ABSTRACT
Active immunotherapy of cancer with therapeutic vaccines has been the subject of experimental and clinical studies for at least 50 years. Our approach has employed 1) autologous, human cancer cells because of extensive evidence that tumor rejection antigens may differ between multiple tumors of the same histology; 2) the immunopotentiating drug, cyclophosphamide; and 3) haptens, particularly dinitrophenyl. Multiple clinical trials in 455 patients with melanoma and ovarian cancer have shown that administration of haptenized vaccines at the proper dosage-schedule regularly induces T cell-mediated immunity to autologous tumor cells as measured by delayed-type hypersensitivity. Moreover, the vaccine causes changes in the tumor site suggestive of an immune reaction, including inflammation and infiltration with CD8+ T lymphocytes that are activated and produce cytokines. The T cell response is oligoclonal, and dominant Vβ families differ between patients. Studies of measurable metastases show clinically important tumor regression. Commercial development of this technology is clearly feasible.
Clinical trials of therapeutic cancer vaccines were first reported almost 50 years ago. It is important to understand that when these trials began, cellular immunology was still cowering in its Dark Age. In 1968, as a fourth-year medical student, I was assigned a paper on the thymus (then usually referred to as “thymus gland”). I could find no more than 20 references, all of them concluding that the purpose of the thymus was unknown. Until Robert Good’s landmark work,Citation1 the term “T cell” was not used. Unheard of was the differentiation of CD4+ and CD8+ T cells.Citation2 “Suppressor” cells were a phenomenon that most clinical cancer researchers viewed as witchcraft.Citation3 Dendritic cells were years away from being discovered.Citation4 There were no reliable methods for studying T cell function in vitro.
Budding, young (and all were young) clinical tumor immunotherapists were considered freaks. They were driven by the work of Richmond Prehn. In 1957, Prehn and MainCitation5 astounded the world of cancer research by reporting that inbred mice immunized with cells extracted from methylcholanthrene-induced sarcomas were completely protected from challenge with live sarcoma cells. A key observation of these experiments was that there was no cross-reactive protection between different sarcomas induced by the same carcinogen: Mice immunized with and protected from sarcoma A failed to inhibit the growth of sarcoma B and vice-versa. And so, the idea that only autologous cancer vaccines could work was born. It is remarkable that support for this model of human immunotherapy has persisted for 65 years and is verified by recent studies.Citation6
In these early days, the attention of immunotherapists was focused on melanoma. Spontaneous regression of metastatic disease occurred more commonly with melanoma than with any other cancer, i.e., 1 in 800 cases.Citation7 Injection of immunological adjuvants, e.g., bacille Calmette-Guérin (BCG), into superficial melanoma metastases was in some cases an effective treatment. Primary cutaneous melanomas often showed signs of partial regression, and this was usually associated with local vitiligo, thought to be mediated by a cellular immune response.Citation8 A secondary target was renal cell carcinoma,Citation9 but other human cancers were ignored.
My colleague, Michael J. Mastrangelo, was an early believer in immunotherapy who wandered through the desert for 40 years seeking the promised land of cancer vaccines that worked. Laucius et al.Citation10 reported patients with surgically incurable melanoma treated with a vaccine consisting of autologous, irradiated melanoma cells mixed with adjuvant BCG. Remarkably, 4/18 patients showed anti-tumor responses, partial or complete and of short duration. Our group, then working at the Fox Chase Cancer Center, sought to improve this result by adding the alkylating agent, cyclophosphamide (CY). Henry Maguire, a dermatologist turned cellular immunologist, demonstrated in 1967 that pretreatment of guinea pigs with CY augmented contact hypersensitivity to dinitrochlorobenzene, a response mediated by T cells.Citation11 Turk and ParkerCitation12 showed that over a wide range of dosages, CY could potentiate the development of cell-mediated immunity to a variety of antigens, providing that the drug was administered 1–3 days prior to injection of antigen. There are hundreds of other papers showing CY immunopotentiation of cellular immunity in experimental systems.Citation13–15
We extended these findings to humans: Administration of CY in either of two doses, 300 mg/M2 or 1000 mg/M2, 3 days before challenge with the primary antigen, keyhole limpet hemocyanin greatly augmented the acquisition of delayed type hypersensitivity (DTH) to that antigen.Citation16–18 Moreover, CY could augment the immune response to tumor-associated antigens in patients with malignant melanoma: Administration of CY 3 days before an autologous melanoma vaccine potentiated the development of DTH to autologous melanoma cells when compared to treatment with vaccine alone. Two of 9 patients treated with CY + vaccine, but none of the 10 treated with vaccine alone, exhibited complete regression of metastatic melanoma.Citation19
CY pretreatment was incorporated into all of our subsequent studies of cancer vaccines. The mechanism of immunopotentiation by CY has never been clearly delineated, and most of this work was performed before the physiology and biochemistry of regulatory T cells was understood.Citation20 In this age of specific inhibitors of regulatory activity, e.g., anti-CTLA-4 and anti-PDL1 monoclonal antibodies, does CY still have a role in vaccine regimens? Since there is no answer to this question, we have continued to use CY prior to vaccine administration in our clinical trials with the intention of adding anti-PDL1 antibodies following administration of vaccine.
In our initial study, we treated 43 patients with metastatic melanoma with an autologous, whole-cell vaccine 3 days after the administration of low dose CY, 300 mg/M2 iv.Citation19 The vaccine consisted of cryopreserved, irradiated autologous melanoma cells, obtained from metastatic masses and mixed with BCG just before intradermal injection. The CY + vaccine combination was repeated every 28 days. DTH was tested by injecting 1 × 106 irradiated melanoma cells intradermally and measuring the diameter of the induration at 48 hours. Most patients had minimal pre-treatment DTH responses to melanoma cells. After two vaccine treatments, the responses increased significantly (mean increase ± SE = 12.1 mm ±1.6 mm, p < .001), and that level of response was maintained after 4, 6 and 8 treatments. Thus, it appeared that patients receiving CY + vaccine developed DTH to undefined tumor-associated antigens. Thirty-three patients could be evaluated for anti-tumor effects. There were three complete remissions, one partial remission, and two minor responses. The toxicity of the immunotherapy program was minimal and consisted of a local inflammatory response at the site of vaccine injection and mild nausea or vomiting in 25% and 10% of patients, respectively.
DTH has been our primary measurement of T cell responses. This cutaneous response has long been known to reflect T cell-mediated immunity.Citation21 It became the standard indicator of cellular immunity to microorganisms.Citation22 The test has practical advantages: It is inexpensive to perform, rapid, and reproducible. Our subsequent work (see below) suggested that the magnitude of DTH to autologous tumor cells was strongly correlated with important clinical parameters, such as overall survival.
Haptenized cancer vaccines
History and rationale
Haptens are tiny lights that illuminate the dark recesses of the immune system. They were discovered by Karl Landsteiner, who used haptens to explore the breadth and fine sensitivity of antibody responses. LandsteinerCitation23 worked with a variety of simple chemicals, including nitrophenyls and phenyl arsonates, that were incapable of inducing an immune response by themselves, but became immunogenic when they were attached covalently to a protein carrier. He coined the term “hapten” from the Greek haptein, meaning to fasten.
Landsteiner made what was at the time an astounding observation: Rabbits immunized with a haptenized protein produced three sets of antibodies: (a) to the hapten itself, (b) to the carrier protein, and (c) to hapten–protein conjugate. The anti-hapten antibodies were highly specific in that they did not react to structurally different molecules of the same origin. For example, antibodies elicited by immunization with meta-aminobenzene sulfonate showed minimal reactivity to para-aminobenzene sulfonate and no reactivity to aminobenzene arsonate or aminobenzene carboxylate. These experiments undermined the “almost dogmatic belief … that a special chemical constitution, peculiar to proteins and not even to all of them, is necessary for the production of antibodies.”
