ABSTRACT
The risk for acquiring human papillomavirus (HPV) infections and associated diseases is lifelong. An important part of prophylactic HPV vaccine development is durable protection against infection and disease. With comprehensive long-term follow-up (LTFU) in adolescents, men, and women, the quadrivalent HPV (qHPV) vaccine demonstrated durable effectiveness, immunogenicity, and safety, with almost no breakthrough disease. Those who received a placebo during initial trials were offered the qHPV vaccine at study conclusion and continued to be followed in LTFU extensions. In this catch-up vaccination group, LTFU demonstrated protection even in individuals with current or prior HPV infection after approximately 3 years. The initial efficacy and durable long-term effectiveness of the qHPV vaccine have already translated to a real-world reduction in cancer and cancer precursors. To date, there is no evidence of waning protection; evidence suggests that vaccination ultimately provides strong protection against future disease, with effective prophylaxis even among those with past infections.
Human papillomavirus (HPV) causes cancers and other diseases. An estimated 85% of sexually active women and 91% of sexually active men acquire HPV at some point during their lifetime.Citation1 Clearly, vaccination prior to HPV exposure provides the broadest protection, but given the lifetime risk of infection, the HPV vaccine should also provide durable protection to maximize benefit. To address the durability of protection, the World Health Organization (WHO) considers long-term follow-up (LTFU) evaluation of effectiveness, immunogenicity, and safety an integral part of prophylactic HPV vaccine development.Citation2
Some older adults remain at risk for HPV infection throughout their lives and might benefit from vaccination later in life (catch-up) if they were not vaccinated as adolescents.Citation3 Indeed, in per-protocol analyses, the quadrivalent HPV (qHPV) vaccine demonstrated 94.1% efficacy against cervical dysplasia (any grade) related to vaccine HPV types 6/11/16/18 in women up to age 45 years in the global FUTURE III studyCitation4 and 100% efficacy in a separate study in China.Citation5
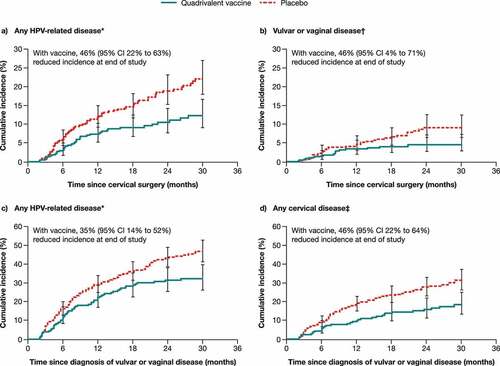
Data from men and women with a history of HPV-related diseases further support the potential for HPV vaccination to protect adults.Citation6–9 In a retrospective analysis of data from two qHPV vaccine efficacy studies in young women who underwent cervical surgery or were diagnosed with genital warts or vulvar/vaginal disease related to infection present before vaccination (), prior qHPV vaccination was associated with a significant reduction (46.2−64.9% for those who underwent cervical surgery; 35.2% for those diagnosed with infection-related disease) in any subsequent HPV-related disease, including high-grade disease.Citation6 Other studies demonstrated that HPV vaccination before and after surgical treatment for cervical lesions reduced the risk of subsequent cervical intraepithelial neoplasia (CIN) grade 2 or higher, related to HPV16/18 (88.2% efficacy 60 days or more post-surgery),Citation8 and the risk of recurrent CIN 2–3 post-surgery was higher in qHPV vaccine non-recipients compared with recipients (hazard ratio [HR] 2.840).Citation9 In a nonconcurrent cohort study of young men who have sex with men (MSM) with a history of anal high-grade squamous intraepithelial lesions (HSIL), a positive oncogenic HPV test was associated with an increased risk of recurrent anal HSIL, while qHPV vaccination was associated with a significant reduction in anal HSIL recurrence within 2 years post-treatment (HR 0.47) when compared with unvaccinated MSM.Citation7
Figure 1. Time to detection of any HPV-related disease (a) or vulvar or vaginal disease (b) after cervical surgery; and of any HPV-related disease (c) or any cervical disease (d) after diagnosis of vulvar or vaginal disease. Case counting began 60 days after surgery or diagnosis.
