?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Rotavirus vaccination is the most effective means to prevent rotavirus gastroenteritis, but its coverage in China is not ideal. We aimed to explore parental preferences for rotavirus vaccination for their children under 5years old to improve vaccination coverage. A Discrete Choice Experiment was conducted online on 415 parents with at least one child under 5years old in 3 cities. Five attributes including vaccine effectiveness, protection duration, risk of mild side-effects, out-of-pocket costs, and time required for vaccination were identified. Each attribute was set at three levels. Mixed-logit models were used to measure parental preferences and the relative importance of vaccine attributes. The optimal vaccination strategy was also explored. 359 samples were included in the analysis. The impacts of the vaccine attribute levels on vaccine choice were all statistically significant (p < .01), except for 1-hour vaccination time. The risk of mild side-effects was the most important factor influencing vaccination. The time required for vaccination was the least important attribute. The largest increase in vaccination uptake (74.45%) occurred with decreased the vaccine risk of mild side-effects from 1/10 to 1/50. The predicted vaccination uptake of the optimal vaccination scenario was 91.79%. When deciding about vaccination, parents preferred the rotavirus vaccination with lower risk of mild side-effects, higher effectiveness, longer protection duration, 2-hour vaccination time and lower cost. The authorities should support enterprises to develop vaccines with lower side-effects, higher effectiveness and longer protection duration in the future. We call for appropriate government subsidies for the rotavirus vaccine.
Introduction
Rotavirus (RV) is the most common pathogen of severe acute diarrhea. Almost every child will be infected more than once with RV before the age of 5.Citation1 In 2016, rotavirus caused more than 258 million episodes of diarrhea among children aged under five years worldwide, an incidence of 0.42 cases per child-year, resulting in 1.537 million hospitalizations and 128,000 deaths.Citation2 In northern and southern China, the annual incidence of rotavirus gastroenteritis (RVGE) in children under 5 years old is 54.7 and 45.6 per 1,000, respectively.Citation3 China has the highest annual social cost (US$365 million) for treating RVGE in Asia.Citation4 The disease seriously affects children’s health and causes a heavy disease and economic burden on individuals, health systems and society. Vaccination is currently regarded as the most effective method for reducing disease and economic burden.Citation5 In countries where the RV vaccine has been introduced, mortality and hospitalization rate for children with acute gastroenteritis caused by RV have reduced significantly.Citation6 Between 2006 and 2019, hospitalization rate of RVGE among children under 5 years old decreased by 59% in 49 countries.Citation7 From 1990 to 2016, there was a 48.2% decrease in RV-related deaths.Citation2 Surveillance of RVGE incidence and RV vaccination in children in Guangzhou, China, from 2007 to 2016 revealed a negative correlation between incidence risk and vaccination coverage.Citation8
The World Health Organization (WHO) recommends all countries to include the RV vaccine in immunization programs, and 109 countries and territories worldwide have included it by 2020.Citation9 The RV vaccination coverage in the United States, United Kingdom, and Canada is approximately 70.75%, 87.4%, and 85%, respectively.Citation10–12 The RV vaccines currently marketed in China include the Lanzhou lamb rotavirus vaccine (LLR) and the pentavalent human-bovine reassortment rotavirus vaccine (RV5), neither of which is included in the national immunization program. From 2008 to 2012, 32.8% of children in six Chinese provinces vaccinated LLR,Citation5 with 45%, 37.7%, and 15.5% of children in high-, middle-, and low-income regions, respectively. Overall, RV vaccination coverage in China is low and significantly varies by region. Understanding the parental preferences for RV vaccination to assess factors influencing vaccination decisions, and to further identify vaccination strategy with high preferences is an important guide for improving vaccination coverage. A discrete choice experiment (DCE) is a quantitative research method for scientifically measuring preferences that have been increasingly applied in vaccination studies.Citation13 In recent years, more and more scholars studied vaccine preferences of children’s parents using DCE. In 2017, Christine reviewed the application progress of DCE in existing studies on vaccination preference.Citation14 Some scholars have also applied the DCE to the study of rotavirus vaccination preference.Citation15–17 By analyzing the choice trade-offs of the study participants based on attribute levels, the quantitative effect of relevant factors influencing the study participants’ choice of vaccination is extracted.Citation18 There are more relevant studies in European and American countries, fewer in China.Citation14,Citation19 At present, studies on vaccination behavior preference internationally mainly focus on COVID-19, influenza, HPV and other vaccines with high public attention. However, the rotavirus vaccine is less well known than other vaccines. Parental preference for rotavirus vaccination has not been studied in China.
