra M, Reddy T, Yende N, Seocharan I, Takalani A, et al. Vaccine effectiveness against hospital admission in South African health care workers who received a homologous booster of Ad26.COV2 during an Omicron COVID-19 wave: preliminary results of the Sisonke 2 study. medRxiv. 2021; PG-. doi:10.1101/2021.12.28.21268436.
- Klein NP, Stockwell MS, Demarco M, Gaglani M, Kharbanda AB, Irving SA, Rao S, Grannis SJ, Dascomb K, Murthy K, et al. Effectiveness of COVID-19 PG-352-358 Pfizer-BioNtech BNT162b2 mRNA vaccination in preventing COVID-19–associated emergency department and urgent care encounters and hospitalizations among nonimmunocompromised children and adolescents aged 5–17 years — VISION network, 10 states, April 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(9):352–58. doi:10.15585/mmwr.mm7109e3.
- Chenchula S, Karunakaran P, Sharma S, Chavan M. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: a systematic review. J Med Virol. 2022;94(7):2969–76. doi:10.1002/jmv.27697.
- Du Y, Chen L, Shi Y. Booster COVID-19 vaccination against the SARS-CoV-2 Omicron variant: a systematic review. Hum Vaccin Immunother. 2022;18(5):2062983. doi:10.1080/21645515.2022.2062983.
- Zeng B, Gao L, Zhou Q, Yu K, Sun F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: a systematic review and meta-analysis. BMC Med. 2022;20(1):200. doi:10.1186/s12916-022-02397-y.
- SARS-CoV-2 variants in analyzed sequences, United Kingdom. [accessed 2022 Jul 31]. https://ourworldindata.org/grapher/covid-variants-area?country=~GBR.
- WHO. International guidelines for certification and classification (coding) of COVID-19 as cause of death. World Heal Organ. 2020 Apr:14. [Accessed 2022 Aug 5]. https://www.paho.org/en/documents/international-guidelines-certification-and-classification-coding-covid-19-cause-death.
- Organization WH. Living guidance for clinical management of COVID-19. World Heal Organ. 2021 Nov:63. [Accessed 2022 Aug 5]. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2.
- Centers for Disease Control and Prevention. Summary document for interim clinical considerations for use of COVID-19 vaccines currently authorized or approved in the United States - FactSheet. Published online 2022. [Accessed 2022 Aug 6]. https://www.cdc.gov/vaccines/covid-19/downloads/summary-interim-clinical-considerations.pdf.
- COVID-19 Variant Data | Department of Health. [accessed 2022 Aug 6]. https://coronavirus.health.ny.gov/covid-19-variant-data#.
- CDC COVID Data. [Accessed 2022 Aug 6]. https://covid.cdc.gov/covid-data-tracker/#variant-proportions.
- Sabu JM, Zahid I, Jacob N, Alele FO, Malau-Aduli BS. Effectiveness of the BNT162b2 (Pfizer-BioNtech) vaccine in children and adolescents: a systematic review and meta-analysis. Vaccines. 2022;10(11):1880. doi:10.3390/vaccines10111880.
- Au WY, Cheung P-H. Effectiveness of heterologous and homologous COVID-19 vaccine regimens: living systematic review with network meta-analysis. BMJ. 2022;377:e069989. doi:10.1136/bmj-2022-069989.
- Lazarus JV, Romero D, Kopka CJ, Karim SA, Abu-Raddad LJ, Almeida G, Baptista-Leite R, Barocas JA, Barreto ML, Bar-Yam Y, et al. A multinational Delphi consensus to end the COVID-19 public health threat. Nature. 2022;611(7935):332–45. doi:10.1038/s41586-022-05398-2.
Appendix A
Detailed discussion
Our systematic review included 51 studies with at least 10,581,027 subjects. Since the emergence of the Omicron variant, it was not clear how effective the COVID-19 vaccines are against the Omicron infection as compared to the other variants, such as the Delta variant. In this review, we compiled all reported data about the effectiveness of the COVID-19 vaccines against the Omicron-related infections, hospitalization, severity, or death. In addition, we also reported any data comparing the OR, HR or RR of Omicron or other variants related infections, hospitalization, severity, or death in vaccinated populations.
How were variants and subvariants identified?
Variants were confirmed as Omicron using whole-genome sequencing or a novel variant-specific PCR test and assumed Delta otherwise. Other studies implemented S-gene target failure (SGTF), identified by genotyping by detecting the del69/70 mutation in the S-gene, as a proxy for Omicron (BA.1) variant. A minority of studies designated variant by solely matching dates of PCR-test and national variant-trends. In countries such as Qatar, viral genome sequencing and multiplex RT-PCR variant screening of random positive clinical samples are the basis of variant screening; this is also complemented by deep sequencing of wastewater samples. Well into surge of the Omicron variant, non-SGTF genotyped cases were proxied for the Omicron BA.2 subvariant.
COVID-19 vaccine effectiveness against Omicron and other variants in reducing infection
Pfizer/BioNTech
The Pfizer/BioNTech vaccine has been the most widely investigated vaccine across the world given its status as one of the first-approved and most efficacious vaccines against the SARS-CoV-2 wildtype. Its waning effectiveness against the Delta variant was earlier called into questionCitation6, although recent studies reveal that its protection against Omicron infection is much more scrutinized. Across studies that investigate this relationship, the conclusion points toward the booster being a necessity in preventing further Omicron spread given the lower vaccine effectiveness against Omicron just months following primary course completion; naturally, partial vaccination has been shown to be even less effective. In their early investigation of the Danish national database between November 20 and December 12, 2021, Hansen et al.Citation42 studied 5,767 Omicron cases, largely in young individuals (median age 28, 93% <60 years). The authors noted VEs of 55.2% (95% CI: 23.5 to 73.7) against Omicron infection in the 1st month after primary vaccine protection, which waned substantially to −76.5% (95% CI: −95.3 to −59.5) which is reflected in the negative VE values obtained during the 4th and 5th months. Although VE against the Delta variant also decreased, it was not as substantial (86.7% [95% CI: 84.6 to 88.6] during 1st month to 53.8% [95% CI: 52.9 to 54.6] during the 4th and 5th months). Booster vaccination reestablished VE to 54.6% (95% CI: 30.4 to 70.4) against Omicron and to 81.2% (95% CI: 79.2 to 82.9) against Delta during a 1-month follow-up, providing an early hint for the need of boosters to counter the Omicron variant. Similar inter-variant conclusions were reached by Šmíd et al.Citation35 in their study of the national database of the Czech Republic between December 7, 2021, to February 13, 2022, with data from 18,045,537 residents. With respect to protection against Delta infection, compared to unvaccinated, the study reported that partial Pfizer/BioNTech vaccination was associated with HRs of 0.336 (95% CI: 0.315–0.358) and 0.397 (95% CI: 0.318–0.495) at 1 and 2 months from inoculation. Similarly, full vaccination was associated with HRs of 0.183 (95% CI:0.171–0.196), 0.303 (95% CI: 0.292–0.313) and 0.462 (95% CI: 0.454–0.470) at 1, 2 and 3 months from 2nd dose. In stark contrast, with respect to Omicron infections, HRs were found to be drastically increased at 0.69 (95% CI: 0.66–0.72) and 0.47 (95% CI: 0.41–0.54) at 1 and 2 months since partial vaccination, but 0.51 (95% CI: 0.50–0.52), 0.73 (95% CI: 0.71–0.75) and 0.89 (95% CI: 0.88–0.90) at 1, 2 and 3 months following full vaccination. These findings reaffirm two observations: protection against Omicron infection from partial and full courses is both less effective and shorter lasting against Omicron compared to Delta.
Findings from the UK also reveal that protection from symptomatic disease against Delta is both initially stronger and lasts longer compared to Omicron, with respect to partial, full, and booster Pfizer/BioNTech vaccination. In one of the largest test-negative case-control designed studies included in this review, Andrews et al.Citation61 investigated the VE of primary and booster courses of multiple vaccines against the Omicron BA.1 and the Delta variants in England between November 27, 2021, and January 12, 2022Citation64, including data from 886,774 Omicron cases, 204,154 Delta cases, and 1,572,621 test-negative controls. Partial vaccination with Pfizer/BioNTech protects against symptomatic Delta infection with a peak VE of 72.3% (95% CI: 69.4–74.9) at ≥4 weeks; in contrast, protection against symptomatic Omicron infection was less robust at 31.5% (95% CI: 29.9–33.1) at the same time from the 1st dose. A second dose, thus completing primary course, reinvigorated the VE against both variants, although to a greater extent against Delta by reaching a peak of 90.9% (95% CI: 89.6–92.0), compared to only 65.5% (95% CI: 63.9–67.0) against Omicron, both at 2–4 weeks after 2nd dose. However, by 25 weeks, effectiveness against both variants had dropped, to 62.7% (95% CI: 71.6–63.7) against Delta and 8.8% (95% CI: 7.0–10.5) against Omicron. A booster Pfizer/BioNTech dose once again reestablished VE to levels on-par with or greater than that conferred by primary course; VE: 92.3% (95% CI: 91.6–92.9) against Delta and 66.9% (95% CI: 66.1–67.6) against Omicron. Further, although protection against Delta remained consistent at 89.9% (95% CI: 89.2–90.5) even at ≥10 weeks, it waned considerably to 45.7% (95% CI: 44.7–46.7) against Omicron. Moreover, boosters provide vulnerable individuals with comparable protection as the general population despite a faster waning of the primary course protection, as shown by a prospective longitudinal surveillance study in a cohort of 1,121 hemodialysis patients in the UK by Spensley et al.Citation41 Between December 1, 2021, and January 16, 2022, compared to unvaccinated, the authors report VE of 66% (95% CI: 36–81) or an HR of 0.34 (95% CI: 0.19–0.64) against Omicron infection after completion of a booster Pfizer/BioNTech course, compared to a non-efficacious HR of 0.83 (95% CI: 0.43–1.62) from only full vaccination.
