ABSTRACT
Herpes zoster (HZ) results from waning immunity following childhood infection with varicella zoster virus (VZV) but is preventable by vaccination with recombinant HZ vaccine or live HZ vaccine (two doses or one dose, respectively). Vaccine efficacy declines with age, live HZ vaccine is contraindicated in immunosuppressed individuals, and severe local reactogenicity of recombinant HZ vaccine is seen in up to 20% of older adults, indicating a potential need for new vaccines. Nonreplicating chimpanzee adenovirus (ChAd) vectors combine potent immunogenicity with well-established reactogenicity and safety profiles. We evaluated the cellular and humoral immunogenicity of ChAdOx1 encoding VZV envelope glycoprotein E (ChAdOx1-VZVgE) in mice using IFN-γ ELISpot, flow cytometry with intracellular cytokine staining, and ELISA. In outbred CD-1 mice, one dose of ChAdOx1-VZVgE (1 × 107 infectious units) elicited higher gE-specific T cell responses than two doses of recombinant HZ vaccine (1 µg) or one dose of live HZ vaccine (1.3 × 103 plaque-forming units). Antibody responses were higher with two doses of recombinant HZ vaccine than with two doses of ChAdOx1-VZVgE or one dose of live HZ vaccine. ChAdOx1-VZVgE boosted T cell and antibody responses following live HZ vaccine priming. The frequencies of polyfunctional CD4+ and CD8+ T cells expressing more than one cytokine (IFN-γ, TNF-α and IL-2) were higher with ChAdOx1-VZVgE than with the conventional vaccines. Results were similar in young and aged BALB/c mice. These findings support the clinical development of ChAdOx1-VZVgE for prevention of HZ in adults aged 50 years or over, including those who have already received conventional vaccines.
Introduction
Herpes zoster (shingles) is one of the three most important vaccine-preventable diseases of aging in terms of disability-adjusted life-years, together with influenza and pneumococcal disease.Citation1 This painful neurocutaneous disease is caused by reactivation of latent varicella zoster virus (VZV) in adults who have had chickenpox.Citation2 The characteristic dermatomal skin-blistering rash is often accompanied by debilitating and prolonged pain that severely impairs health-related quality of life.Citation3 Pain persists as post-herpetic neuralgia for months or years after resolution of the rash in up to approximately 20% of cases.Citation4 Most adults worldwide are at risk of developing herpes zoster because about 95% have had previous exposure to VZV and therefore have latent infections in sensory ganglia.Citation3 The economic burden of herpes zoster is substantial, with annual direct medical costs estimated at USD 29 million in the United Kingdom in 2008 and USD 2.6 billion in the US in 2020.Citation3,Citation5,Citation6
The lifetime risk of developing herpes zoster increases dramatically with age after the age of 50 years, reaching 50% in unvaccinated people aged 80 years or older, but herpes zoster can also affect immunocompromised people of any age.Citation7 Evidence indicates that herpes zoster occurs when immunity to VZV falls below a critical level owing to immunosenescence or immunosuppression resulting from disease or medication.Citation8–10 VZV-specific CD4+ and CD8+ T cell memory responses developed in childhood after recovery from varicella normally limit reactivation of VZV and prevent herpes zoster in adulthood.Citation11 VZV-specific immune responses wane with age and eventually fail to prevent herpes zoster following VZV reactivation, but protection can be restored by vaccination.Citation12–14
Following the results of pivotal randomized controlled trials,Citation15–17 national guidelines in 12 countries support vaccination against herpes zoster in adults aged 50 or over, but differ in recommended target age and vaccine type.Citation18,Citation19 Worldwide, two principal HZ vaccines have received regulatory approval.Citation3 ‘Shingles (herpes zoster) vaccine (live)’ comprises a high dose of the attenuated Oka strain of VZV given as a single injection and is marketed as Zostavax® by Merck & Co.Citation18,Citation20 ‘Herpes zoster vaccine (recombinant, adjuvanted)’ comprises recombinant VZV envelope glycoprotein E (gE) in AS01B adjuvant given as two doses 2–6 months apart, and is marketed as Shingrix® by GlaxoSmithKline.Citation18,Citation21 We refer to these vaccines herein as live HZ vaccine and recombinant HZ vaccine, respectively.
The real-world effectiveness of current vaccines in preventing herpes zoster was estimated at 45.9% (95% confidence interval [CI]: 42.2, 49.4) for live HZ vaccine and 79.3% (95% CI: 57.6, 89.7) for recombinant HZ vaccine in a recent meta-analysis of 22 studies in over 9 million participants.Citation19 These values were lower than those observed in meta-analyses of efficacy in randomized controlled trials.Citation22–25 Both the effectiveness and efficacy of live HZ vaccine decrease with age at immunization and wane with time since immunization.Citation19,Citation23,Citation24 Furthermore, live HZ vaccine is contraindicated in immunocompromised individuals, and recombinant HZ vaccine is associated with severe local reactogenicity in up to 20% of recipients.Citation26–31 These findings indicate an unmet need for new vaccines and regimens with improved safety, tolerability, immunogenicity, efficacy and effectiveness.