The main goal of this study is to examine the parental preferences for RV vaccination for their children under 5 years old. The second purpose is to predict the potential vaccination coverage of different vaccine scenarios. Based on this, we propose policy recommendations to improve the current coverage of RV vaccination and provide a basis for further improving immunization program and related strategies, so as to promote sustainable improvement of children’s health.
Methods
DCE can extract quantitative information about the inter-substitution of factors, and also has causal inference capabilities unavailable in general cross-sectional studies. Moreover, it can predict the choice probability of participants in different scenarios that provide forward-looking information for policy improvement. The method adopted in this study is based on the checklist and reports of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Conjoint Analysis Good Research Practices Task Force.Citation20–22
Identification of attributes and levels
Determining vaccine attributes and levels is crucial to ensure DCE efficacy. The selection of attributes should consider: (1) the correlation between attributes and the research problem; (2) The correlation between attributes and decision-making environment or background; (3) Whether an attribute is related to an attribute not included. The levels of this study were determined using real-world data on Chinese population rotavirus vaccines. Taking the actual situation as the reference level, other levels can be obtained by raising or lowering the level within the appropriate interval. First, nine attributes and respective levels were preliminary identified through literature review of studies on vaccination with DCE, RV vaccine and factors influencing vaccination. The nine attributes included vaccine effectiveness, out-of-pocket costs, risk of side-effects, protection duration, number of doses, vaccination sites, severity of disease, susceptibility to the disease, vaccine origin. Then, to determine the final attributes and levels, we consulted 4 experts studying vaccination with DCE, 3 experts studying RV vaccine, and conducted key informant interview with 12 pediatricians, 18 immunization service providers, 3 immunization policymaker and 17 parents of children under five years old from Jinan, Zhengzhou and Lanzhou. Attributes were ranked, increase and decrease. Finally, the top 5 recognized attributes were chosen. And, the reasonableness of levels setting were reviewed. Opinions were finally agreed after several discussions. Thereafter, a pilot survey was conducted with 30 parents. The pilot study found that some phrases and attribute definitions need to be precise, and problem descriptions need to be detailed. Based on the pilot, the questionnaire was subsequently revised. Five attributes and three levels for each attribute were finally included in the present study ().
Table 1. Attributes and levels used in the discrete choice experiment.
Experimental design
After determining the attributes and levels, we need to combine the attributes and levels into choice sets and present them to interviewees. Based on the attributes and levels set in this study (5 attributes, with 3 levels for each attribute), a total of 243 possible vaccination schemes would be generated, then randomly combining the two schemes into a choice set would yield (243 × 242)/2 = 29,403 choice sets. Obviously, it is impossible to present such a large number of choice sets to the respondents. Therefore, in order to solve this problem, we adopted Fractional Factorial Design. D-efficiency Design is one of the design methods of Fractional Factorial Design which emerged in recent years and has been applied more and more widely. It can give consideration to the balance and analysis of attribute level and obtain the most representative experimental combination. Specifically,18 choice sets were generated through D-efficient design using SAS 9.4. To reduce the cognitive load of respondents, according to the practice of previous DCE studies (choice sets were evenly divided into 2–3 blocks), the 18 choice sets were divided into 2 blocks, and each block of nine choice sets was randomly distributed to half of the study population. In addition, a choice set which one option significantly was superior to another was added to the DCE design(not included in regression analysis) to observe whether the respondents made a rational choice. If the respondent did not choose the optimal option, it indicated that the respondent did not really understand the experiment content, so this questionnaire should be excluded during the result analysis. Since the vaccine is not mandatory, we set up an “opt-out” option to simulate the real-world scenario better. Eventually, each questionnaire consisted of 10 choice sets and each choice set comprised three options, with Vaccine A, Vaccine B and no vaccination. An example of a choice set in the present study is displayed in .
