ABSTRACT
The objective of this study was to critically review the cost-effectiveness (CE) of the recombinant zoster vaccine (RZV) against herpes zoster (HZ). A literature review was conducted in PubMed, Embase, and Cochrane between January 1, 2017, and February 28, 2022, and on select public healthcare agency websites to identify and collect data from CE studies comparing RZV to zoster vaccine live (ZVL) or to no vaccination. Study characteristics, inputs, and outputs were collected. The overall CE of RZV was assessed. RZV vaccination against HZ is cost-effective in 15 out of 18 studies included in the present review. Varying incremental cost-effectiveness ratios (ICERs) observed may be associated with different assumptions on the duration of protection of RZV, as well as different combinations of structural and disease-related study (model) inputs driving the estimation of ICERs.
Plain Language Summary
What is the context?
• Herpes zoster, also known as shingles, may cause painful rashes and skin alterations.
• Chronic pain, also referred to as post-herpetic neuralgia, may persist for months or even years after the initial rash.
• The disease is caused by reactivation of the varicella zoster virus.
• The recombinant zoster vaccine (RZV) and the zoster vaccine live (ZVL) are approved for the prevention of herpes zoster and post-herpetic neuralgia.
• We reviewed published evidence from the past 5 years on RZV.
What is new?
• Out of 18 selected studies, RZV vaccination against herpes zoster and post-herpetic neuralgia is cost-effective in 15.
• In the 15 studies establishing RZV cost-effectiveness, RZV is always cost-effective or frequently cost-saving in direct comparisons to ZVL, when applicable.
• RZV was found cost-saving in several immune-compromised populations.
What is the impact?
• The overview of the currently available body of evidence related to cost-effectiveness of RZV may help informing decision makers about the value of vaccination against herpes zoster.
Introduction
Herpes zoster (HZ), commonly referred to as shingles, is caused by the reactivation of latent varicella-zoster virus (which causes both varicella and herpes zoster). HZ frequently presents as a painful debilitating rash, including skin inflammation and blisters, and sometimes causes scarring and permanent pigment changes. Anyone with a previous record of varicella (chickenpox) is at risk of developing herpes zoster. The frequency of HZ increases with age due to age-related decline in immunity.Citation1 The cumulative incidence of HZ was recently estimated between 2.9 and 19.5 cases per 1000 population worldwide, and the HZ incidence rate was estimated between 5.23 and 10.9 cases per 1000 person-years.Citation2
Chronic pain persisting 3 months after initial rash detection or HZ diagnosis with an average pain score above 3 on the Likert scaleCitation3 is commonly defined as post-herpetic neuralgia (PHN) and may continue for months or even years.Citation4 HZ and PHN have been shown to adversely affect healthy aging and quality of life (QoL).Citation5
The recombinant zoster vaccine (RZV, Shingrix, GSK, Belgium) was approved by the Food and Drug Administration (FDA) in 2017Citation6 and was preferentially recommended by the Advisory Committee on Immunization Practices (ACIP)Citation7 shortly thereafter for the prevention of HZ in immunocompetent adults aged 50 years and above and in immunocompetent adults who were previously vaccinated with zoster vaccine live (ZVL, Zostavax, Merck Sharp & Dohme Co, United States). It is currently also recommended by ACIP for immunocompromised adults aged 19 years and above.Citation8 RZV was approved by the European Medicines Agency (EMA) in 2018Citation9 for the prevention of HZ and PHN in older adults aged 50 years and above and in younger adults aged 18 years and above who are at increased risk of HZ. The vaccine is recommended and reimbursed in a number of countries worldwide.Citation10 ZVL was approved by the FDA in 2006Citation11 and by the EMACitation12 the same year. ZVL is no longer available for use in the United States (US) as of November 2020.Citation13
Many health technology assessment (HTA) bodies and national immunization technical advisory groups are currently evaluating RZV worldwide. As such, it is relevant to provide an overview of the inputs, assumptions, and results presented in cost-effectiveness (CE) of RZV publications to date.
Methods
Study selection
The main literature search was performed on PubMed, Embase, and Cochrane reviews with additional queries on select governmental public health online repositories. Publication dates of interest were between January 1, 2017, and February 28, 2022. The exact search queries are shown in Appendix A. Results were analyzed individually to determine inclusion eligibility into the present study. Only CE or equivalent types of studies (i.e., cost-utility analyses) involving RZV were considered. Cost-of-illness and budget impact studies were excluded by default. Conference abstracts (indexed in Embase) were also excluded by default. Studies deemed eligible for inclusion into the present critical review were further searched manually (references) for potential identification of additional sources.
A summary of the search and screening strategy and its outcomes is depicted graphically in an adapted PRISMA-2020Citation14 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart suitable for reviews including records from databases, registries, and other sources ().
Figure 1. PRISMA flowchart.
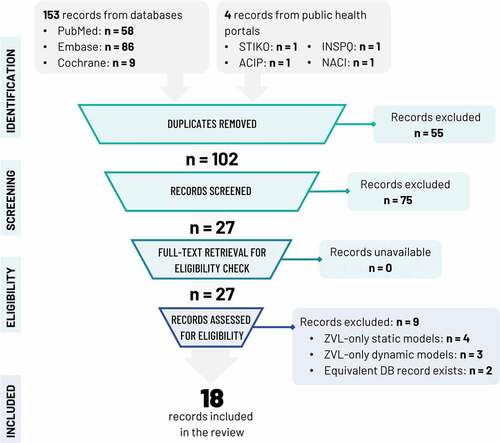
Known governmental public health online repositories searched manually for additional sources included the Centers for Disease Control and Prevention (CDC)/ACIP (US), the National Advisory Committee on Immunization (Canada), the Institut National de Santé Publique du Québec (Canada), and the Standing Committee on Vaccination (STIKO, Germany).
Data extraction
For each study included in the review, the extracted information was study characteristics, model attributes, and CE parameters. Detailed input data were collected by type (epidemiological, vaccine profiles, costs, QoL and utilities, adverse events). Top-level and detailed CE results were also extracted.
Quality assessment
The latest version of the Consolidated Health Economic Evaluation Reporting Standards (CHEERS)Citation15 was used to objectify and formally assess the quality of each study, incorporating a total of 28 items recommended for best reporting of health economic evaluations.
Further elaboration on the quality of selected studies forms part of the Discussion of the present review.