Nonreplicating chimpanzee adenovirus (ChAd) vaccine vectors induce potent cellular and humoral immune responses.Citation32 During the COVID-19 pandemic, the ChAdOx1 nCoV-19 vaccine offered excellent protection against disease, a well-established safety profile and scalable global manufacturing.Citation33,Citation34 The immunogenicity and safety profiles of ChAdOx1-based vaccines have also been established in clinical trials in older adults and people living with HIV.Citation35–40 ChAdOx1 therefore represents a suitable platform for a herpes zoster vaccine in adults aged over 50 years, including those with compromised immune systems.
We have developed a novel herpes zoster vaccine utilizing the ChAdOx1 vector. Here, we evaluate the cellular and humoral immunogenicity of ChAdOx1 expressing VZV glycoprotein E (gE) in mice, compared with the licensed live HZ vaccine and recombinant HZ vaccine in a variety of dosing regimens.
Methods
Vaccines
A DNA sequence encoding VZV gE (Oka strain; locus GE_VZVO; UniProtKB/Swiss-Prot Q9J3M8.1) was optimized for human codon usage, synthesized and cloned into the ChAdOx1 E1 locus under the control of the cytomegalovirus immediate-early promoter and the bovine growth hormone polyadenylation sequence, as previously described.Citation41 The resulting ChAdOx1-VZVgE vaccine was propagated, purified and titered as previously described.Citation41 Live HZ vaccine (‘Shingles [herpes zoster] vaccine [live]’; Zostavax®) and recombinant HZ vaccine (‘Herpes zoster vaccine [recombinant, adjuvanted]’; Shingrix®) were obtained from Clinigen. Doses were based on infectious units (IU) for ChAdOx1-VZVgE, plaque-forming units (PFU) for live HZ vaccine, and amount of protein (in µg) for recombinant HZ vaccine. For reconstitution of Shingrix®, manufacturer’s guidelines were followed as per product insert, and the ratio of gE/MPL/QS21 was maintained. When required, products were diluted in PBS prior to administration to achieve appropriate doses.
Western blotting
To confirm gE expression by ChAdOx1-VZVgE, sub-confluent HEK293T cells were transduced with ChAdOx1-VZVgE at a multiplicity of infection of 1 or 5 infectious units per cell. Cells were harvested and lysed 18 h later. Clarified lysates were mixed with Laemmli sample buffer, heat-denatured, and separated by electrophoresis on a 4–20% precast gradient sodium dodecyl sulfate polyacrylamide gel (BioRad). Proteins were transferred to nitrocellulose membranes by western blotting. Blots were probed with anti-gE primary antibody 9C8 (Abcam 52,549) and horseradish peroxidase-conjugated secondary antibody, both diluted in phosphate-buffered saline (PBS) containing 0.05% Tween-20 (PBS-T). Blots were visualized using Pierce enhanced chemiluminescent western blotting substrate (ThermoFisher) and a Chemidoc imaging system (BioRad). Recombinant HZ vaccine was used as a positive control.
Animals
All mice were used in accordance with the UK Animals (Scientific Procedures) Act 1986 under project license number 30/2889 and P9804B4F1 granted by the UK Home Office. Female inbred BALB/c/cOlaHsd (BALB/c) and outbred Crl:CD1 (CD-1) mice were obtained from Envigo and Charles River Laboratories, respectively, and were randomly distributed into experimental groups on arrival. Mice were housed in individually ventilated cages under specific pathogen-free conditions, with constant temperature and humidity and a 12-h light/dark cycle (8 am to 8 pm). Mice received immunizations with the doses and regimens described in Results and Figure legends in the musculus tibialis in a volume of 50 µL using PBS as a diluent. All mice were humanely sacrificed at the end of each experiment by a method approved under Schedule 1 of the above Act. Serum and spleens were collected for analyses of humoral and cell-mediated immunity.
Immune assays
Synthetic peptides
Crude synthetic 15-mer peptides overlapping by 11 amino acids and spanning the same gE protein sequence used in ChAdOx1-VZVgE were obtained from Mimotopes. The 154 peptides were dissolved in dimethyl sulfoxide to 20 µg/mL and combined into three pools (pool 1, peptides 1–5 and 134–154; pool 2, peptides 6–69; pool 3, peptides 70–133).
Enzyme-linked immuno-spot assay
Cellular immune responses were assessed by interferon-γ (IFN-γ) enzyme-linked immuno-spot (ELISpot) assay, as previously described.Citation42 Briefly, splenocyte suspensions were prepared by passing spleens through 70 μM cell strainers, followed by treatment with ammonium chloride potassium lysis buffer and resuspension in complete minimum essential medium (Sigma). Splenocytes were restimulated separately with the three gE peptide pools at a final concentration of 2 μg/ml in IPVH-membrane 96-well plates (Millipore) previously coated with 5 µg/mL anti-mouse IFN-γ AN18 (Mabtech). Plates were incubated for 18–20 h at 37°C with 5% CO2 in a humidified incubator. Spot-forming cells (SFCs) were detected with biotinylated anti-mouse IFN-γ (1 mg/mL) followed by streptavidin-conjugated alkaline phosphatase (1 mg/mL) and alkaline phosphate substrate kit (BioRad). Spots were counted using an AID ELISpot reader and software (Autoimmun Diagnostika) and responses to each peptide pool were summed for analysis. Background responses (from splenocytes cultured with media alone, without peptide stimulation) were subtracted from responses in stimulated wells.