Table 2. Example of choice sets.
Study sample and data collection
According to the economic development level and geographical area, Jinan City, Zhengzhou City and Lanzhou City were selected as the study areas. Jinan is located in eastern China and has a high level of economic development. Zhengzhou is located in central China, with a moderate level of economic development. Lanzhou is located in western China and has a low level of economic development. The sample was well representative. Questionnaires were collected by random sampling from parents of children under 5 years old in Jinan, Zhengzhou, and Lanzhou by distributing electronic questionnaires online between July 14 and July 31, 2022. Respondents met the following criteria: (1) They are parents of children under the age of 5; (2) They have normal cognitive abilities; (3) They volunteer to participate in this study. At the same time, we excluded parents who dropped out of the survey. The questionnaire consisted of two parts: the first part was a survey of the basic personal information, including age, gender, education, occupation, and annual family income, etc. The second section comprised of DCE questions. According to Orme’s rule of thumb,Citation23 the study required a minimum of 84 participants (500 × 3)/(9 × 2). Considering the precision and reliability of sample estimation in the three regions and possibility of the existence of missing values, we expanded the sample size. So, we intended to investigate 140 participants in each region. In practice, a total of 415 parents completed the questionnaire. We removed 35 questionnaires that did not pass the consistency test, and 21 questionnaires that always selected one option or were incomplete or did not meet the filling time requirement. Finally, 56 invalid questionnaires were eliminated and 359 valid questionnaires were obtained. Each block accounted for 49.58% and 50.42%, respectively. This study has been approved by the Medical Ethics Committee of the Center for Health Management and Policy Research, Shandong University (No. ECCHMPSDU20210402). The participants were briefed on the purpose of the study and their consent was obtained prior to the questionnaire.
Statistical analysis
Data analysis was performed with STATA 15.0. The descriptive analysis results on the participants’ socio-demographic characteristics are presented as numbers and percentages. We set out-of-pocket costs as a continuous variable and other attributes as categorical variables. The data were analyzed using Mixed-logit (MIXL) models to measure the parental preferences for different vaccine attributes. It fits the utility function as follows:
In this study, all coefficients of attribute levels were assumed to be the random normal distribution and independent. represents the utility value obtained after the parents made choices.
is a specific constant coefficient for the scenario (Alternative Specific Constant, ASC) to represent preferences that are inherent and independent of specific attribute values.
-
are the attribute coefficient representing the weight of each attribute level. By analyzing the sign (plus or minus), magnitude and significance of the coefficient
estimates, we can determine which attribute levels really influence choice behavior, as well as the direction and importance of the effects.
We then predicted uptake rates of RV vaccination under various scenarios to estimate individual’s preferred vaccination strategy. In this study, the hypothetical base vaccination strategy is 50% vaccine effectiveness, 1-year protection duration of vaccination, 1/10 risk of mild side-effects, CNY 900 as the vaccination cost, and 3-hour time for vaccination.
Where, is the probability of choosing alternative
among a set of
alternatives;
is the overall utility of the vaccination strategy;
is the utility of vaccination and non-vaccination.
Results
Participants’ characteristics
The sample was evenly distributed across the three regions. More than half (55.99%) of the parents’ children were boys, and 82.17% of the children live in urban areas. The percentage of children aged 3–5 years old was 47.63%. The majority of children (98.33%) had very good or good health condition. More than 90% of parents had a high education level (college or above), and 43.45% of the parents were between 30 and 34 years old. Most parents were employees of enterprise and government (72.70%). Almost 70% of the families were in the>100,000 income category (). Compared with the national census data, our sample showed a similar sex ratio, but higher educational attainment and proportion of living in the urban area.
Table 3. Characteristics of the study sample (n = 359).
