ABSTRACT
For years, prospective randomized clinical trials excluded patients with non-conventional histologies of renal cell carcinoma (RCC). The paucity of data has led to adopting the same treatment strategies used for clear-cell RCC (ccRCC). In the present narrative review, we explored state of the art about use of immune checkpoint inhibitors (ICIs) in variant histologies of RCC. According to the results collected, ICIs as monotherapy showed promising antitumor activity in advanced non-clear cell (ncc)RCC. The objective response rate (ORR) was similar to that observed with single-agent anti-PD-1 in the ccRCC population, either in the first-line or the second-line setting, and responder patients experienced an early and durable benefit. Combined ICI-based strategies have shown increasing evidence in nccRCC and robust results in the sarcomatoid variants of RCC. A definitive recommendation about treating non-conventional histologies, either in adjuvant or metastatic settings, should be supported by more extensive dedicated trials.
Introduction
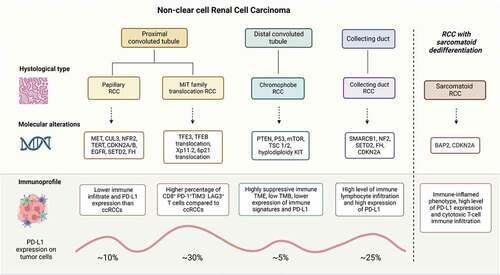
The primary representative histology in adult kidney cancer is clear-cell renal cell carcinoma (ccRCC), accounting for about 80% of all renal cell carcinoma cases. Under the name of non-clear cell renal cell carcinoma (nccRCC), the current classification includes different entities, such as papillary renal cell carcinoma (pRCC), chromophobe renal cell carcinoma (chRCC), MiTF translocation-associated renal cell carcinoma (tRCC), and collecting duct carcinoma (CDC) among the classified subtypes.Citation1 In addition, the sarcomatoid variant represents a feature potentially characterizing all the other primary histologies.Citation2
Many pivotal prospective randomized clinical trials excluded patients with non-clear cell renal cell carcinoma (nccRCC) from recruitment for years. In most recent cases, non-clear cell histology is no longer considered an exclusion criterion but a stratification factor. On the other hand, the low incidence of nccRCC limits in the planning of dedicated large randomized trials. Therefore, most available data are obtained from subgroup analyses of phase III trials including mainly clear-cell renal cell carcinoma (ccRCC), single-arm phase II studies, nominal therapeutic use programs, and retrospective analyses. The paucity of data has led to adopting, for patients with nccRCC, the same treatment strategies used for ccRCC, often without solid evidence. Instead, genome sequencing studies have shown the importance of approaching the different subtypes of nccRCC as single entities with a specific spectrum of altered molecular pathways and immune context,Citation3 with distinct potential therapeutic targets ().
In clinical guidelines, the preferred regimens for metastatic nccRCC are still tyrosine kinase inhibitors (TKI) targeted on vascular endothelial growth factor receptor (VEGFR). However, nccRCC subtypes are often resistant to VEGFR-inhibition and characterized by heterogeneous clinical and biological behaviors, so new and different therapeutic strategies, especially with immune checkpoint inhibitors (ICIs), are under investigation.Citation4
Two randomized phase II trials (the ESPN and ASPEN trials) and a subsequent meta-analysis highlighted that patients with nccRCC, compared to those with ccRCC, benefit less from systemic therapy with mTORi and VEGFRi in terms of both radiological responses and PFS and OS.Citation5–7
Papillary Renal Cell Carcinoma (pRCC) is the most frequent subtype of non-clear cell Renal Cell Carcinomas (nccRCCs), accounting for 10–15% of all Renal Cell Carcinomas (RCCs). The fifth edition of the WHO classification of cancers of the male urinary and genital tract abolished the subcategorization of pRCC into types 1 and 2, considering that mixed phenotypes are increasingly common and differentiation has no therapeutic implications.Citation1
pRCC is heterogeneous in clinical manifestations, including indolent multifocal and aggressive solitary tumors. Prognosis is strongly related to histopathological features, including grade and the presence of sarcomatoid dedifferentiation. Recent studies confirm that sarcomatoid and/or rhabdoid dedifferentiation leads to a poor prognosis and poor response to targeted therapies. First-generation targeted therapy approved for ccRCC has shown limited efficacy in pRCC (ORR <15%).Citation4 More convincing data emerged from dedicated trials in this specific histology, especially with TKIs targeting MET.Citation8,Citation9
pRCCs are characterized by a lower immune infiltrate than ccRCCs, and only 10% of pRCCs express PD-L1 on tumor cells.Citation10 Therefore, immunotherapy strategies could increase the number of activated antitumor immune cells, such as cytotoxic T lymphocytes (CTL).
