ABSTRACT
In accordance with European directives, each year the enhanced safety surveillance (ESS) of seasonal influenza vaccines should be conducted in order to detect any potential increase in reactogenicity when the vaccine composition is updated or a new formulation becomes available. The objective of this passive ESS (EPSS) was to assess the frequency of spontaneously reported adverse events (AEs) following vaccination with the 2021/22 formulation of the MF59-adjuvanted quadrivalent influenza vaccine (aQIV) among older adults in Italy through the collection of data within a short time period (start of seasonal influenza vaccination) in order to monitor the reactogenicity of aQIV early in the season. All AEs reported within seven days following vaccination were analyzed by type and seriousness. In all, 1,059 vaccination cards were distributed to individuals aged ≥65 years. Only one, non-serious, spontaneous individual case safety report was submitted, yielding an overall rate of 0.9 per 1,000 doses administered. This report consisted of a reactogenic AE of pyrexia. The EPSS confirmed that the reactogenicity profile of aQIV was consistent with the known safety profile of the previous trivalent formulation. These optimal safety data could bolster public confidence in influenza vaccination and help to improve vaccination coverage.
Introduction
Worldwide, seasonal influenza epidemics of variable severity cause an enormous socioeconomic burden.Citation1–3 Indeed, during the winter months, seasonal influenza can infect up to 20% of the population and cause substantial morbidity, mortality and economic and social disruption.Citation4 For instance, in Europe, each year 4–50 million symptomatic infections occur and 15,000–70,000 citizens die of influenza-related complications.Citation5 Subjects who are at greatest risk of serious illness are: children, pregnant women, older adults and those with certain chronic diseases (e.g., diabetes, immunodeficiency, cardiovascular, respiratory, kidney and liver diseases, neurological pathologies, etc.).Citation6–10
Vaccination is the most efficacious means of limiting the burden of seasonal influenza and in recent years, its value has further increased in light of the current pandemic caused by SARS-CoV-2.Citation1,Citation6,Citation11
The constant evolution of influenza viruses requires continuous global monitoring and annual updating of the composition of influenza vaccines, in order to increase the probability of match between the vaccine strains and the strains most likely to be circulating in the upcoming season.Citation12,Citation13 Moreover, specific tasks must be undertaken in order to ensure effective monitoring of vaccine safety.Citation14 Pharmacovigilance activities for seasonal influenza vaccines present particular challenges, as these vaccines are administered yearly to large population cohorts in a short and fixed period of time.Citation15 In the European Union, pharmacovigilance activities are performed under guidance issued by the European Medicines Agency (EMA), which, since 2014, has required the implementation of enhanced surveillance. This may include the following options: active surveillance, passive surveillance and data mining or other use of electronically recorded health data.Citation16,Citation17
Enhanced passive safety surveillance (EPSS) is a prospective observational study and is conducted in the routine clinical care setting, as the decision to be vaccinated is left entirely to the individual and the choice of influenza vaccine type or brand to be administered is solely at the discretion of vaccinating physicians [general practitioner (GP) or public health physician]. EPSS collects adverse events (AEs) spontaneously reported by vaccinees, as opposed to the solicited collection of safety data (active surveillance).