Parental preferences
The impacts of the vaccine attribute levels on vaccine choice were all statistically significant (p < .01), except for 1-hour time for vaccination. The negative coefficient suggested that the parents preferred RV vaccination at a lower cost. The positive coefficients indicated that the parents preferred RV vaccination with higher effectiveness, longer protection duration, lower risk of mild side-effects and 2-hour time for vaccination. 1/50 risk of mild side-effects had the greatest impact on vaccine choice (β = 1.921, p < .001), followed by 90% vaccine effectiveness (β = 1.751, p < .001). The duration of protection and time required for vaccination also affected vaccine choice, but to a lesser extent than the risk of mild side-effects and effectiveness. In contrast, out-of-pocket costs had the smallest effect(β=-0.3 × 10−3, p < .01). The positive sign of the constant coefficient indicated that parents preferred to vaccinate their children to prevent RV ().
Table 4. Mixed logit model of respondent preferences (n = 359).
Relative importance of attributes
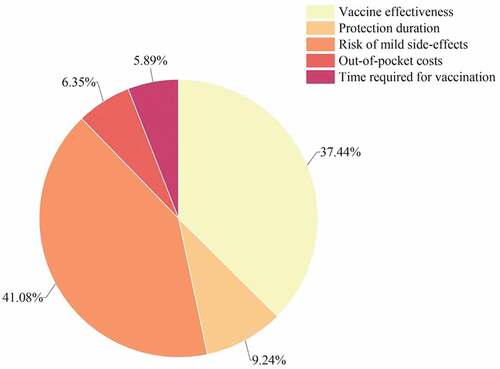
According to the results of relative importance of attributes, the risk of mild side-effects was the most important attribute influencing parents’ choice to vaccinate their children against RV, with the highest score (41.08%). The second was vaccine effectiveness (37.44%). The time required for vaccination was the least important attribute (5.89%), out-of-pocket costs also have a little effect on vaccination (6.35%) ().
Probability of vaccine uptake
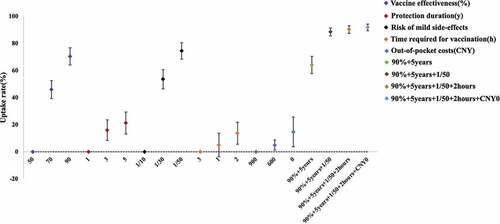
The probability of vaccination choices increased 45.94% and 70.41%, respectively, when vaccine effectiveness increased from 50% to 70% and 90%, respectively. When the protection duration increased from 1 year to 3 and 5 years, vaccine uptake increased 16.01% and 21.27%, respectively. The vaccine uptake would increase 53.56% and 74.45%, respectively, decreasing the vaccine risk of mild side-effects from 1/10 to 1/30 and 1/50. If the vaccine were free, vaccine uptake would increase 14.73%. Similarly, when the time required for vaccination decreased to 2 hours, the probability of vaccination choices increased 13.69%. In addition, we predicted the impacts of different combinations of vaccine choices. Compared to base vaccination strategy, the probability of vaccination choices can reach 91.79% when vaccination became the optimal vaccination strategy (90% vaccine effectiveness, 5-year duration of protection, 1/50 risk of mild side-effects, 2-hour time for vaccination, free vaccination) ().
Discussion
It is the first study to examine parental preferences for RV vaccination for their children younger than five years old in China using a discrete choice experiment. It was found that parents preferred RV vaccine with higher effectiveness, longer protection duration, lower risk of mild side-effects, 2-hour time for vaccination and lower cost. Parents were most concerned about the risk of mild side-effects. The time required for vaccination had the least effect on parents’ choice to vaccinate their children against RV. This study discovered that if attributes and levels were adjusted to the parents’ preferred vaccination choice, their probability of vaccination choice would vary, with the “90% vaccine effectiveness, 5-year duration of protection, 1/50 risk of mild side-effects, 2-hour time for vaccination, free vaccination” combination having the highest probability of choice.