WeigleCitation24 extended these observations to proteins that were not immunogenic in their native state. Rabbits that had been rendered tolerant to bovine serum albumin (BSA) by neonatal injections of this protein failed to produce anti-BSA antibody even after injection with Freund’s adjuvant. In contrast, unresponsive rabbits injected with BSA conjugated to sulfanilic acid (SA) produced antibody not only to SA-conjugated BSA but to native BSA as well. Thus, immunization with a hapten-modified protein could break established immunological tolerance to that protein.
Even more surprising was Weigle’s observation that hapten conjugation could break natural tolerance. The injection of rabbits with homologous thyroglobulin in incomplete Freund’s adjuvant produced, as expected, little or no antibody to thyroglobulin. However, rabbits injected with thyroglobulin that had been modified with arsanilic acid plus SA or with trinitrophenyl produced precipitating antibody to both modified and native thyroglobulin. Moreover, some of the rabbits developed histological evidence of autoimmune thyroiditis. Once the animals had been immunized, the height of their antibody titers and the severity of the thyroiditis could be increased by administering booster injections of native thyroglobulin.
Hapten modification affects T cell immunity as well. ShearerCitation25 demonstrated that cell-mediated cytotoxicity could be induced in vitro to trinitrophenyl-modified syngeneic spleen cells and that the effector T cells were directed against these “new antigenic determinants.” The targets could be trinitrophenyl-modified normal spleen cells or trinitrophenyl-modified P815 mastocytoma cells providing that the stimulator and target cells were from mice of the same strain. Thus, the T-cell response was directed to trinitrophenyl (TNP)-modified cell-surface proteins, and the response was H2 restricted.
Finally, the autoreactivity induced by hapten-modified immunogens was also T-cell-mediated. Immunization of mice with trinitrophenyl-conjugated syngeneic spleen cells resulted in the development of DTH to unmodified syngeneic lymphoblasts.Citation26 This phenomenon was H2 restricted. The stimulating moiety was contained within a low molecular weight fragment of the H2 heavy chain that was extracted from haptenized cells but did not contain the hapten – further evidence for the importance of the “new antigenic determinant.”
Chemistry
The number of synthetic compounds that can function as haptens is limited only by the imagination of the chemist. However, most of the published experiments have utilized six haptens: dinitrophenyl (DNP), TNP, arsanilic acid, SA, phosphorylcholine, and N-iodoacetyl-N’-(5-sulfonic-1-naphthyl) ethylene diamine (AED). The reason for focusing on such a small sampling of haptens is that the immunological responses appear to depend less on the structure of the hapten than on the chemistry of its conjugation to protein.
Two of the most intensely studied haptens, DNP and TNP, are attached to proteins by covalent bonding; apparently, “the DNP-NH bond in proteins is even more stable than the peptide bond.”Citation27 Other haptens, such as SA, must be introduced into proteins by a diazonium reaction, i.e., a diazonium salt is made by treatment with sodium nitrate.Citation28 DNP (and TNP) couple to the hydrophilic portions of membrane molecules that are rich in lysine and leucine, whereas the diazonium conjugates have an affinity for hydrophobic portions containing tyrosine and histidine.
Induction of autoimmunity
Haptens have been intentionally applied to develop animal models of what are presumed to be human autoimmune diseases. Neurath et al.Citation29 applied small amounts of the hapten, trinitrobenzene sulfonic acid (TNBS) (a derivative of TNP), to the rectal mucosa of mice. This resulted in the development of a chronic transmural colitis accompanied by diarrhea and weight loss that mimicked human Crohn’s disease. Histologic examination showed abundant CD4+ T cells that produced mRNA for gamma interferon in situ. Of great interest was the observation that the colitis persisted for at least 2 months, obviously long after all hapten-modified cells had been shed and cleared. Therefore, the T cells, initially induced by hapten-modified mucosa, were able to recognize and react against unidentified proteins on normal mucosa.
Augmentation of tumor immunity
The idea that haptenization could augment tumor immunity seems to have been first proposed by Mitchison,Citation30 who in 1970 discussed a number of ways of inducing “helper determinants” onto tumor cells. The most convincing experimental validation of this hypothesis remains the work of Mullen et al.Citation31 They produced two variants of the fibrosarcoma 1591—a regressor tumor that was highly immunogenic and always rejected by normal mice and a progressor tumor that had become nonimmunogenic as a result of losing a tumor rejection antigen. Surprisingly, animals that had been implanted with the progressor tumor were unable to reject even a small challenge of the regressor tumor. These progressor tumorbearing mice were not generally immunosuppressed since they generated a normal immune response to allogeneic tumors and certain other syngeneic tumors. Their immunological tolerance was tumor-specific. However, it was possible to break tolerance against the progressor tumor by haptenization. Thus, mice immunized with TNP-modified regressor tumor rejected a challenge with TNP-modified progressor tumor. Moreover, 28 days later they were able to reject a challenge with unmodified progressor tumor.Citation32 Protection against the progressor tumor could also be transferred by splenic T cells obtained from TNP-regressor-immunized mice.
Before and after this work was published, a number of other investigators demonstrated that modification of tumor cells with DNP or TNP increased the efficacy of vaccines. For example, Cavallo and ForniCitation33 found that mice immunized with DNP-modified mammary adenocarcinoma cells exhibited delayed tumor appearance and slower tumor growth after challenge with unmodified tumor cells. Galili et al.Citation34 performed a similar experiment with a virally induced lymphoma with more impressive results: Immunization with TNP-modified tumor appeared to be effective in increasing the percentage of long-term survivors even in animals in whom the unmodified tumor was nonimmunogenic. Fujiwara et al.Citation35 took a somewhat different approach. They found that a fairly large (8-mm diameter) plasmacytoma could be made to regress completely by intratumoral injection of TNCB, provided that the mice had been previously sensitized by topical application of TNCB. As a result, these animals became resistant to challenges with unmodified tumor cells. This immunotherapy was particularly interesting because it worked not only with a transplantable tumor, but also with an autochthonous tumor that was induced in the mice by inoculation of methylcholanthrene.
Finally, Sojka et al.Citation36 used a highly metastatic 410.4 tumor that had originated from a spontaneous murine mammary carcinoma. The tumor was injected into the mammary fat pad and was allowed to grow to 6 mm to 8 mm diameter and then excised. Following surgery, mice were treated with multiple injections of a vaccine consisting of irradiated tumor cells haptenized with DNP and then mixed with BCG. Low-dose CY was administered 3 d prior to each vaccine injection. Control mice received the identical treatment regimen except that the tumor cells in the vaccine were irradiated but not hapten modified. These experimental conditions were designed to mimic the postsurgical adjuvant protocols frequently used in clinical vaccine studies, and, specifically, to experimentally reproduce our observations in melanoma patients, which are described below. The result was positive and highly reproducible: mice that received DNP-modified vaccine had significantly longer relapse-free survival than animals receiving the unmodified vaccine, which, incidentally, was no better than saline. The protective effect of the haptenized vaccine was dependent on both CD4+ and CD8+ T cells. Moreover, both gamma interferon and tumor necrosis factor were essential since in vivo depletion of either with a monoclonal antibody abrogated the protective effect.
Autologous haptenized vaccine in human melanoma
Rationale
Our clinical studies were stimulated by the promising results that had been reported in animal models up to that time and by the seminal observations on hapten immunology made over the prior 50 years that have been described above. We reasoned that the failure of human cancer vaccines to immunize cancer-bearing patients was due to immunological tolerance to one or many rejection antigens. Since haptenization could break tolerance even to normal cells, it might be able to make tumor antigens immunogenic enough to see clinical effects.