From the initial approval of the qHPV vaccine, individuals and clinicians have rightfully questioned the duration of qHPV vaccine effectiveness as well as its utility in those with prior HPV infection. Some delayed vaccination until post-adolescence to prevent waning immunity in later years or forwent vaccination entirely because of prior infection. Long-term follow-up of effectiveness and safety was critical to inform clinical decisions. Of note, induction of durable effectiveness following a complete regimen of qHPV vaccine was deemed possible since vaccination with three doses of qHPV vaccine was shown to induce long-term immune memory.Citation10 Moreover, vaccination with three or four doses of hepatitis B vaccine, another recombinant protein vaccine, demonstrated long-term protection.Citation11
Overview of qHPV vaccine LTFU data
Four qHPV vaccine studies were designed to evaluate the long-term effectiveness of the qHPV vaccine in clinical trial participants over 10–14 years follow-up ().Citation12,Citation14,Citation17,Citation20 The qHPV vaccine LTFU study recently reported by Maldonado et al. (Study 019) in this journal involved women aged 27–45 years who participated in the FUTURE III base study at sites in Colombia.Citation12 Two prior open-label LTFU extensions of randomized, placebo-controlled, Phase 3 efficacy, immunogenicity, and safety studies have been reported: Study 020 had a similar design as the Maldonado et al. study and included young men, both heterosexual and MSM,Citation14 while Study 015 leveraged Nordic national registries to evaluate vaccine efficacy in young women compared with historical rates in an unvaccinated population.Citation17 Study 018 involved LTFU extension of a placebo-controlled immunogenicity and safety study in boys and girls vaccinated at the age of 9–15 years.Citation20 While effectiveness could not be evaluated in that base study, given young adolescents are not typically exposed to HPV, the LTFU included an evaluation of effectiveness during the LTFU period, as the participants reached age ≥16 years. The LTFU extensions from all four baseline studies were carried out in the same rigorous fashion as the initial studies. Individuals were seen yearly and lesions suspicious for HPV-related disease were biopsied. Pathology panel evaluation was performed on all biopsy specimens, and HPV typing was conducted to determine endpoint attribution.
Table 1. Summary of qHPV vaccine LTFU studies.
Across all four studies, no breakthrough HSIL was observed in per-protocol analyses during LTFU among participants vaccinated at the beginning of the base study (). In women who received the qHPV vaccine at age 27–45 years at the start of FUTURE III, there were no cases of HPV6/11/16/18-related CIN or condyloma after a maximum follow-up of 10.1 years (median: 8.9 years) after the third vaccine dose.Citation12 Similarly, in women who received the qHPV vaccine at age 16–23 years in FUTURE II, there were no cases of HPV16/18-related high-grade CIN after a maximum follow-up of 14.0 years (median: 11.9 years); vaccine effectiveness was 100% (95% CI: 94.7–100.0) compared with an unvaccinated population.Citation17 Among the young men vaccinated with the qHPV vaccine at the start of Study 020, there were no cases of HPV6/11/16/18-related external genital lesions (EGL) and no cases of HPV6/11/16/18-related anal HSIL during LTFU after a maximum follow-up of 11.5 years (median: 9.5 years).Citation14 There was a single case of breakthrough anal low-grade dysplasia (LSIL) among MSM during LTFU for Study 020. Two HPV types, HPV6/58, were identified in the lesion, of which HPV6 was a vaccine type, hence the LSIL was defined as a breakthrough case.Citation14 Finally, in Study 018, no cases of HPV6/11/16/18-related cervical or external genital neoplasia were observed during LTFU after a maximum follow-up of 10 years (median: 9.9 years).Citation20 The rates of HPV6/11/16/18-related persistent infection among Study 018 participants vaccinated at age 9–15 years were low and within the ranges observed in the vaccinated cohorts in previous efficacy studies.
Table 2. Summary of the effectiveness endpoints in the qHPV vaccine LTFU studies (per-protocol analyses).