Chromophobe RCC (chRCC) is the second most common subtype of nccRCC, after papillary RCC, accounting for about 5% of nccRCC.Citation11 chRCC generally has an indolent behavior and are considered immunologically cold, with a highly suppressive immune microenvironment, low tumor mutational burden, lower expression of immune signatures, and PD-L1.Citation12
MiTF translocation-associated RCC (tRCC) is a rare nccRCC subtype accounting for about 1–4% of adult RCCs (15% when considering young adults, <45 years) and 20–40% of pediatric RCCs.Citation13 It represents a distinct genetic entity, namely molecularly defined RCC, introduced in the 2004 WHO classification of renal tumors. It is more frequent in females (2:1), and its incidence is likely underestimated due to the frequent morphological overlap with other histologies and the lack of standard techniques for molecular testing. The translocations (or alterations, such as amplifications) include transcription factors from the MiT family, such as TFE3 (Xp.11.2), TFEB (6p21.1), and MITF (3p13).Citation14 This subgroup of RCC has significantly poor outcomes compared to other RCC subtypes, both in the localized and metastatic setting. Its resistance to VEGFR-TKI is well known despite the lack of dedicated trials. On the other hand, it emerged that these tumors are infiltrated with CD8+ T cells, though the T cells harbor an exhaustion immunophenotype distinct from that of clear cell RCC. Together with the evidence of a heightened NRF2-driven antioxidant response that is associated with resistance to targeted therapies, these findings may inspire tailored therapeutic proposals for tRCC.Citation15 Interestingly, tRCC belongs to the proliferative cluster according to the IMmotion 151 classification, showing low angiogenesis and low PD-L1 expression in most cases.Citation3 Moreover, higher CD8+ T cells were reported in MED15-TFE3 fusions, and increased expression of PD-L1 was reported in TFEB amplified RCC, suggesting that some tRCC subtypes may be more immune-cold.Citation16
Collecting duct carcinoma (CDC) represents less than 1% of all renal tumors. CDCs are aggressive tumors, 70% of cases are metastatic at diagnosis, and the median OS does not exceed 13 months.Citation17
Sarcomatoid RCC mainly represents a variant of clear-cell renal carcinoma. Sarcomatoid features are found in 5%−15% of all RCCs and can be associated with any histology (5–13% ccRCC, 2–7% in pRCC, 29% in collecting duct RCC). They are more common in metastatic (15–20%) than the localized disease (5–6%). RCC with sarcomatoid features (sRCC) is characterized by mesenchymal dedifferentiation, high biological aggressiveness, and poor prognosis, especially when the disease is metastatic.Citation2 Distinctive molecular characteristics were reported for sRCC, which is less linked to the VEGF pathway when compared to conventional RCC and often harbors BAP1 mutations and CDKN2A deletions. Genes involved in the immune response are also more frequently altered in this RCC subtype.Citation18 Notably, sRCC is characterized by an immune-inflamed phenotype, with increased PD-L1 expression and cytotoxic T-cell immune infiltration.Citation3 These molecular characteristics could favor the sensitivity of these tumors to immune checkpoint blockade.Citation19 On the other hand, it is known that patients with this histology are less likely to benefit from treatment with TKIs.Citation20
In the present narrative review, we explored state of the art about the use of ICIs in these non-conventional histologies of RCC, both from clinical trials and real-life reports.
Methods
We searched PubMed for studies published in the English language from the inception of the database to 31 August 2022. NCCN guidelines (Version 2.2022), AIOM guidelines (Version 2021), and meeting libraries from the American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) websites were also screened for further relevant publications.
Three investigators (MeB, SER, and MR) independently performed the research, then shared the entire pool of potentially relevant publications about the treatment of nccRCC with ICIs. All the references of the included articles were then screened for the recovery of any further eligible publications.
The search was focused on papillary RCC, chromophobe RCC, collecting duct RCC, translocated RCC, and sarcomatoid RCC (intended as a variant of any histology). We included prospective trials designed explicitly for nccRCC and the more relevant retrospective studies or case series reporting patients with nccRCC treated with ICI-based schedules. MeB extracted data for sRCC, MR for papillary and CDC, and SER for ChRCC and tRCC. In addition, MeB screened all the publications about recent pivotal trials of ICI-based therapeutic schedules in mRCC patients, analyzing the availability of data about subgroups with sRCC from the overall population included in each study.
As a separate explorative search, clinicaltrials.gov was checked for any trial ongoing with ICIs specifically designed for nccRCC patient populations.
Results
The evidence available about published studies with ICI-based treatments conducted explicitly in patients with nccRCC is summarized in for prospective trials and for the most representative retrospective studies (see Supplementary file 1 for the references).
Table 1. Prospective trials with immunotherapy-based treatments specifically conducted in patient populations with non-clear cell renal cell carcinoma.
Table 2. Representative retrospective studies with immunotherapy-based treatments in patient populations with non-clear cell renal cell carcinoma.
Phase III randomized trials of ICI-based combinations reporting data for the subgroup of patients with sRCC are included in , describing the outcomes of patients treated in the ICI-based (experimental) arm vs. the TKI monotherapy (control) arm.
Table 3. Results for the subgroup of patients with sarcomatoid renal cell carcinoma included in pivotal trials with immune-checkpoint inhibitor-based combinations.
Data from single studies are discussed in separate paragraphs according to histology.
reports ongoing clinical trials with ICIs specifically designed for patients with nccRCC.
Table 4. Trials ongoing specifically investigating immune-checkpoint inhibitors in patient populations with non-clear cell renal cell carcinoma (nccRCC).