Aggregated and detailed CE results were compiled under separate tables. Detailed public health impact outcomes were not in scope of the review.
Results
The literature search on PubMed, Embase, and Cochrane reviews yielded 153 records; 16 studies were admitted to review.
Manual search of the public health online repositories mentioned in the Study Selection revealed four additional studies.Citation16–19 TwoCitation16,Citation17 were deemed equivalent to Drolet et al.Citation20 and were excluded from further review.
The PRISMA flowchart of identification and screening is shown in . In total, 18 studies were subjected to detailed review.
All studies explicitly stated the study objective, target population, setting, comparators, study perspective, time horizon, discount rates, and choice of health outcomes. All but oneCitation18 study explicitly described analytic methods, reported input parameters in detail, and characterized uncertainty via sensitivity analyses. In view of its outline nature, the report of Ortega-Sanchez et al.Citation18 recapping two original CEAs on immunocompromised populations did not follow CHEERS closely; this observation does in no way speak to the quality of the original research summarized therein.
Study Characteristics
The study characteristics are summarized in . Of the 18 studies included, nine studies were performed in North America,Citation18,Citation20–27 five in Europe,Citation19,Citation28–31 and four in Asia.Citation32–35
Table 1. Studies included in the review and main study characteristics.
All but oneCitation18 study examined the CE of RZV and/or ZVL on populations of older adults 50 years and above.
All studies compared RZV to no vaccination; four studiesCitation21,Citation22,Citation26,Citation32 additionally directly compared RZV to ZVL and twoCitation29,Citation30 compared a boosting strategy for ZVL to no vaccination. Three studiesCitation21,Citation23,Citation24 examined the CE of RZV on cohorts previously vaccinated with ZVL. Many studiesCitation20,Citation24,Citation25,Citation29,Citation30,Citation32 additionally performed CE analyses of ZVL vaccination vs no vaccinationCitation13.
Most studies examined cohorts aged 50 years and above; fourCitation19,Citation26,Citation27,Citation31 focused on 60 years and above, and twoCitation32,Citation33 on 65 years and above. The immunocompromised cost-effectiveness analysis (CEA)Citation18 was performed on immunocompromised adults older than 18 years of age (YOA).
Eleven studiesCitation19–21,Citation24,Citation25,Citation27,Citation29,Citation30,Citation32,Citation34,Citation35 were funded independently; six studiesCitation22,Citation23,Citation26,Citation28,Citation31,Citation33 were funded by the vaccine manufacturer. One studyCitation18 compared the results of two separate CEA analyses on immunocompromised populations <50 YOA (one conducted by the CDC, the other by the vaccine manufacturer).
All studies used the quality-adjusted life year (QALY) to measure health benefits and a monetary value (MV) to express costs in a currency appropriate to the study locale, and all studies reported incremental cost-effectiveness ratios (ICERs) defined as the ratio of incremental costs over incremental benefits between two interventions. One studyCitation32 additionally applied the net monetary benefit (NMB) metric. NMB is calculated as NMB = [Incremental Benefits] × WTP – [Incremental Costs], with WTP representing a known willingness-to-pay thresholdCitation36 for one unit of incremental benefit.
Model design
The studied model design parameters are shown in .
Table 2. Core model characteristics.
All studies utilized a combination of decision tree (for the different vaccination strategies under consideration) and Markov state-transition models deployed within each branch of the decision tree representing a distinct vaccination strategy or no vaccination.
Most modelsCitation22,Citation23,Citation25,Citation26,Citation28,Citation29,Citation33 were implemented in Excel but a fewCitation24,Citation32,Citation35 relied on various versions of TreeAge Pro.Citation37 Two modelsCitation19,Citation30 were implemented in R.Citation38
All but one study utilized a lifetime horizon; de Boer et al.Citation29 utilized a time horizon of 15 years. A few studies limited the time horizon to 100Citation25,Citation32,Citation35 or 103Citation30 years, and oneCitation34 imposed an upper limit on the follow-up period equal to 50 years.
Only a few studiesCitation19,Citation21,Citation34,Citation35 incorporated state-transition diagrams distinguishing between health states for male and female (M/F) subjects. The study of Hoshi et al.Citation32 reported core model input data differentiated between M/F subjects but no M/F health state differentiation was evident from the state transition diagram.
Model cycle time was generally 1 year; You et al.Citation34,Citation35 utilized a cycle time of 1 month, and Ultsch et al.Citation19 a cycle time of 3 months.
An examination of the state transition diagrams for each study under consideration, cross-checked against model and methods description, revealed that all but one studyCitation29 associated PHN with a distinct health state. Complications were generally also taken into account with the exception of Hoshi et al.,Citation32 Pieters et al.Citation30 and Ultsch et al.Citation19
The modeling of recurrent HZ varied between studies. Many analysesCitation21–23,Citation25,Citation26,Citation28,Citation33 assumed a recurrent HZ incidence rate equal to that of first time HZ incidence. Recurrence was not implemented in the studies of de Boer et al.Citation29 and You et al.Citation34 or was restricted to a one-time event in the studies of You et al.Citation35 and Hoshi et al.Citation32 In one study,Citation24 a cumulative recurrence rate was applied. The implementation of recurrence in the studies of Drolet et al.Citation20 and Pieters et al.Citation30 is unclear; the state transition diagram of Drolet et al.Citation20 implies inclusion of recurrent HZ.
Most studies kept track of healthcare resources such as hospitalizations and general practitioner (GP) visits; in two studiesCitation34,Citation35 a more general classification under inpatient/outpatient was preferred.
Finally, all model implementations enabled the conduct of deterministic and probabilistic sensitivity analyses (DSA/PSA). The CEA of Drolet et al.Citation20 was probabilistic by design; all outcomes were presented as median values and percentiles from an extensive Monte-Carlo simulation encompassing 30,000 runs. A few studiesCitation24,Citation25,Citation29 performed 1- and 2-way DSAs; one studyCitation21 presented 1-, 2-, and 3-way DSAs.
CE parameters
CE parameters are summarized in .
Table 3. Cost-effectiveness model parameters.
Most studies were conducted from the societal costing perspective; four studiesCitation20,Citation26,Citation30,Citation32 were performed under the healthcare payer perspective, and fourCitation19,Citation22,Citation24,Citation33 investigated both. The RZV manufacturer CEA on immunocompromised populationsCitation18 was also conducted from both perspectives.