In each of the placebo-controlled qHPV vaccine trials, participants randomized to placebo were offered catch-up qHPV vaccination at the end of the base study.Citation12,Citation14,Citation17,Citation20 Such catch-up vaccination groups (CVGs) were followed during LTFU in men aged 16–26 years (Study 020), women aged 24–45 years (Study 019), and boys and girls aged 9–15 years (Study 018) and can provide some information about the effects of delayed vaccination.Citation12,Citation14,Citation20 Given the delay of vaccination by 3–4 years, CVG participants in Studies 019 and 020 were older and had more sexual partners at the time of qHPV vaccination compared with their counterparts who were randomized to qHPV vaccine at the start of the base study. Consistent with this, some individuals became infected with HPV during the base studies. In the CVG of Study 020, 15% of the men were seropositive for any vaccine HPV type prior to catch-up vaccination, while only 7% were seropositive at Day 1 of the base study.Citation14 Women in the CVG who were 27–45 years of age at baseline of Study 019 were age 32–50 years prior to catch-up vaccination and 31.7% and 32.4% were seropositive for any vaccine HPV type at baseline and before vaccination, respectively.Citation12 Despite this, the incidence rates of HPV6/11/16/18-related disease endpoints were markedly lower among both women (Study 019) and men (Study 020) in the CVG during the LTFU period (i.e., after qHPV vaccination) in comparison to the base study period (i.e., before qHPV vaccination) (). Furthermore, in the LTFU extension of the FUTURE II study,Citation17 a vaccine effectiveness analysis was conducted among women with serologic evidence of prior HPV infection (serology-positive), but without active infection (PCR-negative) prior to vaccination in the base study. No cases of HPV6/11/16/18-related cervical/vulvar/vaginal disease were observed over 14 years based on 4064.6 person-years of follow-up.Citation17 Accordingly, HPV vaccination may be effective in preventing lesions in individuals with serologic evidence of prior HPV infection.
Table 3. Summary of the effectiveness endpoints in the qHPV vaccine LTFU studies (intention-to-prevent analyses).
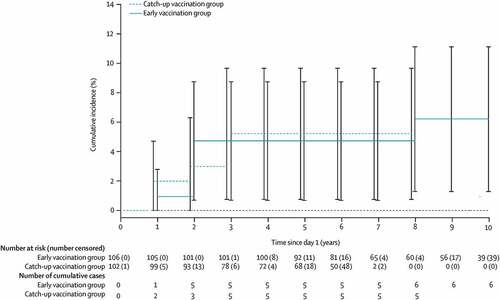
In the analysis of HPV6/11/16/18-related anal intraepithelial neoplasia (AIN) among MSM from the CVG of Study 020, a ‘washout’ phase was observed in the number of events over time from vaccination, with most events occurring early ().Citation14 Indeed, all five of the cases of AIN in the CVG occurred in the first 3 years after vaccination. These early cases were likely due to a prevalent infection, followed by a plateau in event occurrence with increased time since vaccination.Citation14
Figure 2. Cumulative incidence of anal intraepithelial neoplasia and anal cancer related to HPV6, 11, 16, and 18 in men who have sex with men vaccinated with the quadrivalent HPV vaccine in the long-term follow-up study.