All of our work has been performed with an autologous vaccine because of the large body of experimental work supporting this approach described above. In addition, all of our protocols have incorporated low-dose CY to take advantage of its ability to potentiate cell-mediated immune responses.
Finally, we chose DNP as the hapten because of its long record of clinical use without significant toxicity. Most normal subjects and the majority of cancer-bearing patients can be sensitized by topical application of dinitrochlorobenzene (DNCB), and their PBL proliferate in response to DNP-modified autologous lymphocytes.Citation37 Moreover, DNP is easy to conjugate to tumor cells since it does not require chemical modification. All of the studies described here and below were approved by the Institutional Review Board of Thomas Jefferson University, and informed consent was obtained from all patients.
Preparation and administration of the vaccine
Metastatic tumor was excised, maintained at 4°C, and delivered to the laboratory within 48 hours of excision. Tumor cells were extracted by enzymatic dissociation with collagenase, aliquoted, frozen in a controlled-rate freezer, and stored in liquid nitrogen in a medium containing human albumin and 10% dimethyl sulfoxide until needed. On the day that a patient was to be treated, an aliquot of cells was thawed, washed, and irradiated to 2500 cGy. The cells were then washed again and modified with DNP by the method of Miller and Claman.Citation38 This involved a 30-min incubation of tumor cells with DNFB, followed by washing with saline. After washing, the cells were counted, suspended in 0.2-mL Hanks solution with human albumin, and maintained at 4°C until administered.
Just prior to the injection, 0.1 mL of Tice BCG was added to the vaccine. The mixture was then drawn up into a 1-mL syringe and injected intradermally, usually into the upper arm. We chose BCG because it is a potent adjuvant and an approved drug as well. BCG has been continuously used as an adjuvant throughout the clinical testing of our technologies. To minimize the local reaction to BCG, the dose was progressively attenuated 10-fold through a series of vaccine administrations. The initial BCG dose was a 1:10 dilution (1–8 × 106 CFU) and the subsequent BCG doses were a 1:100 dilution (1–8 × 105 CFU) and a 1:1000 dilution (1–8 × 104 CFU).
The animal tumor models prompted us to pre-sensitize patients with DNFB by topical application of a 1% solution in acetone-corn oil, and this was done in the initial studies. Subsequently, the presensitization was found to be unnecessary (but not deleterious) for the induction of maximum DTH to autologous melanoma cells. All of the DNP-vaccine injections were given into one area, usually on the dorsal, upper arm that had not been subjected to a lymph node dissection. Five vaccine dosage schedules have been tested; the optimal and most convenient schedule was one or two series of six weekly DNP-modified vaccines. A single dose of CY 300 mg/M2 iv was administered 3 days before the first vaccine.
provides a summary of the patient populations treated with the DNP-modified autologous vaccine by our group at Thomas Jefferson University. The observations of immunological and clinical responses described below were performed in the melanoma subjects. The results obtained for ovarian carcinoma will be presented in a separate section.
Table 1. Summary of patients treated with DNP-modified autologous vaccine.
Toxicity of DNP-modified autologous vaccine
In most patients, the toxicity was limited to the reaction at the vaccine injection site. All patients developed pruritic papules that progressed to pustules, sometimes with small ulcerations, which resolved into small white or pink scars. The intensity of the local reactions was ameliorated by reducing the dose of BCG. As expected, about one-third of patients developed nausea, 10% with vomiting, following the administration of CY, but systemic toxicity caused by the vaccine was uncommon: Less than 5% of patients noted fever or chills following vaccine administration, and no patient experienced a decrease in performance status. One patient developed generalized urticaria 15 min after injection of her fourth dose of DNP-vaccine; this was treated with an antihistamine and resolved in 5 days without sequelae. Three patients developed erythema around their lymphadenectomy sites following vaccine administration, which was asymptomatic and spontaneously abated. Skin biopsy of one of these patients showed a nonspecific vasculitis. There were no significant changes in blood counts or routine serum chemistries. We observed no clinical evidence of autoimmunity. Specifically, no patients developed vitiligo following treatment.
Delayed-type hypersensitivity responses
Patients were tested for DTH by modification of standard methodology.Citation19 Cryopreserved melanoma cell suspensions and PBL were thawed, washed, and irradiated (2500 cGy). DNP modification of melanoma cells and PBL was performed as described above. Melanoma cells (1 × 106) and PBL (3 × 106), each either DNP modified or unmodified, were suspended in Hanks balanced salt solution without serum, phenol red, or antibiotics and injected intradermally into the ventral forearm. The mean diameter of induration was measured after 48 h. A positive response was defined as maximum diameter of induration ≥5 mm. Patients were also skin tested with intermediate strength purified protein derivative (PPD) (5 TU). DTH testing was performed before the treatment program was initiated and at various times post-treatment. Analyses were performed by determining the maximum DTH response exhibited by each patient to each of the test reagents.
Pretreatment-positive DTH responses to autologous melanoma cells, either DNP modified or unmodified, were observed in 8% of patients and were generally small (median = 6 mm, range = 5–18 mm).
A summary of the DTH responses observed following administration of DNP-vaccine is shown in . The responses of patients with measurable metastases were similar to those in the postsurgical adjuvant group (resection of clinically evident disease). Almost all patients developed positive responses to DNP-modified autologous melanoma cells and to PPD; these responses were usually at least 10 mm diameter. Responses to unmodified autologous tumor cells were induced only in a subset of these patients and were smaller (usually 5–10 mm diameter).
Table 2. Delayed-type hypersensitivity responses following DNP-vaccine administration.
Following vaccine treatment, five patients (2%) exhibited a small (5–6 mm) DTH response to autologous, unmodified PBL after treatment. This could have represented a T-cell response against normal-tissue antigens but is more likely to be artifactual since no other manifestations of autoimmunity were observed.
These results may be interpreted as follows: (a) Modification of melanoma cells with DNP produces “new antigenic determinants” that induce strong and consistent cell-mediated immune responses, even in cancer-bearing subjects. (b) Evidently, these responses are sometimes associated with cell-mediated immunity against one or more determinants on unmodified melanoma cells. Such determinants appear to be melanoma-associated since they are not present on normal lymphocytes. This interpretation is consistent with the large body of work on hapten responses in experimental systems that preceded it.
Corresponding in vitro T-cell response to DNP-modified cells
The agreement between our results and the animal studies is strengthened by experiments that we performed with T cells obtained from DNP-vaccine-treated subjects. PBL obtained from 8 of 11 patients proliferated, and in 5 of 11 produced gamma interferon, upon stimulation with DNP-modified autologous lymphocytes or melanoma cells. The response was human leukocyte antigen (HLA) restricted and hapten restricted, since no responses were elicited by unmatched DNP-modified allogeneic cells or by TNP-modified autologous cells. Finally, a CD8+ cell line obtained from one of these responding PBL killed DNP-modified autologous melanoma cells but was not cytotoxic for DNP-modified allogeneic tumor cells.Citation39
Furthermore, we were able to identify MHC-associated, DNP-modified peptides that reproduced these responses.Citation40 Peptides, extracted from DNP-modified melanoma cells by an acid elution technique and fractionated by high-performance liquid chromatography (HPLC), were loaded onto autologous B lymphoblasts, which were tested as stimulators to the cell line described above. Surprisingly, the stimulatory capacity of peptides was confined to a single HPLC fraction; spectrophotometric analysis of this fraction showed that the peptides were conjugated to DNP. Perhaps, this peak represented a family of peptides correspon ding with Weltzien’s dominant peptides with the hapten-modified lysine in position 4.