Discussion
According to the results, ICIs as monotherapy showed promising antitumor activity in nccRCC (see ). The objective response rate (ORR) was similar to that observed with single-agent checkpoint inhibitors in the ccRCC population, either in the first-line (36.5% with pembrolizumab) or the second-line setting (23% with nivolumab). Objective response rates were lower in pre-treated nccRCC patients; nevertheless, responder patients experience an early and durable benefit.
Compared with first-generation TKI data, these results are outstanding, considering the ORR obtained with sunitinib in the ASPEN and ESPN phase II trials, respectively, of 18% and 9%. Also, with the meaningful limitation of a likely very different patient population, mPFS reached with ICIs seems better than that obtained with such TKIs.Citation5,Citation6 On the other hand, more recent data with new-generation TKI, such as cabozantinib and savolitinib (used in the PAPMET and SAVOIR trials), together with ICI-monotherapy data, led to the update of the first-line treatment recommendations at least for papillary RCC.Citation21
Papillary renal cell carcinoma
The population of patients with pRCC, likely due to their epidemiology, is often the principal representative in nccRCC clinical trials.
The single-arm phase II study Keynote-427 was the first clinical trial that evaluated a first-line single-agent checkpoint inhibitor in naïve patients with advanced nccRCC (Cohort B): 165 nccRCC, including 118 pRCC (71.5%), received open-label pembrolizumab (200 mg every three weeks) for ≤24 months. At a median follow-up of 34 months, in the total population, ORR was 26.7% (95% CI, 20.1 to 34.1), DCR 43.0%, median PFS 4.2 months (95% CI, 2.9 to 5.6), median OS was 28.9 months (95% CI, 24.3 months to not reached). For patients with papillary histology, ORR was 28.8% (95% CI, 20.8% to 37.9%), disease control rate (DCR) was 47.5% (95% CI, 38.2% to 56.9%), median PFS 5.5 months (95% CI, 3.9 to 6.9), and median OS was 31.5 (95% CI, 25.5 to not reached).
In the CheckMate 374 trial, an open-label phase IIIb/IV study, the safety and efficacy of nivolumab were verified in previously treated advanced/metastatic nccRCC. An analysis of 44 patients, including 24 pRCC, who received nivolumab up to the third line of treatment showed an ORR of 13.6% and a median OS of 16.3 months. No complete responses were observed in pRCC patients, nine patients had stable disease, and two patients had a partial response for an ORR of 8.3%, a DCR of 45%, and a DOR of 10 months.
The potential and durable efficacy of single-agent ICI in pRCC, although in a limited percentage of patients, has stimulated studies testing combination therapies (ICI plus ICI and ICI plus VEGFR-TKi).
The multicohort phase IIIb/IV CheckMate-920 study tested the combination of nivolumab and ipilimumab in nccRCC patients (52 patients), showing an ORR of 27% in the 18 enrolled pRCCs and 36% in the presence of sarcomatoid features. 90% of patients with pRCC who achieved a radiological response remained progression-free at a follow-up of 24 months. Median PFS was 3.7 (95% CI 2.7 to 4.6) months, and median OS was 21.2 (95% CI 16.6 to not estimable) months.
The cohort B of the HCRN GU16–260 trial, a phase II study of nivolumab and salvage nivolumab plus ipilimumab in treatment-naïve patients with advanced nccRCC, included 18 patients with pRCC, reporting an ORR of 5.3% in this subgroup, with no complete responses. In this study, neither nivolumab monotherapy, nor salvage strategy with ipilimumab combinations, obtained meaningful results in nccRCC.
The results of several single-arm studies with the combination of ICIs and TKI-VEGFR/MET-inhibitors were recently presented.
The phase I/II CALYPSO study tested the combination of a MET inhibitor, savolitinib, plus an ICI, durvalumab, in 41 patients with treatment-naïve (n = 28) or previously treated (n = 13) metastatic pRCC. The ORR was 27%, with a median PFS of 5.3 months and no correlation between tumor response and PD-L1 status and MET biomarker analysis.
A phase II study evaluated atezolizumab plus bevacizumab in patients with advanced renal cell carcinoma (RCC) with variant histology (42 patients, including 12 pRCC) or any RCC histology with ≥20% sarcomatoid differentiation (18 patients). In this trial, ORR was 33% for the overall population, 50% for ccRCC with sarcomatoid differentiation, and 26% for patients with variant histology RCC, with a median PFS of 8.3 months.
The phase 1b study COSMIC 021 evaluated cabozantinib plus atezolizumab in patients with solid tumors, including ccRCC (70 patients) and nccRCC (32 patients). In nccRCC (n = 32), ORR was 31% (80% CI, 20 to 44), all partial responses, and median PFS was 9.5 months. Responses were observed across subtypes of nccRCC and irrespective of PD-L1 status. The median time to response was 2.7 months, DCR was 94%, and median DOR was 8.3 months (95% CI, 2.4 to NE). The highest ORR was among patients with papillary RCC at 47% (7 of 15).