This study showed that non-economic factors such as vaccine risk of mild side-effects and effectiveness substantially impacted vaccination. In contrast, the time required for vaccination and out-of-pocket costs had a small influence, similar to the findings of most studies.Citation24–26 However, this differs from a Netherlands study on parental preferences for RV vaccination in young children, which found that parental vaccination decisions were primarily driven by out-of-pocket costs, followed by vaccine effectiveness. This difference may be related to China’s child-centered cultural background. We also confirmed it through interviewing parents.
The study found the vaccine risk of mild side-effects was the most important factor, followed by effectiveness. A study from China supported that the probability of vaccination increased as vaccine effectiveness increased and side-effects decreased, with vaccine safety being considered more important than vaccine effectiveness.Citation27 A study of individual decisions to vaccinate their children or themselves found that vaccination is a multi-factor decision dominated by side-effects.Citation28 Vaccine safety and effectiveness have always been a concern of the public, organizations and governments,Citation29 and are the primary reasons for vaccination in all countries. Vaccine safety (24%) and effectiveness (21%) were also considered as the most common attributes in similar studies.Citation14 In recent years, China has experienced vaccine-related scandals that severely eroded parental confidence in vaccines. Concerns about vaccine safety can reduce or even eliminate the parents’ vaccination willingness,Citation30 and parents are more concerned about vaccine safety than ever. Our study predicted that RV vaccination rates would increase by 45.94% and 70.41% if vaccine effectiveness improved from 50% to 70 and 90%. Randomized controlled trials on Chinese children have shown that the effectiveness of domestic and imported RV vaccines for the Chinese population was 56.6% and 69.3%, respectively.Citation31,Citation32 The effectiveness of RV vaccines needs to be enhanced. Therefore, companies should pay more attention to improving safety and effectiveness in vaccine research and development. Similarly, healthcare professionals involved in immunization should also take parents’ perspective and explain in detail the risk of side-effects and the effectiveness of vaccines to allay their concerns. The above two points may play a specific role in promoting immunization coverage.
A Meta-analysis of randomized controlled trials of rotavirus vaccination evaluated the combined efficacy for severe RVGE at 2 weeks and 12 months after the last dose of vaccine. In high mortality areas, the combined efficacy was low at 2 weeks (66%) and declined rapidly to 44% at 12 months.Citation33 Vaccine manufacturers should make efforts to develop RV vaccines with long-term protective immunity. To our surprise, parents were more likely to prefer the 2-hour vaccination time, unlike other studies where parents preferred a shorter waiting time.Citation34,Citation35 The possible reason for this is that the attribute we included was the time required for vaccination, including travel time to and from the vaccination site, waiting time for vaccination, injection time, and observation time. Different from adults, immunization providers need to reassure children, provide more attentive services and be more patient, which may take longer. Several DCE studies on vaccination preferences showed that vaccination rates increased as vaccination costs decreased.Citation36 A survey in Australia showed that the inclusion of pneumococcal vaccines in a publicly funded program increased vaccination coverage from 7% to 51%.Citation37 Developed countries like Brazil and South Korea have also found that free vaccination improves vaccination rates.Citation38,Citation39 In China, RV vaccines are non-immunization programs, and vaccinators must reap the benefits of vaccination at the expense of other living expenses. This affects the willingness to vaccinate, especially among low-income people. We continue to call on the government to provide appropriate subsidies. For example, local governments allow RV payments from parents’ individual medical saving accounts, or provide free vaccinations to promote vaccination for more low-income families.
There are some limitations of this study. (1) Our study was conducted online as it was difficult to conduct an on-site questionnaire during the COVID-19 pandemic. The authenticity of personal information in online surveys cannot be tracked. And the survey population was relatively concentrated in the highly educated group, the youth group, and the urban area, which may underestimate the impact of costs on preferences. (2) Due to the impact of COVID-19 and financial constraints, a total of 415 parents completed the questionnaire. However, in the context of a large population in China, this sample size is still insufficient. Due to the small sample size, we presented demographics, but not about the demographic disparities in vaccination preferences or the demographics of study participants. In the future, we will expand the sample to explore further the interaction effects on individual characteristics and vaccination attribute preferences.