Large-scale studies across the US have confirmed and reproduced the results described in the abovementioned studies across Europe. Accorsi et al.Citation18 conducted a test-negative case–control study with 23,391 cases (13,098 Omicron and 10,293 Delta) and 46,764 controls that were tested for COVID-19-like illness between December 10, 2021, and January 1, 2022, across 49 US states. The study reported that compared to unvaccinated, being boosted with Pfizer/BioNTech was associated with an adjusted OR (aOR) of 0.35 (95% CI: 0.32–0.38) against Omicron and 0.077 (95% CI: 0.070–0.086) against Delta symptomatic infection, thereby highlighting Omicron’s increased immune evasion versus Delta, but also the booster’s ability to protect against mass waves of infection. Secondly, a similar difference between the variants was present, although to a lesser degree, when comparing the odds of symptomatic infection in booster versus primary series recipients; aOR: 0.35 (95% CI: 0.32–0.37) for Omicron and 0.17 (95% CI: 0.16–0.19) for Delta, thereby highlighting the importance of the booster dose against the newer variants of concern. The study ascertained the similarity of ORs against Omicron infection in both comparisons (3 doses versus unvaccinated and versus 2 doses) to immune evasion; further, this could mean that protection conferred by primary series is only effective against Omicron if taken recently. Further, the authors hypothesized that boosters may reduce transmissibility potential after detecting higher cycle threshold (Ct) values in Omicron viral load in the boosted cases.
Despite the immediate success of booster vaccination against Omicron-infection reported by the above studies, it is also important to note that a few have hinted toward quick waning of this protection. Patalon et al.Citation27 conducted a retrospective test-negative case–control study among 546,924 PCR tests performed between January 1 and 21, 2022 by the Maccabi Healthcare Services in Israel. Compared to boosting 5 months prior to testing, Pfizer/BioNTech booster-VE against Omicron infection decreased each month from vaccination, with a peak VE of 53.4% (95% CI: 47.7–58.6) for those boosted just a month prior to testing versus a VE of 3.6% (95% CI: 0.6–6.5) for those boosted 4 months prior to testing. Using two-dose (primary series) vaccinees as comparator, however, the VE ranged from 59.4% (95% CI: 54.9–63.5) to 16% (95% CI: 12.3–19.5) for those boosted 1 month to 5 months prior to testing, respectively, thus highlighting the marginal effectiveness of boosters over the second dose, although still affected by time since vaccination.
Given similar early vaccine rollout and existence of national SARS-COV-2 vaccine and infection surveillance programs in Qatar, Abu-Raddad et al.Citation17 conducted a large, matched retrospective cohort study including 2,232,224 individuals during a period of Omicron predominance between December 19, 2021, and January 26, 2022. The authors show that, compared to primary series, booster Pfizer/BioNTech doses were 49.4% (95% CI: 47.1–51.6) effective against symptomatic Omicron infection; in contrast, the booster was more effective in preventing symptomatic Delta-infection, with VE of 86.1% (95% CI: 67.3–94.1) compared to primary series. These results reaffirm and quantify findings regarding the marginal effectiveness of boosters over only primary series, against both variants. In a parallel but substantially extended Qatar-based study by Chemaitelly et al.Citation28, data from 1,308,926 individuals with at least two doses of the vaccine, 355,979 of which reported a booster, was investigated between December 23, 2021, and February 28, 2022, during a large Omicron wave consisting of both BA.1 and BA.2 subvariant. Partial Pfizer/BioNTech vaccination was only 39.2% (95% CI: 2.3–62.1) and 36.1% (95% CI: 12.1–53.5) effective against BA.1 and BA.2 symptomatic infection ≥14 days after 1st dose compared to unvaccinated, respectively. Effectiveness of full vaccination against symptomatic Omicron BA.1 and BA.2 infection peaked in the first 3 months after receiving the 2nd dose (46.6% [95% CI: 33.4–57.2] for BA.1 and 51.7% [95% CI: 43.2–58.9] for BA.2), which waned significantly over 7 months to below −10% for both subvariants. Although booster doses increased effectiveness discriminately against the two subvariants, i.e., to 59.9% (95% CI: 51.2–67.9; BA.1) and 43.7% (95% CI: 36.5–50.0; BA.2) recently after 3rd dose, it waned quickly to ~40% against both subvariants after ≥1 month. In conclusion, full courses Pfizer/BioNTech provide moderate protection against Delta-, but not Omicron-associated infections; this reduction is further accelerated by time since last dose. Although boosters significantly reestablish protection against both variants, it is less robust and again subject to accelerated waning with time against Omicron.
Moderna
Most of the studies discussed above also investigated the effectiveness of partial, full, and/or booster Moderna vaccination given their similar distribution owing to comparable efficacies and legislative approval time frames. Although most studies showed that the protection provided by the two mRNA vaccines were comparable, a few have reported contrasting findings. For instance, Hansen et al.Citation42 reported non-significant VEs of 36.7% (95% CI: −69.9 to 6.4) against Omicron in just the 1st month after primary vaccination, which further waned considerably to −39.3% (95% CI: −61.6 to −20.0) during the 4th and 5th months. Although VE of the primary course also warned against the Delta variant over time, the decline was less substantial (88.2% [95% CI: 83.1–91.8] during 1st month to 65.0% [95% CI: 63.6 to 66.3] during the 4th-5th months). Booster vaccination re-established VE against Delta to 82.8% (95% CI: 58.8–92.9) reaffirming the significance of the booster against Delta-associated infection; however, insufficient number of Omicron cases disallowed Moderna booster VE estimation against Omicron, which can perhaps also explain the wide confidence interval in the preceding analyses. Hence, a comparison can be made to the results by Šmíd et al.Citation35 who reported the effectiveness of Moderna vaccines using a large national database in the Czech Republic. Regarding protection against Delta variant infection, compared to unvaccinated, partial Moderna vaccination was associated with HRs of 0.325 (95% CI: 0.269–0.393) and 0.330 (95% CI: 0.165–0.661) at 1 and 2 months from first dose. Similarly, full Moderna vaccination was associated with HRs of 0.295 (95% CI: 0.244–0.358), 0.248 (95% CI: 0.209–0.295), and 0.327 (95% CI: 0.310–0.346) at 1, 2 and 3 months from last dose, respectively. At the same time, with respect to Omicron infections, HRs were found to be modest at 0.51 (95% CI: 0.45–0.58) and 0.40 (95% CI: 0.25–0.64) at 1 and 2 months since partial vaccination, but 0.51 (95% CI: 0.45–0.58), 0.59 (95% CI: 0.54–0.64) and 0.80 (95% CI: 0.78–0.83) at 1, 2 and 3 months following full vaccination, respectively. These observations reaffirm previous inter-variant findings, in addition to hinting toward slightly better protection compared to Pfizer/BioNTech.
Andrews et al.Citation61 reaffirm the protection provided by Moderna vaccination in other parts of Europe, specifically England, revealing that partial vaccination protects against symptomatic Delta infection similar to Pfizer/BioNTech, with mean VEs of 60.1% (95% CI: 51.8–66.9) in the first 3 weeks after 1st dose to 57.4% (95% CI: 52.6–61.8) at ≥4 weeks, revealing limited decline. In contrast, the VEs against symptomatic Omicron infection ranged from 47.9% (95% CI: 43.1–52.3) to 31.9% (95% CI: 27.3–36.1) in similar respective time frames. Completion of the primary course increased the VE against both variants, although to a greater extent against Delta (94.5% [95% CI: 90.5–96.9] versus Delta compared to 75.1% [95% CI: 70.8–78.7] versus Omicron, both 2–4 weeks after 2nd dose). However, by the third-dose eligibility period, i.e., ≥25 weeks, protection against both variants had dropped, although to a slightly lesser degree than seen with Pfizer/BioNTech; VE: 80.4% (95% CI: 67.3–88.2) against Delta and 14.9% (95% CI: 3.9–24.7) against Omicron. A booster Moderna dose reestablished VE to 96.4% (95% CI: 91.4–98.5) against Delta and 66.3% (95% CI: 63.7–68.8) against Omicron by 2–4 weeks after 3rd dose. These results greatly align with those seen from the use of Pfizer/BioNTech, showing that although protection is limited and shorter lasting against Omicron compared to Delta, outcomes fare much better when compared to those unvaccinated.