Across the qHPV vaccine LTFU studies, anti-HPV6/11/16/18 antibodies peaked at Month 7, declined sharply through Month 12–24 and more gradually thereafter, and persisted through at least 10 years of follow-up.Citation12,Citation14,Citation17,Citation20
Consistent with the established safety profile of the qHPV vaccine, no vaccine-related serious adverse events (AEs) were reported during LTFU in the studies that included clinical follow-up visits.Citation12,Citation14,Citation20
Commentary
The described LTFU data from clinical trials show that the qHPV vaccine provides durable prevention of high-grade precancers across female and male populations.Citation12,Citation14,Citation17,Citation20 The results were remarkably consistent across the LTFU studies, which included substantially different populations, not only in terms of gender and age but also in terms of geographic regions and income levels. While the LTFU study in women 27–45 years of age included participants from a single middle-income country (Colombia),Citation12 the vaccine was similarly effective in younger women aged 16–23 years from high-income countries (Nordic),Citation17 and the young men and adolescents from multiple countries in global studies.Citation14,Citation20 Observed discontinuation rates in Studies 018, 019, and 020 (which involved periodic clinical assessments of study participants) during LTFU were approximately 4–6% per year, which was similar to base study rates; discontinuation was primarily due to loss to follow-up or withdrawn consent.Citation12,Citation14,Citation20 In Study 015, which was a registry-based, passive follow-up for effectiveness, only 1 participant (<0.1%) withdrew consent.Citation17
The results from CVG participants provide evidence that unvaccinated adults continued to be at risk for new HPV infection and disease related to the four vaccine types. However, once vaccinated, these individuals derived subsequent protection from the disease after a “wash-out” period of approximately 2 years.Citation14 Furthermore, post-hoc analyses of qHPV vaccine clinical trial participants who underwent surgeries to remove lesions showed that prior qHPV vaccination was effective at preventing subsequent disease in men and women.Citation6–9 These findings, together with demonstration of durable effectiveness across the LTFU studies, suggest that vaccinated adult populations can continue to benefit from reduced disease risk over the long term. While it is preferable to vaccinate individuals prior to sexual debut and exposure to HPV, individuals who were not vaccinated as young adolescents may still benefit from delayed HPV vaccination, even in the face of active disease and infection. This harkens back to the baseline studies in females where even those previously infected with vaccine types and without active infection at the time of qHPV vaccination developed significant protection from vaccine (efficacy against HPV6/11/16/18-related cervical dysplasia: 100% [95% CI: 12.7–100]; in this group of study participants, efficacy against HPV6/11/16/18-related condyloma 100% [95% CI: 28.3–100]; efficacy against HPV6/11/16/18-related persistent infection: 66.8% [95% CI: 3.8–90.5]) when compared to those who received placebo.Citation4,Citation22 The delay in significant efficacy is probably also related to this “wash-out” phenomenon of clearance of prevalent vaccine viral types followed by protection from vaccine against reinfection and possible reactivation. HPV based on L1 virus-like particle vaccines are prophylactic and do not have therapeutic effects.
The four LTFU studies all involved a 3-dose regimen of the qHPV vaccine.Citation12,Citation14,Citation17,Citation20 Moreover, robust immune memory responses were observed following the antigen challenge at 5 years post-primary dose series.Citation10 There is interest in alternative dosing regimens to facilitate broad implementation of HPV vaccination.Citation23 HPV vaccination is now widely licensed and recommended as a 2-dose series in young adolescents, administered 6–12 months apart.Citation24–26 Persistent antibody responses to a 2-dose regimen of the qHPV vaccine through 10 years have been shown in a clinical trial in girls vaccinated at age 9–13 years of age, which were non-inferior to those observed in women aged 16–26 years who received a 3-dose regimen.Citation27 The long-term effectiveness of the 2-dose regimen was inferred based on these results. In 2019, the WHO proposed that some countries with operational and programmatic issues may adopt an extended interval (3–5 years) between the two doses;Citation28 a clinical study is ongoing to rigorously assess the immunogenicity and safety of extended interval 2-dose regimens.Citation29 In 2022, the WHO proposed that each country could decide to implement alternative, off-label regimens, including a 2-dose schedule in all age groups or a 1-dose schedule for individuals aged 9–20 years.Citation23 Studies have demonstrated that the licensed 2- and 3-dose regimens elicit high-level, long-term protection in multiple demographic groups. It will be critical to rigorously assess whether a single-dose HPV vaccine regimen induces similar long-term protection.
While efficacy against subsequent invasive cancers could not be evaluated directly in the context of a clinical trial due to the rarity of disease and length of time between infection and cancer development, there is strong evidence that treating high-grade precursor lesions prevents cancer. It is well established that definitive treatment of high-grade cervical disease can prevent progression to invasive cervical cancer.Citation30 The ANCHOR trial similarly showed that in people living with HIV, treating anal HSIL reduced the risk of anal cancer by approximately 60%, compared with close monitoring alone.Citation31 Indeed, emerging real-world observations in the decades following HPV vaccine licensure demonstrate decreases in incidence of invasive cervical cancer in vaccinated populations.Citation32–36 Thus, it is reasonable to expect that decreases in HPV-related surrogate endpoints (e.g., precancers) observed in LTFU studies will correspondingly impact associated cancers.