Although there are some limitations, this online survey has a representative sample from multiple regions in China. Furthermore, we quantitatively analyzed that vaccine-related attributes influence parental preferences to vaccinate with the RV vaccine. The results provide evidence of the effect of rotavirus vaccine characteristics on the vaccination behavior of children’s parents. This study provides an empirical basis for addressing the problem of actual vaccination rates that still fall short of the target and reluctance to vaccinate. It can be a reference for relevant government agencies and will guide policymakers and vaccine manufacturers to implement interventions to improve RV vaccination rates in children.
Conclusions
Chinese parents considered the risk of mild side-effects as the most important attribute of RV vaccination, followed by vaccine effectiveness, protection duration and out-of-pocket costs. Time required for vaccination was the least important attribute affecting parental preferences in RV vaccine choice. The authorities should support the production and development of high-quality vaccines by investing capital and resources and creating a healthy and favorable policy environment. The regulatory bureau should carry out strong supervision on vaccine production, storage, transportation, temperature monitoring and other aspects to ensure vaccine quality and safety. Immunization service providers should improve the quality of vaccination service. Furthermore, to improve the vaccination rate in China by reducing the cost, it is necessary to combine the results of disease burden research and health economic evaluation of the RV vaccine. Each province and city should decide based on their financial level.
Author contributions
All authors have contributed to the production of the article.
Acknowledgments
The authors are grateful to the Centre for Health Management and Policy Research, School of Public Health, Cheeloo College of Medicine, Shandong University. The authors thank editor and reviewers for suggestions that have significantly improved the paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- World Health Organization. Rotavirus vaccines. WHO position paper-January 2013. Wkly Epidemiol Rec. 2013;88(5):49–8.
- Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, Armah G, Bines JE, Brewer TG, Colombara DV, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018;172(10):958–65. doi:10.1001/jamapediatrics.2018.1960.
- Wang JX, Zhou HL, Mo ZJ, Wang S-M, Hao Z-Y, Li Y, Zhen S-S, Zhang C-J, Zhang X-J, Ma J-C, et al. Burden of viral gastroenteritis in children living in rural China: population-based surveillance. Int J Infect Dis. 2020;90:151–60. doi:10.1016/j.ijid.2019.10.029.
- Kawai K, O’brien MA, Goveia MG, Mast TC, El Khoury AC. Burden of rotavirus gastroenteritis and distribution of rotavirus strains in Asia: a systematic review. Vaccine. 2012;30(7):1244–54. doi:10.1016/j.vaccine.2011.12.092.
- Liu Y, Yue CY, Li Y, Liu C, Li S-L, Jiang X-Y. Analysis of vaccination situation of orial live attenuated rotavirus vaccine (LLR strain) among children in 6 provinces of China. Chin J prev med. 2018;52(3):282–86. doi:10.3760/cma.j.issn.0253-9624.2018.03.012.
- Aliabadi N, Antoni S, Mwenda JM, Weldegebriel G, Biey JNM, Cheikh D, Fahmy K, Teleb N, Ashmony HA, Ahmed H, et al. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008–16: findings from the global rotavirus surveillance network. Lancet Glob Health. 2019;7(7):e893–903. doi:10.1016/S2214-109X(19)30207-4.
- Burnett E, Parashar UD, Tate JE. Global impact of rotavirus vaccination on diarrhea hospitalizations and deaths among children <5 years old: 2006-2019. J Infect Dis. 2020;222(10):1731–39. doi:10.1093/infdis/jiaa081.
- Fu C, Dong Z, Shen J, Yang Z, Liao Y, Hu W, Pei S, Shaman J. Rotavirus gastroenteritis infection among children vaccinated and unvaccinated with rotavirus vaccine in Southern China. JAMA Netw Open. 2018;1(4):e181382. doi:10.1001/jamanetworkopen.2018.1382.
- World Health Organization. Map production Immunization Vaccines and Biologicals (IVB). WHO/IVB Database. [accessed 2022 Sep 15]. https://www.who.int/immunization/monitoring_surveillance/VaccineIntroStatus.pptx.