Flow cytometry
Cellular immune responses were also assessed by flow cytometry with intracellular cytokine staining for tumor necrosis factor alpha (TNF-α), interleukin-2 (IL-2) and IFN-γ, as previously described.Citation42 Briefly, splenocytes were placed in 96-well round-bottom plates and restimulated with the three gE peptide pools at a final concentration of 5 µg/mL. Plates were incubated for 2 h at 37°C with 5% CO2 in a humidified incubator, before the addition of 0.2 μL per well of GolgiPlug and GolgiStop (BD Biosciences), incubation for a further 4 hours, and overnight storage at 4°C. Cells were stained with Live/Dead Aqua (ThermoFisher) and the following fluorescent antibody conjugates: CD4–eFluor 650, CD8–peridinin-chlorophyll-protein-cyanine 5.5, CD62 L – allophycocyanin-eFluor 780 and CD127–phycoerythrin-cyanine 7 (Invitrogen, ThermoFisher, eBioscience). Cells were then fixed with 4% paraformaldehyde and stained intracellularly with TNF-α–fluorescein isothiocyanate, IL-2–phycoerythrin and IFN-γ–allophycocyanin, diluted in Perm/Wash buffer (BD Biosciences). Data were acquired using an LSR II flow cytometer (BD Biosciences) and analyzed in FlowJo (TreeStar). Background responses (from splenocytes cultured with media alone, without peptide stimulation) were subtracted from responses in stimulated wells.
Enzyme-linked immunosorbent assay
Humoral immune responses were assessed by enzyme-linked immunosorbent assay (ELISA). MaxiSorp ELISA plates (Nunc) were coated overnight at 4°C with 50 μL per well of 2 μg/mL VZV glycoproteins (Serion Immunologics) diluted in PBS. Plates were washed six times with PBS-T and blocked for 1 h at room temperature with 100 μL per well of blocking buffer (10% skimmed milk in PBS-T). Mouse sera were initially diluted appropriately in PBS, then serially diluted three-fold down the plate. Plates were incubated for 2 h, washed, then incubated for 1 h with alkaline phosphatase-conjugated goat anti-mouse whole IgG (Sigma A3562) diluted in blocking buffer, all at room temperature. Plates were developed with 1 mg/mL p-nitrophenyl phosphate (Sigma) in diethanolamine substrate buffer (ThermoFisher). Optical density at 405 nm was measured using an ELx800 microplate reader (Bio-Tek). Reciprocal titers were calculated using the dilution curves and expressed in arbitrary units (AU).
Statistical analyses
No inferential statistical tests were performed because there was no predefined approach to limiting study-wide type I error or controlling for multiple comparisons. Data were summarized as mean and 95% confidence interval (CI) to enable exploratory noninferential comparisons between experimental groups. Nonoverlapping confidence intervals were considered indicative of potentially meaningful differences.
Results
Antigen expression in vitro
Western blotting confirmed the expression of gE by ChAdOx1-VZVgE in vitro, with the expected apparent molecular weight observed following transduction of HEK293T cells (Supplementary Figure S1).
Immunogenicity in mice
Single-dose regimens in BALB/c mice
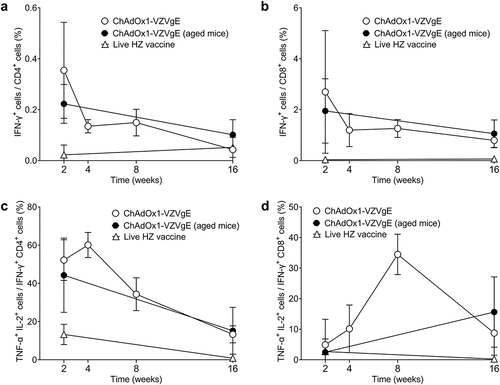
One intramuscular dose of ChAdOx1-VZVgE (1 × 107 IU) elicited higher gE-specific T cell responses than one dose of live HZ vaccine (1.3 × 103 PFU), as assessed by IFN-γ ELISpot assay 2 weeks after vaccination of young BALB/c mice, in two separate experiments (). T cell responses to gE were highest 2 weeks after ChAdOx1-VZVgE administration, then declined gradually at weeks 4, 8 and 16, but remained higher than responses to live HZ vaccine throughout this time period (). ChAdOx1-VZVgE elicited both gE-specific CD4+ T cells and gE-specific CD8+ T cells, as assessed by flow cytometry and intracellular cytokine staining for IFN-γ (). The majority of IFN-γ+ CD4+ T cells and IFN-γ+ CD8+ T cells were also positive for both TNF-α and IL-2 ().
Figure 1. Cellular (a, b) and humoral (c) immunogenicity of a single dose of ChAdOx1-VZVgE in BALB/c mice.
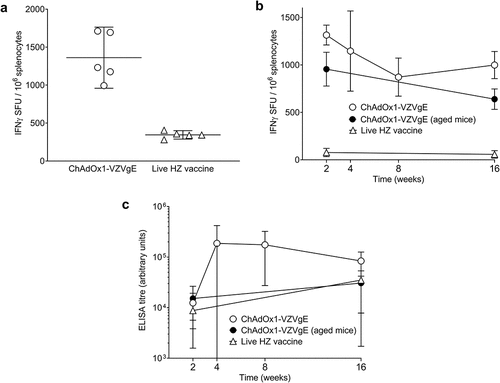
Figure 2. Flow cytometric characterization of (a, c) CD4+ (b, d) CD8+ T cell responses following a single dose of ChAdOx1-VZVgE in BALB/c mice.