In the 2021/22 influenza season, EPSS on the quadrivalent formulation of the MF59-adjuvanted influenza vaccine (aQIV) (Fluad Tetra®, CSL Seqirus UK Limited) was conducted in Italy, the aim being to record any increase in AEs of interest and to compare the results with the safety profile reported in the summary of product characteristics (SPC).Citation18,Citation19
aQIV has recently been authorized in Europe (including Italy), the United States and Australia; it is currently indicated for older adults aged ≥65 years, as it contains the MF59 adjuvant, which is able to stimulate a greater immune response in these individuals, who are subject to immunosenescence.Citation18–24
Materials and methods
The EPSS protocol was approved by the Ethics Committee of the Liguria Region (Genoa, Italy) (N. CER Liguria 346/2020), complied with the EMA guidelines, and was carried out in full accordance with good pharmacovigilance practices.Citation16,Citation17 Briefly, surveillance involved older adults aged ≥65 years and was implemented in the Liguria and Apulia Regions (Italy). Surveillance was planned to include a total of 1,000 routine exposures to aQIV, as required by the current interim EPSS guidelines on seasonal influenza vaccines in the EU. These guidelines are designed to detect common AEs, i.e. those expected to be reported at a rate of ≥1%. Therefore, to meet this requirement, 1,000 individuals vaccinated within four to six weeks following the start of the influenza vaccination season are needed.Citation17 Furthermore, by vaccinating 1,000 individuals, the cumulative Poisson law probability of observing at least one AE is >99.9%.Citation17
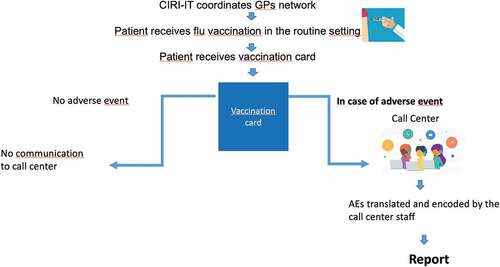
Specifically, the EPSS was conducted through a network of 15 GPs, coordinated by the Interuniversity Research Center on Influenza and other Transmissible Infections (CIRI-IT). The start of EPSS was scheduled in such a way as to coincide with the start of the seasonal influenza vaccination campaign (October 6, 2021).
During the surveillance period, information on vaccine exposure was recorded by CIRI-IT on an electronic surveillance case-form. Following routine vaccination, GPs instructed vaccinees to report any AE in general, and those occurring during the first seven days in particular. Vaccinees were given a standardized and uniquely numbered vaccination card with specific information on the batch, brand, date of vaccine administration and the contact number for reporting AEs.
Vaccinees were encouraged to report (by phoning a dedicated toll-free call center and/or by reporting directly to the health authorities, as indicated on the vaccination card) any AE occurring within seven days post-vaccination. AE reports were collected and processed through a structured interview in Italian. Multiple reports involving the same vaccination card number (either reports of different AEs or of the same event reported by different reporters) were handled as separate individual case safety reports (ICSR), each with its own unique reference number.
The EPSS utilized spontaneously reported AEs, as opposed to solicited safety data. All ICSRs received by the call center were entered in an electronic safety report capture tool. All AEs were coded in accordance with the current version of the medical dictionary for regulatory activities (MedDRA®).
ICSRs that occurred more than seven days after vaccine administration fell outside the EPSS protocol and were therefore not considered in the current analysis.
In the present report, reactogenic adverse events of interest (rAEIs) consisted of systemic events [fever (all grades) and fever ≥39°C, nausea and vomiting, malaise, headache, decreased appetite, myalgia and/or arthralgia], injection-site reactions [pain, erythema (redness), swelling and induration] and events indicative of allergic and hypersensitivity reactions (including rash and eye symptoms).
depicts the overall study flowchart.
Data analysis
Exposure data (date of vaccination, brand of vaccine, batch number and vaccinee’s age) were used to estimate the number of eligible vaccine exposures in the surveillance cohort.
The primary outcome of the safety analyses was the estimated rAEI reporting rate (number of rAEIs per 1,000 doses administered).
The denominator corresponded to the total number of aQIV doses administered (eligible vaccine exposures). To calculate the reporting rate, the total number of eligible AEs (valid ICSRs which contained a unique vaccination card number and corresponded to eligible exposure data) was divided by the total number of eligible exposures over the full surveillance period. ICSRs indicating at least one serious AE were analyzed separately and reported as a proportion of the total number of ICSRs. rAEIs were also described according to their seriousness (serious or non-serious), and their observed reporting rates were compared with the expected rates, as per the SPC.Citation18,Citation19 Finally, other spontaneous AEs were also described.