Studies conducted in the US further add to the increasing literature investigating the Moderna vaccine effectiveness against both variants. Accorsi et al.Citation18 show that compared to unvaccinated, being boosted with Moderna was associated with an aOR of 0.28 (95% CI: 0.26–0.31) against Omicron and 0.045 (95% CI: 0.038–0.053) against Delta symptomatic infection. Similarly, comparing booster versus primary series, the authors show aOR of 0.31 (95% CI: 0.28–0.34) against Omicron and 0.13 (95% CI: 0.11–0.15) against Delta, both results aligning with Pfizer/BioNTech vaccination findings that mRNA boosters are an important tool against Omicron symptomatic infection and transmissibility. Tseng et al.Citation13 report comparable findings based on their early test-negative case–control study across Southern California, US, between December 6 and 31, 2021. Partial vaccination only provided protection of 56.7% (95% CI: 40.7–68.4) and 20.4% (95% CI: 9.5–30.0) against Delta and Omicron variants, respectively. The primary course effectiveness against Delta infection peaked and dipped at 80.2% (95% CI: 68.2–87.7) and 61.3% (95% CI: 55.0–66.7) at 14–90 and >270 days, respectively, showing modest reliability over time. Booster doses further increased VE to 93.7% (95% CI: 92.2–94.9) and 86.0% (95% CI: 78.1–91.1) at 14–90 days and >60 days, respectively. In comparison, the protection against infection with the Omicron variant for two doses peaked at merely 44% (95% CI: 35.1–51.6) at 14–90 days and demonstrated a rapid decline, reaching 5.9% (95% CI: 0.4–11.0) at >270 days since 2nd dose. Boosters revamped protection, but also waned quickly, from 71.6% (95% CI: 69.7–73.4) 14–60 days to 47.4% (95% CI: 40.5–53.5) at >60 days.
The studies from Qatar further contribute to this discussion given the widespread distribution of both mRNA vaccines across the city-state. In a study focused on investigating the marginal effectiveness of dose 3 compared to dose 2, Abu-Raddad et al.Citation17 show that Moderna boosters were 47.3% (95% CI: 40.7–53.3) effective against symptomatic Omicron infection compared to primary series only. This aligns with the comparison of the Pfizer/BioNTech booster, compared to its primary course. Further, Chemaitelly et al.Citation28 showed that partial Moderna vaccination was not reliable in their protection against both BA.1 and BA.2 symptomatic infection. Full vaccination against Omicron BA.1 was 71% (95% CI: 24.0–89.0) effective in the first 3 months following 2nd dose compared to unvaccinated, but this protection rapidly waned by ≥7 months to −10.2%. (95% CI: −23.1 to 1.3). Against BA.2 subvariant, the full course of Moderna was not effective at any point from 1 to ≥7 months after 2nd dose. This finding is in contrast to what the authors report for Pfizer/BioNTech vaccination, hence signaling lower protection of Moderna primary series against this subvariant. However, booster doses greatly increased effectiveness to 51.5% (95% CI: 32.3–65.2; BA.1) and 39.4% (95% CI: 24.8–51.2; BA.2) recently within a month after 3rd dose, once again revealing a slightly greater predisposition of BA.2 to escape Moderna-induced immune response, although a similar pattern was also observed with Pfizer/BioNTech protection between BA.1 and BA.2.
Janssen
Although many studies have reported the use of Janssen along with other vaccines in their populations, very few have reported on Janssen efficacies independent of other manufacturers, making it difficult to comment on its efficacy alone. However, owing to their large national database, Šmíd et al.Citation35 have shown that Janssen vaccines were associated with HRs of 0.403 (95% CI: 0.377–0.430), 0.427 (95% CI: 0,391–0.465) and 0.467 (95% CI: 0.435–0.501) at 1, 2 and 3 months from full vaccination against Delta variant infection, showing modest and long-lasting protection. Against Omicron, these figures were 0.53 (95% CI: 0.51–0.55), 0.63 (95% CI: 0.60–0.65) and 0.65 (95% CI: 0.62–0.67), respectively, highlighting that viral vector vaccines such as Janssen are also susceptible to waning VE with time and Omicron-specific immune evasion. Notably, Janssen appears to be comparable in effectiveness to full vaccination with either mRNA vaccine against Omicron, but not Delta, where mRNA vaccines provide greater protection. Lewnard et al.Citation24 conducted a prospective cohort study in South California, US, to report characteristic and clinical outcomes among Delta and Omicron BA.1/BA.2 cases detected in an outpatient setting between November 1, 2021, and March 17, 2022. The authors show that Janssen full vaccination was associated with an aOR of 1.56 (95% CI: 1.44–1.70) with respect to outpatient Omicron versus Delta infection compared to unvaccinated, highlighting Omicron’s increased tendency to escape infection fighting immune protection compared to Delta. There was no difference in the odds of being infected with Omicron BA.2 over BA.1 when vaccinated with Janssen (aOR: 0.84 [95% CI: 0.57–1.25]).
Oxford/AstraZeneca
Given the lack of legislative approval in the US, only few studies across Europe have reported on the protection provided by the Oxford/AstraZeneca vaccine independently of others. In the previously described large England-based study by Andrews et al.Citation61, the authors show that partial (1-dose) vaccination with Oxford/AstraZeneca protects against symptomatic Delta infection much less compared to both mRNA vaccines, with mean VEs of 42.9% (95% CI: 39.8–45.9) and 17.7% (95% CI: 14.3–21.0) against symptomatic Delta and Omicron variants at ≥4 weeks, respectively. Completion of primary series increased the VE against both variants, although to a greater extent against Delta (82.8% [95% CI: 74.5–88.4] versus Delta, compared to 48.9% [95% CI: 39.2–57.1] vs Omicron, both 2–4 weeks after 2nd dose). However, by the third-dose eligibility period, at ≥25 weeks, effectiveness of 2-doses against both variants had dropped, to 43.5% (95% CI: 42.4–44.5) against Delta and −2.7% (95% CI: 04.2 to −1.2) against Omicron. These trends have also been reproduced from the large Czech study by Šmíd et al.Citation35, where full vaccination with Oxford/AstraZeneca was protective against Delta infection with HRs of 0.345 (95% CI: 0.288–0.435) and 0.551 (95% CI: 0.524–0.579) at 2 and 3 months post completion of full vaccination series, respectively. Against Omicron infection, HRs were higher at 0.49 (95% CI: 0.31–0.77) and 0.95 (95% CI: 0.91–0.99; practically no protection), respectively, at 2 and 3 months from full vaccination. Similar findings were reported by Spensley et al.Citation41 where primary Oxford/AstraZeneca vaccination against Omicron infection in a cohort of hemodialysis patients, who were likely vaccinated early on given their comorbidities, was statistically non-protective, with an HR of 1.04 (95% CI: 0.57–1.97). However, Andrews et al.Citation61 further reveal that a booster Oxford/AstraZeneca dose reestablished VE to 77.1% (95% CI: 55.1–88.3) against Delta and 57.7% (95% CI: 37.6–71.3) against Omicron; however, these were the lowest VEs among all booster-primary course combinations (combinations with mRNA vaccines, either as primary course or boosters, have been discussed in the following section). Hence, a novel indication of these findings is that both primary series and booster protection with purely Oxford/AstraZeneca vaccines are less effective at preventing symptomatic Delta and Omicron infection compared to a combination with mRNA or Janssen vaccines; their effect against Delta was more comparable, although still less protective.
Heterogenous or mixed vaccine regimens
Pfizer/BioNTech, Moderna, and/or Oxford/AstraZeneca
In the previously discussed large, England-based, test-negative case–control study Andrews et al.Citation61, the vast majority of individuals who had completed primary series with either Pfizer/BioNTech, Moderna, or Oxford/AstraZeneca were administered mRNA boosters, as per national policy. Regimens composed of purely one type of vaccine have been discussed in their respective sections above; here we discuss heterogenous combinations. Against Delta variant, both mRNA-boosters resulted in mean VE > 95% by 2nd–4th week of inoculation, regardless of primary course with either of the three vaccines and remained high (>88%) over the longest follow-up period of ≥10 weeks. However, against Omicron, the results are more concerning. Among Oxford/AstraZeneca primary recipients, Pfizer/BioNTech boosters increased VE to 62.4% (95% CI: 61.8–63.0) at 2nd-4th week, dropping to 39.6% (95% CI: 38.0–41.1) by ≥10 weeks, whereas Moderna boosters after Oxford/AstraZeneca primary series increased VE to 70.1% (95% CI: 67.0–68.9) at 2nd–4th weeks, dropping to 60.9% (95% CI: 59.7–62.1) at 5th–9th weeks. Among Pfizer/BioNTech and Moderna primary course recipients, corresponding 2nd–4th week VEs were 73.9% (95% CI: 73.1–74.6) and 64.9% (95% CI: 62.3–67.3) in those receiving Moderna and Pfizer/BioNTech boosters, respectively, which were similar to VEs observed from homogenous mRNA-vaccine boosting.