The results of a phase II study evaluating nivolumab plus cabozantinib in metastatic pRCC (cohort 1) have recently been published (NCT03635892). Twenty-six (65%) were previously untreated, and 14 (35%) had one last line. At a median follow-up of 13.1 months, ORR was 48%, DCR 98%, PFS 12.5 months (95% CI 6.3–16.4), and OS 28 months (95% CI 16.3-NR).
Preliminary results of the Keynote-B61 study, a single-arm phase 2 study (NCT04704219) evaluating the combination of pembrolizumab plus lenvatinib as first-line treatment for nccRCC, were presented at the ESMO Congress 2022. Of 82 patients with a follow-up ≥24 weeks, 51 were papillary renal carcinomas. Confirmed ORR was 47.6% (95% CI, 36.4–58.9; 3 CRs [3.7%]; 36 PRs [43.9%]), DCR was 79.3% (95% CI, 68.9–87.4) a median DOR was not reached (range, 1.4+ to 7.2+ mo). The 6-month PFS rate was 72.3% (95% CI, 60.7–81.0), and the 6-month OS rate was 87.8% (95% CI, 78.5–93.2).
All the studies described in this section (see ) showed no new signs of toxicity compared to those conducted in ccRCCs.
Although these results are based on phase II trials with a limited number of enrolled patients and often immature data for PFS and OS, as also happened for VEGFR-TKIs, the physician is supported in using ICI combinations as the first-choice therapy for patients with pRCC.
The ongoing phase II trial will provide additional data in this histologic subtype ().
Regarding the adjuvant setting, a single study with ICI (IMmotion010) included patients with nccRCC, in particular 18 pRCC, of which only 6 were treated in the experimental arm with atezolizumab (2% of the overall population receiving ICI).Citation22 The trial was negative, and the paucity of data about non-clear subgroups represents an unmet medical need to be taken into account for future adjuvant trials.
Chromophobe renal cell carcinoma
In the literature, no retrospective or prospective studies are available specifically on chRCC patients treated with ICIs, and only a few case reports have been published.Citation23–25 The main data available are extrapolated from nccRCC studies, including different histology subtypes (see and ).
The chRCC populations enrolled in the Keynote-427 trial with pembrolizumab, the COSMIC-021 trial with atezolizumab plus cabozantinib, and the phase II trial with nivolumab plus cabozantinib, achieved ORR of 9.5%, 11%, and 0% respectively ().
In the recently reported Keynote-B61 study (), patients with chRCC (18.3% of the overall study population) had poor outcomes, with the lowest ORR among the included histologies (13.3%). Nevertheless, 80% of patients evaluable for response (15 chRCC cases) obtained a reduction in tumor burden, reaching a pretty good disease control rate.
Three case reports are available on chRCC patients treated with ICIs, two with nivolumab with disease response, and one patient resistant to nivolumab plus ipilimumab. The two patients treated with nivolumab had sarcomatoid differentiation, while the other had a PD-L1 expression of 80%.Citation23–25
In a prospective and retrospective analysis of nccRCC patients treated with ICIs, chRCC accounted for about 16% (range 10%–28%) of the whole nccRCC population (N range: 19–165) and was generally associated with poor response and survival outcomes compared with the other nccRCC patients, especially papillary and sarcomatoid nccRCC.Citation11
For chRCC, future research should focus on identifying biomarkers for patient selection or combining therapy strategies to improve the ICI antitumor effect.
MiTF translocation-associated renal cell carcinoma
According to tRCC rarity, only a few data are available on the efficacy of ICI in this RCC histology. The retrospective analysis conducted by Boilève et al. () was the first case series analysis in tRCC patients treated with ICIs, showing relatively poor response (ORR 16.7%) and survival outcomes (mPFS 2.5 months, range 1–40 months).
A subsequent report by the MSKCC cohort reported extensive molecular data of tRCC patients receiving multiple lines of therapy, including ICI in eight cases. In the intra-patient clinical timeline, the most prolonged duration of responses was reported for ICI treatment.Citation26
More recently, Thouvenin et al. () reported data from a retrospective, multicenter study on tRCC, in which 18 patients treated with I-line ICI-based therapy did not achieve benefit when compared to 38 patients receiving TKIs or other treatments: mOS was 13.5 months (95% CI: 3.9-NA) for pts treated with ICI combinations in versus 36.2 months (95% CI: 27.7-NA) for others (p = .001). These data suggest that some tRCC patients might not benefit from a first-line ICI-based strategy.
On the contrary, with the combination of lenvatinib and pembrolizumab, the five patients with tRCC evaluable for response (of 15 tRCC included) in the Keynote-B61 trial () showed meaningful results, with ORR 60% and DCR 80%.
In addition, only some case reports were published, with contrasting results. Zhao et al. reported good disease-free survival in stage III tRCC patients who underwent surgery and then received one year of ICI (camrelizumab) in combination with tyrosine kinase inhibitor (axitinib), with no sign of recurrence after 18 months of follow-up.Citation27
Yan et al. recently reported two patients with metastatic tRCC treated with VEGFR-TKI plus ICI as first-line therapy, reaching a PFS of 16.6 and 25.6 months, respectively.Citation28
On the other hand, Masago et al. showed that the immune combination of nivolumab plus ipilimumab was ineffective for a single patient with metastatic tRCC.Citation29
tRCC tumors have a highly variable clinical behavior with distinct gene mutations and are characterized by a permissive immune microenvironment, high mutational heterogeneity, and PD-L1 expression.Citation11,Citation13 Given their rarity, there is no consensus on the optimal therapy, especially on the role of immunotherapy, for these nccRCC histologies. However, according to the abovementioned characteristics, tRCC tumors seem more potentially sensible to ICIs, even if more extensive studies are needed to see relevant results.