Clinical importance of DTH responses
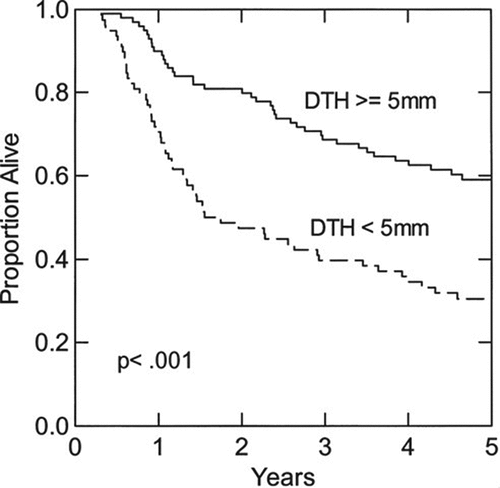
An important observation that has been sustained over the course of these studies is the association of a positive DTH response to unmodified, autologous melanoma cells with prolonged survival. Thus, in the measurable metastases group, the survival of patients who developed a positive DTH to unmodified tumor cells was significantly longer than the survival of those who did not: 16.5 months vs 8.4 months, respectively (p = .023, log-rank test).Citation41 In the postsurgical adjuvant group,Citation42 the development of a positive response to unmodified tumor cells was associated with significantly greater 5-yr survival (p < .001, log-rank test) (). This effect remained significant in a multivariate analysis that included important clinical prognostic variables, such as the number of positive lymph nodes. In contrast, the magnitude of DTH response to DNP-modified autologous melanoma cells had no significant impact on survival.
Figure 1. Effect of DTH to unmodified autologous melanoma cells on overall survival. Patients had clinical stage III melanoma and were disease-free following lymphadenectomy. The overall survival of patients who developed DTH ≥5 mm induration (solid line, N = 99) is compared with that of patients whose maximum DTH response was <5 mm (dashed line, N = 78). Difference between the curves: p < .001, log-rank test. Reprinted fromCitation41 with permission.
T-cell responses at the tumor site
A rather surprising observation was made early into the first clinical trials of DNP-modified autologous vaccine: the development of inflammatory responses at metastatic sites.Citation43 These responses were initially observed in superficial (nodal or subcutaneous) metastases, and consisted of marked erythema, warmth, and tenderness of the tumors and the overlying skin. Responding metastatic lesions varied in size from 5 mm diameter skin metastases to 10 cm lymph node masses. The number of inflamed tumors on a single patient ranged from 1 to >100. In some patients who had multiple superficial metastases, the inflammatory response involved all of the observable lesions, whereas others had inflammation in 25–75% of their visible tumors. The time from the beginning of vaccine treatment to an observable inflammatory response was fairly long—2–4 months.
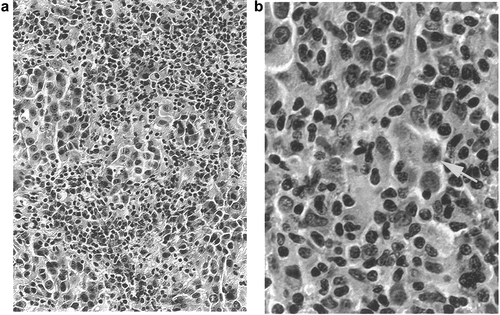
Biopsy of superficial metastases excised following treatment with DNP-vaccine showed a striking histologic change: the tumors had become infiltrated with T lymphocytes.Citation44 Such an infiltration is not observed in subcutaneous metastases obtained from patients prior to immunotherapy; only occasionally were significant numbers of lymphocytes identified, and they were usually limited to perivascular areas. The histology is illustrated in .
Figure 2. Photomicrograph showing inflammatory response in subcutaneous metastasis. The clinically inflamed tumor was excised 5 months after beginning treatment with DNP-vaccine. (a) (200×) the tumor tissue is intensely infiltrated with lymphocytes. (b) (600×) Necrosis of individual tumor cells is apparent (arrow).
We quantified the inflammatory responses by flow cytometric analysis of T lymphocytes in cell suspensions made from subcutaneous metastases excised before and after DNP-vaccine treatment. The median number of T cells in 15 post-vaccine tumors was 41%, with some tumors containing more than 50% T cells. In contrast, in subcutaneous metastases excised without prior immunotherapy, T cells were sparse (median = 9%), and significantly less frequent than in post-DNP-vaccine-treated tumors. Lymphocytes other than T cells were rare; the mean proportion of CD19(+) B cells and CD56(+) natural killer cells in tumor suspensions was 1% and 4%, respectively. T cells were predominantly CD8(+); the mean CD8/CD4 ratio was 5.0 vs 1.0 in matched PBL. Infiltrating T cells strongly expressed CD69, an early marker of T-cell activation.Citation45
The production of cytokines by lymphocytes infiltrating the metastases was studied by analyzing the tissues using a standard RT-PCR technique.Citation46 Post-vaccine, inflamed biopsies contained messenger RNA for gamma interferon (five of eight), IL-4 (four of eight), or both (three of eight), and for tumor necrosis factor (TNF) (four of seven). In contrast, gamma interferon mRNA was detected in only 1 of 17 and TNF mRNA in 2 of 16 control specimens (pretreatment lymph node metastases or noninflamed subcutaneous metastases). Since gamma interferon and TNF are critical cytokines in the initiation and perpetuation of cell-mediated immune responses, their presence within these tumors has biological significance.
Clonal expansion of T cells at the tumor site
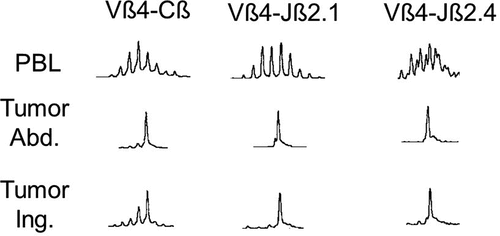
We investigated the rearrangement of the TCR beta chain (TCR-Vβ) as well as the junctional diversity in T cells infiltrating melanoma metastases following treatment with DNP-vaccine.Citation47,Citation48 In 19 of 26 control specimens, V-D-J length analysis showed the expected polyclonal patterns. In contrast, post-vaccine tumors from 9 of 10 patients showed dominant peaks of V-D-J junction size in one or more Vβ families. Dominant peaks were seen most frequently in six Vβ families (Vβ7, 12, 13, 14, 16, 23) and were never seen in seven others. Further analysis of the oligoclonal Vβ products showed dominant peaks in the J-region as well. Of particular interest was the finding that Vβ and Jβ peaks were similar in inflamed metastases obtained at different times or from different sites from the same patient. An example of the V-D-J size distribution histograms for one patient (20297) is shown in . The dominant peaks seen in the post-vaccine tumors do not appear to be present in the matched PBL.
Figure 3. Examples of histograms of V-D-J junction size distributions from two inflamed metastases excised from one patient. Amplification with Vβ primers revealed a clonal peak in Vβ-4 for both abdominal (Abd) and inguinal (Ing) metastases, both of which became inflamed after treatment of the patient with DNP-vaccine, but a polyclonal distribution in matched PBLs. Amplification with Jβ primers showed clonal peaks in Jβ-2.1 and 2.4 for both tumors but not in PBLs. Reprinted fromCitation47 with permission.
Finally, the amplified PCR products from seven of these specimens were cloned and sequenced and the amino acid sequence of the CDR3 deduced. In six of seven specimens, the same CDR3 sequence was repeated in at least two clones and, in five of seven in at least three clones. That these novel clones are functional was shown by isolating T-cell lines from two infiltrated skin metastases by enrichment for TCR-Vβ14 T cells. This line displayed HLA-class I–restricted lysis of the autologous melanoma cells.Citation47
These results indicate that vaccination with autologous, DNP-modified melanoma cells can expand selected clones of T cells that migrate to the tumor site that were not detectable prior to immunization. Moreover, such clones are potentially destructive to the tumor. This is an observation that has not been made with other human tumor vaccines.
