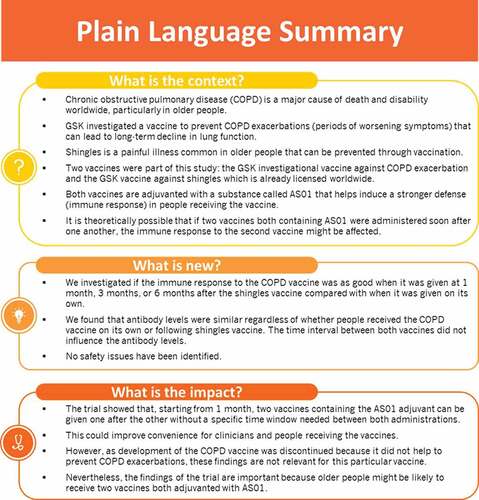
ABSTRACT
A candidate AS01-adjuvanted vaccine containing four surface proteins from non-typable Haemophilus influenzae and Moraxella catarrhalis (NTHi-Mcat) has been developed to help prevent exacerbations of chronic obstructive pulmonary disease (COPD). Sequential administration of different vaccines containing the same AS01-adjuvant system could lead to immune interference. We compared administration of NTHi-Mcat following AS01-adjuvanted recombinant zoster vaccine (RZV) versus NTHi-Mcat alone. This phase 2a, open-label trial (NCT03894969) randomized healthy current or former smokers (50–80 years) without COPD to administration of NTHi-Mcat at 1, 3 or 6 months after RZV or to NTHi-Mcat alone (2-dose for both vaccines). Primary outcome was non-inferiority of the humoral immune response to NTHi-Mcat administered 1 month after RZV versus NTHi-Mcat alone, evaluated by specific antibody geometric mean concentration (GMC) ratio with 95% confidence intervals (CIs). The per-protocol set included 411 participants. Primary objective was met; lower limit of the 95%CI for the GMC ratio above 0.667 for all four vaccine antigens, 1 month after the second NTHi-Mcat dose. NTHi-Mcat induced similar immune response regardless of whether administered alone or 1, 3 or 6 months following RZV. Safety and reactogenicity profiles were acceptable; adverse event frequency was similar among study groups. Injection site pain was the most common symptom. No new safety concerns were identified. The study demonstrated non-inferiority of the immune response elicited by NTHi-Mcat administered sequentially to RZV versus NTHi-Mcat alone, indicating no immune interference. Starting from 1 month, no specific interval is required between RZV and NTHi-Mcat containing the same AS01-adjuvant system components in different quantities.
Introduction
The Global Burden of Disease Study estimates that chronic obstructive pulmonary disease (COPD) was the sixth leading cause of loss of disability-adjusted life years in 2019 and the third leading cause in people ≥75 years of age.Citation1 Exacerbations, defined as acute worsening of respiratory symptoms resulting in additional treatment, are an important feature of COPD, associated with deteriorating health status, increases in hospitalization and disease progression.Citation2 Preventing exacerbations is an important management goal for patients with COPD.Citation2 Exacerbations are usually associated with viral or bacterial infections or environmental factors such as pollution.Citation2 Non-typable Haemophilus influenzae (NTHi) and Moraxella catarrhalis (Mcat) are among the most common bacteria identified in the sputum of patients with COPD during exacerbation events and are often isolated as co-pathogens.Citation3,Citation4
A novel area of research into prevention of COPD exacerbations is the development of vaccines against associated bacterial infections. GSK has developed a multicomponent vaccine (NTHi-Mcat vaccine) consisting of three surface proteins from NTHi and one surface protein from Mcat, adjuvanted with the Adjuvant System AS01E. The vaccine has been shown to be immunogenic and have an acceptable safety profile in phase 1 and 2 studies, but did not demonstrate efficacy against moderate and severe COPD exacerbations in a phase 2 study.Citation5,Citation6 Development of the vaccine has been discontinued.