Collecting duct carcinoma
Given the histopathological similarities with urothelial carcinoma, platinum-based cytotoxic chemotherapy has traditionally been considered the first-choice treatment option for metastatic CDC. However, with the combination of gemcitabine plus cisplatin or gemcitabine plus carboplatin, ORR was 26%, median PFS 7.1 months, and median OS 10.5 months.Citation30 The results of the phase II BONSAI study evaluating the activity of cabozantinib in first-line in 23 CDCs were recently published and showed encouraging results with an ORR of 35% (1 CR andPR) and median PFS of 6 months.Citation31
Currently, no prospective studies evaluate the efficacy of ICI in first- or subsequent lines of therapy specifically for CDCs.
A retrospective study evaluated the effectiveness of treatments after first-line chemotherapy in 57 patients, of which 35 had metastatic CDC (and the other 22 had metastatic renal medullary carcinoma) treated between 2010 and 2019 in 11 French centers. All patients received first-line chemotherapy with platinum ± bevacizumab, with a median time to progression of 7.27 (95% CI, 7–100 months) and an objective response rate (ORR) of 39% (95% CI, 26–52%). Subsequent treatments included tyrosine kinase inhibitors, chemotherapy, and checkpoint inhibitors, with ORRs ranging from 10 to 15% and disease control rates from 24 to 50%. The median duration of response for all treatments was two months. After a median follow-up of 13 months, the median overall survival was 12 (95% CI, 11–16) months. In total, 20 patients received ICI, including anti-PD-1)/PD-L1) monotherapy for all patients, except for two who received an anti-PD-1 plus an anti-CTLA4. The ICI-associated ORR and DCR values were 10% (n = 2/20) and 30% (n = 6/20), respectively.Citation32
Single case reports have described the activity of ICI in previously treated mCDC patients.Citation33–35
Given the poor results obtained with chemotherapy and target therapies for patients with this rare variant, we look forward to furthering data supporting dual ICI or ICI/TKI combination in CDC cases from ongoing phase II clinical trials ().
Clear-cell renal carcinoma with sarcomatoid features
Therapeutic combinations based on immune checkpoint inhibitors (ICI) are the new standard of care as the first-line treatment of patients with metastatic renal cell carcinoma (mRCC). In the pivotal trials of ICI-based combinations, patients with clear-cell histology RCC with sarcomatoid features were usually included. The results for these subgroups were separately reported in dedicated publications or congress presentations. reports the outcomes of 569 patients with sRCC, representing 12.9% of the population enrolled in 5 trials, treated in the first-line setting with an ICI-based combination. The amplitude of benefit obtained in terms of objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) in patients with sRCC was likely due to the poor efficacy of the anti-VEGFR monotherapy used in the control arm. Indeed, it is well known that sRCC is resistant to conventional antiangiogenic treatment.Citation3,Citation18
In the randomized phase III CheckMate-214 trial (), investigating the only ICI–ICI combination available in this setting, namely nivolumab plus ipilimumab, the sRCC subgroup obtained outstanding outcomes, despite considering intermediate and poor-risk patients only according to the IMDC model. ORR was 61%, complete responses 23%, median PFS 26.5 months, and median OS 48.6 months at the last update. At the indirect comparison, these results are the best obtained in sRCC, suggesting that an antiangiogenic drug may not be necessary to reach efficacy in this population. This trial was also the only one providing an independent central review of the histologic specimens for the histology definition.
On the other hand, recent meta-analyses offered either overall outcome and network comparisons of the different ICI-based combinations, strongly supporting the efficacy of ICI-based combinations for sRCC therapy and suggesting the ICI-TKI combination nivolumab plus cabozantinib as the preferred first-line combination for the treatment of patients with sRCC, at least in terms of efficacy, despite without an overwhelming superiority above the others.Citation36
In all cases, as reported in , the benefit of the ICI-based combination strategy above the TKI monotherapy emerged irrespectively of the specific associated drug, reinforcing the need for immunotherapy in the systemic approach to sRCC and suggesting its investigations in other settings for this particular population. Moreover, the efficacy seems unrelated to the IMDC stratification, reinforcing the concept that the IMDC score is prognostic more than predictive, and it should not be used as a relevant parameter for the clinical choice in this patient subgroup.
Looking at the adjuvant setting, data reported for the sarcomatoid subgroup treated with pembrolizumab in the Keynote-564 trial (11% of the overall population) showed a meaningful amplitude of benefit in this population, with an HR of 0.54 (95%CI 0.29–1.00) in the case of sarcomatoid features presence vs. HR of 0.63 (95%CI 0.48–0.83) in the non-sarcomatoid population. In sRCC population enrolled, 71.8% of patients were disease-free at 24 months with pembrolizumab vs. 52% in the control arm (mDFS not reached vs. 40.5 months).Citation37
Ongoing trials
Several clinical trials are still ongoing on the role of ICIs in patients with advanced or metastatic nccRCC ().