Results
In total, 1,059 vaccination cards were distributed to individuals aged ≥65 years. All subjects were vaccinated between October 6, 2021 and November 17, 2021.
During the surveillance period, only one ICSR was received, yielding an overall rate of 0.9 per 1,000 doses administered. This ICSR, which was submitted by a 71-year-old woman who suffered from lymphoma and had discontinued her chemotherapy treatment, was deemed non-serious. The report consisted of five individual AEs, one of which was an rAEI (pyrexia with a measured body temperature of 37.7°C). The overall reporting rate of rAEIs was therefore equal to that of ICSRs (0.09%). Other AEs were reported in conjunction with pyrexia, and included feeling hot, pain in the extremities, generalized pain and insomnia.
Discussion
The composition of seasonal influenza vaccines is updated annually and these vaccines are administered to large numbers of healthy individuals within a short time. Consequently, continuous post-marketing monitoring of their safety is essential. Indeed, at the start of every seasonal influenza vaccination campaign, pharmaceutical companies are called upon to carry out ESS and to record any AEs that occur within seven days post-vaccination.
In the present EPSS, no safety issues regarding aQIV were identified.
These results are in line with those obtained during the previous EPSS carried out by our research group during the 2015/16, 2016/17 and 2017/18 influenza seasons, when the trivalent formulation was used.Citation24 Specifically, only one, non-serious, spontaneous ICSR, containing one rAEI (pyrexia), was received during the current surveillance program, while five, seven and five ICSRs were received during the seasons 2015/16, 2016/17 and 2017/18, respectively. If we include fever as an rAEI, two, four and three cases were reported during the previous EPSS.Citation24
Our findings are consistent with the data from the Italian system of AE monitoring following vaccination with any available influenza vaccine in all age-classes. In the 2021/2022 influenza season, an overall rate of 0.049 per 1,000 doses was reported. Of the 595 reports, 20% concerned the over-65 age-class. Severe AEs decreased markedly in the 2021/22 influenza season in comparison with the 2020/21 season.Citation25
Other data on the post-marketing safety of the trivalent formulation have been collected by means of different types of surveillance.Citation26–28 During the period July 2016–June 2018, the US Vaccine Adverse Event Reporting System (VAERS) (national spontaneous reporting system) did not identify any unexpected health condition and reported that the most common AEs were injection-site pain (21%) and erythema (18%).Citation26
Two studies used the active approach.Citation27,Citation28 In the first, Yoo BW et al. performed open-label, multi-center, post-marketing surveillance in South Korean subjects and collected solicited local and systemic AEs from day 1 to 4 of the study. All unsolicited AEs and serious AEs were recorded from day 1 until study termination (day 29). As expected, the most commonly reported reactions were mild and local [injection-site pain (30%) and tenderness (27%)].Citation27 The second study was a cohort study that used data from a solicited short-message-service – based self-reported survey to evaluate AEs occurring within three to five days after vaccination. The most commonly reported AEs were injection-site pain (1.3%) and injection-site swelling or redness (0.9%). Fever was reported in 0.6% of participants.Citation28
Our findings, together with those of other post-marketing studies, are consistent with the well-established safety and tolerability profile of seasonal MF59-adjuvanted vaccines observed in Randomized Clinical Trials (RCTs) and with that reported in the current SPC of aQIV.Citation18,Citation19,Citation29 The safety of aQIV has mainly been evaluated in two RCTs (V118_20 and V118_18), in which 4269 subjects received aQIV. Solicited local and systemic AEs were collected for 7 days after vaccination, and unsolicited adverse reactions were collected for 21 days after vaccination. Commonly reported (≥10%) AEs across both studies were injection-site pain (16.3% and 31.9%), fatigue (10.5% and 16.0%) and headache (10.8% and 12.0%) in V118_18 and V118_20, respectively. Most solicited reactions were mild or moderate in intensity and resolved within the first three days after vaccination.Citation18
This EPSS has several strong-points. First, the surveillance program collected data within a short period at the start of the influenza vaccination campaign, thereby enabling vaccine exposure to be estimated almost in real time, in order to identify potential risks before the peak of influenza vaccination. Second, adopting a user-friendly spontaneous AE reporting method (issuing a vaccination card to each vaccinee) may have improved the quality of spontaneous AE reports.