Herpes zoster, or shingles, is a painful condition that usually manifests as a blistering rash and can be associated with complications including long-term neuropathic pain.Citation7 The incidence of herpes zoster varies by age, gender and geographical location, and has been estimated at between 5.23 and 10.9 cases per 100,000 population in adults ≥50 years of age.Citation8 The incidence appears to be increasing over time and rises with older age.Citation8 A recombinant zoster vaccine (RZV), adjuvanted with the Adjuvant System AS01B, has been shown to prevent herpes zoster infection in adults ≥50 years of age and is licensed in many countries for that indication, as well as in adults who are at increased risk of herpes zoster infection because of immunodeficiency or immunosuppression.Citation9,Citation10
Although development of the NTHi-Mcat vaccine has been halted, it is likely that other AS01E-adjuvanted and AS01B-adjuvanted vaccines could be administered in the same populations. The use of an adjuvant is intended to induce higher and more sustained immunogenicity notably in elderly individuals and those with an impaired immune response.Citation11,Citation12 AS01B and AS01E contain the same components (3-O-desacyl-4'-monophosphoryl lipid A [MPL], Quillaja saponaria Molina, fraction 21 [QS21] licensed by GSK from Antigenics LLC, a wholly owned subsidiary of Agenus Inc., a Delaware, USA corporation and liposomes) although in different quantities. There is a theoretical possibility that sequential administration of different vaccines containing the same AS01 Adjuvant System could lead to a tolerance effect that would decrease the immunogenicity of the second vaccine (immune interference). More recently, there has been a hypothesis that AS01 might induce trained immunity, resulting in an increase in the immune response to subsequent antigens.Citation13 A key objective of the present study was to evaluate whether immune interference occurs with sequential administration of two different vaccines adjuvanted with the same AS01, the first study to evaluate this theoretical concern. In this phase 2a non-inferiority study, NTHi-Mcat vaccine was administered in healthy adults 50–80 years of age either following RZV or without previous RZV administration. The immunogenicity and safety of NTHi-Mcat vaccine when given sequentially to RZV and when given alone were compared. The key findings of the study are summarized in .
Material and methods
The study was a phase 2a, randomized, open-label study done in 14 centers in Estonia, Finland, France, Italy and Spain (NCT03894969). The study was approved by independent ethics committees or institutional review boards, conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice (ICH-GCP) guidelines, and regulatory requirements of participating countries. Participants provided written informed consent.
Participants, study design and vaccines
The study included healthy adults 50–80 years of age who were either a current or former smoker with a smoking history of ≥10 pack-years. Because cigarette smoking is the most common risk factor for COPD, participants with this smoking history were chosen to immunologically represent the vaccine’s target population as closely as possible. Participants with a COPD diagnosis of any severity were excluded. Other exclusion criteria are shown in the Appendices. Participants received the licensed RZV in a 2-dose schedule, 2 months apart, followed by the first dose of the NTHi-Mcat vaccine at 1, 3 or 6 months after the second RZV dose. The NTHi-Mcat vaccine was given in a 2-dose schedule, 2 months apart. A control group received only the NTHi-Mcat vaccine. The first RZV dose was given on Day 1 of the study and participants were followed up until Day 661.
GSK supplied both study vaccines. RZV consists of a recombinant subunit varicella-zoster virus glycoprotein E antigen (50 µg) and the AS01B Adjuvant System (50 µg MPL, 50 µg QS21 and liposomes). The NTHi-Mcat vaccine is a multicomponent vaccine consisting of four surface proteins: three proteins from NTHi, a free recombinant protein D (PD, 10 µg) and a recombinant fusion protein combining protein E and pilin A (PE-PilA, 10 µg), and the Mcat ubiquitous surface protein A2 (UspA2, 3.3 µg). The NTHi-Mcat vaccine is adjuvanted with the AS01E Adjuvant System, consisting of 25 µg MPL, 25 µg QS21 and liposomes. Both vaccines were administered as a 2-dose series as an intramuscular injection (0.5 mL) in the deltoid of the non-dominant arm.
Participants were randomized equally to one of four study groups: (1) first dose of NTHi-Mcat vaccine administered 1 month after the second dose of RZV (NTHi-Mcat_1 group); (2) first dose of NTHi-Mcat vaccine administered 3 months after the second dose of RZV (NTHi-Mcat_3 group); (3) first dose of NTHi-Mcat vaccine administered 6 months after the second dose of RZV (NTHi-Mcat_6 group); (4) NTHi-Mcat alone. Vaccines were randomized within blocks by GSK using the MATerial EXcellence (MatEx) program. The investigators allocated participants to a study group using a randomization system on internet (SBIR), with a minimization procedure accounting for age category (50–59, 60–69, 70–80 years), smoking status (current or former) and center. Minimization factors had equal weight in the algorithm.