Antitumor activity – melanoma
Patients with measurable metastases
We have reported the results of a series of clinical trials of patients with surgically incurable metastatic melanoma with measurable metastases;Citation41 there were 97 patients, of whom 83 were evaluable. Among the 83 evaluable patients, there were 11 responses—2 complete, 4 partial, and 5 mixed; 2 patients were judged to have stable disease. Both complete responses and two of the four partial responses occurred in patients with lung metastases. Response durations were as follows: partial responses— 5, 6, 8, and 47+ months; complete responses—12, 29 months. Two examples of antitumor responses are described below:
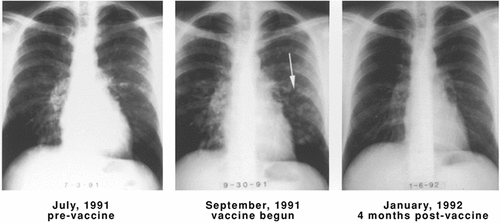
Patient #20063 (CR), a 28-yr-old man, developed multiple bilateral lung metastases shortly after tumor tissue had been obtained from a regional lymph node metastasis (, first panel). These metastases increased in size and number just prior to the start of DNP-vaccine treatment (, second panel). After a course of DNP-vaccine administration, the appearance of the metastatic nodules was unchanged (not shown). However, 2 months later, the metastases had completely regressed (, third panel). The patient remained tumor-free until 1 year later when mediastinal and hilar recurrence was noted. His survival from beginning of DNP-vaccine treatment was 34.5 months.
Figure 4. Regression of lung metastases in patient #20063 after administration of DNP-modified, autologous vaccine. Multiple lung metastases are seen in July 1991, most prominently in the left lower lobe, which increased in size and number in the September 1991 X-ray. Four months after beginning DNP-vaccine treatment (January 1992), the metastases had completely regressed. Reprinted fromCitation40 with permission.
Patient #20254 (CR), a 77-yr-old man, presented simultaneously with a regional lymph node metastasis in the neck and 2-cm diameter mass in the lung adjacent to the cardiac border that increased in size over 2 months of observation. At 5 months after beginning DNP-vaccine treatment, the same mass was thought to be slightly smaller. The mass continued to slowly regress and by the 2-year point it had regressed completely. After a remission of 29 months, the patient developed recurrent melanoma in the anterior mediastinum, which was incompletely resected. His overall survival was 48.4 months.
These examples of regression of lung metastases are noteworthy because the regressions developed slowly and only after a latent period. Evidence of response required at least 4 months, and in two of these cases, maximum regression required at least 1 year. Patients who had an antitumor response (complete, partial, or mixed) had a significantly longer survival time – about 2.5 times that of nonresponders (p < .01, log-rank test and by multivariate analysis).
Postsurgical adjuvant patients
We hypothesized that the ability of autologous DNP-vaccine to produce antitumor effects was limited by the large tumor burden of patients with clinically evident metastases. Therefore, it seemed reasonable to test the vaccine in a setting in which the tumor burden was much lower. Patients with bulky but resectable regional lymph node metastases constitute an ideal group since the metastatic masses provide a source of cells for preparing vaccine, but the postsurgical tumor burden is below the level of clinical detection.
Therefore, we conducted a series of trials in this patient population. All of the study subjects had regional lymph node metastases with a palpable mass of at least 3 cm diameter. They were treated by standard lymphadenectomy, and tumor cells were extracted and cryopreserved from the nodal tissue. To be eligible, the patients had to be tumor-free by postoperative evaluation, and the vaccine treatments had to be started within a month of surgery.
Of 214 patients participating in the trials, 20 had in-transit metastases as well, and 40 had clinically evident metastases to two nodal sites. The 5-year overall survival rate of the 214 patients participating was 44%.Citation42 As noted above, the development of a positive DTH response to autologous, unmodified melanoma cells was a highly significant determinant of overall survival, even in a multivariate analysis. Unexpectedly, 5-year post-relapse survival was also significantly greater in patients who had developed a positive DTH response to autologous, unmodified melanoma cells after the original vaccine treatment (positive DTH, 25.2%; negative DTH, 12.3%; p < .001, log-rank test). This effect remained significant in a multivariate analysis that included the other significant variables (hazard ratio for positive vs. negative DTH = 0.488; range, 0.274 to 0.869; p = .015). This finding can be at least partially explained by the observation that patients with positive DTH to unmodified melanoma cells had a significantly higher likelihood of experiencing relapse with tumors that were resectable (positive DTH, 62% resectable; negative DTH, 41% resectable; p = .022, Fisher’s exact test). Post relapse survival was not affected by the magnitude of the DTH response to DNP-modified autologous tumor cells.
Timing of an “Induction Dose”: In these studies, four dosage schedules were tested;Citation42 two schedules (referred to as A and D) were associated with significantly lower overall survival than the other two (referred to as B and C). Because the development of positive DTH to unmodified melanoma cells was very important to clinical outcome, we performed retrospective analyses to explain this observation. All four dosage schedules included the following procedures: 1) baseline skin testing performed by intradermal injection of 1 × 106 irradiated, autologous, DNP-modified tumor cells without BCG into the ventral forearm; 2) administration of low-dose CY; and 3) multiple intradermal injections of DNP-modified melanoma cells mixed with BCG beginning 3 days after CY administration. One difference between schedules was the timing of the baseline skin tests, either 3–8 days before CY administration or the same day or one day later. This key DTH response was significantly larger in patients who had received baseline skin testing on day −3 to −8 than in those who had been skin-tested on day 0 or day+1 (p < .001). The percentage of patients who developed a positive DTH was as follows: day −3 to −8, 73%; day 0 or+1, 20% (p < .01; Fisher’s exact test). The difference in timing of the baseline skin tests explains the differential. The timing of baseline skin testing had no significant effects on the development of DTH to DNP-modified melanoma cells or to PPD.
Surprisingly, this variable influenced survival as well. Five-year overall survival was 51% in the group that had baseline skin testing on day −3 to −8 versus 33% in the group with baseline skin testing on days 0 or +1 (p = .007) (). A similar dichotomy was observed for 5-year relapse-free survival (40% v 22%, respectively; p = .005). This effect remained significant in a multivariate analysis that included the known prognostic variables: number of positive nodes, presence of extranodal extension, and sex.
Figure 5. Overall survival of patients with stage III melanoma treated with autologous, dinitrophenyl-modified vaccine stratified by timing of the baseline skin test (induction dose, ID). Reprinted fromCitation41 with permission.
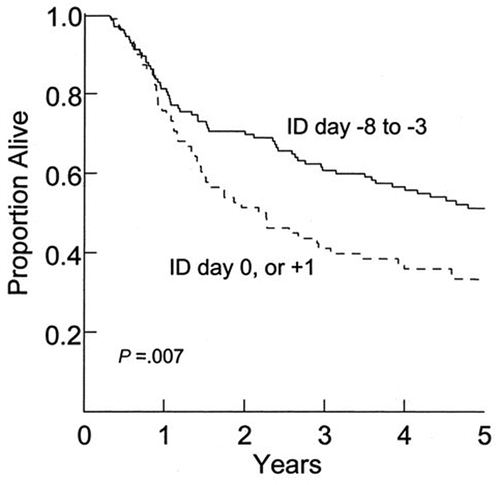
These data indicate that what we have considered merely a baseline skin test may serve as an induction dose of vaccine. The timing of the induction dose relative to the administration of CY apparently determines whether the subsequent course of DNP vaccine results in tumor immunity or unresponsiveness.
Summary of clinical studies in melanoma
It appears that autologous, DNP-modified melanoma vaccine has clinical activity. Well-documented regression of metastatic disease has been demonstrated, and overall survival in resected advanced stage III patients looks promising.