The observed response durability together with the clear connection between vaccine efficacy against surrogate endpoints (e.g., precancers) in clinical trials and decreases in invasive cervical cancer in real-world studies is promising for vaccine use in the context of HPV-related head and neck cancer prevention. The burden of HPV-related head and neck cancer is substantial.Citation37 Routine screening is lacking, and treatment of precancers is not possible in this context. As such, HPV vaccination currently represents the only approach for cancer prevention. While qHPV vaccine efficacy against oral HPV infection and oropharyngeal cancer has not been directly evaluated in large prospectively designed clinical trials, lower rates of oral infection with qHPV vaccine HPV types have been reported in vaccinated cohorts.Citation38–41 A clinical trial (Study 049) was recently initiated to directly evaluate 9-valent HPV vaccine efficacy against HPV oral persistent infection, a possible endpoint for HPV-related head and neck cancer.Citation42
Editorial opinion summary
In clinical trials, the qHPV vaccine demonstrates durable effectiveness through 10+ years LTFU in women aged 27–45 years, consistent with observations in younger populations of women and men. Vaccination of young adolescents is ideal, but age and history of HPV infection should not be a barrier to vaccination.
The US Advisory Committee on Immunization Practices (ACIP) recommends routine catch-up HPV vaccination for individuals through age 26 years.Citation3 Blanket catch-up vaccination of all adults aged >26 years is not currently recommended, although it is recognized that some adults aged 27–45 years who are not adequately vaccinated might benefit from vaccination.Citation3 For this age group, shared clinical decision-making is recommended between provider and patient to determine if vaccination is warranted. Certain populations are at even higher risk for HPV-related disease, including immunocompromised individuals (e.g., people living with HIV, transplant recipients, and people receiving immunotherapy or chemotherapy), MSM, and transgender people, and are most likely to benefit from catch-up vaccination.
Questions remain regarding the best use of screening to reduce HPV-related disease burden alongside vaccination. For cervical and anal cancer, it is unclear whether screening practices will shift toward testing for HPV infection and away from cytological testing. Patients experience some morbidity and negative impact on quality of life related to screening procedures. Moreover, once detected during screening, further care is needed to deal with infection and treat HPV-related precancers and cancers. The durable effectiveness of HPV vaccines could lead to significant benefits over screening alone in terms of both psychosocial and physical well-being and cost savings as such post-screening care would not be required.
The efficacy seen in the CVG after a “wash-out” period has multiple clinical implications that clinicians and individuals should consider when discussing the utility of catch-up vaccination. A recommendation for catch-up vaccination should not be withheld even if there is evidence of prior infection or HPV-related disease. In fact, the LTFU studiesCitation12,Citation14,Citation17,Citation20 as well as other prior studiesCitation6–9 demonstrated post-treatment reduction in disease recurrence for women and men. This also has potential implication in post-treatment surveillance algorithms (possibly lengthening intervals in those disease-free 3 years post-vaccination), but real-world data will be required before any such changes.
While HPV vaccines are indicated in individuals 9 years and above (with no upper age limit) in certain countries, they are only indicated in individuals 9–45 years of age in other countries. In the latter situation, off-label catch-up vaccination may be considered even in those who might suddenly find themselves at increased risk for HPV-related disease and post-treatment recurrence because of immune compromise. Individuals in this category could include those living with or at increased risk for HIV infection (intravenous drug users, sex workers, and MSM with multiple partners), those placed on solid-organ transplant lists, and those requiring immune modulating medication or chemotherapy. Individuals at risk for new HPV infection and possible disease, including those having new or multiple sexual partners, might also benefit from protection afforded by catch-up vaccination irrespective of age.
The LTFU data pointing to continued vaccine effectiveness should not hinder vaccination in those between the recommended 9–11 years of age because of a fear of waning immunity by the time of sexual debut. Moreover, there are no current data supporting the need for booster vaccination.