ICERs were generally measured against known or assumed WTP thresholds. In one case,Citation29 the WTP threshold was defined unambiguously in national health technology assessment (HTA) recommendations.Citation39 WTPs were otherwise chosen empirically relying on either World Health Organization (WHO) guidelinesCitation40 or unofficial precedent.
Cost and benefits were generally discounted in agreement with prior health economic practice for the locale of interest. Notably, two CEAs conducted in JapanCitation32,Citation33 employed different discounting factors (2% vs 3% for both costs and benefits, respectively). Discounting rates of 2% have been recommended by the Japanese Ministry of Health, Labor and Welfare.Citation41
In a similar fashion for two Canadian CEAs, Drolet et al.Citation20 employed 3% for costs and benefits while McGirr et al.Citation26 applied a rate of 1.5% for costs and benefits. Discounting factors of 1.5% in the base-case are recommended by the Canadian Agency for Drugs and Technologies in Health.Citation42
Model inputs
Detailed model inputs are organized under Tables B1 to B7 of Appendix B.
Epidemiology
Epidemiological model inputs are compiled under Table B1.
All but twoCitation34,Citation35 analyses relied on one or more local epidemiological sources for the incidence rate of HZ. The CEAs of You et al.Citation34,Citation35 reused international data sources excluding case reports. The study of Pieters et al.Citation30 used local HZ incidence rates derived from medically attended HZ rates (ambulatory and hospitalized) but relative PHN incidence from a retrospective database analysis performed in the UK.Citation43 The Canadian CEA by McGirr et al.Citation26 utilized data from the province of British Columbia.Citation44
Vaccine efficacy – RZV
Vaccine efficacy (VE) and waning model inputs are summarized in Table B2 for RZV and Table B3 for ZVL.
All studies relied on ZOE50 and ZOE70 clinical trial dataCitation45,Citation46 to model RZV initial efficacy and waning over time. One recent analysisCitation28 used updated efficacy data from the long-term follow-up (LTFU) study investigating the efficacy of RZV up to 8 years post-vaccination.Citation47
One CEACitation34 reused the RZV efficacy model presented to ACIP in 2017.Citation48
Most studies implemented simple linear modeling of RZV efficacy over time, with the exception of Drolet et al.Citation20 and Pieters et al.,Citation30 who investigated multiple non-linear efficacy models in addition to the linear one. In Pieters et al.,Citation30 base-case results were reported for two distinct models: (a) an optimistic logarithmic model potentially underestimating waning effects and, (b) a pessimistic 1-minus exponential model resulting in rapid waning of efficacy over time. The CEA of Drolet et al.Citation20 is unique in that it reports median (and percentile) ICERs from a total of 30,000 simulations, during which the VE model is sampled stochastically amongst a family of six frequently used VE model types. Model parameter values are not stated explicitly in Drolet et al.Citation20
Vaccine efficacy – ZVL
All but oneCitation32 study modeled ZVL VE based on the Shingles Prevention Study (SPS)Citation49 and its follow-ups.Citation50,Citation51 Some studiesCitation21–23 explicitly took Zoster Efficacy, Safety, and Tolerability (ZEST)Citation52 into account. The study of Hoshi et al.Citation32 relied on real-world evidence data from Baxter et al.Citation53 The study of Carpenter et al.Citation25 reused the ZVL VE modeling of a previous CEA.Citation54
Top-up efficacy (ZVL) was implemented for the burden of illness (BOI) in the study of Le and Rothberg.Citation27 Top-up efficacies for PHN were implemented in the studies of Le and Rothberg,Citation21 McGirr et al,Citation26 Ultsch et al.Citation19 and Curran et al.Citation22,Citation23
Most studies resorted to linear modeling of ZVL efficacy, with the exception of the studies of de Boer et al.,Citation29 Drolet et al.,Citation20 Pieters et al.,Citation30 and Hoshi et al.Citation32 The 1-minus exponential VE model was reported to be the best fit in the studies of de Boer et al.Citation29 and Pieters et al.Citation30 The CEA of Drolet et al.Citation20 reported median outcomes from a large number of stochastic simulations (30,000), during which the ZVL VE model type was sampled uniformly from a superset of six different non-linear models. Model parameter values were not reported explicitly in Drolet et al.,Citation20 but may be inferred from earlier work.Citation55,Citation56
Direct costs
Direct HZ treatment costs are reported in Table B4.
All studies incorporated local sources for estimating the direct costs of treating a case of HZ and PHN. With the exception of Le and Rothberg.,Citation21 You et al.,Citation34,Citation35 Shiragami et al.,Citation33 Carpenter et al.,Citation25 and Drolet et al.,Citation20 direct costs were stratified by age or age group.
For the Dutch CEA,Citation29 hospitalization rates, one-day hospitalization rates, GP visit rates, and over-the-counter medication costs per HZ case were combined to derive our own estimates of the aggregate HZ treatment costs.
In the Belgian CEA,Citation30 costs were reported for hospitalized and ambulatory cases of HZ and PHN based on (pain) severity; an estimation of the aggregate costs of treating HZ and/or PHN is included in Table B4. Similarly in You et al.,Citation34,Citation35 direct costs per PHN case were estimated from a reported flat cost per month and a PHN persistence (duration) model detailed in the original reports.
Several studiesCitation21–24,Citation34,Citation35 reported costs for complications explicitly, and a fewCitation22,Citation23,Citation26,Citation28,Citation33 took into account the costs of treating adverse events due to vaccination.
None of the studies included other costs such as training, communication about the vaccine or logistic costs.
Indirect costs
Indirect costs are shown in Table B5.
All but four studiesCitation20,Citation26,Citation30,Citation32 reported indirect costs in line with the costing perspective chosen for the analysis (see also ). Three studiesCitation29,Citation34,Citation35 added lifetime earning losses (attributable to HZ death) to productivity losses due to HZ illness; one studyCitation29 relied on the friction approachCitation57 for estimating productivity losses due to HZ death specifying a friction period of 84 days.
In two studies,Citation34,Citation35 indirect costs were not reported explicitly but background data relating to labor force participation by gender, unemployment rates, median monthly income, and length of hospitalization by complication type were reported; the estimates shown in Table B5 are based on the same data.