Antibody responses to gE were highest 4 weeks after ChAdOx1-VZVgE administration and were similar in magnitude to those elicited by live HZ vaccine, as assessed 2 weeks and 16 weeks after vaccination (). Both the cellular and humoral immunogenicity of ChAdOx1-VZVgE were similar in young and old BALB/c mice, aged 8–10 weeks and at least 24 weeks, respectively (; ).
Single-dose regimens in outbred mice
In a dose-finding experiment in CD-1 mice, the magnitude of gE-specific humoral and cellular responses increased across low, medium and high single intramuscular doses of ChAdOx1-VZVgE (1 × 106, 1 × 107 and 1 × 108 IU, respectively) and recombinant HZ vaccine (0.2 µg, 1 µg and 5 µg, respectively) (Supplementary Figure S2). Antibody responses were similar for ChAdOx1-VZVgE and recombinant HZ vaccine at each dose level, but IFN-γ ELISpot responses were higher for ChAdOx1-VZVgE than recombinant HZ vaccine at all dose levels (Supplementary Figure S2). Based on these results, we selected a ChAdOx1-VZVgE dose of 1 × 107 IU and a recombinant HZ vaccine dose of 1 µg for subsequent experiments.
Single-dose and double-dose regimens in outbred mice
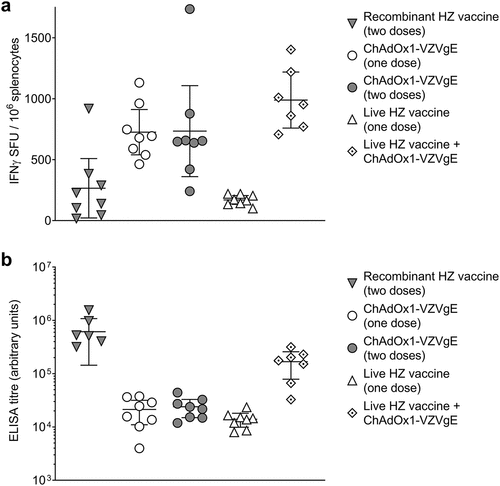
We next investigated five VZV vaccination regimens (), comprising recombinant HZ vaccine (two doses, as per clinical practice), ChAdOx1-VZVgE (one or two doses), live HZ vaccine (one dose, as per clinical practice) and a heterologous prime-boost with live HZ vaccine and ChAdOx1-VZVgE (one dose of each). The interval between doses was 4 weeks, with immunogenicity assessments 4 weeks after the last vaccination.
Table 1. Experimental design for assessment of five HZ vaccination regimens in CD-1 mice.
One dose of ChAdOx1-VZVgE elicited higher gE-specific T cell responses than both conventional regimens, as assessed by IFN-γ ELISpot assay (). When given as the second dose in the regimen, ChAdOx1-VZVgE did not boost T cell responses following homologous priming but did boost T cell responses following heterologous live HZ vaccine priming, with the highest mean IFN-γ ELISpot response observed in this group ().
Figure 3. Cellular (a) and humoral (b) immunogenicity of five VZV vaccination regimens in CD-1 mice.
All five regimens induced similar levels of gE-specific CD4+ T cells as assessed by flow cytometry and intracellular cytokine staining for IFN-γ (). The proportion of IFN-γ+ CD4+ T cells that were also positive for TNF-α and IL-2 was highest for the heterologous prime-boost regimen (live HZ vaccine plus ChAdOx1-VZVgE), followed by the ChAdOx1-VZVgE single-dose and double-dose regimens, followed by the conventional vaccine regimens (). Regarding gE-specific CD8+ T cells, the proportions positive for IFN-γ, for TNF-α and for both IFN-γ and TNF-α were highest for the two regimens involving homologous or heterologous bosting with ChAdOx1-VZVgE and were lowest for the conventional vaccine regimens ().
Figure 4. Flow cytometric characterization of CD4+ T cell responses following five VZV vaccination regimens in CD-1 mice.
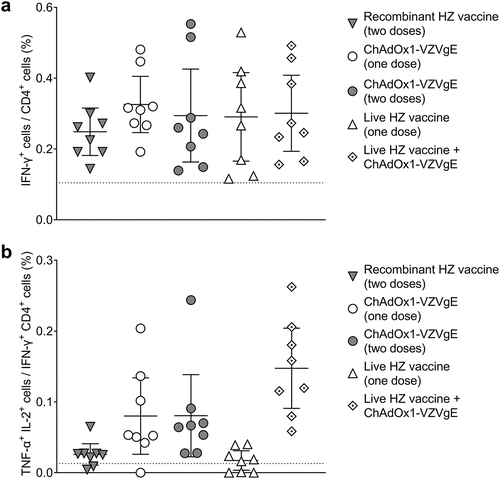
Figure 5. Flow cytometric characterization of CD8+ T cell responses following five VZV vaccination regimens in CD-1 mice.
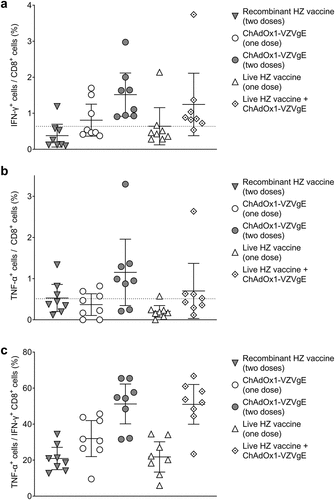
Antibody responses to gE were higher in mice receiving two doses of recombinant HZ vaccine than in those receiving one or two doses of ChAdOx1-VZVgE or one dose of live HZ vaccine (). Boosting live HZ vaccine with ChAdOx1-VZVgE led to increased antibody responses compared with live HZ vaccine alone ().