Equally important are insights into what we call the “immunopharmacology” of this vaccine that were provided by analysis of the clinical trials. It seems clear that DTH is an important measure of the T cell response to autologous melanoma cells induced by DNP-vaccine. DTH is strongly predictive of positive clinical outcomes. Also, analysis of DTH responses has provided an understanding of the impact of dosage-schedule on effectiveness. The induction dose phenomenon might be applicable to other cancer vaccines, particularly when immunomodulators, such as CY and checkpoint inhibitors, are co-administered. Cancer vaccines, regardless of their composition or technical sophistication, are drugs to which pharmacological principles apply.
Autologous haptenized vaccine in ovarian cancer
There are no practical impediments to trials of the DNP-modified, autologous vaccine in other cancers. However, given melanoma’s reputation as being particularly immunogenic among human malignancies, some would speculate that the applicability of this immunological trick would be limited.
We have conducted two phase I-II trials of autologous, DNP-modified vaccine in patients with advanced ovarian cancer. In the first trial, 13 evaluable patients with bulky, chemotherapy-refractory disease and 9 who had undergone complete resection were treated. Positive DTH to autologous DNP-modified tumor cells was elicited in 19 of 22 patients (median = 14 mm induration). More importantly, DTH to unmodified tumor cells was induced in 17 of 22 patients (median = 6 mm). DTH to autologous DNP-modified lymphocytes and to autologous unmodified lymphocytes was observed in only 6 of 22 and 0 of 22 patients, respectively.
Unexpectedly, a clinical response was observed as well: one patient, who had previously had excision of a peritoneal tumor, exhibited complete regression of a residual peritoneal mass by computed tomography and a concomitant fall in serum CA-125 from 65 to 6. Both the CT and CA-125 responses were maintained for 6 months.
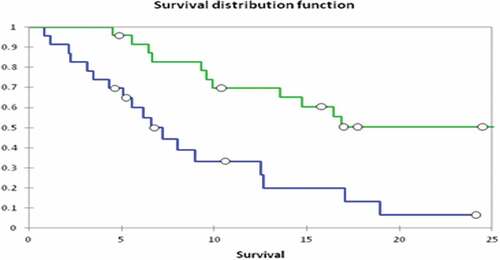
In a second study,Citation49 26 subjects with recurrent platinum resistant ovarian cancer were enrolled. Each was randomly assigned using a double-blind method to receive one of three dose levels of DNP modified cells on a 6-month schedule. Vaccine was prepared for, but not administered to, 25 additional subjects for reasons including undergoing splenectomy during debulking, subject anxiety and request for additional chemotherapy before vaccine availability, progression before vaccine availability, post-op complications precluding study, physician choice, and others. All toxicities were grade 1–2 except for six grade 3 injection site reactions. In six vaccinated subjects, CA-125 levels became normal following surgery plus vaccine and remained normal throughout the 9-month duration of the protocol. Median overall survival by Kaplan – Meier method after surgery in the vaccinated group (n = 24, 2 lost to follow up) was 25.4 months compared to 6.5 months in the vaccine-prepared but not administered group ().
Bihaptenized cancer vaccine
The idea that modifying a cell with two haptens may make it more immunogenic than a single haptenization comes from the work of Weigle.Citation50 He found that rabbit thyroglobulin modified with two haptens induced a much higher titer antibody response against native thyroglobulin than thyroglobulin modified with only one of those haptens. Since DNP modifies hydrophilic residues of MHC-bound peptides (mainly ε-amino groups of lysine),Citation51 it seems logical that the second hapten should conjugate hydrophobic residues (mainly tyrosine and histidine). Haptens in that category, which bind proteins through an azo linkage, include SA, arsanilic acid, and phosphorylcholine.
It is known that immunization of mice with syngeneic lymphocytes modified with arsanilic acid induces strong T cell responses against those modified cells, including DTH and cytolytic T cells.Citation28 Obviously, the administration of even minute amounts of arsanilic acid into human is not acceptable, but SA, a nontoxic compound in small amounts, should have the same immunological effect. Both compounds bind to tyrosine and histidine after being diazotized by treatment with sodium nitrite. Moreover, immunization of animals with SA-modified protein can induce autoimmunity.Citation24
We have developed a second-generation autologous tumor vaccine in which half the cells are modified with DNP and half are modified with a second hapten, SA. Moreover, the bihaptenized vaccines are fixed with a low concentration of ethanol and frozen so that all the vaccines required for a course of treatment can be made at the same time.
Melanoma cells were thawed and divided into two aliquots. Half were modified with DNP by our standard method. The other half were modified with SA by a method adapted from established techniques.Citation52
Patients with stage IV melanoma were administered the vaccine at one of three dosage levels (0.05 × 106, 0.50 × 106, or 5.0 × 106 cells/dose) using a previously determined optimal schedule of administration.Citation53 Before and after a 10-week course of vaccine, patients were tested for DTH to autologous melanoma cells, hapten-modified and unmodified, and to control materials.
Twenty-three patients were studied. No serious adverse events causally related to the vaccine were observed. At the highest dose tested, the bihaptenized vaccine induced significant DTH responses: DNP-modified melanoma cells = 8/10, SA-modified melanoma cells = 8/10, unmodified melanoma cells = 6/10.
Three patients had documented tumor regression, one partial response and two mixed responses. One response was in pelvic lymph nodes and the other two were subcutaneous metastases. Of eight patients who had all metastatic tumor resected prior to vaccine, seven remained free of melanoma.
Preparation of an autologous, bihaptenized cancer vaccine is feasible and administration appears to be safe with signs of effectiveness.
Commercialization of autologous, haptenized vaccines
In 1997, Thomas Jefferson University licensed the DNP-modified vaccine technology to a startup company, AVAX Technologies. During the 15 years of its existence, AVAX was successful in working with the FDA to develop several company-sponsored INDs. That accomplishment required development of the vaccine manufacturing process to GMP specifications and to the point at which commercialization of the product was feasible. AVAX demonstrated that the optimal dosage range for each vaccine administration was 12 ± 8 × 106 tumor cells.Citation54 It was also determined that eight vaccine injections should be administered over a six-month period, requiring a minimum of 32 × 10Citation6 tumor cells. Since tumor cells were also needed for quality control testing and considering the loss of tumor cells in the manufacturing process, the minimum number of tumor cells was estimated to be 100 × 106. To obtain that number by mechanical dissociation it was necessary to start with a tumor mass of at least 2.5 cm diameter.
AVAX completed two clinical trials of the DNP-modified melanoma vaccines.Citation55 The first was a post-surgical adjuvant study in patients with stage IIIB and IIIC melanoma. Subjects were randomized at a 2:1 ratio to receive MVAX® or high-dose alpha interferon, the treatment of choice at that time. The trial had to be closed after 10% accrual, because some vaccine batches were found to be bacterially contaminated. Interestingly, the most common contaminant was the anaerobic skin contaminant, Propionibacterium acnes, probably acquired from surgical skin flaps. To address this problem, a frozen version of MVAX® was developed by combining the tumor dissociation and vaccine production and freezing the final product, giving it a shelf life measured in months rather than hours. All quality control tests could be completed before each batch of vaccine was released; batches that were not sterile were discarded.
The new formulation was considered by the FDA to be a new product that required a safety trial.Citation56 Accordingly, 64 patients with surgically incurable melanoma were assigned to receive one of four vaccine dosage groups (0, 0.5, 2.5, and 5.0. 106). The readout was DTH to DNP-modified and unmodified, autologous melanoma cells. Only the high-dose arm (5 × 106 melanoma cells per dose) was positive. After a preplanned extension of that arm, 23/30 (77%) of those patients developed (+) DTH to autologous DNP-modified melanoma cells. Moreover, 9/16 tested (56%) developed (+) DTH to autologous unmodified melanoma cells. These results are indistinguishable from the DTH results obtained with “fresh” vaccine cells in previous studies. Importantly, there were no safety issues with frozen MVAX®.