Pfizer/BioNTech and/or Oxford/AstraZeneca
In addition to homogenous full courses of Pfizer/BioNTech and Oxford/AstraZeneca, as well as 3-dose course with Pfizer/BioNTech, Spensley et al.Citation41 also reported that a Pfizer/BioNTech booster dose following a primary series of Oxford/AstraZeneca was protective in preventing Omicron infection in hemodialysis patients in the UK with a VE of 47% (95% CI: 2–70) or an HR of 0.53 (95% CI: 0.30–0.98), whereas primary series using either of the two were not effective. VE was also reestablished in the cohort, which received Pfizer/BioNTech as both primary and booster vaccination, further adding to the growing evidence that mRNA-boosting plays a significant part in preventing infection, irrespective of priming vaccine.
Pfizer/BioNTech, Moderna, and/or Janssen
Lewnard et al.Citation24 show that when vaccinated with Janssen primary series and further mRNA vaccine-boosted, the (adjusted) odds of Omicron infection was about 2.32 (95% CI: 1.97–2.72) times more likely than Delta infection compared to when unvaccinated. However, there was no difference in the odds of Omicron BA.2 vs BA.1 infection (aOR: 11.4 [95% CI: 0.78–1.67]) compared to unvaccinated.
Data for different vaccine types
At the time of writing, only mRNA vaccines have been administered as boosters as they are the only ones investigated and approved for the purpose; this is true regardless of primary course. Other studies have not differentiated on the vaccine utilized for vaccination but have instead mentioned what vaccines are administered in the national population and their respective proportions; here we discuss all such mixed regimens.
Pfizer/BioNTech and/or Moderna
In a Norway-based registry cohort study of 1,122 primary SARS-CoV-2 cases (Delta, n = 460; Omicron, n = 662), and their 2,169 household contacts (Delta, n = 1301; Omicron, n = 868), Jalali et al.Citation19 investigated VE of contact between December 14, 2021, and January 23, 2022, a time where only the Pfizer/BioNTech and the Moderna vaccines were suppliedCitation65,Citation66. The authors report that VE for partial, full, and booster vaccinations were 22% (95% CI: 0–46), 27% (95% CI: 6–49) and 45% (95% CI: 26–57), respectively, compared to unvaccinated for Omicron variant infection. Corresponding VEs against Delta were substantially higher at 31% (95% CI: 0–72), 42% (95% CI: 23–55), and 65% (95% CI: 42–80), respectively. Further, primary infected cases with boosters were shown to have a considerably higher risk (RR: 4.34 [95% CI 1.52–25.16]) of transmitting Omicron infection to household contacts compared to Delta, highlighting that the variant’s inherent greater transmissibility is fueled by immune evasion.
Two studies from the VISION collaboration network across 10 states in the US, between August 2021 and January 2022 provide good estimates to study mRNA vaccine effectiveness in the country against Delta in comparison with Omicron. Ferdinands et al.Citation32 investigated 241,204 emergency department (ED) and urgent care (UC) encounters with ‘COVID-like illness’ between August 26, 2021, and January 22, 2022. Primary course mRNA VE against laboratory-confirmed SARS-CoV-2 Delta infection ranged between 92% (95% CI: 91–94) at <2 months from last dose to 77% (95% CI: 76–78) at ≥5 months, compared to unvaccinated. Booster doses increased protection to 97% (95% CI: 96–97) at <2 months from 3rd dose, waning to 89% (95% CI: 64–97) by ≥4 months. In comparison, the primary course VE against infection during Omicron-predominant periods ranged from 69% (95% CI: 62–75) at <2 months to 37% (95% CI: 34–40) at ≥5 months, which raised to 87% (95% CI: 85–88) at <2 months following booster dose; however, this dropped to non-significant levels by ≥5 months (31% [95% CI: −50 to 68]), although this could be an artificial bias due to low number of cases. These reveal that primary course VE against Delta somewhat wanes with time, but this is recharged by longer-lasting boosters, in contrast, decline is seen on a quicker and larger scale with Omicron. In an earlier study using the same collaborative dataset, Thompson et al.Citation58 analyzed 222,772 ED/UC encounters from August 26, 2021, to January 5, 2022. mRNA vaccines were 86% (95% CI: 85–87), 76% (95% CI: 75–77) and 94% (95% CI: 93–94) effective against laboratory-confirmed ED/UC visits during Delta-predominance at 14–179 or ≥180 days after 2nd dose and 3 doses, respectively. Protection against Omicron infection was less promising, with VEs of 52% (95% CI: 46–58), 38% (95% CI:32–43) and 82% (95% CI: 79–84), respectively. The studies conclude that VE against ED/UC visits after receipt of both 2- and 3-doses of mRNA vaccines was lower in Omicron-predominated periods compared to Delta-predominated periods, after adjusting for time since vaccination. Additionally, similar to previous studies, booster doses reestablished VEs, but also waned with time since inoculation.
Pfizer/BioNTech, Moderna, and/or Oxford/AstraZeneca
In an UK-based cohort study, Nguyen et al.Citation39 compared the rates of infection among 19,692 infection-naïve, mRNA vaccine-boosted individuals who had received either the Oxford/AstraZeneca (n = 12,036) or the Pfizer/BioNTech (n = 7,656) vaccines as primary series. Reporting of effectiveness was based on date of booster administration, divided into eight 14-day periods between September 16, 2021, and January 1, 2022, with follow-up until January 23, 2022, a maximum of 129 days. In the UK, Delta was the predominant variant until December 6, 2021, and Omicron was the predominant variant from January 6, 2022Citation64. Interestingly, the mean adjusted HR revealed no difference stemming from administration of primary Oxford/AstraZeneca series compared to primary Pfizer/BioNTech series (HR: 0.99 [95% CI: 0.88–1.11]) among individuals who have been mRNA vaccine boosted. Further, this conclusion holds in periods of both Delta and Omicron predominance. The authors also show that mRNA vaccine-booster vaccination decreases infection rates in both cohorts; from 13% to 7.2% in the Oxford/AstraZeneca group, and from 12% to 7.6% in the Pfizer/BioNTech group. Finally, Buchan et al.Citation44 studied 4,261 Delta positive and 16,087 Omicron positive cases, as well as 114,087 test-negative controls, between December 6 and 26, 2021 in Ontario, Canada. The authors reported that primary series with ≥1 mRNA vaccine (2 mRNA doses, or 1 mRNA and 1 Oxford/AstraZeneca dose) provided consistent and long-lasting protection against symptomatic Delta infection, with VEs ranging from 89% (95% CI: 87–90) at 7 days to 80% (95% CI: 74–84) at ≥240 days since 2nd dose; nevertheless, administration of an mRNA booster further increased protection to 97% (95% CI: 96–98) by ≥7 days, irrespective of whether Pfizer/BioNTech or Moderna. In stark contrast, but similar to observed trends, VE against Omicron infection was 36% (95% CI: 24–45) at 7–59 days after completion of a primary series of ≥ 1 mRNA vaccine, which was no longer protective by 180 days (VE: 1% [95% CI: −8 to 10]); administration of Pfizer/BioNTech and Moderna boosters raised VE to 60% (95% CI: 55–65) and 65% (95% CI: 55–72) at ≥7 days after inoculation. In conclusion, those receiving homogeneous 3-dose course of Oxford/AstraZeneca were less protected against both variants, especially Omicron; notably, however, receiving an mRNA booster offered protection at similar levels regardless of whether primary course was composed of either mRNA vaccines or Oxford/AstraZeneca, which could serve an integral role in building up basal levels of immunity in a future era of vaccine shortage. Further, it appears the primary Pfizer/BioNTech series followed by Moderna boosters are most protective against Omicron infection.
Pfizer/BioNTech, Moderna, Oxford/AstraZeneca, and/or Janssen
Šmíd et al.Citation35 have investigated the protection conferred by mRNA boosters regardless of primary vaccination with Pfizer/BioNTech, Moderna, Oxford/AstraZeneca, or Janssen, as mandated by policy in the Czech RepublicCitation68. Overall, full vaccination protection against Omicron infection was 43% (95% CI: 42–44) just <2 months after the latest dose; this declined to as low as 9% (95% CI: 8–10) after >2 months. Receiving an mRNA booster dose increased protection to 56% (95% CI: 55–56) recently but declined to 21% (95% CI: 19–23) after >2 months. The authors also comment that these numbers greatly contrasted to that of Delta-infection protection, which were 73% (95% CI: 72–74), 57% (95% CI: 56–58), 90%, (95% CI: 90–91) and 82% (95% CI: 79–84), respectively. With respect to individual manufacturers’ boosters, Pfizer/BioNTech boosters greatly reduced the risk of Delta variant infection, with HRs of 0.101 (95% CI: 0.096–0.105) and 0.201 (95% CI: 0.178–0.0.227) at 1 and 2 months since inoculation, respectively. HRs at similar time periods were 0.078 (95% CI: 0.069–0.089) and 0.090 (95% CI: 0.047–0.173) for the Moderna booster, highlighting its slightly higher effectiveness against this variant. Against Omicron-infection however, the authors report that Pfizer/BioNTech boosters at 1 and 2 months since inoculation were associated with HRs of 0.42 (95% CI: 0.41–0.42) and 0.76 (95% CI: 0.74–0.78) and were 0.39 (95% CI: 0.38–0.40) and 0.67 (95% CI: 0.62–0.71) at the same time frames for Moderna boosters. Hence, although protection of both mRNA boosters was robust and comparable, Omicron was associated with great immune evasion even following recent vaccination, further deteriorating with time.