Similarly for one USA CEA,Citation25 indirect cost data per HZ and PHN case were deduced by lost time reported in hours by severity of pain and a flat average hourly wage.
QoL and utilities
QoL and utility inputs are reported in Table B6.
Several studiesCitation20–26,Citation28,Citation29,Citation32,Citation33 reported QALY losses per case of HZ directly.
In most cases QALY losses were age-specific, but two studiesCitation25,Citation26 reported aggregate losses averaged across all age groups. Only two studiesCitation22,Citation23 reported different QALY losses for vaccinated and unvaccinated subjects in line with the observations of the SPSCitation49 and following the QALY loss implementation in a previous CEA for ZVL.Citation58
The Dutch CEACitation29 did not distinguish between a case of HZ with or without PHN; average values were employed and reported.
In the Hong Kong CEAs,Citation34,Citation35 disutility values were reported for outpatient & inpatient cases of HZ with or without complications, along with a complex non-linear model for estimating the persistence of PHN beyond 12 months. These data formed the basis of HZ and PHN QALY loss estimation by case shown in Table B6.
The CEAs by Le and RothbergCitation21 reported QALY losses for HZ explicitly but refrained from providing concrete values for PHN. Additional pain data from a previous epidemiological study performed in the UKCitation59 were reported by the authors for PHN, and those formed the basis of our own estimates of age-specific QALY losses for PHN included in Table B6.
HZ and PHN QALY losses in Pieters et al.Citation30 relied on previous workCitation60 demonstrating QALY/utility loss factors by severity of pain as well as data on the proportion of subjects in each pain state from an older epidemiological study conducted in the UK.Citation43 These were combined to complete the estimates under Table B6.
Finally, QALY losses per HZ and PHN case were not stated explicitly in Ultsch et al.Citation19 despite explicit reference to a previous prospective QoL study in Canada;Citation61 the estimations shown in Table B6 were based on the same source.
Adverse events (AEs)
Model input parameters related to AEs post-vaccination are compiled in Table B7, including information on frequency by AE type, treatment costs by type, as well as utility/QALY losses by type when available. The aggregated treatment costs of AEs per inoculation are shown in Table B4 (direct costs).
Model outputs
Top-level CE results are summarized in . A more detailed list of ICERs is compiled under Table B8.
Table 4. Cost-effectiveness results.
Top-level CE results
All but three studiesCitation29,Citation30,Citation32 demonstrated the cost-effectiveness of RZV vaccination vs no vaccination or vaccination with ZVL.
Three studies established the cost-effectiveness of RZV revaccination for subjects previously vaccinated with ZVL.Citation21,Citation23,Citation24
Many studiesCitation19–22,Citation24–26,Citation28,Citation33 demonstrated the CE of RZV across all age groups investigated.
In immunocompromised populations,Citation18 RZV was found cost-saving in hematopoietic stem cell transplantation (HSCT) patients by two models (industry/CDC). RZV was also found cost-saving in renal transplant (industry model) and multiple myeloma (CDC model); RZV was cost-effective in hematologic malignancy and human immunodeficiency virus (HIV) patients (CDC model), and HIV, breast cancer, Hogkin lymphoma patients (industry model).
Sensitivity analyses
All studies provided a number of model input parameters with the greatest effect on CE. Those included structural model inputs such as discount rates, but also vaccination costs (i.e., vaccine prices per dose), vaccine efficacy and waning parameters, HZ and PHN incidence, QALY losses, and direct treatment costs. A non-exhaustive list by study is shown in .
Detailed CE results
A non-exhaustive list of ICERs reported by each study by age group, costing perspective, com-parison type (i.e., RZV vs no vaccination or RZV vs ZVL, etc.), and corresponding vaccine price per dose is compiled under Table B8.
Discussion
The CE of HZ vaccination in older adults has been reviewed in the past.Citation62–64 The present study focuses on the CE of HZ vaccination with RZV. While one and nine manuscripts on the CE of RZV vaccination were identified in the systematic reviews of Chiyaka et al.Citation62 and Udayachalerm et al.,Citation64 respectively, our search has in the meantime identified an additional nine records, for a total of 18 studies included in the present review. All studies were performed in high-income countries, consistent with the observation of Chiyaka et al.Citation62 The CHEERS checklist indicates studies of high quality, with the exception of the presentation to the CDC,Citation18 which does not reflect on the quality of the original research contained within (two unrelated CE models on select immunocompromised cohorts, one by the CDC, the other by the vaccine manufacturer). Our CE findings are generally in good agreement with previous reports.Citation62,Citation64 Overall, RZV was found cost-effective in 15 out of 18 studies, cost-effective or cost-saving in the subset of the aforementioned 15 studies where a direct comparison to ZVL was applicable, and cost-effective in revaccinating cohorts previously vaccinated with ZVL. RZV was additionally found cost-effective or cost-saving in a variety of immunocompromising conditions.
A quantitative exploration of CE outcomes such as the meta-analysis of net monetary benefits in Udayachalerm et al.Citation64 was not attempted. Instead, a critical review of variations in CE levels based on an in-depth look into modeling structure and model input data was undertaken, and main findings are discussed below.
All 18 models in this review were static models, i.e., no dynamic models evaluating RZV were identified. The comparison of CE analyses performed under varying assumptions is difficult in view of variation in methodological approaches. While a typical static multi-cohort health economic model using state transition probabilities (Markov model) is straightforward to construct for HZ and PHN, ICER estimation is invariably non-linear in nature and remains sensitive to the range of inputs. Structural parameters such as discounting rates and model time horizon (follow-up period) are known to have a pronounced effect on ICERs, rendering the direct comparison of models developed for different locales under different prevailing HTA guidelines challenging. In addition, ICERs are not directly comparable when key inputs vary between models, including RZV price per dose and 2nd dose RZV series compliance.
Because vaccine efficacy and waning over time coupled with HZ incidence rates determine the number of incident HZ cases as a function of time, the RZV vaccine efficacy model chosen by each study (and the corresponding ZVL vaccine efficacy model for direct comparisons) was an important input in the reviewed CE analyses. As an example, three CEAs conducted in the USCitation22,Citation24,Citation25 predicted widely varying ICERs at 50 (or 50–59) YOA: $14.9K, $46.8K, and $91.2K, respectively. A simple estimation of the RZV duration of protection (from initial efficacy to zero) in the three studies from the VE data of Table B2 suggests 35, 19.4, and 17.8 years respectively. However, updated clinical trial dataCitation28,Citation47 estimate a vaccine efficacy of 84.1% eight-year post-vaccination with 2-doses of RZV, indicating that waning of efficacy to zero after 17.8 or even 19.4 years is unlikely.