Study outcomes and assessments
The primary outcome was the demonstration of non-inferiority of the humoral immune response to the NTHi-Mcat vaccine administered 1 month after RZV administration versus NTHi-Mcat administration alone, measured 1 month after the second dose of NTHi-Mcat vaccine. Blood samples for evaluation of immunogenicity of the NTHi-Mcat vaccine were taken pre-vaccination and 1 month after the second dose. Humoral immunogenicity was evaluated in terms of anti-PD, anti-PE, anti-PilA and anti-UspA2 IgG antibodies using an enzyme-linked immunosorbent assay (ELISA) developed by GSK using an in-house reference serum. The assay cutoff values corresponded to the assay’s lower limit of quantification (LLOQ) and were 153 EU/mL for anti-PD, 16 EU/mL for anti-PE, 8 EU/mL for anti-PilA and 28 EU/mL for anti-UspA2. Geometric mean concentrations (GMCs) and the proportion of participants with antibody concentrations above the assay cutoff level were calculated.
Secondary outcomes were a description of the humoral immune response, cell mediated immunity (CMI) in terms of CD4+ T-cells, as well as safety and reactogenicity of the NTHi-Mcat vaccine. Tertiary (exploratory) outcomes were the description of the CMI response of the NTHi-Mcat vaccine in terms of CD8+ T-cells and the T-helper profile of the vaccine antigen-specific CD4+ and CD8+ T-cell responses.
The CMI response was evaluated by flow cytometry using the intracellular cell staining assay, adapted from a previously described method.Citation14 Following incubation, cells were washed and stained with anti-CD4-PerCP and anti-CD8-APC-Cy7. The frequencies of vaccine antigen-specific CD4+ and CD8+ T-cells expressing at least two different markers from CD40 ligand (CD40L), interleukin-2 (IL-2), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-13 (IL-13) and interleukin-17 (IL-17) upon in vitro stimulation with each antigen were computed (per 106 cells). In addition, the T-helper profile of the specific T-cell response in T-helper-1, T-helper-2 and T-helper-17 cells was characterized based on specific expression of IFN-γ, IL-13 and IL-17, respectively.
Participants recorded solicited injection site and general symptoms on the day of each NTHi-Mcat vaccination and for the next 6 days. Unsolicited (spontaneously reported) adverse events (AEs) were recorded until 30 days after vaccination. Serious adverse events (SAEs), potential immune-mediated diseases (pIMDs), and AEs leading to discontinuation were recorded until the final study contact.
Statistics
The primary endpoint of non-inferiority was considered for the group that received NTHi-Mcat vaccine 1 month after the second dose of RZV (NTHi-Mcat_1 group) versus NTHi-Mcat administration alone. Descriptive analyses were done for the groups that received NTHi-Mcat vaccine 3 and 6 months following RZV (NTHi-Mcat_3 and NTHi-Mcat_6 groups). The primary objective was changed by protocol amendment from a sequential assessment of non-inferiority in the NTHi-Mcat_6 group, followed by the NTHi-Mcat_3 group and lastly by the NTHi-Mcat_1 group due to the impact of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic on study procedures (e.g. missed study visits or visits performed out of the allowed interval) that caused the NTHi-Mcat_3 and NTHi-Mcat_6 groups to not achieve the minimum sample size for the per protocol set (PPS).
Two-sided 95% confidence intervals (CIs) were computed for the antibody GMC ratio (NTHi-Mcat vaccine administered following RZV over NTHi-Mcat vaccine alone) using an analysis of covariance (ANCOVA) model on the log10 transformations of the antibody concentrations. The ANCOVA model included study group, smoking status (current or former), age category (50−59, 60−69, 70–80 years) and center as factors and antibody concentration before the first dose of NTHi-Mcat vaccine as a covariate. Non-inferiority criteria were met if the lower limit (LL) of the 2-sided 95% CIs for the GMC ratio was above 0.667 for all four antibodies.
The study sample size was defined to ensure a global 80% power to conclude non-inferiority for the response to all four antigens. The target response-wise power was 94.6%, assuming independence, for the response to all four antigens. The sample size calculation was based on a standard deviation of antibody log10 concentrations of 0.36 and a null true difference on the log10 scale. Based on these calculations, the target sample size was 432 participants evaluable for immunogenicity (108 per group). Assuming that 20% of participants might withdraw, be non-evaluable or excluded due to protocol deviation, the target for randomization was 540 participants (135 per group).
The primary immunogenicity analysis was done in the PPS, comprizing all participants who received the full course of study vaccine to which they were randomized and had post-vaccination data, excluding participants with protocol deviations that led to exclusion. CMI was evaluated in a sub-cohort of 60 participants. Safety was evaluated in the safety set which included all participants who received at least one NTHi-Mcat vaccine dose.
Results
The first participant was enrolled on 23 April 2019 and the last study visit took place on 13 August 2021. A total of 541 participants were enrolled and randomized, all of whom received at least one dose of NTHi-Mcat vaccine or RZV; 16 participants received only RZV and were excluded from the safety set which therefore comprized 525 participants (). The PPS included 411 participants (). Baseline demographic characteristics were similar between study groups. In the safety set, mean age was approximately 60 years, approximately three-quarters were aged ≤64 years, there were similar proportions of men and women and similar proportions of current and former smokers (). Most participants were White. Participant baseline characteristics in the PPS were similar across the four vaccine groups (data not shown).