Last but not least, these new data further emphasize the strong benefit of vaccination in low resource locations. Vaccinating children in areas where routine screening for HPV-related disease is limited can obviously reduce the number of individuals at risk for cancer who would not be identified at a precancerous stage. For those with the disease, catch-up vaccination at the time of treatment can potentially reduce the risk for recurrent disease and cancer, especially in settings with low potential for post-treatment evaluation.
The LTFU data show that the qHPV vaccine (and probably the 9-valent HPV vaccine by extension) supports continued effectiveness and safety for up to over 10 years without the need for booster vaccination. While qHPV vaccination should occur before potential exposure, catch-up vaccination is effective even in those with prior disease and should be offered. Other individuals at increased risk of HPV-related diseases should be offered catch-up vaccination, potentially even if they are over 45 years of age.
Acknowledgments
Medical writing assistance, under the direction of the author, was provided by Erin Bekes, PhD, and Derick Osakunor, PhD, of CMC AFFINITY, a division of IPG Health Medical Communications Ltd., in accordance with Good Publication Practice (GPP 2022) guidelines. This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Disclosure statement
Dr Goldstone reports speaker honoraria from, and being an investigator for, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; being an investigator for Inovio; receiving research support from Medtronic; and being a consultant for THD Solutions.
Additional information
Funding
References
- Chesson HW, Dunne EF, Hariri S, Markowitz LE. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41(11):660–8. doi:10.1097/OLQ.0000000000000193.
- World Health Organization (WHO). Expert committee on biological standardization. Guidelines to assure the quality, safety, and efficacy of recombinant HPV virus-like particle vaccines. Geneva, Switzerland: World Health Organization; 2006.
- Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the advisory committee on immunization practices. Morb Mortal Wkly Rep. 2019;68(32):698–702. doi:10.15585/mmwr.mm6832a3.
- Castellsagué X, Muñoz N, Pitisuttithum P, Ferris D, Monsonego J, Ault K, Luna J, Myers E, Mallary S, Bautista OM, et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age. Br J Cancer. 2011;105(1):28–37. doi:10.1038/bjc.2011.185.
- Wei L, Xie X, Liu J, Zhao Y, Chen W, Zhao C, Wang S, Liao X, Shou Q, Qiu Y, et al. Efficacy of quadrivalent human papillomavirus vaccine against persistent infection and genital disease in Chinese women: a randomized, placebo-controlled trial with 78-month follow-up. Vaccine. 2019;37(27):3617–24. doi:10.1016/j.vaccine.2018.08.009.
- Joura EA, Garland SM, Paavonen J, Ferris DG, Perez G, Ault KA, Huh WK, Sings HL, James MK, Haupt RM. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data. BMJ. 2012;344:e1401. doi:10.1136/bmj.e1401.
- Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis. 2012;54(7):891–98. doi:10.1093/cid/cir1036.
- Garland SM, Paavonen J, Jaisamrarn U, Naud P, Salmerón J, Chow SN, Apter D, Castellsagué X, Teixeira JC, Skinner SR, et al. Prior human papillomavirus-16/18 AS04-adjuvanted vaccination prevents recurrent high grade cervical intraepithelial neoplasia after definitive surgical therapy: post-hoc analysis from a randomized controlled trial. Int J Cancer. 2016;139(12):2812–26. doi:10.1002/ijc.30391.
- Kang WD, Choi HS, Kim SM. Is vaccination with quadrivalent HPV vaccine after loop electrosurgical excision procedure effective in preventing recurrence in patients with high-grade cervical intraepithelial neoplasia (CIN2–3)? Gynecol Oncol. 2013;130(2):264–68. doi:10.1016/j.ygyno.2013.04.050.
- Olsson S-E, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, Iversen O-E, Høye J, Steinwall M, Riis-Johannessen G, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007;25(26):4931–39. doi:10.1016/j.vaccine.2007.03.049.
- Van Damme P, Dionne M, Leroux-Roels G, Der Meeren OV, Di Paolo E, Salaun B, Surya Kiran P, Folschweiller N. Persistence of HBsAg-specific antibodies and immune memory two to three decades after hepatitis B vaccination in adults. J Viral Hepat. 2019;26(9):1066–75. doi:10.1111/jvh.13125.