A quick comparison of other inputs between Curran et al.Citation22 and Prosser et al.Citation24 reveals slightly lower HZ incidence rates in Prosser et al.,Citation24 lower QALY losses per HZ case in Curran et al.,Citation22 and higher costs per HZ case in Prosser et al.Citation24 (but lower for PHN). Similarly, the estimated QALY losses per HZ and PHN case are higher in Carpenter et al.Citation25 than in Curran et al.,Citation22 and HZ incidence is identical. Yet ICER in Carpenter et al.Citation25 is several times higher than in Curran et al.,Citation22 specifically for the 50–59-year-old age group, due to the differences in the duration of protection as outlined above.
The optimal age of vaccination with RZV varied between studies. In the German CEA of Curran et al.,Citation28 which made use of 8-year long VE data for RZV, the cohort of 50+ YOA was established as the most cost-effective under the societal perspective, while the independent investigation of Prosser et al.Citation48 estimated the CE-optimal vaccination cohort at 60+ YOA.
Two alternative CEAs conducted in the setting of JapanCitation32,Citation33 also deserve a more detailed comparative analysis and interpretation. The study of Shiragami et al.Citation33 supports RZV CE, while Hoshi et al.Citation32 indicates marginal CE for RZV at 80+ YOA only (see Table B8 for the details). A careful investigation of structural model parameters reveals that Hoshi et al.Citation32 utilized discounting rates of 3% vs 2% in Shiragami et al.Citation33 The RZV price per dose in Hoshi et al.Citation32 was approximately 16% higher than the one quoted in Shiragami et al.Citation33 Costs were comparable across the two studies but QALY losses for HZ and PHN were slightly lower in Hoshi et al.Citation32 Most importantly, RZV efficacy modeling in Hoshi et al.Citation32 assumed faster waning over time resulting in diminished (zero) protection after 19.4 years, which is not supported by the latest RZV vaccine efficacy data.Citation28,Citation47 At the same time, efficacy and waning of ZVL in Hoshi et al.Citation32 followed Baxter et al.,Citation53 which estimates VE at 31.8% (95% confidence interval [CI] 15.1% to 45.2%) after 8 years, while a similar RWE studyCitation65 indicates only 4.2% (95% CI 24.0% to 25.9%) at year 8, and the long-term persistence substudyCitation51 reports a vaccine efficacy for incidence of HZ of 31.1% (22.4% to 36.2%) at year 8, but only 6.8% (−4.9% to 13.4%) at year 9, and already negative at −1.7% (−14.2% to 4.8%) 11 years post-vaccination.
In the case of one CEA conducted in the Netherlands,Citation29 low RZV CE levels may similarly follow as the synergistic effect of the following factors: (a) a modeled time-horizon restricted to 15 years, (b) assumed RZV vaccine efficacy waning of 4.1% annually for 50–69 YOA after 4 years post-vaccination and for 70+ YOA, (c) recurrent HZ incidence not included in the model, (d) adjustment (lowering) of the nationally reported HZ incidence by 10% for possible false-positive diagnoses, and, (e) low relative PHN incidence, which implicitly affects the estimation of QALY losses.Citation66
In the Belgian CEA,Citation30 the atypically low RZV CE outcomes may be traced to adjustments performed on the overall HZ incidence rates to immunocompetent specific incidence rates, using a simplistic calculation (the overall HZ incidence rates are presented in Bilcke et al.Citation67) Appropriate adjustments would rely on knowledge of the true proportion of immunocompetent individuals in the population, as well as on appropriate risk ratios, i.e., the risk of HZ in the immunocompromised population versus the risk of HZ in the immunocompetent population. Consequently, the adjustments performed resulted in artificially low HZ incidence rates leading to low RZV CE outcomes. Moreover, RZV is indicated in individuals who are immunocompromised. As such, an analysis of the cost-effectiveness of RZV could be done on all patients applying overall HZ incidence rates, making the need to perform adjustments on the overall incident rates redundant.
In a previous review of HZ vaccine cost-effectiveness manuscripts, Szucks et al noted that a limitation of most modeling studies was that outdated input data were used.Citation68 The authors further noted that cost-effectiveness models should be updated when new evidence becomes available to support the effect on a potential vaccination recommendation. In the case of RZV, because longer-term follow-up study results on the efficacy of the vaccine become continuously available,Citation69 future studies examining the CE of RZV in different settings should be expected, potentially exhibiting less variability in outcomes as a consequence of reduced uncertainty in vaccine efficacy estimates over time.
Most cost-effectiveness models focused on costs of administering a vaccine (e.g. vaccine and administration costs, costs of treating adverse events) and did not include other costs such as training, communication about the vaccine, and logistics.
As a final point, most analyses did not include differential utility losses for vaccinated and unvaccinated cases. Similar to other vaccine preventable diseases, it has been demonstrated that in breakthrough cases of HZ following vaccination there is an attenuation in the severity of the disease,Citation70 and future studies would be expected to implement utility loss values differentiating between vaccinated and unvaccinated subjects accordingly.
Contributorship
All authors were involved in the design of the study, collected or generated the data, analyzed and/or interpreted the data and participated to the development of this manuscript and in its critical review with important intellectual contributions. All authors had full access to the data and gave approval of the final manuscript before submission. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The work described was carried out in accordance with ICMJE recommendations for conduct, reporting, editing and publications publishing of scholarly work in medical journals. The corresponding author had the final responsibility to submit for publication.
Trademark
Shingrix is a trademark owned by or licensed to GSK.
Zostavax is a trademark of Merck Sharp & Dohme Corp.
Supplemental Material
Download PDF (610.8 KB)Acknowledgements
The authors would like to thank Dr Ioannis Polios (independent consultant) for valuable comments and insightful review during manuscript development. The authors would also like to thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK.
Disclosure statement
NG, CN and DC are employed by GSK. NG and DC hold shares in GSK. All authors declare no other financial and non-financial relationships and activities.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2168952
Additional information
Funding
References
- Schmader K. Herpes Zoster. Ann Intern Med. 2018;169(3):1–13. doi:10.7326/AITC201808070.