However, like all passive surveillance programs, this EPSS has some limitations, the main one being under-reporting (whereby only a fraction of the total number of AEs occurring after vaccination are reported). This limitation is linked to the phenomenon of differential reporting (whereby more serious AEs and those with more rapid onset after vaccination are more likely to be reported during the surveillance period than minor AEs or those with a longer time to onset). This phenomenon has also been described by other authors. de Lusignan S et al. reported that conditions not requiring medical attendance, especially local reactions or minor systemic symptoms, could be subject to under-reporting.Citation30 These authors tried to overcome this limitation by asking vaccinated patients to return their cards even if they did not experience any AE, reporting that no AE occurred. We did not adopt this approach, as vaccinees taking part in the EPSS were encouraged by their GPs or public health physicians to report all AEs. Furthermore, older age could be associated to lower reporting of AEs, as documented in an Australian enhanced passive surveillance program implemented from July 2007 to June 2016. Specifically, fewer AEs per influenza vaccine dose were reported by subjects aged over 65 years than by those aged 18–65 years. This apparent under-reporting may have been due to misinterpretation on the part of older individuals; as they suffered from more co-morbidities, they may have attributed their AEs to their underlying conditions, rather than to vaccination.Citation31
Another limitation of this EPSS is linked to its short-term nature, which makes it difficult to estimate events of interest that are rare, of multifactorial etiology, and/or exhibit long latency.
If merged with the body of available evidence of the advantage of using enhanced formulations to reduce the burden of influenza in older adults, these safety data could bolster public confidence in influenza vaccination and help to improve vaccination coverage.Citation32,Citation33
Acknowledgments
The authors thank all EPSS participants and GPs of the CIRI-IT network (Avanzino Giuseppe, Bosco Edmondo, Mangini Paola Maria, Messina Valeria, Percivale Mauro, Pirino Roberto, Aprile Emilia, Bosco Trentino, D’Errico Giovanni Battista, Fiume Damiano, Lippolis Orazio, Lo Russo Nicola Antonio, Marcellino Gaetano, Mastronuzzi Tecla) who helped to carry out EPSS during the 2021/2022 influenza season. The authors thank Stefano Mosca for his IT support.
Disclosure statement
DA, PLL, AO, GI, DP are members of the scientific committee of CIRI-IT, which received a grant for the EPSS. OT is employed by CSL Seqirus.
Additional information
Funding
References
- Vaccines against influenza: WHO position paper – May 2022. [accessed 2022 Sep 15]. https://www.who.int/publications/i/item/who-wer9719.
- Putri WCWS, Muscatello DJ, Stockwell MS, Newall AT. Economic burden of seasonal influenza in the United States. Vaccine. 2018;36(27):3960–5. doi:10.1016/j.vaccine.2018.05.057.
- Szucs T. The socio-economic burden of influenza. J Antimicrob Chemother. 1999;44(Suppl B):11–15. doi:10.1093/jac/44.suppl_2.11.
- Tyrrell CS, Allen JLY, Gkrania-Klotsas E. Influenza: epidemiology and hospital management. Medicine (Abingdon). 2021;49(12):797–804. doi:10.1016/j.mpmed.2021.09.015.
- Paget J, Danielle Iuliano A, Taylor RJ, Simonsen L, Viboud C, Spreeuwenberg P. Global seasonal influenza-associated mortality collaborator network and GLaMOR collaborating teams. Estimates of mortality associated with seasonal influenza for the European Union from the GLaMOR project. Vaccine. 2022;40(9):1361–9. doi:10.1016/j.vaccine.2021.11.080.