Figure 2. Participant disposition.
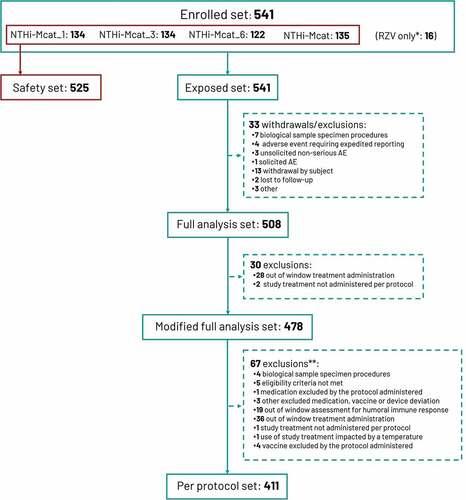
Table 1. Baseline demographics.
Humoral immune response
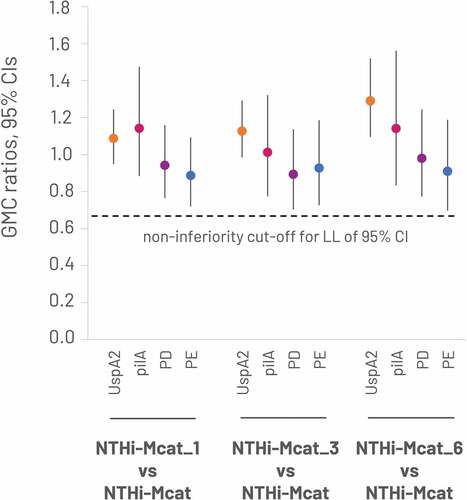
The study met its primary objective by demonstrating non-inferiority of the immune response to NTHi-Mcat vaccine in the NTHi-Mcat_1 group versus the NTHi-Mcat alone group at 1 month after the second dose of NTHi-Mcat vaccine. The LL of the GMC ratio was above 0.667 for the anti-PD, anti-PE, anti-PilA and anti-UspA2 antibodies (). Descriptive analysis of the immune response in the NTHi-Mcat_3 and NTHi-Mcat_6 groups versus the NTHi-Mcat alone group showed similar results ().
Figure 3. Non-inferiority of sequential administration of RZV and NTHi-Mcat vaccine versus NTHi-Mcat alone: GMC ratios at 1 month after the second dose of NTHi-Mcat vaccine (per-protocol set).
The NTHi-Mcat vaccine was immunogenic against all four antigens. Pre-vaccination GMCs increased by 1 month after the second NTHi-Mcat vaccine dose (). Pre-vaccination, the percentage of participants in each study group with antibody titers above the assay LLOQ was 100% for anti-UspA2, 38.1–49.0% for anti-PilA, 16.9–21.8% for anti-PD and 50.7–63.5% for anti-PE. Post-vaccination, all participants had antibody titers above the assay LLOQ for anti-UspA2, anti-PilA and anti-PE antibodies; >97% had antibody titers above the assay LLOQ for anti-PD antibodies. Most participants had an 8-fold increase in anti-PilA (98.4–99.1%), anti-PD (68.8–83.1%) and anti-PE (97.6–100%) antibodies post-vaccination (). For anti-UspA2 antibodies, few participants had an 8-fold increase (2.4–12.3%), but 44.4–64.6% had a 2-fold increase ().
Figure 4. GMCs against each NTHi-Mcat vaccine antigen pre-vaccination and at 1 month after the second vaccine dose (per-protocol set).
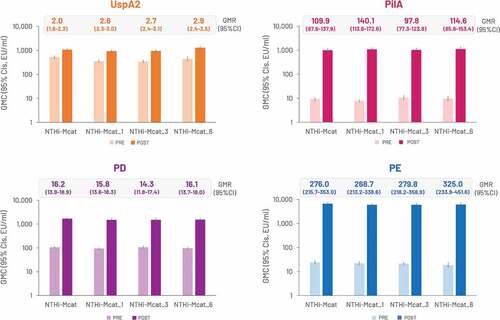
Table 2. Percentage of participants with 2-, 4- and 8-fold increase for each NTHi-Mcat vaccine antigen from pre-vaccination to 1 month after the second vaccine dose (per-protocol set).