- van Oorschot D, Vroling H, Bunge E, Diaz-Decaro J, Curran D, Yawn B. A systematic literature review of herpes zoster incidence worldwide. Hum Vacc Immunotherap. 2021;17(6):1714–32. doi:10.1080/21645515.2020.1847582.
- Coplan PM, Schmader K, Nikas A, Chan ISF, Choo P, Levin MJ, Johnson G, Bauer M, Williams HM, Kaplan KM, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5(6):344–56. doi:10.1016/j.jpain.2004.06.001.
- Johnson RW, Wasner G, Saddier P, Baron R. Postherpetic neuralgia: epidemiology, pathophysiology and management. Expert Rev Neurother. 2007;7(11):1581–95. doi:10.1586/14737175.7.11.1581.
- Lang P-O, Aspinall R. Vaccination for quality of life: herpes–zoster vaccines. Aging Clin Exp Res. 2021;33(4):1113–22. doi:10.1007/s40520-019-01374-5.
- US Food & Drug Administration. Biologics License Application (BLA) approval for Zoster vaccine recombinant. Adjuvanted; 2017 [accessed 2022 Jul 6]. https://www.fda.gov/media/108274/download
- Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, Harpaz R. Recommendations of the advisory committee on immunization practices for use of Herpes Zoster vaccines. Morb Mort Wkly Rep. 2018;67(3):103–08. doi:10.15585/mmwr.mm6703a5.
- Anderson TC, Masters NB, Guo A, Shepersky L, Leidner AJ, Lee GM, Kotton CN, Kotton KL. Use of recombinant Zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices — United States, 2022. Morb Mort Wkly Rep. 2022;71(3):80–84. doi:10.15585/mmwr.mm7103a2.
- European Medicines Agency. Shingrix. Authorisation Details. [ accessed 2022 Jul 6]. https://www.ema.europa.eu/en/medicines/human/EPAR/shingrix#authorisation-details-section
- Parikh R, Widenmaier R, Lecrenier N. A practitioner’s guide to the recombinant zoster vaccine: review of national vaccination recommendations. Expert Rev Vacc. 2021;20(9):1065–75. doi:10.1080/14760584.2021.1956906.
- US Food & Drug Administration. Biologics License Application (BLA) approval for Zoster vaccine. Live; 2006 [ accessed 2022 Jul 6]. http://wayback.archive-it.org/7993/20170722150959/https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm132873.htm
- European Medicines Agency Zostavax. Authorisation Details. [ accessed 2022 Jul 6]. https://www.ema.europa.eu/en/medicines/human/EPAR/zostavax#authorisation-details-section
- Centers for Disease Control and Prevention. Zostavax (Zoster Vaccine Live) recommendations; 2020 [ accessed 2022 Jul 15]. https://www.cdc.gov/vaccines/vpd/shingles/hcp/zostavax/recommendations.html.
- Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C, Shamseer L, Tetzlaff J, Akl E, Brennan S, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. 2021. J Clin Epidemiol. 2021;134:178–89. doi:10.1016/j.jclinepi.2021.03.001.
- Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, Caulley L, Chaiyakunapruk N, Greenberg D, Loder E, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 explanation and elaboration: a report of the ISPOR CHEERS II good practices task force. Value Health. 2022;25(1):10–31. doi:10.1016/j.jval.2021.10.008.
- An Advisory Committee Statement (ACS). National Advisory Committee on Immunization (NACI). Updated recommendations on the use of Herpes Zoster vaccines. 2018 [ accessed 2022 May 10]. https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/healthy-living/updated-recommendations-use-herpes-zoster-vaccines-eng.pdf.
- Institut national de santé publique du Québec. Avis sur la pertinence d’ajouter la vaccination contre le zona au Programme québécois d’immunisation; 2018 [ accessed 2022 May 10]. https://www.inspq.qc.ca/publications/2381.
- Ortega-Sanchez IR Economics of vaccinating immunocompromised 19–49-years-old adults against herpes zoster in the US; 2021 [ accessed 2022 May 10]. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-09-29/02-ZosterVaccines-OrtegaSanchez-508.pdf.
- Ultsch B, Weidemann F, Koch J, Siedler A Modellierung von epidemiologischen und gesundheitsökonomischen: Effekten von Impfungen zur Prävention von Herpes zoster; 2017 [ access 2022 May 10]. https://www.rki.de/DE/Content/Infekt/Impfen/ImpfungenAZ/Zoster/Modellierung_Zoster_Impfung.pdf?__blob=publicationFile.
- Drolet M, Zhou Z, Sauvageau C, DeWals P, Gilca V, Amini R, Bénard É, Brisson M. Effectiveness and cost-effectiveness of vaccination against herpes zoster in Canada: a modelling study. Can Med Assoc J. 2019;191(34):E932–9. doi:10.1503/cmaj.190274.
- Le P, Rothberg MB. Cost-effectiveness of the adjuvanted Herpes Zoster subunit vaccine in older adults. JAMA Intern Med. 2018;178(2):248–58. doi:10.1001/jamainternmed.2017.7431.
- Curran D, Patterson B, Varghese L, Van Oorschot D, Buck P, Carrico J, Hicks K, Lee B, Yawn B. Cost-effectiveness of an adjuvanted recombinant Zoster vaccine in older adults in the United States. Vaccine. 2018;36(33):5037–45. doi:10.1016/j.vaccine.2018.07.005.
- Curran D, Patterson BJ, Van Oorschot D, Buck PO, Carrico J, Hicks KA, Lee B, Yawn BP. Cost-effectiveness of an adjuvanted recombinant zoster vaccine in older adults in the United States who have been previously vaccinated with zoster vaccine live. Hum Vacc Immunotherap. 2019;15(4):765–71. doi:10.1080/21645515.2018.1558689.
- Prosser LA, Harpaz R, Rose AM, Gebremariam A, Guo A, Ortega-Sanchez IR, Zhou F, Dooling K. A cost-effectiveness analysis of vaccination for prevention of Herpes Zoster and related complications: input for national recommendations. Ann Intern Med. 2019;170(6):380–88. doi:10.7326/M18-2347.
- Carpenter CF, Aljassem A, Stassinopoulos J, Pisacreta G, Hutton D. A cost-effectiveness analysis of an adjuvanted subunit vaccine for the prevention of Herpes Zoster and post-herpetic neuralgia. Open Forum Infect Dis. 2019;6(7):ofz219. doi:10.1093/ofid/ofz219.