- Gasparini R, Amicizia D, Lai PL, Panatto D. Clinical and socioeconomic impact of seasonal and pandemic influenza in adults and the elderly. Hum Vaccin Immunother. 2012;8(1):21–28. doi:10.4161/hv.8.1.17622.
- Fallani E, Orsi A, Signori A, Icardi G, Domnich A. An exploratory study to assess patterns of influenza- and pneumonia-related mortality among the Italian elderly. Hum Vaccin Immunother. 2021;17(12):5514–21. doi:10.1080/21645515.2021.2005381.
- Panatto D, Gasparini R, Amicizia D. Influenza vaccination coverage in the elderly and socio-economic inequalities in Italy. J Prev Med Hyg. 2019;59(4 Suppl 2):E1–E2. doi:10.15167/2421-4248/jpmh2018.59.4s2.1198.
- Gasparini R, Amicizia D, Lai PL, Panatto D. Influenza vaccination: from epidemiological aspects and advances in research to dissent and vaccination policies. J Prev Med Hyg. 2016;57:E1–E4.
- Trucchi C, Paganino C, Orsi A, Amicizia D, Tisa V, Piazza MF, Gallo D, Simonetti S, Buonopane B, Icardi G, et al. Hospital and economic burden of influenza-like illness and lower respiratory tract infection in adults ≥50 years-old. BMC Health Serv Res. 2019;19(1):585. doi:10.1186/s12913-019-4412-7.
- European Centre for Disease Prevention and Control. Seasonal influenza. [accessed 2022 Sep 13]. https://www.ecdc.europa.eu/en/seasonal-influenza.
- WHO Global Influenza Programme. [accessed 2023 Jan 30]. https://www.who.int/teams/global-influenza-programme/vaccines.
- ECDC. Seasonal influenza vaccination strategies. [accessed 2023 Jan 30]. https://www.ecdc.europa.eu/en/seasonal-influenza/prevention-and-control/vaccines/vaccination-strategies.
- Dos Santos G. Challenges in implementing yearly enhanced safety surveillance of influenza vaccination in Europe: lessons learned and future perspectives. Hum Vaccin Immunother. 2019;15(11):2624–36. doi:10.1080/21645515.2019.1608745.
- Grohskopf LA, Blanton LH, Ferdinands JM, Chung JR, Broder KR, Talbot HK, Morgan RL, Fry AM. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2022–23 Influenza Season. MMWR Recomm Rep. 2022 Aug 26;71(1):1–28. doi:10.15585/mmwr.rr7101a1.
- European Medicines Agency. Pharmacovigilance Risk Assessment Committee (PRAC). [accessed 2023 Sep 13]. https://www.ema.europa.eu/en/glossary/prac.
- European Medicines Agency (EMA). Interim guidance on enhanced safety surveillance for seasonal influenza vaccines in the EU. [accessed 2022 Sep 13]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/04/WC500165492.pdf.
- European Medicines Agency (EMA). Fluad Tetra. [accessed 2022 Sep 15]. https://www.ema.europa.eu/en/documents/product-information/fluad-tetra-epar-product-information_en.pdf.
- Italian Medicine Agency. Fluad, summary of product characteristics. [accessed 2022 Sep 15]. https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_004166_031840_RCP.pdf&retry=0&sys=m0b1l3.
- de Waure C, Boccalini S, Bonanni P, Amicizia D, Poscia A, Bechini A, Barbieri M, Capri S, Specchia ML, Di Pietro ML, et al. Adjuvanted influenza vaccine for the Italian elderly in the 2018/19 season: an updated health technology assessment. Eur J Public Health. 2019;29(5):900–5. doi:10.1093/eurpub/ckz041.
- Calabrò GE, Boccalini S, Panatto D, Rizzo C, Di Pietro ML, Abreha FM, Ajelli M, Amicizia D, Bechini A, Giacchetta I, et al. The new quadrivalent adjuvanted influenza vaccine for the Italian elderly: a health technology assessment. Int J Environ Res Public Health. 2022;19(7):4166. doi:10.3390/ijerph19074166.