- Maldonado I, Plata M, Gonzalez M, Correa A, Nossa C, Giuliano A, Joura EA, Ferenczy A, Ronnett BM, Stoler MH, et al. Effectiveness, immunogenicity, and safety of the quadrivalent HPV vaccine in women and men aged 27–45 years. Hum Vaccin Immunother. 2022;18(5):2078626. doi:10.1080/21645515.2022.2078626.
- Muñoz N, Manalastas R Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, Clavel C, Luna J, Myers E, Hood S, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009;373(9679):1949–57. doi:10.1016/S0140-6736(09)60691-7.
- Goldstone SE, Giuliano AR, Palefsky JM, Lazcano-Ponce E, Penny ME, Cabello RE, Moreira ED, Baraldi E, Jessen H, Ferenczy A, et al. Efficacy, immunogenicity, and safety of a quadrivalent HPV vaccine in men: results of an open-label, long-term extension of a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2022;22(3):413–25. doi:10.1016/S1473-3099(21)00327-3.
- Giuliano AR, Palefsky JM, Goldstone S, Moreira ED Jr, Penny ME, Aranda C, Vardas E, Moi H, Jessen H, Hillman R, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 2011;364(5):401–11. doi:10.1056/NEJMoa0909537.
- Palefsky JM, Giuliano AR, Goldstone S, Moreira ED Jr, Aranda C, Jessen H, Hillman R, Ferris D, Coutlee F, Stoler MH, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576–85. doi:10.1056/NEJMoa1010971.
- Kjaer SK, Nygård M, Sundström K, Dillner J, Tryggvadottir L, Munk C, Berger S, Enerly E, Hortlund M, Ágústsson ÁI, et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine. 2020;23:100401. doi:10.1016/j.eclinm.2020.100401.
- The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–27. doi:10.1056/NEJMoa061741.
- Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Brown DR, Koutsky LA, Tay EH, García P, et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (Types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev Res (Phila). 2009;2(10):868–78. doi:10.1158/1940-6207.CAPR-09-0031.
- Ferris DG, Samakoses R, Block SL, Lazcano-Ponce E, Restrepo JA, Mehlsen J, Chatterjee A, Iversen O-E, Joshi A, Chu J-L, et al. 4-valent human papillomavirus (4vHPV) vaccine in pre-adolescents and adolescents after 10 years. Pediatrics. 2017;140(6):e20163947. doi:10.1542/peds.2016-3947.
- Reisinger KS, Block SL, Lazcano-Ponce E, Samakoses R, Esser MT, Erick J, Puchalski D, Giacoletti KED, Sings HL, Lukac S, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J. 2007;26(3):201–09. doi:10.1097/01.inf.0000253970.29190.5a.
- Olsson SE, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Brown DR, Koutsky LA, Hseon Tay E, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin. 2009;5(10):696–704. doi:10.4161/hv.5.10.9515.
- World Health Organization (WHO). Human papillomavirus vaccines: WHO position paper (2022 update). Wkly Epidemiol Rec. 2022;97:645–72.
- World Health Organization (WHO). Human papillomavirus vaccines: WHO position paper, May 2017. Wkly Epidemiol Rec. 2017;92(19):241–68.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the advisory committee on immunization practices. Morb Mortal Wkly Rep. 2016;65(49):1405–08. doi:10.15585/mmwr.mm6549a5.
- D’Addario M, Redmond S, Scott P, Egli-Gany D, Riveros-Balta AX, Henao Restrepo AM, Low N. Two-dose schedules for human papillomavirus vaccine: systematic review and meta-analysis. Vaccine. 2017;35(22):2892–901. doi:10.1016/j.vaccine.2017.03.096.
- Donken R, Dobson SRM, Marty KD, Cook D, Sauvageau C, Gilca V, Dionne M, McNeil S, Krajden M, Money D, et al. Immunogenicity of 2 and 3 doses of the quadrivalent human papillomavirus vaccine up to 120 months postvaccination: follow-up of a randomized clinical trial. Clin Infect Dis. 2020;71(4):1022–29. doi:10.1093/cid/ciz887.