- McGirr A, Van Oorschot D, Widenmaier R, Stokes M, Ganz ML, Jung H, Varghese L, Curran D. Public health impact and cost-effectiveness of non-live adjuvanted recombinant Zoster vaccine in Canadian adults. Appl Health Econ Health Policy. 2019;17(5):723–32. doi:10.1007/s40258-019-00491-6.
- Le P, Rothberg MB. Cost-effectiveness of the recommendations of the Advisory Committee on immunization practices for the recombinant adjuvanted Zoster subunit vaccine. JAMA Intern Med. 2018;178(9):1277–78. doi:10.1001/jamainternmed.2018.3200.
- Curran D, Van Oorschot D, Matthews S, Hain J, Salem AE, Schwarz M. Long-term efficacy data for the recombinant zoster vaccine: impact on public health and cost effectiveness in Germany. Hum Vacc Immunotherap. 2021;17(12):5296–303. doi:10.1080/21645515.2021.2002085.
- de Boer PT, van Lier A, de Melker H, van Wijck AJM, Wilschut JC, van Hoek AJ, Postma MJ, de Boer PT, van Wijck AJM, van Hoek AJ. Cost-effectiveness of vaccination of immunocompetent older adults against herpes zoster in the Netherlands: a comparison between the adjuvanted subunit and live-attenuated vaccines. BMC Med. 2018;16(1):228. doi:10.1186/s12916-018-1213-5.
- Pieters Z, Ogunjimi B, Beutels P, Bilcke J. Cost-effectiveness analysis of Herpes Zoster vaccination in 50- to 85-year-old immunocompetent Belgian cohorts: a comparison between no vaccination, the adjuvanted subunit vaccine, and live-attenuated vaccine. PharmacoEconomics. 2022;40(4):461–76. doi:10.1007/s40273-021-01099-2.
- Van Oorschot D, Anastassopoulou A, Poulsen Nautrup B, Varghese L, von Krempelhuber A, Neine M, Lorenc S, Curran D. Cost-effectiveness of the recombinant zoster vaccine in the German population aged ≥60 years old. Hum Vacc Immunotherap. 2019;15(1):34–44. doi:10.1080/21645515.2018.1509645.
- Hoshi S-L, Seposo X, Shono A, Okubo I, Kondo M. Cost-effectiveness of recombinant Zoster vaccine (RZV) and Varicella Vaccine Live (VVL) against herpes zoster and post-herpetic neuralgia among adults aged 65 and over in Japan. Vaccine. 2019;37(27):3588–97. doi:10.1016/j.vaccine.2019.05.006.
- Shiragami M, Mizukami A, Kaise T, Curran D, Van Oorschot D, Bracke B, Watanabe D. Cost-effectiveness of the adjuvant recombinant Zoster vaccine in Japanese adults aged 65 years and older. Dermatol Ther (Heidelb). 2019;9(2):281–97. doi:10.1007/s13555-019-0291-4.
- You JHS, Ming W-K, Lee C-F, Tsang O-Y, Chan P-S. Potential cost-effectiveness of adjuvanted herpes zoster subunit vaccine for older adults in Hong Kong. Vaccine. 2018;36(31):4610–20. doi:10.1016/j.vaccine.2018.06.049.
- You JHS, Ming W-K, Tsang O-Y, Chan P-S. Optimal gender-specific age for cost-effective vaccination with adjuvanted herpes zoster subunit vaccine in Chinese adults. PLOS One. 2019;14(1):e0210005. doi:10.1371/journal.pone.0210005.
- Trippoli S. Incremental cost-effectiveness ratio and net monetary benefit: current use in pharmacoeconomics and future perspectives. Eur J Intern Med. 2017;43:e36. doi:10.1016/j.ejim.2017.05.015.
- TreeAge. 2022. TreeAge Pro. Willamstown, MA: TreeAge Software
- R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013.
- Zorginstituut Nederland. Guideline for economic evaluations in healthcare; 2016 [ accessed 2022 May 10]. https://english.zorginstituutnederland.nl/binaries/zinl-eng/documenten/reports/2016/06/16/guideline-for-economic-evaluations-in-healthcare/Guideline+for+economic+evaluations+in+healthcare.pdf.
- World Health Organization. Making choices in health: wHO guide to cost-effectiveness analysis. In: Tan-Torres Edejer T, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans DB, CJL Murray, editors. 2003. Geneva:World Health Organization.
- Japanese Ministry of Health LaW. Analysis guidelines on cost-effectiveness evaluation in Central Social Insurance Medical Council. 2016. [ accessed 2022 Oct 10]. http://www.mhlw.go.jp/file/05-Shingikai-12404000-Hokenkyoku-Iryouka/0000109789.pdf.
- Canada’s Drug and Health Technology Agency. Guidelines for the economic evaluation of health technologies: Canada; 2017 [ accessed 2022 May 10]. https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf.
- Gauthier A, Breuer J, Carrington D, Martin M, Rémy V. Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the United Kingdom. Epidemiol Infect. 2009;137(1):38–47. doi:10.1017/S0950268808000678.
- Marra F, Chong M, Najafzadeh M. Increasing incidence associated with herpes zoster infection in British Columbia, Canada. BMC Infect Dis. 2016;16(1):589. doi:10.1186/s12879-016-1898-z.
- Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang S-J, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, et al. Efficacy of an adjuvanted Herpes Zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–96. doi:10.1056/NEJMoa1501184.
- Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang S-J, Díez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barberà J, et al. Efficacy of the Herpes Zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–32. doi:10.1056/NEJMoa1603800.
- Boutry C, Hastie A, Diez-Domingo J, Tinoco JC, Yu C-J, Andrews C, Beytout J, Caso C, Cheng H-S, Cheong HJ, et al. The adjuvanted recombinant Zoster vaccine confers long-Term Protection Against Herpes Zoster: interim Results of an Extension Study of the Pivotal phase 3 clinical trials ZOE-50 and ZOE-70. Clin Infect Dis. 2022;74(8):1459–67. doi:10.1093/cid/ciab629.
- Prosser LA. Economic evaluation of vaccination for prevention of Herpes Zoster and related complications. Atlanta, GA: University of M; 2017.