A randomized, control study was begun with the improved technology. However, the study had barely been started when AVAX experienced financial difficulty and had to discontinue the trial.
In 2018, Biovaxys Technology Corp was established. The primary technology of the company is an autologous bihaptenized ovarian cancer vaccine (BVX-0918). The target patient population is patients with advanced, chemotherapy-resistant ovarian cancer. The vaccine will be composed of autologous tumor cells obtained from debulking surgery that are bihaptenized, as described above. A phase I study to begin next year will determine safety, but survival and CA-125 levels also will be monitored. Biovaxys intends to co-develop the vaccine with one of the immune checkpoint inhibitors because of the proven ability of these biologic drugs to enhance immune responses.Citation57
Epilogue
Commercialization of the autologous, bihaptenized ovarian cancer vaccine would be welcomed by the community of patients who have no highly effective, safe treatment for their disease. As there is no obvious reason the technology could not be applied to the treatment of other human cancers, Biovaxys is applying the technology to colon and cervical cancers. Another major goal of Biovaxys is to use T lymphocytes obtained from patients successfully treated with its vaccines as probes for discovering new tumor antigens that are relevant to tumor rejection. Then, the dream of personalized, but off-the-shelf, therapeutic cancer vaccines will have been realized.
Abbreviations
| BCG | = | bacille Calmette-Guérin |
| BSA | = | bovine serum albumin |
| CY | = | cyclophosphamide |
| DNP | = | dinitrophenyl |
| DTH | = | delayed type hypersensitivity |
| PBL | = | peripheral blood lymphocytes |
| SA | = | sulfanilic acid |
| TNP | = | trinitrophenyl |
Disclosure statement
I am Chief Medical Officer and a stockholder in Biovaxys Technology Corp.
Additional information
Funding
References
- Good RA, Cain WA. Relationship between thymus-dependent cells and humoral immunity. Nature. 1970;226(5252):1256–12. doi:10.1038/2261256a0.
- Chess L, Schlossman SF. Functional analysis of distinct human T-cell subsets bearing unique differentiation antigens. In: Stutman O, editor. Contemporary topics in immunobiology. Vol. 7. Boston (MA): Springer; 1977. p. 363–80.
- Gershon RK. T cell control of antibody production. Contemp Top Mol Immunol. 1974;3:1–40.
- Steinman RM, Kaplan G, Witmer MD, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979;149:1–16. doi:10.1084/jem.149.1.1.
- Prehn RT, Main JM. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957;18:769–78.
- Foy SP, Jacoby K, Bota DA, Hunter T, Pan Z, Stawiski E, Ma Y, Lu W, Peng S, Wang CL, et al. Non-viral precision T cell receptor replacement for personalized cell therapy. Nature. 2022. doi:10.1038/s41586-022-05531-1.
- Everson TC, Cole WH. Spontaneous regression of cancer. Philadelphia (PA): Saunders; 1966.
- Bodurtha AJ, Berkelhammer J, Kim YH, Laucius JF, Mastrangelo MJ. A clinical, histologic, and immunologic study of a case of metastatic malignant melanoma undergoing spontaneous remission. Cancer. 1976;37:735–42. doi:10.1002/1097-0142(197602)37:2<735:AID-CNCR2820370221>3.0.CO;2-Z.
- Tykka H, Hjelt L, Oravisto KJ, Turunen M, Tallberg T. Disappearance of lung metastases during immunotherapy in five patients suffering from renal carcinoma. Scand J Resp Dis. 1974;89(Suppl):123–34.
- Laucius JF, Bodurtha AJ, Mastrangelo MJ, Bellet RE. A phase II study of autologous irradiated tumor cells plus BCG in patients with metastatic malignant melanoma. Cancer. 1977;40:2091–93. doi:10.1002/1097-0142(197711)40:5<2091:AID-CNCR2820400517>3.0.CO;2-H.
- Maguire HC Jr., Ettore VL. Enhancement of dinitrochlorobenzene (DNCB) contact sensitization by cyclophosphamide in the guinea pig. J Invest Dermatol. 1967;48:39–42. doi:10.1038/jid.1967.6.
- Turk JL, Parker D. Effect of cyclophosphamide on immunological control mechanisms. Immunol Rev. 1982;65:99–113. doi:10.1111/j.1600-065X.1982.tb00429.x.
- Hengst JCD, Mokyr MB, Dray S. Importance of timing in cyclophosphamide therapy of MOPC- 315 tumor- bearing mice. Cancer Res. 1980;40:2135–41.
- North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;55:1063–74. doi:10.1084/jem.155.4.1063.
- Taieb J, Chaput N, Schartz N, Roux S, Novault S, Menard C, Ghiringhelli F, Terme M, Carpentier AF, Darrasse-Jése, et al. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J Immunol. 2006;176:2722–29. doi:10.4049/jimmunol.176.5.2722.
- Berd D, Mastrangelo MJ, Engstrom PF, Paul A, Maguire H. Augmentation of the human immune response by cyclophosphamide. Cancer Res. 1982;42:4862–66.
- Berd D, Maguire HC Jr, Mastrangelo MJ. Potentiation of human cell-mediated and humoral immunity by low-dose cyclophosphamide. Cancer Res. 1984;44:5439–43.
- Berd D, Mastrangelo MJ. Active immunotherapy of human melanoma exploiting the immunopotentiating effects of cyclophosphamide. Cancer Invest. 1988;6:337–49. doi:10.3109/07357908809080657.
- Berd D, Maguire HC Jr, Mastrangelo MJ. Induction of cell-mediated immunity to autologous melanoma cells and regression of metastases after treatment with a melanoma cell vaccine preceded by cyclophosphamide. Cancer Res. 1986;46:2572–77.
- Greenwald RJ, Latchman YE, Sharpe AH. Negative co-receptors on lymphocytes. Curr Opin Immunol. 2002;14:391–96. doi:10.1016/S0952-7915(02)00341-2.
- Landsteiner K, Chase MW. Experiments on transfer of cutaneous sensitivity to simple compounds. Proc Soc Exp Biol Med. 1942;49:688–90. doi:10.3181/00379727-49-13670.
- Enders JF, Kane LW, Maris EP, Stokes J Jr. Immunity in mumps: v.The correlation of the presence of dermal hypersensitivity and resistance to mumps. J Exp Med. 1946;84:341–64. doi:10.1084/jem.84.4.341.
- Landsteiner K, van der Scheer J. On cross reactions of immune sera to azoproteins. J Exp Med. 1936;63:325–39. doi:10.1084/jem.63.3.325.
- Weigle WO. The production of thyroiditis and antibody following injection of unaltered thyroglobulin without adjuvant into rabbits previously stimulated with altered thyroglobulin. J Exp Med. 1965;122:1049–62. doi:10.1084/jem.122.6.1049.
- Shearer GM. Cell-mediated cytotoxicity to trinitrophenyl-modified syngeneic lymphocytes. Eur J Immunol. 1974;4:527–33. doi:10.1002/eji.1830040802.
- Tarcic N, David CS, Naor D. Auto-delayed-type hypersensitivity induced in immunodeficient mice with modified self-antigens. V.Cellular autoreactivity directed against self-h-2dd subregion mediates the inflammatory responses. Immunology. 1989;67:184–90.
- Little JR, Eisen HN. Preparation of immunogenic 2,4-dinitrophenyl and 2,4,6-trinitrophenyl proteins. In: Williams C, Chase M, editors. Methods in immunology and immunochemistry. New York (NY): Academic Press; 1967. p. 128–32.