- Rogers MAM, Kim C, Hofstetter AM. Geospatial variation in rotavirus vaccination in infants, United States, 2010-2017. Emerg Infect Dis. 2019;25(10):1993–95. doi:10.3201/eid2510.190874.
- Byrne L, Ward C, White JM, Amirthalingam G, Edelstein M. Predictors of coverage of the national maternal pertussis and infant rotavirus vaccination programmes in England. Epidemiol Infect. 2018 Jan;146(2):197–206. doi:10.1017/S0950268817002497.
- Rafferty E, Guo X, McDonald B, Svenson LW, MacDonald SE. Measurement of coverage, compliance and determinants of uptake in a publicly funded rotavirus vaccination programme: a retrospective cohort study. BMJ Open. 2019 Nov 2;9(11):e031718. doi:10.1136/bmjopen-2019-031718.
- De Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ. 2012;21(2):145–72. doi:10.1002/hec.1697.
- Michaels-Igbokwe C, MacDonald S, Currie GR. Individual preferences for child and adolescent vaccine attributes: a systematic review of the stated preference literature. Patient. Dec 2017;10(6):687–700. doi:10.1007/s40271-017-0244-x.
- Veldwijk J, Lambooij MS, Bruijning-Verhagen PC, Smit HA, Wit GAD. Parental preferences for rotavirus vaccination in young children: a discrete choice experiment. Vaccine. 2014 Oct 29;32(47):6277–83. doi:10.1016/j.vaccine.2014.09.004.
- Veldwijk J, van der Heide I, Rademakers J, Schuit AJ, de Wit GA, Uiters E, Lambooij MS. Preferences for vaccination: does health literacy make a difference? Med Decis Making. 2015 Nov;35(8):948–58. doi:10.1177/0272989X15597225.
- Poulos C, Standaert B, Sloesen B, Stryjewska I, Janitsary A, Hauber B. Preferences for vaccines against children’s diarrheal illness among mothers in Poland and Hungary. Vaccine. 2018 Sep 25;36(40):6022–29. doi:10.1016/j.vaccine.2018.08.001.
- Diks ME, Hiligsmann M, van der Putten IM. Vaccine preferences driving vaccine-decision making of different target groups: a systematic review of choice-based experiments. BMC Infect Dis. 2021 Aug 28;21(1):879. doi:10.1186/s12879-021-06398-9.
- Poulos C. A review of conjoint-analysis studies of vaccine preferences. ISPOR 21st annual international meeting; 2016 May 21–25; Washington DC: United States: Research Triangle Park. RTI Health Solutions; 2016
- Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, Johnson FR, Mauskopf J. Conjoint analysis applications in health—a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011 Jun;14(4):403–13. doi:10.1016/j.jval.2010.11.013.
- Reed Johnson F, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, Bresnahan BW, Kanninen B, Bridges JFP. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health. 2013 Jan-Feb;16(1):3–13. doi:10.1016/j.jval.2012.08.2223.
- Hauber AB, González JM, Groothuis-Oudshoorn CG, Prior T, Marshall DA, Cunningham C, IJzerman MJ, Bridges JFP. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016 Jun;19(4):300–15. doi:10.1016/j.jval.2016.04.004.
- Orme B Sample size issues for conjoint analysis studies. Sequim: Sawtooth Software Technical Paper; 1998.
- Gong T, Chen G, Liu P, Lai X, Rong H, Ma X, Hou Z, Fang H, Li S. Parental vaccine preferences for their children in China: a discrete choice experiment. Vaccines (Basel). 2020 Nov 16;8(4):687. doi:10.3390/vaccines8040687.
- Sun X, Wagner AL, Ji J, Huang Z, Zikmund-Fisher BJ, Boulton ML, Ren J, Prosser LA. A conjoint analysis of stated vaccine preferences in Shanghai, China. Vaccine. 2020 Feb 5;38(6):1520–25. doi:10.1016/j.vaccine.2019.11.062.