- Domnich A, Arata L, Amicizia D, Puig-Barberà J, Gasparini R, Panatto D. Effectiveness of MF59-adjuvanted seasonal influenza vaccine in the elderly: a systematic review and meta-analysis. Vaccine. 2017;35(4):513–20. doi:10.1016/j.vaccine.2016.12.011.
- Barbieri M, Capri S, Waure C, Boccalini S, Panatto D. Age- and risk-related appropriateness of the use of available influenza vaccines in the Italian elderly population is advantageous: results from a budget impact analysis. J Prev Med Hyg. 2017;58(4):E279–87. doi:10.15167/2421-4248/jpmh2017.58.4.867.
- Panatto D, Haag M, Lai PL, Tomczyk S, Amicizia D, Lino MM. Enhanced Passive Safety Surveillance (EPSS) confirms an optimal safety profile of the use of MF59® -adjuvanted influenza vaccine in older adults: results from three consecutive seasons. Influenza Other Respir Viruses. 2020;14(1):61–66. doi:10.1111/irv.12685.
- Vaccine report 2021. The Italian postmarketing surveillance of non covid vaccines. [accessed 2023 Feb 10]. https://www.aifa.gov.it/-/aifa-pubblica-il-rapporto-vaccini-2021-1.
- Haber P, Moro PL, Ng C, Dores GM, Lewis P, Cano M. Post-licensure surveillance of trivalent adjuvanted influenza vaccine (aIIV3; Fluad), Vaccine Adverse Event Reporting System (VAERS), United States, July 2016–June 2018. Vaccine. 2019 Mar 7;37(11):1516–20. doi:10.1016/j.vaccine.2019.01.052.
- Yoo BW, Kim CO, Izu A, Arora AK, Heijnen E. Phase 4, post-marketing safety surveillance of the MF59-adjuvanted influenza vaccines FLUAD® and VANTAFLU® in South Korean subjects aged ≥65 years. Infect Chemother. 2018 Dec;50(4):301–10. doi:10.3947/ic.2018.50.4.301.
- Pillsbury AJ, Fathima P, Quinn HE, Cashman P, Blyth CC, Leeb A, Macartney KK. Comparative postmarket safety profile of adjuvanted and high-dose influenza vaccines in individuals 65 years or older. JAMA Netw Open. 2020 May 1;3(5):e204079. doi:10.1001/jamanetworkopen.2020.4079.
- Pellegrini M, Nicolay U, Lindert K, Groth N, Della Cioppa G. MF59-adjuvanted versus non-adjuvanted influenza vaccines: integrated analysis from a large safety database. Vaccine. 2009;27(49):6959–65. doi:10.1016/j.vaccine.2009.08.101.
- de Lusignan S, Dos Santos G, Byford R, Schuind A, Damaso S, Shende V, McGee C, Yonova I, Ferreira F. Enhanced safety surveillance of seasonal quadrivalent influenza vaccines in English primary care: interim analysis. Adv Ther. 2018;35(8):1199–214. doi:10.1007/s12325-018-0747-4.
- Clothier HJ, Crawford NW, Russell M, Buttery JP. Adverse events following vaccination of older people may be under-reported. Med J Aust. 2017 Sep 2;207(7):301–2. doi:10.5694/mja16.01371.
- Domnich A, Panatto D, Pariani E, Napoli C, Chironna M, Manini I, Rizzo C, Orsi A, Icardi G, IT-BIVE-HOSP Network Study Group. Relative effectiveness of the adjuvanted versus non-adjuvanted seasonal influenza vaccines against severe laboratory-confirmed influenza among hospitalized Italian older adults. Int J Infect Dis. 2022;125:164–9. doi:10.1016/j.ijid.2022.10.041.
- WHO. The safety of medicines in public health programmes: pharmacovigilance an essential tool. [accessed 2022 Sep 15]. https://apps.who.int/iris/bitstream/handle/10665/43384/9241593911_eng.pdf.