Some of these are finally focused on specific populations and molecular-driven, as the phase III SAMETA trial, based on prior phase II results with savolitinib and durvalumab, which is explicitly enrolling patients with MET-selected pRCC and comparing three arms: the combination (100 patients), durvalumab alone (50 patients), and sunitinib as the control arm (50 patients).
Other phase III studies are still enrolling heterogeneous populations, allowing the accrual of patients with both ccRCC or nccRCC: for example, the Contact-03 study, which recruitment has been recently concluded, will provide information on the efficacy of cabozantinib + atezolizumab compared to cabozantinib as second/third-line after previous ICI therapy also for papillary or unclassified mRCC [NCT04338269].
Two randomized phase II trials (SUNNIFORECAST – NCT03075423 and AREN1721 – NCT03595124, see ) are explicitly planned in the nccRCC population with a survival outcome as the primary endpoint (12y-OS and PFS, respectively). The SUNNIFORECAST trial compares nivolumab plus ipilimumab with the standard therapy according to the physician’s decision in 306 nccRCC patients in the first-line setting. In contrast, the AREN1721 trial assesses the efficacy of the nivolumab plus axitinib combination compared with nivolumab alone in both untreated and pretreated tRCC patients.
The remaining ongoing studies in the field are mainly single-arm phase II trials on combining an ICI and a TKI, with ORR as the primary endpoint (see ).
Conclusion
The current data for nccRCC suggests that ICI-based therapeutic strategies could also represent the new cornerstone of systemic treatment in these subgroups of mRCC patients, especially in the case of pRCC. A definitive recommendation should be supported by the eagerly awaited results of dedicated trials, still currently ongoing. In light of the sometimes dramatically different outcomes of every drug based on the histologic subtype, the efforts for future trials would be directed to planning multicenter studies tailored to single histology.
The adjuvant setting is a highly unmet clinical need for non-conventional histologies of RCC.
On the other hand, the sarcomatoid variant is a niche with a firmly established new therapeutic standard (at least in the metastatic setting) based on ICI, irrespective of the IMDC risk group or other clinical variables.
Supplemental Material
Download PDF (125.3 KB)Disclosure statement
Melissa Bersanelli received research funding from Seqirus UK, Pfizer, Novartis, BMS, Astra Zeneca, Roche S.p.A., and Sanofi Genzyme; honoraria as a speaker at scientific events by Bristol-Myers Squibb (BMS), Novartis, Astra Zeneca, and Pfizer and as a consultant for the advisory role by Pierre-Fabre, MSD, Sanofi, IPSEN, Novartis, BMS, and Pfizer; she also received fees for copyright transfer by Sciclone Pharmaceuticals, Sanofi, Pierre-Fabre, MSD. Mimma Rizzo received honoraria as a speaker/consultant by MSD, Astra Zeneca, Bristol-Myers Squibb (BMS), Novartis, and Pfizer. Sara Elena Rebuzzi received honoraria as a speaker at scientific events and travel accommodation by Janssen, BMS, Amgen, GSK, Astellas, MSD, and Ipsen. All the other authors declare no conflict of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2171672.
Additional information
Funding
References
- Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol. 2016;70(1):93–11. doi:10.1016/j.eururo.2016.02.029.
- de Peralta-Venturina M, Moch H, Amin M, Tamboli P, Hailemariam S, Mihatsch M, Javidan J, Stricker H, Ro JY, Amin MB. Sarcomatoid differentiation in renal cell carcinoma. Am J Surg Pathol. 2001;25(3). doi:10.1097/00000478-200103000-00001.
- Motzer RJ, Banchereau R, Hamidi H, Powles T, McDermott D, Atkins MB, Escudier B, Liu L-F, Leng N, Abbas AR, et al. Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade. Cancer Cell. 2020;38(6):803–17.e4. doi:10.1016/j.ccell.2020.10.011.
- Zhang T, Gong J, Maia MC, Pal SK. Systemic therapy for non-clear cell renal cell carcinoma. Am Soc Clin Oncol Educ Book. 2017;37:337–42. doi:10.1200/EDBK_175572.
- Armstrong AJ, Halabi S, Eisen T, Broderick S, Stadler WM, Jones RJ, Garcia JA, Vaishampayan UN, Picus J, Hawkins RE, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomized phase 2 trial. Lancet Oncol. 2016;17:(3):378–88. doi:10.1016/S1470-2045(15)00515-X.
- Tannir NM, Jonasch E, Albiges L, Altinmakas E, Ng CS, Matin SF, Wang X, Qiao W, Dubauskas Lim Z, Tamboli P, et al. Everolimus versus Sunitinib prospective evaluation in metastatic non–clear cell renal cell carcinoma (ESPN): a randomized multicenter phase 2 trial. Eur Urol. 2016;69(5):866–74. doi:10.1016/j.eururo.2015.10.049.