Cell-mediated immune response
The analysis was performed in 38 participants from the CMI sub-cohort of 60 participants and measured the frequencies of vaccine antigen-specific CD4+ T-cells expressing at least two different markers from CD40L, IL-2, TNF-α, IFN-γ, IL-13 and IL-17 on in vitro stimulation. Frequencies rose from pre-vaccination to post-vaccination for each antigen-specific CD4+ T-cell in all study groups (). No apparent CD8+ T-cell response was observed (data not shown).
Figure 5. Frequency of antigen-specific CD4+ T-cells expressing at least two activation markers upon in vitro stimulation with each NTHi-Mcat vaccine antigen, pre-vaccination and at 1 month after the second vaccine dose (per-protocol CMI sub-cohort).
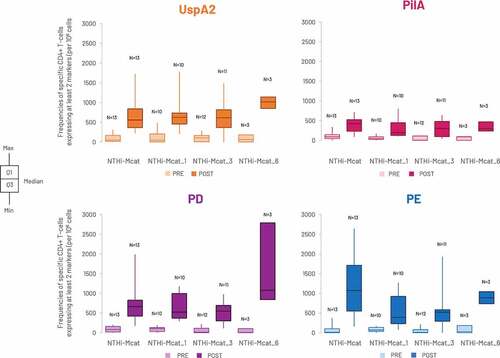
The T-helper profile was explored with the frequencies of antigen-specific CD4+ or CD8+ T-cells expressing IFN-γ, IL-13 or IL-17, which are commonly associated with a T-helper 1, T-helper 2 or T-helper 17 profile, respectively. The frequencies of CD4+ T-cells expressing IFN-γ rose post-vaccination, indicating a T-helper 1 response; however, there was no evidence of a T-helper 2 or T-helper 17 response (Supplement Figure S1; Supplement Table S1). There was no evidence of a CD8+ T-helper response (data not shown).
Safety and reactogenicity
Solicited injection site and general AEs occurring within 7 days of a NTHi-Mcat vaccination were reported in a similar number of participants in each study group (). A slight increase in the proportion of participants reporting solicited local AEs after the second NTHi-Mcat vaccination compared with the first NTHi-Mcat vaccination was observed in all study groups (61.9%, 72.9%, 64.4%, 68.1% after the first vaccination and 70.7%, 77.9%, 74.6%, 77.2% after the second vaccination in the NTHi-Mcat_1, NTHi-Mcat_3, NTHi-Mcat_6 and NTHi-Mcat alone groups, respectively). Pain was the most frequent AE in all groups. Severe solicited AEs were rare (). At least one unsolicited AE occurring during the 30-day period after NTHi-Mcat vaccination was experienced by 25.4% of participants in the NTHi-Mcat_1 group, 24.6% in the NTHi-Mcat_3 group, 16.4% in the NTHi-Mcat_6 group and 23.0% in the NTHi-Mcat alone group. Infections and infestations, and musculoskeletal and connective tissue disorders were the most common unsolicited AEs, occurring in 7.6% and 5.1% of all participants, respectively. With regard to unsolicited AEs reported in the 30-day post-vaccination period, causally-related AEs occurred in a total of 26 (5.0%) participants, severe (grade 3) AEs occurred in 13 (2.5%) participants, and severe, causally-related AEs occurred in two (0.4%) participants.
Table 3. Participants reporting solicited injection site and general adverse events during the 7-day post-vaccination period (safety set).
Over the entire study period (up to Day 661), 32 participants experienced a SAE, including one death. One participant in the NTHi-Mcat_3 group died from a ruptured cerebral aneurysm at Day 324, not considered related to vaccination. Nine participants experienced at least one AE leading to premature withdrawal. Six pIMDs were reported by five participants: polymyalgia rheumatica and giant cell arteritis were experienced by one participant in the NTHi-Mcat_1 group; rheumatoid arthritis, Basedow’s disease and gout were experienced by one participant each in NTHi-Mcat_3 group; and psoriasis was experienced by one participant in the NTHi-Mcat_6 group. The polymyalgia rheumatica and giant cell arteritis were considered as SAEs related to vaccination; the other pIMDs were considered unrelated to vaccination. The participant with polymyalgia rheumatica and giant cell arteritis was a 72-year-old female with a medical history of hypertension, hypercholesterolemia and vaginal dryness who had previously experienced a heart attack and angioplasty. Grade 3 polymyalgia rheumatica occurred 321 days after the second dose of NTHi-Mcat vaccine; 40 days later, the participant was hospitalized and diagnosed with giant cell arteritis and polymyalgia rheumatica.