- World Health Organization Strategic Advisory Group of Experts (WHO/SAGE). Meeting of the strategic advisory group of experts on immunization, October 2019: conclusions and recommendations. Wkly Epidemiol Rec. 2019;94:541–60.
- Teppler H, Bautista O, Flores S, McCauley J, Luxembourg A. Design of a Phase III immunogenicity and safety study evaluating two-dose regimens of 9-valent human papillomavirus (9vHPV) vaccine with extended dosing intervals. Contemp Clin Trials. 2021;105:106403. doi:10.1016/j.cct.2021.106403.
- World Health Organization (WHO). WHO guidelines for treatment of cervical intraepithelial neoplasia 2–3 and adenocarcinoma in situ: cryotherapy, large loop excision of the transformation zone, and cold knife conization; 2014.
- Palefsky JM, Lee JY, Jay N, Goldstone SE, Darragh TM, Dunlevy HA, Rosa-Cunha I, Arons A, Pugliese JC, Vena D, et al. Treatment of anal high-grade squamous intraepithelial lesions to prevent anal cancer. N Engl J Med. 2022;386(24):2273–82. doi:10.1056/NEJMoa2201048.
- Falcaro M, Castañon A, Ndlela B, Checchi M, Soldan K, Lopez-Bernal J, Elliss-Brookes L, Sasieni P. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and Grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet. 2021;398(10316):2084–92. doi:10.1016/S0140-6736(21)02178-4.
- Kjaer SK, Dehlendorff C, Belmonte F, Baandrup L. Real-world effectiveness of human papillomavirus vaccination against cervical cancer. J Natl Cancer Inst. 2021;113(10):1329–35. doi:10.1093/jnci/djab080.
- Lei J, Ploner A, Elfström KM, Wang J, Roth A, Fang F, Sundström K, Dillner J, Sparén P. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383(14):1340–48. doi:10.1056/NEJMoa1917338.
- Luostarinen T, Apter D, Dillner J, Eriksson T, Harjula K, Natunen K, Paavonen J, Pukkala E, Lehtinen M. Vaccination protects against invasive HPV-associated cancers. Int J Cancer. 2018;142(10):2186–87. doi:10.1002/ijc.31231.
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi:10.3322/caac.21763.
- de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8(2):e180–90. doi:10.1016/S2214-109X(19)30488-7.
- Abel MK, Mann AK, Sonawane K, Kapp DS, Deshmukh AA, Chan JK. Prevalence of oral human papillomavirus infection by number of vaccine doses among US adults. JNCI Cancer Spectr. 2021;5(6):pkab086. doi:10.1093/jncics/pkab086.
- Castillo A, Osorio JC, Fernández A, Méndez F, Alarcón L, Arturo G, Herrero R, Bravo LE. Effect of vaccination against oral HPV-16 infection in high school students in the city of Cali, Colombia. Papillomavirus Res. 2019;7:112–17. doi:10.1016/j.pvr.2019.03.001.
- Chaturvedi AK, Graubard BI, Broutian T, Pickard RKL, Tong ZY, Xiao W, Kahle L, Gillison ML. Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J Clin Oncol. 2018;36(3):262–67. doi:10.1200/JCO.2017.75.0141.
- Grün N, Ährlund-Richter A, Franzén J, Mirzaie L, Marions L, Ramqvist T, Dalianis T. Oral human papillomavirus (HPV) prevalence in youth and cervical HPV prevalence in women attending a youth clinic in Sweden, a follow up-study 2013–2014 after gradual introduction of public HPV vaccination. Infect Dis (Lond). 2015;47(1):57–61. doi:10.3109/00365548.2014.964764.
- Giuliano AR, Wilkin T, Bautista OM, Cheon K, Connor L, Dubey S, Luxembourg A, Rawat S, Shaw A, Velicer C, et al. Design of a phase III efficacy, immunogenicity, and safety study of 9-valent human papillomavirus vaccine in prevention of oral persistent infection in men. Contemp Clin Trials. 2022;115:106592. doi:10.1016/j.cct.2021.106592.