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, et al. A vaccine to prevent Herpes Zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–84. doi:10.1056/NEJMoa051016.
- Schmader KE, Oxman MN, Levin MJ, Johnson G, Zhang JH, Betts R, Morrison VA, Gelb L, Guatelli JC, Harbecke R, et al. Persistence of the efficacy of Zoster vccine in the Shingles prevention study and the short-term persistence substudy. Clin Infect Dis. 2012;55(10):1320–28. doi:10.1093/cid/cis638.
- Morrison VA, Johnson GR, Schmader KE, Levin MJ, Zhang JH, Looney DJ, Betts R, Gelb L, Guatelli JC, Harbecke R, et al. Long-term persistence of Zoster vaccine efficacy. Clin Infect Dis. 2015;60(6):900–09. doi:10.1093/cid/ciu918.
- Schmader KE, Levin MJ, Gnann JW Jr., McNeil SA, Vesikari T, Betts RF, Keay S, Stek JE, Bundick ND, S-C S, et al. Efficacy, safety, and tolerability of Herpes Zoster vaccine in persons aged 50–59 years. Clin Infect Dis. 2012;54(7):922–28. doi:10.1093/cid/cir970.
- Baxter R, Bartlett J, Fireman B, Marks M, Hansen J, Lewis E, Aukes L, Chen Y, Klein NP, Saddier P. Long-term effectiveness of the live Zoster vaccine in preventing Shingles: a cohort study. Am J Epidemiol. 2018;187(1):161–69. doi:10.1093/aje/kwx245.
- Le P, Rothberg MB. Cost-effectiveness of Herpes Zoster vaccine for persons aged 50 years. Ann Intern Med. 2015;163(7):489–97. doi:10.7326/M15-0093.
- Brisson M, Pellissier JM, Camden S, Quach C, De Wals P. The potential cost-effectiveness of vaccination against herpes zoster and post-herpetic neuralgia. Hum Vaccin. 2008;4(3):238–45. doi:10.4161/hv.4.3.5686.
- Bilcke J, Ogunjimi B, Hulstaert F, Van Damme P, Hens N, Beutels P. Estimating the age-specific duration of herpes zoster vaccine protection: a matter of model choice? Vaccine. 2012;30(17):2795–800. doi:10.1016/j.vaccine.2011.09.079.
- Kigozi J, Jowett S, Lewis M, Barton P, Coast J. Valuing productivity costs using the friction-cost approach: estimating friction-period estimates by occupational classifications for the UK. Health Econ. 2017;26(12):1862–68. doi:10.1002/hec.3513.
- Pellissier JM, Brisson M, Levin MJ. Evaluation of the cost-effectiveness in the United States of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Vaccine. 2007;25(49):8326–37. doi:10.1016/j.vaccine.2007.09.066.
- Edmunds WJ, Brisson M, Rose JD. The epidemiology of herpes zoster and potential cost-effectiveness of vaccination in England and Wales. Vaccine. 2001;19(23–24):3076–90. doi:10.1016/S0264-410X(01)00044-5.
- van Hoek AJ, Gay N, Melegaro A, Opstelten W, Edmunds WJ. Estimating the cost-effectiveness of vaccination against herpes zoster in England and Wales. Vaccine. 2009;27(9):1454–67. doi:10.1016/j.vaccine.2008.12.024.
- Drolet M, Brisson M, Schmader KE, Levin MJ, Johnson R, Oxman MN, Patrick D, Blanchette C, Mansi JA. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. Can Med Assoc J. 2010;182(16):1731–36. doi:10.1503/cmaj.091711.
- Chiyaka E, Nghiem V, Zhang L, Deshpande A, Mullen P, Le P. Cost-Effectiveness of Herpes Zoster vaccination: a systematic review. Pharmacoeconomics. 2019;37(2):169–200. doi:10.1007/s40273-018-0735-1.
- Kawai K, Preaud E, Baron-Papillon F, Largeron N, Acosta C. Cost-effectiveness of vaccination against herpes zoster and postherpetic neuralgia: a critical review. Vaccine. 2014;32(15):1645–53. doi:10.1016/j.vaccine.2014.01.058.
- Udayachalerm S, Renouard M, Anothaisintawee T, Thakkinstian A, Veettil S, Chaiyakunapruk N. Incremental net monetary benefit of herpes zoster vaccination: a systematic review and meta-analysis of cost-effectiveness evidence. J Med Econ. 2022;25(1):26–37. doi:10.1080/13696998.2021.2008195.
- Tseng HF, Harpaz R, Luo Y, Hales CM, Sy LS, Tartof SY, Bialek S, Hechter RC, Jacobsen SJ. Declining effectiveness of Herpes Zoster vaccine in adults aged ≥60 years. J Infect Dis. 2016;213(12):1872–75. doi:10.1093/infdis/jiw047.
- van Wijck AJM, Aerssens YR. Pain, Itch, quality of life, and costs after Herpes Zoster. Pain Practice. 2017;17(6):738–46. doi:10.1111/papr.12518.
- Bilcke J, Ogunjimi B, Marais C, de Smet F, Callens M, Callaert K, van Kerschaver E, Ramet J, van Damme P, Beutels P. The health and economic burden of chickenpox and herpes zoster in Belgium. Epidemiol Infect. 2012;140(11):2096–109. doi:10.1017/S0950268811002640.
- Szucs TD, Pfeil AM. A systematic review of the cost effectiveness of Herpes Zoster vaccination. PharmacoEconomics. 2013;31(2):125–36. doi:10.1007/s40273-012-0020-7.
- A long-term follow-up study (ZOE-LTFU) of two studies 110390 (ZOSTER-006) and 113077 (ZOSTER-022) to assess the efficacy, safety, and immunogenicity persistence of GSK biologicals’ Herpes Zoster subunit (HZ/su) vaccine and assessment of 1 or 2 additional doses in two subgroups of older adults. 2016. [ accessed 2022 Oct 10]. https://clinicaltrials.gov/ct2/show/record/NCT02723773?view=record.
- Curran D, Oostvogels L, Heineman T, Matthews S, McElhaney J, McNeil S, Diez-Domingo J, Lal H, Andrews C, Athan E, et al. Quality of life impact of an adjuvanted recombinant Zoster vaccine in adults aged 50 years and older. J Gerontol: Ser A. 2019;74:(8):1231–38. doi:10.1093/gerona/gly150.