- Fernández-Pello S, Hofmann F, Tahbaz R, Marconi L, Lam TB, Albiges L, Bensalah K, Canfield SE, Dabestani S, Giles RH, et al. A systematic review and meta-analysis comparing the effectiveness and adverse effects of different systemic treatments for non-clear cell renal cell carcinoma. Eur Urol. 2017;71(3):426–36. doi:10.1016/j.eururo.2016.11.020.
- Choueiri TK, Heng DYC, Lee JL, Cancel M, Verheijen RB, Mellemgaard A, Ottesen LH, Frigault MM, L’Hernault A, Szijgyarto Z, et al. Efficacy of Savolitinib vs Sunitinib in patients with MET -driven papillary renal cell carcinoma. JAMA Oncol. 2020;6(8):1247–55. doi:10.1001/jamaoncol.2020.2218.
- Pal SK, Tangen C, Thompson IM Jr, Balzer-Haas N, George DJ, Heng DYC, Shuch B, Stein M, Tretiakova M, Humphrey P, et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet. 2021;397(10275):695–703. doi:10.1016/S0140-6736(21)00152-5.
- Choueiri TK, Fay AP, Gray KP, Callea M, Ho TH, Albiges L, Bellmunt J, Song J, Carvo I, Lampron M, et al. PD-L1 expression in non-clear-cell renal cell carcinoma. Ann Oncol. 2014;25(11):2178e84. doi:10.1093/annonc/mdu445.
- Zarrabi K, Walzer E, Zibelman M. Immune checkpoint inhibition in advanced non-clear cell renal cell carcinoma: leveraging success from clear cell histology into new opportunities. Cancers (Basel). 2021;13(15):3652. doi:10.3390/cancers13153652.
- Garje R, Elhag D, Yasin HA, Acharya L, Vaena D, Dahmoush L. Comprehensive review of chromophobe renal cell carcinoma. Crit Rev Oncol Hematol. 2021;160:103287. doi:10.1016/j.critrevonc.2021.103287.
- Rizzo M, Pezzicoli G, Santoni M, Caliò A, Martignoni G, Porta C. MiT translocation renal cell carcinoma: a review of the literature from molecular characterization to clinical management. Biochim Biophys Acta Rev Cancer. 2022;1877(6):188823. doi:10.1016/j.bbcan.2022.188823.
- Tretiakova MS. Chameleon TFE3-translocation RCC and how gene partners can change morphology: accurate diagnosis using contemporary modalities. Adv Anat Pathol. 2022;29(3):131–40. doi:10.1097/PAP.0000000000000332.
- Bakouny Z, Sadagopan A, Ravi P, Metaferia NY, Li J, AbuHammad S, Tang S, Denize T, Garner ER, Gao X, et al. Integrative clinical and molecular characterization of translocation renal cell carcinoma. Cell Rep. 2022;38(1):110190. doi:10.1016/j.celrep.2021.110190.
- Sun G, Chen J, Liang J, Yin X, Zhang M, Yao J, He N, Armstrong CM, Zheng L, Zhang X, et al. Integrated exome and RNA sequencing of TFE3-translocation renal cell carcinoma. Nat Commun. 2021;12(1):5262. doi:10.1038/s41467-021-25618-z.
- Sui W, Matulay JT, Robins DJ, James MB, Onyeji IC, RoyChoudhury A, Wenske S, DeCastro GJ. Collecting duct carcinoma of the kidney: disease characteristics and treatment outcomes from the National Cancer Database. Urol Oncol. 2017;35: 540 e513–540 e518. doi:10.1016/j.urolonc.2017.04.010.
- Bakouny Z, Braun DA, Shukla SA, Pan W, Gao X, Hou Y, Flaifel A, Tang S, Bosma-Moody A, He MX, et al. Integrative molecular characterization of sarcomatoid and rhabdoid renal cell carcinoma. Nat Commun. 2021;12(1):808. doi:10.1038/s41467-021-21068-9.
- Iacovelli R, Ciccarese C, Bria E, Bracarda S, Porta C, Procopio G, Tortora G. Patients with sarcomatoid renal cell carcinoma – re-defining the first-line of treatment: a meta-analysis of randomised clinical trials with immune checkpoint inhibitors. Eur J Cancer. 2020;136:195–203. doi:10.1016/j.ejca.2020.06.008.
- Keskin SK, Msaouel P, Hess KR, Yu K-J, Matin SF, Sircar K, Tamboli P, Jonasch E, Wood CG, Karam JA, et al. Outcomes of patients with renal cell carcinoma and sarcomatoid dedifferentiation treated with nephrectomy and systemic therapies: comparison between the cytokine and targeted therapy eras. J Urol. 2017;198(3):530–37. doi:10.1016/j.juro.2017.04.067.
- Powles T, Albiges L, Bex A, Procopio G, Porta C, Schmidinger M, Suarez C, De Velasco G. eUpdate – renal cell carcinoma treatment recommendations. Published 2021 Sep 28.
- Pal SK, Uzzo R, Karam JA, Master VA, Donskov F, Suarez C, Albiges L, Rini B, Tomita Y, Kann AG, et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2022;400(10358):1103–16. doi:10.1016/S0140-6736(22)01658-0.