Two large studies from the Netherlands help us further accurately estimate the protection conferred by a primary series of these four major vaccines, followed by mRNA boosters. Eggink et al.Citation15 studied 174,349 cases (80,615 Omicron and 93,734 Delta) detected between November 22, 2021, and January 19, 2022. Notably, Omicron disproportionately affected younger individuals and those with a travel history. Moreover, compared to unvaccinated, primary vaccination (using either of the four vaccines) was associated more with Omicron infection than Delta (aOR: 3.6 [95% CI: 3.4–3.7]), highlighting the decreased immune protection against the newer variants; this observation was not much affected after excluding those with travel histories, furthering immune evasion as a key contributing factor for its greater transmissibility and rapid spread. A more recent study by the same group, Andeweg et al.Citation30 investigated PCR test results during two key periods: a Delta-Omicron BA.1 period (November 22, 2021 to January 7, 2022; n = 354,653) and an Omicron BA.1-BA.2 period (January 26 to March 31, 2022; n = 317,110). In the Delta-Omicron BA.1 period, compared to unvaccinated, any-VE against Delta infection was found to be 71% (95% CI: 69–73) at 7+ months from last dose of primary vaccination, which increased to 94% (95% CI: 94–96) following mRNA-booster vaccination. In contrast, primary and booster vaccination were associated with substantially lower VEs of 22% (95% CI: 18–26) and 58% (95% CI: 55–62), respectively, against Omicron BA.1 infection. In the second Omicron BA.1-BA.2 period, the authors report that both primary and booster vaccinations protected against BA.1 and BA.2 comparably. At 7+ months after primary vaccination, VE was 39% (95% CI: 36–42) against BA.1 and 32% (95% CI: 29–36) against BA.2. At 1 month after booster, VE was 69% (95% CI: 67–70) against BA.1 and 61% (95% CI: 58–63) against BA.2. The authors also compared VEs of the booster to primary series, reporting VEs of 75% (95% CI: 72–79) against Delta and 46% (95% CI: 42–50) against Omicron-BA.1 in the Delta-Omicron BA.1 period, and 48% (95% CI: 47–49) against Omicron-BA.1 and 40% (95% CI: 38–43) against BA.2 in the Omicron-BA.1-BA.2 period, showing that although less effective against Omicron compared to Delta, vaccination, especially boosters, are very important in reducing infections and provide modestly more protection against infection than primary series alone.
Pfizer/BioNTech and/or Janssen
Rufino et al.Citation34 obtained self-reported confirmation of COVID-19 infection from patients in South Africa (and later, other countries) via online Facebook-mediated surveys, collecting 100,000 responses per day globally. The surveys assessed for active COVID-19, symptoms, testing, and vaccination status in self-declared adult participants. Risk reduction was determined using various infection-positivity criteria present in the literatureCitation69–71 for the periods of mid-June to mid-July and August–September (both Delta-predominated), and December, 2021 (Omicron-predominated). However, since the authors did record vaccine manufacturer data, they could not investigate VE of partial/full/booster vaccinations despite collecting latest dose numbers from vaccinees as South Africa primarily administers the Pfizer/BioNTech and the Janssen vaccines among its population, each having a different definition of full vaccination based on dose numberCitation72. Overall VE against infection waned from 62% (95% CI: 58–65) during Delta predominance to 24% (95% CI: 17–30) during Omicron predominance. Single-dose and double-dose VE ranged from 51% (95% CI: 46–55) and 81% (95% CI: 78–84) against Delta, to 9% (95% CI: 0–18) and 30% (95% CI: 23 to 36) against Omicron, respectively. Similar VE trends were reproduced using specific datasets from the Gauteng province of South Africa and other countries where Omicron was reported by December 2021. In conclusion, although both one and two-dose effectiveness reduced substantially by the advent of Omicron, the two doses of the vaccines provided better protection than a single dose for both variants.
Pfizer/BioNTech, Moderna, and/or Janssen
Tai et al.Citation38 evaluated the protection against SARS-CoV-2 infection in a highly vaccinated and regularly monitored cohort (n = 2,613) of National Basketball Association (NBA) players and staff in the US tested between December 1, 2021, and January 15, 2022, an Omicron-predominated period. mRNA-boosted individuals (n = 2,164) were compared to non-boosted but fully vaccinated (n = 715) individuals, where Pfizer/BioNTech, Moderna or Janssen vaccines were used for primary courses. Statistically significant results showed that mRNA-booster vaccination was associated with an adjusted HR of 0.43 (95% CI: 0.35–0.53) against any confirmed SARS-CoV-2 infection and an adjusted HR of 0.39 (95% CI: 0.30–0.50) against symptomatic infection, compared to fully vaccinated but non-boosted individuals, furthering the importance of boosters. In a registry-based study of 2,225 primary Omicron and 9,712 primary Delta cases recorded between December 9–12, 2021 in Denmark, Lyngse et al.Citation21 reported 1,414 and 4,923 confirmed secondary (transmitted) cases, respectively. Around the time, the Pfizer/BioNTech (85%), Moderna (14%), and Janssen (1%) vaccines were administered among the Danish populationCitation67. The authors show significantly increased odds (OR: 2.31 [95% CI: 2.09–2.55]) of Delta-variant infection among the unvaccinated or partially vaccinated relatively to those fully vaccinated, whereas no significant difference was noted in the odds of Omicron infection between the unvaccinated/partially vaccinated and fully vaccinated, showing that primary vaccination series is not enough to reduce Omicron infection. In comparison, the authors showed that receiving a booster significantly reduced infection-susceptibility to both Omicron (OR: 0.54 [95% CI: 0.40–0.71]) and Delta (OR: 0.38 [95% CI: 0.32–0.46]) variants. The authors also show that, compared to Delta-infected households, the odds of Omicron transmission among fully vaccinated (OR: 2.61 [95% CI: 2.34–2.90]) and boosted (OR: 3.66 [95% CI: 2.65–5.05]) individuals are substantially greater, highlighting Omicron’s increased transmissibility, likely driven by immune evasion.
COVID-19 vaccine effectiveness against Omicron and other variants in reducing hospitalization only
Pfizer/BioNTech
In one of the earliest reports investigating the effectiveness of primary Pfizer/BioNTech vaccination against Omicron-associated hospitalization in South Africa, where the variant was first detected, Collie et al.Citation45 conducted a retrospective test-negative cohort study in comparison to a Delta-predominated period. The team studied 78,173 PCR tests administered during the period from November 15 to December 7, 2021, and 133,437 tests in the period from September 1 and October 30, 2021, with the Omicron and Delta variants, respectively. Compared to a VE of 93% (95% CI: 90–94) compared to unvaccinated in Delta periods, the VE of the primary Pfizer/BioNTech series against Omicron-period hospitalization was 70% (95% CI: 62–76). These early findings showed that although protection ameliorated due to the novel variant, vaccination still played an important role against COVID-19 related hospitalization. Šmíd et al.Citation35 report that similar patterns as seen against infection, but with consistently higher protection, is seen against hospitalization due to vaccination. Specifically, against Delta, partial and full Pfizer/BioNTech vaccination were associated with HRs of 0.47 (95% CI: 0.37–0.60) and 0.20 (95% CI: 0.15–0.28) at 1 month after inoculation and 0.39 (95% CI: 0.13–1.20) and 0.18 (95% CI: 0.14–0.23) after 2 months, respectively. Corresponding figures against Omicron-related hospitalization were 0.59 (95% CI: 0.34–1.03), 0.54 (95% CI: 0.41–0.72), 0.97 (95% CI: 0.24–3.87), and 1.1 (95% CI: 0.81–1.51), respectively. Hence, although time-sensitive waning of effectiveness is also observed against Delta-induced hospitalization, it is dramatically accelerated against Omicron-induced hospitalization. Finally, in their observational and prospective study at 21 hospitals in the US, their Lauring et al.Citation20 report that the Pfizer/BioNTech primary series was effective against both Alpha (82% [95% CI: 77–86]) and Delta-variant (82% [95% CI: 80–84]) associated hospitalization. Other heterogeneous combinations have been discussed in the following sections.