Discussion
Our study demonstrated the safety and immunogenicity of sequential administration at least 1 month apart of two vaccines adjuvanted with the same AS01 Adjuvant System. We found no evidence of immune interference occurring with sequential administration of two AS01-adjuvanted vaccines, the first study to investigate this theoretical concern. The study met its primary objective by demonstrating non-inferiority of the humoral immune response to NTHi-Mcat vaccine components when administered 1 month following the second dose of RZV versus NTHi-Mcat administered alone. Descriptive analysis of the humoral immune response to NTHi-Mcat vaccine administered 3 and 6 months after the second dose of RZV reported similar findings.
All immunogenicity parameters evaluated as secondary endpoints were similar with sequential administration of RZV followed by NTHi-Mcat vaccine and NTHi-Mcat vaccine administered alone. Antibody titers against all four vaccine antigens were low before vaccination but rose sharply by 1 month after the second vaccine dose. Pre-vaccination, all participants had antibody titers above the assay LLOQ for anti-UspA2, while for the other antigens, this ranged from 16.9% for anti-PD to 63.5% for anti-PE. Most participants had an 8-fold increase in antibody concentration against PD, PE and PilA post-vaccination; however, because all participants had pre-vaccination anti-UspA2 antibody titers above the assay LLOQ, the post-vaccination fold increase was limited. Similar robust humoral immune responses have been observed in previous studies of the vaccine when administered alone.Citation5,Citation6,Citation15,Citation16
The frequencies of vaccine antigen-specific CD4+ T-cells expressing at least two activation markers rose from pre-vaccination to post-vaccination, suggesting a CD4+ T-cell response. A T-helper 1 response was also observed. These data should be interpreted with caution as the analysis was done in a small sub-cohort of participants and the data were highly variable within and between study groups. There was no evidence of a CD8+ T-cell response or a T-helper 2 or T-helper 17 response.
The safety and reactogenicity profile of the NTHi-Mcat vaccine was consistent with previous studies.Citation5,Citation6,Citation15,Citation16 Reactogenicity was similar in the study groups who received NTHi-Mcat after RZV and the group who received NTHi-Mcat vaccine alone and there were few severe reactogenicity events. Unsolicited AEs occurred with similar frequency in all study groups and were mainly mild or moderate in severity. There was one death during the study, not considered related to vaccination. One participant experienced two pIMDs, polymyalgia rheumatica and giant cell arteritis, that were considered related to the NTHi-Mcat vaccine. No other SAEs or pIMDs were considered related to vaccination.
This is the first study to evaluate sequential administration of two different AS01-adjuvanted vaccines; importantly, it demonstrates that safety and immunogenicity are not impacted and there was no evidence of immune interference. The NTHi-Mcat vaccine is adjuvanted with AS01E and the RZV is adjuvanted with AS01B; both Adjuvant Systems contain the same components (MPL, QS21 and liposomes), although in different quantities. Before this study was conducted, there was a theoretical concern that sequential administration of different vaccines containing the same AS01 adjuvant might lead to a tolerance effect that would decrease the immunogenicity of the second vaccine (immune interference). An alteration of the effect of the AS01 adjuvant after sequential doses could be due to a modification in innate responsiveness to AS01 or remodeling of the draining lymph node. It was not known whether this theoretical phenomenon would occur in humans, so immune interference between two AS01-adjuvanted vaccines could not be excluded. Our study was designed to evaluate immune interference in a rigorous manner by non-inferiority testing and, accordingly, adequately powered. The results support a statistically sound conclusion that immune tolerance was not observed when these two vaccines adjuvanted with the same AS01 components were sequentially administered. Future studies looking at sequential administration of different vaccines adjuvanted with the same AS01 might be performed in the future.
Adjuvants are a valuable tool in enhancing vaccine immunogenicity in the elderly, whose immune response is often compromised by immunosenescence.Citation11,Citation12 There is increasing interest in the use of vaccines in older adults and the ability to co-administer or sequentially administer different vaccines is important.Citation12 In this study, we confirmed that two vaccines containing the same AS01 components, although in different quantities, can be safely administered sequentially. We also demonstrated that, starting from 1 month, no specific time window is required to ensure an adequate immune response, which could improve convenience for clinics and vaccine recipients.
A limitation of the study is that smokers and former smokers were used as a proxy for patients with COPD who are the target population for the NTHi-Mcat vaccine. The rationale for the selection of the study population was that smokers and former smokers have been shown to have similar immunological characteristics to patients with COPD.Citation17–19 Development of the NTHi-Mcat vaccine has been discontinued following the failure of a previous phase 2b study to show vaccine efficacy in reducing the rate of moderate or severe COPD exacerbations.Citation6 There is therefore no plan to further explore sequential administration of the vaccines in patients with COPD. A further limitation is that the study did not evaluate immunogenicity and safety of the vaccines when administered on the same day.