- Noguchi G, Tsutsumi S, Yasui M, Ohtake S, Umemoto S, Nakaigawa N, Yao M, Kishida T. Significant response to nivolumab for metastatic chromophobe renal cell carcinoma with sarcomatoid differentiation: a case report. BMC Urol. 2018;18(1):26. doi:10.1186/s12894-018-0339-2.
- Rouvinov K, Osyntsov L, Shaco-Levy R, Baram N, Ariad S, Mermershtain W. Rapid response to Nivolumab in a patient with Sarcomatoid transformation of chromophobe renal cell carcinoma. Clin Genitourin Cancer. 2017;15(6):e1127–30. doi:10.1016/j.clgc.2017.05.028.
- Han SY, Jahagirdar BN, Dudek AZ. Two malignancies with differential responses to immune checkpoint inhibitors: a case report. Anticancer Res. 2020;40(5):2821–26. doi:10.21873/anticanres.14255.
- Marcon J, DiNatale RG, Sanchez A, Kotecha RR, Gupta S, Kuo F, Makarov V, Sandhu A, Mano R, Silagy AW, et al. Comprehensive genomic analysis of translocation renal cell carcinoma reveals copy-number variations as drivers of disease progression. Clin Cancer Res. 2020;26(14):3629–40. doi:10.1158/1078-0432.CCR-19-3283.
- Zhao J, Dai K, Xie J, Fang C, Chen N, Dai J, Xu D. Case report: clinical complete response of advanced renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion by treated by camrelizumab and axitinib: a rare case report. Front Pharmacol. 2022;13:927299. doi:10.3389/fphar.2022.927299.
- Yan X, Zhou L, Li S, Wu X, Cui C, Chi Z, Si L, Kong Y, Tang B, Li C, et al. Systemic therapy in patients with metastatic Xp11.2 translocation renal cell carcinoma. Clin Genitourin Cancer. 2022;20(4):354–62. doi:10.1016/j.clgc.2022.03.005.
- Masago T, Kobayakawa S, Ohtani Y, Taniguchi K, Naka T, Kuroda N, Takahashi C, Isoyama T, Sejima T. Xp11.2 translocation renal cell carcinoma with TFE3 gene fusion in the elderly: case report and literature review. Int Cancer Conf J. 2020;9(4):182–86. doi:10.1007/s13691-020-00430-6.
- Oudard S, Banu E, Vieillefond A, Fournier L, Priou F, Medioni J, Banu A, Duclos B, Rolland F, Escudier B, et al. Prospective multicenter phase II study of gemcitabine plus platinum salt for metastatic collecting duct carcinoma: results of a GETUG (Groupe d’Etudes des Tumeurs Uro-Génitales) study. J Urol. 2007;177:1698–702. doi:10.1016/j.juro.2007.01.063.
- Procopio G, Sepe P, Claps M, Buti S, Colecchia M, Giannatempo P, Guadalupi V, Mariani L, Lalli L, Fucà G, et al. Cabozantinib as first-line treatment in patients with metastatic collecting duct renal cell carcinoma: results of the BONSAI trial for the Italian Network for Research in Urologic-Oncology (Meet-URO 2 Study). JAMA Oncol. 2022;8(6):910–13. doi:10.1001/jamaoncol.2022.0238.
- Mee Guillaume Z, Colomba E, Thouvenin J, Saldana C, Campedel L, Dumond C, Laguerre B, Maillet D, Vicier C, Rolland F, et al. Metastatic renal medullary and collecting duct carcinoma in the era of antiangiogenic and immune checkpoint inhibitors: a multicentric retrospective study. Cancers (Basel). 2022;14(7):1678. doi:10.3390/cancers14071678.
- Mizutani K, Horie K, Nagai S, Tsuchiya T, Saigo C, Kobayashi K, Miyazaki T, Deguchi T. Response to nivolumab in metastatic collecting duct carcinoma expressing PD‑L1: a case report. Mol Clin Oncol. 2017;7(6):988–90. doi:10.3892/mco.2017.1449.
- Yasuoka S, Hamasaki T, Kuribayashi E, Nagasawa M, Kawaguchi T, Nagashima Y, Kondo Y. Nivolumab therapy for metastatic collecting duct carcinoma after nephrectomy: a case report. Medicine. 2018;97:e13173. doi:10.1097/MD.0000000000013173.
- Rimar KJ, Meeks JJ, Kuzel TM. Anti-programmed death receptor 1 blockade induces clinical response in a patient with metastatic collecting duct carcinoma. Clin Genitourin Cancer. 2016;14:e431–34. doi:10.1016/j.clgc.2016.02.013.
- Buti S, Bersanelli M, Mazzaschi G, Cattrini C, Brunelli M, Di Maio M. Can we identify a preferred first-line strategy for sarcomatoid renal cell carcinoma? A network meta-analysis. Immunotherapy. 2022;14(2):145–53. doi:10.2217/imt-2021-0157.
- Powles T, Tomczak P, Park SH, Venugopal B, Ferguson T, Symeonides SN, Hajek J, Gurney H, Chang Y-H, Lee JL, et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(9):1133–44. doi:10.1016/S1470-2045(22)00487-9.