Moderna
Similar to the protection conferred by the Pfizer/BioNTech mRNA vaccine, Šmíd et al.Citation35 report that protection from partial and full Moderna vaccinations are both reasonably high and durable against Delta-associated hospitalization, but are much less reliable against Omicron-associated hospitalization. This is evident from HRs of 0.51 (95% CI: 0.29–0.87; partial vaccination, month 1 from vaccination) and 0.20 (95% CI: 0.09–0.45; full, month 2) against Delta-associated hospitalization, and HRs of 0.445 (95% CI: 0.11–1.78; partial, month 1) and 0.496 (95% CI: 0.206–1.20; full, month 1). However, Tseng et al.Citation13 reported that partial vaccination did not offer reliable protection against Delta-associated hospitalization, with a VE of 71.2% (95% CI: −68.7 to 97.4), although this is likely due to the low number of cases as evidenced from the CI. In contrast, two-dose and three-dose regimens were shown to successfully produce effectiveness figures of 99.0% and 99.7% against Delta-variant hospitalization, respectively. Although primary series VE against Omicron-associated hospitalization was lower at 84.5% (95% CI: 23.0–96.9) compared to Delta, a booster dose successfully increased protection to 99.2% (95% CI: 76.3–100.0), rivaling protection against Delta. Lastly, Lauring et al.Citation20 also show that the Moderna primary series was 90% (95% CI: 85–93) and 88% (95% CI: 86–90) effective against Alpha and Delta variant associated hospitalizations, respectively. Notably, both these figures show slightly better protection than the Pfizer/BioNTech primary series for the corresponding variants in question.
Oxford/AstraZeneca
Šmíd et al.Citation35 show that although protection against Delta-associated hospitalizations from Oxford/AstraZeneca full courses are effective with HRs of 0.20 (95% CI: 0.11–0.38) at 2 months and 0.32 (95% CI: 0.29–0.36) at 3 months from latest dose, the same cannot be said for protection against Omicron-associated hospitalization, where effectiveness is statistically non-significant, even at 2 months from primary series (HR: 2.396 [95% CI: 0.59–9.61]).
Janssen
Šmíd et al.Citation35 report that Janssen full vaccination was also effective at preventing Delta variant-associated hospitalizations, with HRs ranging from 0.39 (95% CI: 0.31–0.49) to 0.46 (95% CI: 0.35–0.61) over the course of 1–3 months from vaccination. However, unlike other vaccines, it is interesting to note that the protection from Janssen appears to increase with time, reaching highest protection of HR: 0.627 (95% CI: 0.427–0.92) at 3 months from vaccination; however, the 1-month protection was non-significant, at 0.727 (95% CI: 0.345–1.22). This promising feature of Janssen has been reproduced in another study. Lewnard et al.Citation24 show that compared to unvaccinated, primary Janssen vaccination was effective at preventing both Delta and Omicron-associated symptomatic hospital admissions that were tested in an outpatient setting, although protection against Omicron (aHR: 0.54 [95% CI: 0.36–0.79]) was lower versus Delta (aHR: 0.39 [95% CI: 0.20–0.77]). This was reflected in the comparison of symptomatic hospital admission association between Omicron vs Delta, where aHR of 0.47 (95% CI: 0.22–1.00) reflects that Omicron was less severe compared to Delta in those vaccinated with Janssen.
Heterogenous or mixed vaccine regimens
Pfizer/BioNTech, Moderna, and/or Janssen
Lewnard et al.Citation24 highlight that full Janssen vaccination followed by an mRNA booster was effective at preventing both Delta and Omicron associated symptomatic hospitalizations tested in outpatient settings compared to only the primary series, although protection against Delta (aHR: 0.11 [95% CI: 0.01–0.76]) was greater than that against Omicron (aHR: 0.46 [95% CI: 0.25–0.84]). This was evident from the direct comparison of Omicron vs Delta symptomatic hospitalization revealing that occurrence of symptomatic hospitalization was less likely with Janssen plus mRNA booster regimen (aHR: 0.26 [95% CI: 0.11–0.59]).
Data for different vaccine types
Pfizer/BioNTech and/or Moderna
In their large multistate analysis consisting of 93,408 hospitalizations, Ferdinands et al.Citation32 reported mRNA VEs against hospitalizations associated with laboratory-confirmed COVID-19, where the authors confirm that although mean VE against Omicron-related hospitalization (55% [95% CI: 50–60; average over <2 to ≥5 months, primary course] and 88% [95% CI: 86–90; average over <2 to ≥5 months, booster dose]) is less than VE against Delta (85% [95% CI: 84–85; average over <2 to ≥5 months] for primary course and 95% [95% CI: 95–96; average over <2 to ≥4 months] for booster), it was apparent that boosters play an important role in preventing COVID-19 hospitalizations among adults. Importantly, vaccines showed greater protection and less waning with time with respect to hospitalization compared to ED/UC visits, serving as a reminder that the priority of booster rollout and administration is to prevent overwhelming healthcare systems with severe cases rather than prevent infections. Thompson et al.Citation58 also revealed that primary mRNA vaccine courses offered more robust and long-lasting protection against Delta-associated hospitalization (VE: 90% [95% CI: 89–90] at 14–179 days prior to testing) than against that of Omicron (VE: 81% [95% CI: 65–90]). However, booster doses reestablished VE above the peak protection offered by primary series, which was comparable between both strains at 94% (95% CI: 93–95) against Delta and 90% (95% CI: 80–94) against Omicron. Further, Lauring et al.Citation20 reported that mere partial mRNA vaccination was associated with pan-variant (combination of Alpha, Delta, and Omicron cases) hospitalization-protection measures of 77% (95% CI; 71–81) and 84% (95% CI; 74–89), if 1st dose was administered ≥14 days prior to illness onset and if the 2nd dose was administered 0–13 days prior to illness onset, respectively. In contrast, a single vaccine dose was not found to be effective within 0–13 days after vaccination (VE: 16% [95% CI: −10 to 36]). Further, the authors show that protection measures from primary series at ≥14 days after 2nd dose were 85% (95% CI: 82–88), 85% (95% CI: 83–87) and 65% (95% CI: 51–75) against Alpha, Delta and Omicron associated hospitalizations, respectively. mRNA Boosters increased the effectiveness against Delta and Omicron induced hospitalizations to 94% (95% CI: 92–95) and 86% (95% CI: 77–91), respectively. Interestingly, Lewnard et al.Citation24 show that partial mRNA vaccination was protective against Omicron-associated symptomatic hospitalizations (HR: 0.59 [95% CI: 0.39–0.89]), but not Delta (HR: 0.61 [95% CI: 0.32–1.15]); this translated into an OR of 0.26 (95% CI: 0.11–059) when comparing likelihood of symptomatic hospital admission in those infected by Omicron compared to Delta. Primary mRNA series provided robust but waning protection against Delta symptomatic hospitalization, with aHRs of 0.23 (95% CI: 0.17–0.30), 0.33 (95% CI: 0.19–0.58) and 0.32 (95% CI: 0.13–0.78) at <90 days, 91–180 days, and >180 days since latest dose. Similar measures were also found to be protective, but unreliable, against Omicron, with aHRs of 0.48 (95% CI: 0.33–0.70), 0.50 (9% CI: 0.40–0.64) and 0.60 (95% CI: 0.52–0.69) at similar timestamps from latest dose; however, the comparison between symptomatic infection with Omicron versus Delta was not statistically significant. Booster mRNA series showed high protection against both variants, although considerably more against Delta (aHR: 0.19 [95% CI: 0.12–0.28]) than Omicron (aHR: 0.54 [95% CI: 0.35–0.52]).
Pfizer/BioNTech, Moderna, Oxford/AstraZeneca, and/or Janssen
Šmíd et al.Citation35 also report on the overall VE against COVID-19 related hospitalization from each variant based on all four vaccines. Although full vaccination catered decent protection against Delta-induced hospitalization (VE: 73% [95% CI: 69–76] at <2 months from full course to 77% [95% CI: 76–79] at >2 months from full course), with mRNA boosters, this figure increased to 97% (95% CI: 97–98) immediately after vaccination and did not wane significantly (VE: 96% [95% CI: 94–97]) after 2 months. However, against Omicron-induced hospitalization, full vaccination catered very limited protection (VE: 45% [95% CI: 29–57] recently after inoculation and 29% [95% CI: 21–37] after a period of 2 months). Although mRNA-boosters similarly helped reestablish effectiveness up to 86% (95% CI: 84–88), it waned slightly to 79% [95% CI: 75–82) at >2 months after booster vaccination. With further vaccine specificity, the authors show that, irrespective of primary course (either of four vaccines), those receiving Pfizer/BioNTech boosters were well-protected against Delta infection (HR: 0.028 [95% CI: 0.024–0.032] at 1 month to 0.047 [95% CI: 0.032–0.068] at 2 months), as were those receiving Moderna boosters (HR: 0.026 [95% CI: 0.18–0.038] at 1 month to 0.020 [95% CI: 0.0028–0.14] at 2 months) without much loss of protection with time. Effectiveness against Omicron-associated hospitalizations was less robust and less durable, for both Pfizer/BioNTech (HR: 0.140 [95% CI: 0.120–0.160] at 1 month to 0.216 [95% CI: 0.181–0.260] at 2 months) and Moderna (HRs: 0.113 [95% CI: 0.079–0.160] to 0.167 [95% CI: 0.098–0.280] at 2 months) boosters. Between the two types of mRNA-boosters, Moderna catered slightly greater protection, but no formal and direct assessment was made.