- Verelst F, Kessels R, Willem L, Beutels P. No such thing as a free-rider? Understanding drivers of childhood and adult vaccination through a multicountry discrete choice experiment. Vaccines (Basel). 2021 Mar 16;9(3):264. doi:10.3390/vaccines9030264.
- Liao Q, Ng TWY, Cowling BJ. What influenza vaccination programmes are preferred by healthcare personnel? A discrete choice experiment. Vaccine. 2020 Jun 15;38(29):4557–63. doi:10.1016/j.vaccine.2020.05.012.
- Verelst F, Willem L, Kessels R, Beutels P. Individual decisions to vaccinate one’s child or oneself: a discrete choice experiment rejecting free-riding motives. Soc Sci Med. 2018 Jun;207:106–16. doi:10.1016/j.socscimed.2018.04.038.
- Kaplan RM, Milstein A. Influence of a COVID-19 vaccine’s effectiveness and safety profile on vaccination acceptance. Proc Natl Acad Sci U S A. 2021 Mar 9;118(10):e2021726118. doi:10.1073/pnas.2021726118.
- Zhou M, Qu S, Zhao L, Kong N, Campy KS, Wang S. Trust collapse caused by the Changsheng vaccine crisis in China. Vaccine. 2019;37(26):3419‐3425. doi:10.1016/j.vaccine.2019.05.020.
- Xia S, Du J, Su J, Liu Y, Huang L, Yu Q, Xie Z, Gao J, Xu B, Gao X, et al. Efficacy, immunogenicity and safety of a trivalent live human-lamb reassortant rotavirus vaccine (LLR3) in healthy Chinese infants: a randomized, double-blind, placebo-controlled trial. Vaccine. 2020 Oct 27;38(46):7393–400. doi:10.1016/j.vaccine.2020.04.038.
- Mo Z, Mo Y, Li M, Tao J, Yang X, Kong J, Wei D, Fu B, Liao X, Chu J, et al. Efficacy and safety of a pentavalent live human-bovine reassortant rotavirus vaccine (RV5) in healthy Chinese infants: a randomized, double-blind, placebo-controlled trial. Vaccine. 2017 Oct 13;35(43):5897–904. doi:10.1016/j.vaccine.2017.08.081.
- World Health Organization. Rotavirus vaccines: WHO position paper-July 2021. Wkly Epidemiol Rec. 2021;96(28):301–20.
- Wang Y, Hu Y, Chen Y, Liang H. Preference and willingness to pay of female college students for human papillomavirus vaccination in Zhejiang Province, China: a discrete choice experiment. Hum Vaccin Immunother. 2021 Oct 3;17(10):3595–602. doi:10.1080/21645515.2021.1932215.
- Eshun-Wilson I, Mody A, Tram KH, Bradley C, Sheve A, Fox B, Thompson V, Geng EH. Preferences for COVID-19 vaccine distribution strategies in the US: a discrete choice survey. PLoS One. 2021 Aug 20;16(8):e0256394. doi:10.1371/journal.pone.0256394.
- Guo N, Wang J, Nicholas S, Maitland E, Zhu D. Behavioral differences in the preference for hepatitis B virus vaccination: a discrete choice experiment. Vaccines (Basel). 2020 Sep 14;8(3):527. doi:10.3390/vaccines8030527.
- Andrews RM. Assessment of vaccine coverage following the introduction of a publicly funded pneumococcal vaccine program for the elderly in Victoria, Australia. Vaccine. 2005;23(21):2756–61. doi:10.1016/j.vaccine.2004.11.039.
- Hunan Provincial Preventive Medicine Association. Expert consensus on developing publicly-funded influenza vaccination project plan. Chin J Prev Med. 2020;54:1364–77. doi:10.3760/cma.j.cn112150-20200806-01009.
- Yun JW, Noh JY, Song JY, Chun C, Kim Y, Cheong HJ. The Korean influenza national immunization program: history and present status. Infect Chemother. 2017;49(4):247–54. doi:10.3947/ic.2017.49.4.247.