Discussion
ChAdOx1-VZVgE elicited robust and long-lasting gE-specific cellular and humoral immune responses in aged and outbred mice in this study. Compared with conventional recombinant HZ vaccine and live HZ vaccine regimens, antibody and IFN-γ+ CD4+ T cell responses were similar, and IFN-γ+ CD8+ T cell responses were higher in magnitude following two doses of ChAdOx1-VZVgE. Furthermore, frequencies of gE-specific polyfunctional T cells were higher following ChAdOx1-VZVgE than following the conventional vaccines, as assessed by expression of TNF-α and IL-2 as well as IFN-γ. Polyfunctional T cells have been associated with successful vaccination and improved outcomes after infection.Citation43 These findings indicate that ChAdOx1-VZVgE could be deployed as a stand-alone vaccine in adults aged 50 years, potentially in countries where take-up of the existing vaccines has been poor. ChAdOx1-VZVgE was also able to boost cellular and humoral responses following priming with live HZ vaccine, indicating further potential utility as a booster in those who have already received a conventional herpes zoster vaccine. This population varies among high-income countries, with the majority of the UK target population, for example, having already received live HZ vaccine and the majority of the US target population having already received recombinant HZ vaccine.Citation18,Citation19
Waning of cell-mediated immunity to VZV is thought to be responsible for the increasing risk of developing herpes zoster with advancing age.Citation12–14 The greater efficacy of recombinant HZ vaccine over live HZ vaccine has been attributed to higher peak Th1 CD4+ T cell memory responses, although antibodies and cytotoxic CD8+ T cells may also play a role in protection.Citation14–44–Citation46 A fourfold rise in gE-specific antibody levels following vaccination with live HZ vaccine is the most widely accepted correlate of protection against herpes zoster but is thought to reflect an underlying boosting of Th1 responses.Citation47 Hence, the lower anti-gE antibody response seen preclinically after two doses of ChAdOx1-VZVgE compared to two doses of recombinant HZ vaccine is not thought to negatively impact on efficacy, since Th1 responses were similar or superior (). Indeed, ChAdOx1-VZVgE offers the potential for improved efficacy over conventional vaccines by providing similar induction of humoral and CD4+ T cell responses plus enhanced induction of CD8+ T cell responses.
The reactogenicity of recombinant HZ vaccine can limit compliance, with up to 20% of older adults missing their second dose.Citation26–31 The reactogenicity profile of ChAdOx1 has been thoroughly established during the COVID-19 pandemic, including its use as a heterologous boost.Citation36–38,Citation48,Citation49 ChAdOx1-VZVgE may offer an improved reactogenicity profile over recombinant HZ vaccine owing to the absence of adjuvant, which may improve compliance with multi-dose regimens. ChAdOx1-VZVgE may also offer an improved safety profile over live HZ vaccine in immunocompromised individuals.
Robust data exist on very rare severe adverse events after vaccination with ChAdOx1 nCoV-19, including how this varies with geography and ethnicity.Citation34,Citation50 Specifically, ChAdOx1 nCoV-19 has been associated with an extremely rare but serious blood clot condition, vaccine-induced thrombotic thrombocytopenia (VITT), a sub-group of thrombosis with thrombocytopenia syndrome (TTS). The pathophysiological mechanism behind VITT remains unclear. The majority of VITT cases occurred within the first 3 weeks after the first vaccination, and there is no increase above background rates of TTS, after second vaccination. The condition is not predictable and has occurred in previously healthy people, although diagnosis, management and treatment options have significantly improved outcomes. VITT appears to be slightly less common in older people, and reporting rates have varied markedly depending on geographical location, with 10.0 cases per million doses in the UK and 0.2 cases per million doses in Brazil, South Korea and the Philippines.Citation50 There is a risk that VITT might also be associated with other ChAdOx1-based vaccines, such as the ChAdOx1-VZVgE vaccine described here. However, it is important to acknowledge that VITT is an extremely rare condition, that TTS also occurs in the background population outside of those who have been vaccinated and that middle-income target markets for the ChAdOx1-VZVgE product have the appropriate medical infrastructure and surveillance mechanisms allowing for rapid treatment of VITT.
Furthermore, the immunogenicity and safety profiles of ChAdOx1-based vaccines in people living with HIV and aged adults were similar to those in young HIV-negative adult populations in clinical trials of COVID-19, influenza and tuberculosis vaccines.Citation35–40 Taken together, these findings indicate that ChAdOx1-VZVgE is likely to be suitable for use in the target population for a herpes zoster vaccine, including those who have already received a conventional vaccine.
Strengths of the present study include the use of aged and outbred mice and the comparison with conventional vaccines using multiple immunogenicity assays. The lack of standardization across preclinical and clinical studies prohibits comparisons of cell-mediated immunity assay results. We did not aim to assess efficacy pre-clinically because no small-animal model of VZV infection accurately recapitulates human herpes zoster.Citation51 Together with the extensive experience in early clinical translation of ChAd-based vaccines for multiple indications,Citation32–54 the immunogenicity findings of the present study support moving ChAdOx1-VZVgE into human trials as a vaccine for the prevention of herpes zoster in adults aged 50 years or over.