COVID-19 vaccine effectiveness against Omicron and other variants in reducing infection, hospitalization, severity, and/or death
Pfizer/BioNTech
Abu-Raddad et al.Citation17 investigated the effects of Pfizer/BioNTech boosters on COVID-19 related severe, critical, or fatal disease, defined as acute-care hospitalizations, ICU admissions, and deaths, respectively (WHO definitionCitation75,Citation76). Earlier, we have described how both mRNA-boosters are approximately 50% more effective in preventing symptomatic Omicron infections compared to the primary series. However, Pfizer/BioNTech was also shown to prevent COVID-19 related severe or fatal disease with greater VE (76.5% [95% CI: 55.9–87.5]) compared to full vaccination, which adds to the observation that despite the great incidence of infection among the individuals who received the booster, there were very few severe cases and no critical or fatal cases. Additionally, such severe cases were low in number in both booster and non-booster cohorts despite the large number of infections, reaffirming that primary series are still durable against severe disease months after receipt of second dose. Findings form Chemaitelly et al.Citation28 further show that partial vaccination provided no protection (VE: 40.9% [95% CI: −199.1 to 88.3]), whereas effectiveness of full vaccination was 70.4% (95% CI: 45.0–84.0) at 1–6 months after 2nd dose and 77.5% (95% CI: 67.8–84.3) by ≥7 months, whereas VE of booster was 90.0% (95% CI: 78.6–96.1) at 1–6 weeks after 3rd dose and 90.1% (80.6–95.0) by ≥7 weeks, without immediate signs of waning. Patalon et al.Citation27 note that the odds of Omicron-associated hospitalization or death were not significantly affected by time from the 3rd dose, highlighting the relatively long-lasting protection against severe disease compared to protection against infection only. Lastly, compared to primary series vaccinees, the authors show significant marginal VE of boosters against hospitalization and death, although it also waned slightly over time, from 72.2% (95% CI: 37.8–87.6%) at 3 months after booster to 54.5% (95% CI: 13.4–76.1) 5 months after booster, revealing the clinically significant superiority of Pfizer/BioNTech boosters to prevent severe Omicron-induced disease compared to primary course alone. Further, the presence of cardiovascular disease, hypertension, COPD, and immunosuppression increased the odds of hospitalization significantly, whereas diabetes and obesity did not.
Moderna
Remarkably, Chemaitelly et al.Citation28 report that partial Moderna could successfully prevent (VE: 100.0%) all Omicron-associated severe, critical, or fatal disease (WHO definitionsCitation75,Citation76); however, this was likely a result of insufficient cases in the cohort. Full vaccination provided good protection (87.1% [95% CI: 40.2–97.2]), however, this quickly decreased to 68.4% [95% CI: 46.1–81.5] by ≥7 months after 2nd dose. Protection from Moderna boosters was difficult to deduce due to low number of severe cases.
Data for different vaccine types
Pfizer/BioNTech and/or Moderna
Lauring et al.Citation20 further estimate the effectiveness of mRNA vaccination in preventing progression to severe disease, characterized by invasive mechanical ventilation (IMV) or death among individuals hospitalized with COVID-19. Full or booster mRNA vaccination was associated with protection of 76% (95% CI: 53–88), 44% (95% CI: 32–54) and 46% (95% CI: 12–67) from Alpha, Delta or Omicron associated severity/fatality, respectively, compared to unvaccinated and hospitalized individuals. Although these values at first glance may seem lower compared to other studies, a difference in methodology must be considered: here the comparators are those unvaccinated who are also hospitalized with COVID-19, compared to all unvaccinated in other studies.
Pfizer/BioNTech, Moderna, and/or Janssen
Although Lewnard et al.Citation24 also report that progression of clinical severity was significantly dependent on variant among the unvaccinated (Omicron was less hazardous for severity), they found no difference among risk for ICU admission (aHR: 0.95 [95% CI: 0.33–2.76]) and mechanical ventilation (aHR: 1.50 [0.56–3.99]) among those who had been vaccinated (with any of the three vaccines); revealing that vaccination does not provide unique, variant-sensitive protection against these forms of severity. However, among vaccinated individuals, the Omicron variant was associated with lower mortality compared to Delta (aHR: 0.25 [95% CI: 0.09–0.70]).
Pfizer/BioNTech, Moderna, Oxford/AstraZeneca, and/or Janssen
Šmíd et al.Citation35 estimated the protection conferred by both primary course with Pfizer/BioNTech, Moderna, Oxford/AstraZeneca, or Janssen vaccines and mRNA-booster vaccines against Delta- and Omicron-associated need for oxygen therapy or admission to intensive care unit (ICU). In brief, against Delta variant, a full course conferred considerable protection against both the need for oxygen therapy (VE: 82% [95% CI: 76–87] at <2 months and 82% [95% CI: 80–83] at >2 months) and ICU admission (VE: 84 [95% CI: 72–91] at <2 months and 86% [95% CI: 83–88] at >2 months) without waning over time. Nevertheless, boosters greatly reestablished VE to 98% (95% CI: 98–98) at <2 months and 97% (95% CI: 95–98) by >2 months against the need for oxygen therapy, and to 98% (95% CI: 97–99) at <2 months and to 97% (95% CI: 92–99) by >2 months against ICU admission, without any signs of waning. In contrast, against the Omicron variant, full vaccination protection was not only subpar, but also non-durable, for both the need for oxygen therapy (VE: 57 [95% CI: 32–72] at <2 months and 32% [95% CI: 20–43] at >2 months) and ICU admission (VE: 58% [95% CI: 3–82] at <2 months and 37% [95% CI: 12–55] at >2 months). Although boosters greatly reestablished protection to 90% (95% CI: 87–92) and 83% (95% CI: 75–89), respectively, they showed considerable signs of waning within the follow-up periods, to 85% (95% CI: 80–88) and 60% (95% CI: 37–74), respectively. However, an additional analysis also revealed that once infected with Omicron variant, patients are much less likely to be hospitalized (OR 0.36 [95% CI: 0.34–0.38]), need oxygen therapy (OR: 0.24 [95% CI: 0.22–0.26]) or ICU admission (OR: 0.24 [95% CI: 0.21–0.28]) compared to Delta, revealing Omicron’s less severe form of disease despite increased immunity escape.
Pfizer/BioNTech, Moderna, and/or Oxford/AstraZeneca
Buchan et al.Citation44 also investigated the effectiveness of primary series (using ≥1 mRNA vaccine, with or without Oxford/AstraZeneca) and booster (mRNA vaccines only) series against severe COVID-19 disease, defined as hospital admission or death, compared to unvaccinated participants. The authors reported that primary 2-dose series with ≥1 mRNA vaccine provided robust and reliable protection against Delta-associated hospitalization, with VEs ranging from a low of 94% (95% CI: 84–98) to a high of 99% (95% CI: 98–99) within a follow-up period of 7 to ≥240 days; Pfizer/BioNTech and Moderna boosters could reestablish and sustain this protection to 99% (95% CI: 98–99) and 100% (95% CI: 98–100) until ≥7 days after 3rd dose, respectively. Primary series protection against Omicron-associated severe disease was less convincing compared to Delta, fluctuating between as low as 37% (95% CI: −71 to 77) to a peak of 82% (62–91) over a follow-up period of 7 to ≥240 days. Pfizer/BioNTech and Moderna boosters convincingly reestablished protection against severe Omicron-disease, raising VE to 95% (95% CI: 87–98) and 93% (74–98), by ≥7 days after 3rd dose, respectively, thus catering similar protection as against severe Delta-associated disease.
COVID-19 vaccine effectiveness against Omicron and other variants in reducing infection, hospitalization, severity, and/or death in children
Based on clinical trial data, FDA authorizations and US CDC recommendation, compared to children aged 12–17 years who are administered the same dose of Pfizer/BioNTech as adults (30 µg), those aged 5–11 years are administered a smaller dose of 10 µg; those aged 6 months–4 years are given 3 µg per doseCitation77. For Moderna, similar limitations apply, with doses of 100 µg, 50 µg and 25 µg, respectively. Other vaccines have not yet been approved in children.