In conclusion, the study demonstrated immunological non-inferiority of sequential administration of the NTHi-Mcat vaccine following RZV administration versus administration of the NTHi-Mcat vaccine alone. No specific time window was required between the two vaccines, which contain the same AS01 components in different quantities.
Contributorship
IG, MT, DC, LG, AT, and AKA contributed to the study design and methods. AP, RJ, V-JA, SM, AMB, and OL were responsible for the oversight of the study at their respective sites and contributed to the recruitment of participants. IG, MT, DC, LG, AT, MD, and AKA were responsible for data acquisition, and/or data analysis, and/or data interpretation. All authors read and edited the manuscript. All authors approved the final version and the decision to submit the manuscript. All authors had full access to all the data and had final responsibility for the decision to submit for publication.
Data sharing
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Trademark
MF59 is a trademark of Novartis.
Supplemental Material
Download PDF (388.7 KB)Supplemental Material
Download PDF (287.9 KB)Supplemental Material
Download PDF (231.5 KB)Acknowledgments
The authors thank the study participants and their families, and thank the members of the NTHi-Mcat-009 study group who are not part of the authorship for their contributions to the study including: FINLAND – Eija-Riitta Salomaa, Peter Csonka; FRANCE – Florence Galtier, Fabrice Laine; ITALY – Ferdinando De Negri. Thanks also to Bertrand Colignon (Laboratory manager responsible for the supervision of the COPD ELISA testing in Clinical Laboratory Sciences; GSK), Camilla D’Eramo (Study Delivery Lead; GSK), Yashodhan Deshpande (Study Delivery Lead; GSK), Sophie Eugène (Clinical Laboratory study manager in Clinical Laboratory Sciences; GSK), Michel Janssens (Expert Scientist responsible for CMI in Clinical Laboratory Sciences; GSK), Sonia Schoonbroodt (Clinical readout team leader in Clinical Laboratory Sciences; GSK), Magali Traskine (Senior Biostatistician; GSK). The authors also thank Business & Decision Life Sciences platform for editorial assistance, manuscript coordination, and writing support, on behalf of GSK. Mary Greenacre (independent medical writer, on behalf of Business & Decision Life Sciences) provided medical writing support.
Disclosure statement
IG, LG, AT, MD, and AKA are employed by GSK. MD is also married to an employee of GSK and both own shares in GSK. AKA is an inventor named on patent applications filed in the name of GSK. RJ reports personal fees from GSK, during the conduct of the study. RJ also discloses personal fees and non-financial support from GSK and Boehringer Ingelheim, and personal fees from Novartis, outside the submitted work. MT was an employee of GSK when this study was conducted and is now an employee of Janssen-Cilag S.p.A. Cologno Monzese, Italy. MT is an inventor named on patent applications filed in the name of GSK. DC was an employee of GSK when this study was conducted and is now an employee of Seqirus srl. DC is an inventor named on patent applications filed in the name of GSK. V-JA reports consulting fees from MSD and Pfizer, and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from MSD, Pfizer, Astellas, Roche, Biogen, Unimed, Astra-Zeneca, and GSK, outside the submitted work. AP and SM have nothing to disclose. OL reports payment or honoraria for educational events from MSD, Pfizer, Sanofi Pasteur, Janssen and GSK, outside the submitted work and DMSB participation from Sanofi and MSD. AMB has received advisory fees and speaker fees from Janssen and Pfizer. All authors declare no other financial and non-financial relationships and activities.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2187194.
Additional information
Funding
References
- Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, Abbasi-Kangevari M, Abbastabar H, Abd-Allah F, Abdelalim A, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–10. doi:10.1016/S0140-6736(20)30925-9.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for prevention, diagnosis and management of COPD: 2023 report. Global Initiative for Chronic Obstructive Lung Disease, Inc.; 2022.
- Wilkinson TMA, Aris E, Bourne S, Clarke SC, Peeters M, Pascal TG, Schoonbroodt S, Tuck AC, Kim V, Ostridge K, et al. A prospective, observational cohort study of the seasonal dynamics of airway pathogens in the aetiology of exacerbations in COPD. Thorax. 2017;72(10):919–27. doi:10.1136/thoraxjnl-2016-209023.
- Mayhew D, Devos N, Lambert C, Brown JR, Clarke SC, Kim VL, Magid-Slav M, Miller BE, Ostridge KK, Patel R, et al. Longitudinal profiling of the lung microbiome in the AERIS study demonstrates repeatability of bacterial and eosinophilic COPD exacerbations. Thorax. 2018;73(5):422–30. doi:10.1136/thoraxjnl-2017-210408.