There are several expected challenges in terms of clinical and regulatory development of a ChAdOx1-VZVgE product. Firstly, it would be difficult to perform placebo-controlled trials in countries in which a shingles vaccine is already licensed, as these would require very large participant numbers. For initial clinical development, it would therefore be advantageous to focus on countries with no licensed shingles vaccine and conduct placebo-controlled trials there, which is estimated to require as many as 30,000 participants followed for 2 years. In terms of regulatory development, there is no presently accepted biomarker upon which to base licensure of a shingles vaccine, although both binding anti-gE antibody and gE-specific CD4+ T cells are believed to be involved in protective efficacy.Citation12 Future Phase 3 studies with ChAdOx1-VZVgE should aim to assess anti-gE antibodies as a correlate of protection, but a multimodal combination of gE-specific CD4+ T cells, CD8+ T cells and antibodies will likely have a role in the mechanism of protection.
Supplementary Figures
Download PDF (665.3 KB)Acknowledgments
We thank the Vector Core Facility of the Jenner Institute, University of Oxford (at the time the work was conducted) for technical assistance.
Disclosure statement
MU and SM declare they have no conflicts of interest. SS is an employee of Vaccitech (UK) Limited. TL has received consultancy fees from Vaccitech and CSL Seqirus and holds intellectual property relating to viral vectored vaccines.
Data availability statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2175558.
Additional information
Funding
References
- Cunningham AL, McIntyre P, Subbarao K, Booy R, Levin MJ. Vaccines for older adults. BMJ. 2021;372:1. doi:10.1136/bmj.n188.
- Cohen JI. Herpes zoster. N Engl J Med. 2013;369:1766–10. doi:10.1056/NEJMcp1302674.
- Pan CX, Lee MS, Nambudiri VE. Global herpes zoster incidence, burden of disease, and vaccine availability: a narrative review. Ther Adv Vaccines Immunother. 2022;10:25151355221084535. doi:10.1177/25151355221084535.
- Johnson RW, Rice AS. Clinical practice. Postherpetic neuralgia. N Engl J Med. 2014;371(16):1526–33. doi:10.1056/NEJMcp1403062.
- Harvey M, Prosser LA, Rose AM, Ortega-Sanchez IR, Harpaz R. Aggregate health and economic burden of herpes zoster in the United States: illustrative example of a pain condition. Pain. 2020;161(2):361–68. doi:10.1097/j.pain.0000000000001718.
- Gater A, Uhart M, McCool R, Preaud E. The humanistic, economic and societal burden of herpes zoster in Europe: a critical review. BMC Public Health. 2015;15(1):193. doi:10.1186/s12889-015-1514-y.
- Lang PO, Aspinall R. Vaccination for quality of life: herpes–zoster vaccines. Aging Clin Exp Res. 2021;33(4):1113–22. doi:10.1007/s40520-019-01374-5.
- Weinberg A, Zhang JH, Oxman MN, Johnson G, Hayward A, Caulfield M, Irwin M, Clair J, Smith J, Stanley H, et al. Varicella-zoster virus–specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009;200(7):1068–77. doi:10.1086/605611.
- Arvin AM, Pollard RB, Rasmussen LE, Merigan TC. Cellular and humoral immunity in the pathogenesis of recurrent herpes viral infections in patients with lymphoma. J Clin Invest. 1980;65(4):869–78. doi:10.1172/JCI109739.
- Weinberg A, Lazar AA, Zerbe GO, Hayward A, Chan I, Vessey R, Silber J, MacGregor R, Chan K, Gershon A, et al. Influence of age and nature of primary infection on varicella-zoster virus–specific cell-mediated immune responses. J Infect Dis. 2010;201(7):1024–30. doi:10.1086/651199.
- Ouwendijk WJ, Laing KJ, Verjans GM, Koelle DM. T-cell immunity to human alphaherpesviruses. Curr Opin Virol. 2013;3(4):452–60. doi:10.1016/j.coviro.2013.04.004.
- Levin MJ, Weinberg A. Immune responses to zoster vaccines. Hum Vaccin Immunother. 2019;15(4):772–77. doi:10.1080/21645515.2018.1560918.
- Levin MJ, Weinberg A. Immune responses to varicella-zoster virus vaccines. Curr Top Microbiol Immunol. 2023;438:223–246. doi:10.1007/82_2021_245.
- Levin MJ, Oxman MN, Zhang JH, Johnson G, Stanley H, Hayward A, Caulfield M, Irwin M, Smith J, Clair J, et al. Varicella-zoster virus–specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197(6):825–35. doi:10.1086/528696.
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–84. doi:10.1056/NEJMoa051016.
- Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang S-J, Díez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barberà J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–32. doi:10.1056/NEJMoa1603800.
- Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang S-J, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–96. doi:10.1056/NEJMoa1501184.
- Parikh R, Widenmaier R, Lecrenier N. A practitioner’s guide to the recombinant zoster vaccine: review of national vaccination recommendations. Expert Rev Vaccines. 2021;20(9):1065–75. doi:10.1080/14760584.2021.1956906.
- Mbinta JF, Nguyen BP, Awuni PAA, Paynter J, Simpson CR. Post-licensure zoster vaccine effectiveness against herpes zoster and postherpetic neuralgia in older adults: a systematic review and meta-analysis. Lancet Healthy Longev. 2022;3(4):E263–75. doi:10.1016/S2666-7568(22)00039-3.
- Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet. 1974;2(7892):1288–90. doi:10.1016/S0140-6736(74)90144-5.
- Didierlaurent AM, Laupeze B, Di Pasquale A, Hergli N, Collignon C, Garcon N. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines. 2017;16(1):55–63. doi:10.1080/14760584.2016.1213632.
- Tricco AC, Zarin W, Cardoso R, Veroniki A-A, Khan PA, Nincic V, Ghassemi M, Warren R, Sharpe JP, Page AV, et al. Efficacy, effectiveness, and safety of herpes zoster vaccines in adults aged 50 and older: systematic review and network meta-analysis. BMJ. 2018;363:k4029. doi:10.1136/bmj.k4029.
- Gagliardi AM, Andriolo BN, Torloni MR, Soares BG, de Oliveira Gomes J, Andriolo RB, Canteiro Cruz E. Vaccines for preventing herpes zoster in older adults. Cochrane Database Syst Rev. 2019;2019(11):CD008858. doi:10.1002/14651858.CD008858.pub4.
- McGirr A, Widenmaier R, Curran D, Espié E, Mrkvan T, Oostvogels L, Simone B, McElhaney JE, Burnett H, Haeussler K, et al. The comparative efficacy and safety of herpes zoster vaccines: a network meta-analysis. Vaccine. 2019;37(22):2896–909. doi:10.1016/j.vaccine.2019.04.014.
- Senderovich H, Grewal J, Mujtaba M. Herpes zoster vaccination efficacy in the long-term care facility population: a qualitative systematic review. Curr Med Res Opin. 2019;35(8):1451–62. doi:10.1080/03007995.2019.1600482.
- Patterson BJ, Chen CC, McGuiness CB, Glasser LI, Sun K, Buck PO. Early examination of real-world uptake and second-dose completion of recombinant zoster vaccine in the United States from October 2017 to September 2019. Hum Vaccin Immunother. 2021;17(8):2482–87. doi:10.1080/21645515.2021.1879579.
- Colindres R, Wascotte V, Brecx A, Clarke C, Hervé C, Kim JH, Levin MJ, Oostvogels L, Zahaf T, Schuind A, et al. Post hoc analysis of reactogenicity trends between dose 1 and dose 2 of the adjuvanted recombinant zoster vaccine in two parallel randomized trials. Hum Vaccin Immunother. 2020;16(11):2628–33. doi:10.1080/21645515.2020.1741312.
- Schmader KE, Levin MJ, Grupping K, Matthews S, Butuk D, Chen M, Idrissi ME, Fissette LA, Fogarty C, Hartley P, et al. The impact of reactogenicity after the first dose of recombinant zoster vaccine on the physical functioning and quality of life of older adults: an open-label, phase III trial. J Gerontol A Biol Sci Med Sci. 2019;74(8):1217–24. doi:10.1093/gerona/gly218.
- Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, Harpaz R. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67(3):103–08. doi:10.15585/mmwr.mm6703a5.
- Willis ED, Woodward M, Brown E, Popmihajlov Z, Saddier P, Annunziato PW, Halsey NA, Gershon AA. Herpes zoster vaccine live: a 10 year review of post-marketing safety experience. Vaccine. 2017;35(52):7231–39. doi:10.1016/j.vaccine.2017.11.013.
- Fiore J, Co-van der Mee MM, Maldonado A, Glasser L, Watson P. Safety and reactogenicity of the adjuvanted recombinant zoster vaccine: experience from clinical trials and post-marketing surveillance. Ther Adv Vaccines Immunother. 2021;9:25151355211057479. doi:10.1177/25151355211057479.
- Ewer K, Sebastian S, Spencer AJ, Gilbert S, Hill AVS, Lambe T. Chimpanzee adenoviral vectors as vaccines for outbreak pathogens. Hum Vaccin Immunother. 2017;13(12):3020–32. doi:10.1080/21645515.2017.1383575.
- Joe CCD, Jiang J, Linke T, Li Y, Fedosyuk S, Gupta G, Berg A, Segireddy RR, Mainwaring D, Joshi A, et al. Manufacturing a chimpanzee adenovirus-vectored SARS-CoV-2 vaccine to meet global needs. Biotechnol Bioeng. 2022;119(1):48–58. doi:10.1002/bit.27945.
- Abrams CS, Barnes GD. SARS-CoV-2 vaccination-induced thrombotic thrombocytopenia: a rare but serious immunologic complication. Annu Rev Med. 2022;74(1):65–74. ePub ahead of print. doi:10.1146/annurev-med-043021-015237.
- Ogbe A, Pace M, Bittaye M, Tipoe T, Adele S, Alagaratnam J, Aley PK, Ansari MA, Bara A, Broadhead S, et al. Durability of ChAdOx1 nCov-19 vaccination in people living with HIV. JCI Insight. 2022;7(7):e157031. doi:10.1172/jci.insight.157031.
- Madhi SA, Koen AL, Izu A, Fairlie L, Cutland CL, Baillie V, Padayachee SD, Dheda K, Barnabas SL, Bhorat QE, et al. Safety and immunogenicity of the ChAdOx1 nCov-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. Lancet HIV. 2021;8(9):e568–80. doi:10.1016/S2352-3018(21)00157-0.