Pfizer/BioNTech
In yet another VISION network US multistate study, Klein et al.Citation63 investigated 39,217 ED/UC encounters and 1,699 hospitalizations of children aged 5–17 years with COVID-19–like illness from April 9, 2021, to January 29, 2022, thus involving periods of both Delta and Omicron predominance. During periods of Delta predominance, initial VE of primary series in children aged 12–15 years was 92% (95% CI: 89–94) but waned quickly over time to 79% (95% CI: 68086) by ≥150 days. In the 16-17-year group, this was 85% (95% CI: 81–89) and 77% (95% CI: 67–84), respectively. Booster dose effectiveness could not be calculated owing to small number of cases. However, against Omicron-infection, VE was 51% (95% CI: 30–65), 45% (95% CI: 30–57) and 34% (95% CI: 8–53) recently after receiving the 2nd dose in the 5-11-, 12-15- and 16-17-year age groups, respectively. The primary series offered no protection against Omicron infection by ≥150 days in both the 12-15- (VE: −2 [95% CI: −25 to 17]) and 16-17-year-old (VE: −3 (95% CI: −30 to 18) cohorts. Boosters reestablished efficacy back to 81% (95% CI: 59–91) against Omicron ≥7 days after vaccination. Hence, the effectiveness of the vaccine seemed to wane over time even in children, and additionally, the effectiveness of the vaccine was significantly lower amongst children aged 5–11 years compared adolescents aged 12–17 years. However, it must be noted that most of the infections in the 5–11-year-old age group occurred during omicron period where the effectiveness of the vaccine was lower in all age groups. Finally, the authors also show that primary Pfizer/BioNTech vaccination provides robust, long-lasting, and effective protection against ‘Omicron or Delta’ hospitalization in all age groups, except 5–11. For instance, in 16–17-year-olds, receiving two doses of the vaccine was 94% (95% CI: 87–97) effective in reducing COVID-19 associated hospitalizations within 14–149 days postvaccination and was not significantly lower in those at ≥150 days postvaccination (VE: 88% [95% CI: 72-95]). However, there was virtually no protection in the 5-11-year-old group, with a non-significant VE measure of 74% (95% CI: −35 to 95), which could be attributed to low level of vases and thus statistical bias, but is nonetheless, a concerning statistic.
A study by Dorabawila et al.Citation43 also probed into the effectiveness of the Pfizer/BioNTech vaccine against infection and hospitalization in children aged 5–17 between December 13, 2021, to January 30, 2022, in New York, US in a large cohort of 852,384 children aged 12–17 and another 365,502 aged 5–11, all of whom had been fully vaccinated. Since the authors did not utilize laboratory testing for population-level cases to determine the variant, it is difficult to link the effectiveness to particular strains. Nevertheless, using data published by the New York State GISAIDCitation73 and the CDCCitation74, it is clear that a vast majority (>80%) of SARS-COV-2 cases sequenced by 1st January 2022 were of Omicron variant. The authors show that VE of the primary series against infection amongst the 12–17 years old children was 85% (95% CI: 84–86) during Delta variant predominance. This quickly declined to 51% (95% CI: 48–54) during a period of Omicron predominance. For those aged 5–11 years, VE against infection waned from 68% (95% CI: 63–72) at a period when Omicron constituted 1/5th of all cases, to merely 12% (95% CI: 6–16) during Omicron predominance. Although reduced from a peak of 94% (95% CI: 76–99) during Delta predominance, VE against hospitalization remained relatively high during Omicron predominance in the 12–17-year-old group, with a protection rate of 73% (95% CI: 53–87). However, protection against Omicron-induced hospitalization in the 5-11-year-old group was non-significant at 48% (95% CI: −12 to 75). The authors also show that VE waned significantly with time since vaccination among both age groups; among 12-17-year olds, VE ranged from 76% (95% CI: 71–81; ≤13 days since 2nd dose) to 46% (95% CI: 18–65; 42–48 days since 2nd dose), whereas among 5-11-year olds, the figures were 65% (95% CI: 62–68) and −41% (95% CI: −56 to −29), respectively. In fact, VE against infection was consistently higher in the older cohort. Further, by 35–41 days from 2nd dose, the primary series no longer provided any protection in those aged 5–11 years. These concerning findings among the 5-11-year-old group is likely due to the lower vaccine dose administered in this population, this calling for a study of the optimal numbers of doses, amount per dose, dose timing, and/or antigens targeted for children in this age group with respect to Pfizer/BioNTech vaccination.
Powell et al.Citation37 conducted a test-negative case–control study across the UK aiming to estimate VE of partial and full Pfizer/BioNTech vaccination against symptomatic Omicron and Delta variant infection and hospitalization in adolescents. The study utilized data from 617,259 eligible tests for 12–15-year-olds, collecting data from week 37, 2021 to Jan. 12, 2022, and from 225,670 tests for 16–17-year-olds, collecting data from week 32, 2021 to Jan. 12, 2022. Due to a unique UK-policy at the time, the study could investigate protection of partial vaccination from the mRNA vaccine in adolescents over a large follow-up period. Among 12-15-year olds, one-dose protection was highest at days 14–20 against both variants, although VE against Delta was much higher (74.5% [95% CI: 73.2–75.6]) compared to Omicron (49.6% [95% CI: 43.9–54.8]). Protection waned drastically over time, reaching lows of 45.9% (95% CI: 41.2–50.1) and 16.1% (95% CI: 12.1–20.0) against Delta and Omicron, respectively, both at days 70–83. Full vaccination reestablished protection against both variants, reached peaks of 93.2% (95% CI: 81.5–97.5) and 83.1% (95% CI: 78.2–86.9), respectively, at days 7–13 after 2nd dose. Similar results were obtained for the 16-17-year-old group, with partial vaccination protection peaking at 75.9% (95% CI: 74.3–77.3; day 14–20) and 52.7% (95% CI: 43.4–60.5; day 21–27) against Delta and Omicron, respectively, and being the lowest at 29.3% (95% CI: 25.9–32.6; day 84–104) and 12.5% (955 CI: 6.9–17.8; day 105+) against Delta and Omicron, respectively. 2nd doses reestablished protection, peaking at 96.1% (95% CI: 95.2–96.8; day 14–34) and 76.1% (95% CI: 73.4–78.6; day 7–13), and being lowest by the end of the follow-up period. Although protection against Omicron could not be assessed due to insufficient follow-up, the authors report that partial Pfizer/BioNTech vaccination protected against Delta-associated hospitalization following infection only at days 28+ (VE: 83.4% [95% CI: 54.0–94.0]) in the 12-15-year-old cohort and at day 0–27 and 28+ with VEs of 64.6% (95% CI: 40.7–78.9) and 76.3% (95% CI: 61.1–85.6) in the 16-17-year-old cohort, respectively. The authors conclude that the adolescent immunization program at the time would not sustain protection as a standalone intervention in the face of newer strains without regular boosters.
Data for different vaccine types
Pfizer/BioNTech, Moderna, Oxford/AstraZeneca, and/or Janssen
Eggink et al.Citation15 report that of 80,615 Omicron cases and 93,734 Delta cases across the Netherlands, children aged 12–19 comprised a greater proportion of Omicron cases (26.3%) than among Delta cases (12.1%). As a similar trend was observed in the next age group (20–29) the authors compiled data from the two groups and reported that aOR for primary vaccination using Pfizer/BioNTech, Moderna, Oxford/AstraZeneca, or Janssen was 4.1 (95% CI: 3.9–4.4) against Omicron infection compared to Delta in the 12–29 age group; corresponding aORs were 3.2 (95% CI: 3.0–3.4) and 2.8 (95% CI: 2.3–3.2) in older age groups, showing that difference in vaccine effectiveness against the two variants is more apparent in younger individuals; however, no explanation was presented for this observation.
How long does the immunity last after vaccination?
Pfizer/BioNTech
An early report by Hansen et al.Citation42 show that VE for Pfizer/BioNTech against the Omicron variant wanes as quickly as 31–60 days after primary 2-dose series vaccination. The authors suggest that this decreased VE against Omicron is likely the result of rapid spread via super-spreading events causing various infections among young and vaccinated individuals, however, this is also the fastest waning estimate among all studies, likely attributable to statistical bias stemming from low number of cases due to its early timeframe. Consensus among other large studies show that immunity from the Pfizer/BioNTech primary series lasts for a period of between 3 and 6 months against Omicron BA.1 subvariant infection, and about ≥7 months against Omicron BA.2 subvariant, based on findings from Accorsi et al.Citation18 and Chemaitelly et al.Citation28. Šmíd et al.Citation35 report that protection from Omicron-induced hospitalization waned by the first month and against Delta-associated hospitalization by the 2nd month, with respect to partial Pfizer/BioNTech vaccination.
Moderna
Based on findings by Accorsi et al.Citation18 and Chemaitelly et al.Citation28, protection catered by the 2-dose primary Moderna series against Omicron BA.1 infection is no longer significant after 6–7 months, but this protection does not last even 1–3 months against Omicron BA.2
Oxford/AstraZeneca
Andrews et al.Citation61 report that primary Oxford/AstraZeneca vaccination is no longer protective by 25+ weeks, while Šmíd et al.Citation35 report that for protection against Omicron-induced hospitalization swayed by 2 months for the same 2-dose series.
Janssen
Šmíd et al.Citation35 report that for protection from Omicron-associated hospitalization, full Janssen vaccination effectiveness does not last the first month.
Data for different vaccine types
Pfizer/BioNTech and/or Moderna Only
Buchan et al.Citation44 show that protection against symptomatic Omicron infection by a primary series consisting of only mRNA vaccines or a combination of an mRNA vaccine with Oxford/AstraZeneca was no longer effective by 6 months after 2nd dose. Further, Ferdinands et al.Citation32 show that mRNA booster VE against laboratory-confirmed ED/UC visits during Omicron-predominated periods was no longer effective by ≥5 months since 3rd dose; however, the finding concerning mRNA boosters is contested by the conclusions of Patalon et al.Citation27, which show marginal effectiveness of 16% over the primary series.