- Van Damme P, Leroux-Roels G, Vandermeulen C, De Ryck I, Tasciotti A, Dozot M, Moraschini L, Testa M, Arora AK. Safety and immunogenicity of non-typeable Haemophilus influenzae-Moraxella catarrhalis vaccine. Vaccine. 2019;37(23):3113–22. doi:10.1016/j.vaccine.2019.04.041.
- Andreas S, Testa M, Boyer L, Brusselle G, Janssens W, Kerwin E, Papi A, Pek B, Puente-Maestu L, Saralaya D, et al. Non-typeable Haemophilus influenzae–Moraxella catarrhalis vaccine for the prevention of exacerbations in chronic obstructive pulmonary disease: a multicentre, randomised, placebo-controlled, observer-blinded, proof-of-concept, phase 2b trial. Lancet Respir Med. 2022;10(5):435–46. doi:10.1016/S2213-2600(21)00502-6.
- Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, Harpaz R. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. Morb Mortal Wkly Rep. 2018;67(3):103–08. doi:10.15585/mmwr.mm6703a5.
- van Oorschot D, Vroling H, Bunge E, Diaz-Decaro J, Curran D, Yawn B. A systematic literature review of herpes zoster incidence worldwide. Hum Vaccin Immunother. 2021;17(6):1714–32. doi:10.1080/21645515.2020.1847582.
- Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–96. doi:10.1056/NEJMoa1501184.
- Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Diez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barberà J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–32. doi:10.1056/NEJMoa1603800.
- Didierlaurent AM, Laupèze B, Di Pasquale A, Hergli N, Collignon C, Garçon N. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines. 2017;16(1):55–63. doi:10.1080/14760584.2016.1213632.
- Cunningham AL, McIntyre P, Subbarao K, Booy R, Levin MJ. Vaccines for older adults. BMJ. 2021;372:n188. doi:10.1136/bmj.n188.
- Bruxvoort KJ, Ackerson B, Sy LS, Bhavsar A, Tseng HF, Florea A, Luo Y, Tian Y, Solano Z, Widenmaier R, et al. Recombinant adjuvanted zoster vaccine and reduced risk of coronavirus disease 2019 diagnosis and hospitalization in older adults. J Infect Dis. 2022;225(11):1915–22. doi:10.1093/infdis/jiab633.
- Moris P, van der Most R, Leroux-Roels I, Clement F, Dramé M, Hanon E, Leroux-Roels GG, Van Mechelen M. H5N1 influenza vaccine formulated with AS03 a induces strong cross-reactive and polyfunctional CD4 T-cell responses. J Clin Immunol. 2011;31(3):443–54. doi:10.1007/s10875-010-9490-6.
- Galgani I, Annaratone M, Casula D, Di Maro G, Janssens M, Tasciotti A, Schwarz T, Ferguson M, Arora AK. Safety and immunogenicity of three doses of non-typeable Haemophilus influenzae-Moraxella catarrhalis (NTHi-Mcat) vaccine when administered according to two different schedules: a phase 2, randomised, observer-blind study. Respir Res. 2022;23(1):114. doi:10.1186/s12931-022-02019-4.
- De Smedt P, Leroux-Roels G, Vandermeulen C, Tasciotti A, Di Maro G, Dozot M, Casula D, Annaratone M, Riccucci D, Arora AK. Long-term immunogenicity and safety of a non-typeable Haemophilus influenzae-Moraxella catarrhalis vaccine: 4-year follow-up of a phase 1 multicentre trial. Vaccine X. 2021;9:100124. doi:10.1016/j.jvacx.2021.100124.
- Takanashi S, Hasegawa Y, Kanehira Y, Yamamoto K, Fujimoto K, Satoh K, Okamura K. Interleukin-10 level in sputum is reduced in bronchial asthma, COPD and in smokers. Eur Respir J. 1999;14(2):309–14. doi:10.1183/09031936.99.14230999.
- Droemann D, Goldmann T, Tiedje T, Zabel P, Dalhoff K, Schaaf B. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir Res. 2005;6(1):68. doi:10.1186/1465-9921-6-68.
- Barceló B, Pons J, Ferrer JM, Sauleda J, Fuster A, Agustí AG. Phenotypic characterisation of T-lymphocytes in COPD: abnormal CD4+CD25+ regulatory T-lymphocyte response to tobacco smoking. Eur Respir J. 2008;31(3):555–62. doi:10.1183/09031936.00010407.