- Sherman LA, Burakoff SJ, Benaceraff B. The induction of cytolytic T lymphocytes with specificity for p-azophenylarsonate coupled syngeneic cells. J Immunol. 1978;121:1432–36. doi:10.4049/jimmunol.121.4.1432.
- Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–90. doi:10.1084/jem.182.5.1281.
- Mitchison NA. Immunologic approach to cancer. Transplant Proc. 1970;11:92–103.
- Mullen CA, Urban JL, Van Waes C, Rowley DA, Schreiber H. Multiple cancers. Tumor burden permits the outgrowth of other cancers. J Exp Med. 1985;162:1665–82. doi:10.1084/jem.162.5.1665.
- Flood PM, Schreiber H, Ron Y. Protective immunity to progressive tumors can be induced by antigen presented on regressor tumors. J Immunol. 1987;138:3573–79. doi:10.4049/jimmunol.138.10.3573.
- Cavallo G, Forni G. Cell reactivity toward syngeneic neoplastic cells in mice hypersensitized to DNP. Eur J Cancer. 1974;10:103–06. doi:10.1016/0014-2964(74)90060-7.
- Galili N, Naor D, Asjo B, Klein G. Induction of immune responsiveness in a genetically low- responsive tumor- host combination by chemical modification of the immunogen. Eur J Immunol. 1976;6:473–76. doi:10.1002/eji.1830060705.
- Fujiwara H, Aoki H, Yoshioka T, Tomita S, Ikegami R, Hamaoka T. Establishment of a tumor-specific immunotherapy model utilizing tnp- reactive helper cell activity and its application to the autochthonous tumor system. J Immunol. 1984;133:509–14. doi:10.4049/jimmunol.133.1.509.
- Sojka DK, Felnerova D, Mokyr MB. Anti-metastatic activity of hapten-modified autologous tumor cell vaccine in an animal tumor model. Cancer Immunol Immunother. 2002;51:200–08. doi:10.1007/s00262-002-0271-9.
- Miller AE, Levis WR. Lymphocyte transformation during dinitrochlorobenzene contact sensitization. J Clin Invest. 1973;52:1925–30. doi:10.1172/JCI107376.
- Miller SD, Claman HN. The induction of hapten-specific T cell tolerance by using hapten-modified lymphoid cells. I.Characteristics of tolerance induction. J Immunol. 1976;117:1519–26. doi:10.4049/jimmunol.117.5_Part_1.1519.
- Sato T, Maguire HC Jr., Mastrangelo MJ, Berd D. Human immune response to DNP-modified autologous cells after treatment with a DNP-conjugated melanoma vaccine. Clin Immunol Immunopathol. 1995;74:35–43. doi:10.1006/clin.1995.1006.
- Sato T, Bullock TNJ, Eisenlohr LC, Mastrangelo MJ, Berd D. Dinitrophenyl-modified autologous melanoma vaccine induces a T cell response to hapten-modified, melanoma peptides. Clin Immunol Immunopathol. 1997;85:265–72. doi:10.1006/clin.1997.4419.
- Berd D, Sato T, Cohn H, Maguire HC Jr., Mastrangelo MJ. Treatment of metastatic melanoma with autologous, hapten-modified melanoma vaccine: regression of pulmonary metastases. Int J Cancer. 2001;94:531–39. doi:10.1002/ijc.1506.abs.
- Berd D, Sato T, Maguire HC Jr., Kairys J, Mastrangelo MJ. Immunopharmacological analysis of an autologous, hapten-modified human melanoma vaccine. J Clin Oncol. 2004;22:403–15. doi:10.1200/JCO.2004.06.043.
- Berd D, Murphy G, Maguire HC Jr., Mastrangelo MJ. Immunization with haptenized, autologous tumor cells induces inflammation of human melanoma metastases. Cancer Res. 1991;51:2731–34.
- Berd D, Maguire HC Jr., Mastrangelo MJ, Murphy GF. Activation markers on t cells infiltrating melanoma metastases after therapy with dinitrophenyl-conjugated vaccine. Cancer Immunol Immunother. 1994;39:141–47. doi:10.1007/BF01533378.
- Testi R, Phillips JH, Lanier LL. Leu23 induction as an early marker of functional CD3/T cell antigen receptor triggering: requirement for receptor crosslinking, prolonged elevation of intracellular ca2++ and activation of pkc. J Immunol. 1989;142:1854–60. doi:10.4049/jimmunol.142.6.1854.
- Lattime EC, Mastrangelo MJ, Bagasra O, Li W, Berd D. Expression of cytokine mRNA in human melanoma tissues. Cancer Immunol Immunother. 1995;41:151–56. doi:10.1007/BF01521340.
- Sensi ML, Farina C, Maccalli C, Lupetti R, Nicolini G, Anichini A, Parmiani G, Berd D. Clonal expansion of T lymphocytes in human melanoma metastases after treatment with a hapten-modified autologous tumor vaccine. J Clin Invest. 1997;99:710–17. doi:10.1172/JCI119215.
- Manne J, Mastrangelo MJ, Sato T, Berd D. T cell receptor rearrangement in lymphocytes infiltrating melanoma metastases after administration of autologous dinitrophenyl - modified vaccine. J Immunol. 2002;169:3407–12. doi:10.4049/jimmunol.169.6.3407.
- Taha M, Berd D, Williams S, Del Priore G, Standiford S, Brown K, Pollock T, Jaggernauth S. Autologous ovarian tumor cells vaccine, modified with the hapten, dinitrophenyl (DNP), in platinum resistant ovarian cancer. Gynecol Oncol. 2014;134:428–37. Abstract 25. doi:10.1016/j.ygyno.2014.04.015.
- Weigle WO. The relationship among acquired immunological tolerance and its termination and tolerance to self and autoimmunity. Int Arch Allergy Appl Immunol. 1965;27:368–69.
- Nahas F, Leskowitz S. The ability of hapten-conjugated cells to induce cell-mediated cytotoxicity is affected by the mode of hapten linkage. Cell Immunol. 1980;54:241–47. doi:10.1016/0008-8749(80)90205-1.
- Bach BA, Sherman L, Benaceraff B, Greene MI. Mechanism of regulation of cell-mediated immunity. Ii.Induction and suppression of delayed-type hypersensitivity to azobenzenearsonate-coupled syngeneic cells. J Imunnol. 1978;121:1460–68. doi:10.4049/jimmunol.121.4.1460.
- Berd D, Sato T, Kuchar J, Riley T, Mastrangelo MJ. Phase I-II trial of a novel mixed haptenized autologous melanoma vaccine. Proc Amer Soc Clin Oncol. 2004;23:2563. doi:10.1200/jco.2004.22.90140.2563.
- Berd D, Sato T, Mastrangelo MJ. Effect of the dose and composition of an autologous, hapten-modified melanoma vaccine on the development of delayed-type hypersensitivity responses. Cancer Immunol Immunother. 2002;51:320–26. doi:10.1007/s00262-002-0285-3.
- Berd D. A tale of two pities: autologous melanoma vaccines on the brink. Hum Vaccin Immunother. 2012;8:1146–51. doi:10.4161/hv.20923.
- Berd D, Bloome E, Schea H, Pinteur B, Fossiez F, Garcia E, Chalus L. Dose-response study of a cryopreserved, autologous, hapten-modified melanoma vaccine (MVAX®). Proc Amer Soc Clin Oncol. 2008;26:1462 (Abstract 3059). doi:10.1200/jco.2008.26.15_suppl.3059.
- Beaver JA, Pander R. The wild west of checkpoint inhibitor development. New Eng J Med. 2021;386:1297–301. doi:10.1056/NEJMp2116863.