- Frater J, Ewer KJ, Ogbe A, Pace M, Adele S, Adland E, Alagaratnam J, Aley PK, Ali M, Ansari MA, et al. Safety and immunogenicity of the ChAdOx1 nCov-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV. 2021;8(8):e474–85. doi:10.1016/S2352-3018(21)00103-X.
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, Voysey M, Aley PK, Angus B, Babbage G, et al. Safety and immunogenicity of ChAdOx1 nCov-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–93. doi:10.1016/S0140-6736(20)32466-1.
- Antrobus RD, Lillie PJ, Berthoud TK, Spencer AJ, McLaren JE, Ladell K, Lambe T, Milicic A, Price DA, Hill AVS, et al. A T cell-inducing influenza vaccine for the elderly: safety and immunogenicity of MVA-NP+M1 in adults aged over 50 years. PLoS One. 2012;7(10):e48322. doi:10.1371/journal.pone.0048322.
- Minassian AM, Rowland R, Beveridge NE, Poulton ID, Satti I, Harris S, Poyntz H, Hamill M, Griffiths K, Sander CR, et al. A Phase I study evaluating the safety and immunogenicity of MVA85A, a candidate TB vaccine, in HIV-infected adults. BMJ Open. 2011;1(2):e000223. doi:10.1136/bmjopen-2011-000223.
- Dicks MD, Spencer AJ, Edwards NJ, Wadell G, Bojang K, Gilbert SC, Hill AV, Cottingham MG. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS One. 2012;7(7):e40385. doi:10.1371/journal.pone.0040385.
- Lambe T, Carey JB, Li Y, Spencer AJ, van Laarhoven A, Mullarkey CE, Vrdoljak A, Moore AC, Gilbert SC. Immunity against heterosubtypic influenza virus induced by adenovirus and MVA expressing nucleoprotein and matrix protein-1. Sci Rep. 2013;3(1):1443. doi:10.1038/srep01443.
- Heineman TC, Cunningham A, Levin M. Understanding the immunology of Shingrix, a recombinant glycoprotein E adjuvanted herpes zoster vaccine. Curr Opin Immunol. 2019;59:42–48. doi:10.1016/j.coi.2019.02.009.
- Levin MJ, Kroehl ME, Johnson MJ, Hammes A, Reinhold D, Lang N, Weinberg A. Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest. 2018;128(10):4429–40. doi:10.1172/JCI121484.
- Johnson MJ, Liu C, Ghosh D, Lang N, Levin MJ, Weinberg A. Cell-mediated immune responses after administration of the live or the recombinant zoster vaccine: 5-year persistence. J Infect Dis. 2022;225(8):1477–81. doi:10.1093/infdis/jiab580.
- Tovar Salazar A, McKhann A, Chen H, Bosch RJ, Weinberg A. Immune correlates of herpes zoster in people living with HIV on effective antiretroviral therapy. AIDS Res Hum Retroviruses. 2019;35(10):890–95. doi:10.1089/aid.2019.0053.
- Gilbert PB, Gabriel EE, Miao X, Li X, Su SC, Parrino J, Chan IS. Fold rise in antibody titers by measured by glycoprotein-based enzyme-linked immunosorbent assay is an excellent correlate of protection for a herpes zoster vaccine, demonstrated via the vaccine efficacy curve. J Infect Dis. 2014;210(10):1573–81. doi:10.1093/infdis/jiu279.
- Costa Clemens SA, Weckx L, Clemens R, Almeida Mendes AV, Ramos Souza A, Silveira MBV, da Guarda SNF, de Nobrega MM, de Moraes Pinto MI, Gonzalez IGS, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399(10324):521–29. doi:10.1016/S0140-6736(22)00094-0.
- Flaxman A, Marchevsky NG, Jenkin D, Aboagye J, Aley PK, Angus B, Belij-Rammerstorfer S, Bibi S, Bittaye M, Cappuccini F, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCov-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002). Lancet. 2021;398(10304):981–90. doi:10.1016/S0140-6736(21)01699-8.
- Soboleva K, Shankar NK, Yadavalli M, Ferreira C, Foskett N, Putsepp K, Ferstenberg LB, Nord M, da Silva HG, Bhuyan P. Geographical distribution of TTS cases following AZD1222 (ChAdox1 nCov-19) vaccination. Lancet Glob Health. 2022;10(1):e33–34. doi:10.1016/S2214-109X(21)00545-3.
- Mahalingam R, Gershon A, Gershon M, Cohen JI, Arvin A, Zerboni L, Zhu H, Gray W, Messaoudi I, Traina-Dorge V. Current in vivo models of varicella-zoster virus neurotropism. Viruses. 2019;11(6):v11060502. doi:10.3390/v11060502.
- Ewer KJ, Lambe T, Rollier CS, Spencer AJ, Hill AV, Dorrell L. Viral vectors as vaccine platforms: from immunogenicity to impact. Curr Opin Immunol. 2016;41:47–54. doi:10.1016/j.coi.2016.05.014.
- Gilbert SC, Warimwe GM. Rapid development of vaccines against emerging pathogens: the replication-deficient simian adenovirus platform technology. Vaccine. 2017;35(35):4461–64. doi:10.1016/j.vaccine.2017.04.085.
- Morris SJ, Sebastian S, Spencer AJ, Gilbert SC. Simian adenoviruses as vaccine vectors. Future Virol. 2016;11(9):649–59. doi:10.2217/fvl-2016-0070.
