ABSTRACT
Debate regarding vaccinating high-risk infants with penta- and hexavalent vaccines persists, despite their good immunogenicity and acceptable safety profile in healthy full-term infants. We report the findings of a systematic literature search that aimed to present data on the immunogenicity, efficacy, effectiveness, safety, impact, compliance and completion of penta- and hexavalent vaccination in high-risk infants, including premature newborns. Data from the 14 studies included in the review showed that the immunogenicity and the safety profile of penta- and hexavalent vaccines in preterm infants was generally similar to those seen in full-term infants, with the exception of an increase in cardiorespiratory adverse events such as apnea, bradycardia and desaturation following vaccination in preterm infants. Despite recommendations of vaccinating preterm infants according to their actual age, and the relatively high completion rate of the primary immunization schedule, vaccination was often delayed, increasing the vulnerability of this high-risk population to vaccine-preventable diseases.
Plain Language Summary
Combined vaccines such as penta- and hexavalent vaccines against multiple childhood diseases are widely used in healthy babies born at term. However, it is still debated whether these vaccines act the same way in babies considered to be high-risk: babies born prematurely at <34 weeks of pregnancy, those with a birthweight of <1500 g or babies with chronic diseases. We did a systematic literature search to find studies on such high-risk babies vaccinated with penta- or hexavalent vaccines; we focused on their antibody levels following vaccination, side effects, and protection from the diseases against which they were vaccinated. We also analyzed whether they were vaccinated on time and with all the doses recommended for healthy full-term babies. We found 14 studies that included premature babies. The results of these studies suggest that premature babies’ immune systems respond to penta- and hexavalent vaccines in largely the same way as those of full-term babies; side effects of penta- and hexavalent vaccines are also mostly similar to those seen in full-term babies. However, side effects like pauses in breathing, slow heart rate or low blood oxygen levels seem to be more common in preterm babies; for safety, these babies should be monitored closely after vaccination. Preterm babies are often vaccinated with a delay compared to the recommended schedule. No studies reported data on protection from the diseases covered by penta- and hexavalent vaccinations in preterm babies. More research is needed on penta- and hexavalent vaccination of other high-risk babies besides those born prematurely.
Introduction
Combination vaccines, including penta- and hexavalent vaccines that offer protection against diphtheria, tetanus and pertussis along with poliomyelitis, Haemophilus influenzae type b (Hib) and/or hepatitis B virus (HBV) are increasingly becoming the standard of care in terms of pediatric immunization programs worldwide.Citation1–4 The advantages of combination vaccines are well-recognized and include fewer medical visits, injections and adverse events (AEs), as well as increased simplicity of and better compliance with the recommended vaccination schedules.Citation1,Citation5 Currently, several types of penta- and hexavalent vaccines are available, with differences in terms of the type (e.g., whole-cell pertussis-containing vaccines [wP] or acellular pertussis-containing vaccines [aP]) and the number of pertussis antigens included (e.g., pertussis toxoid [PT], filamentous hemagglutinin, pertactin, fimbriae) as well as in terms of the antigen quantity contained.Citation4
The body of evidence confirming that combination vaccines have a good immunogenicity and acceptable safety profile in infants is extensive.Citation5,Citation6 However, concerns still exist regarding their administration to infants who are considered to be less likely to mount an adequate immune response and to be at a higher risk of vaccine-related AEs; this includes premature infants (born at <37 weeks of gestation), those with a low birthweight (LBW, <2500 g) and children with special conditions such as cardiovascular malformations or chronic pulmonary disease.Citation7–9 Such infants are generally excluded from clinical trials; therefore, data regarding any particularities related to penta- and hexavalent vaccine use in these populations are scarce. Nevertheless, these high-risk children are more vulnerable to vaccine-preventable diseases (VPDs) than their full-term counterparts, so timely and complete vaccination is even more important for them.Citation10
The largest group of high-risk children is represented by preterm infants: worldwide, on average one out of every ten infants is born prematurely.Citation11 Based on data from 184 countries, 5–18% of children were born prematurely in 2010, and the rates of premature births have been increasing in many countries.Citation11 The World Health Organization defines preterm infants as babies born alive before 37 weeks of pregnancy. Based on gestational age at birth, three sub-categories of preterm infants can be distinguished: extremely preterm (<28 weeks), very preterm (28 to <32 weeks) and moderate to late preterm (32 to <37 weeks),Citation8 with infants born between 32 and <34 weeks being considered as moderate preterm.Citation12 Moderate-to-late preterm infants represent over 80% of all premature babies, and about 10% are very premature.Citation11
Preterm birth is associated with increased risk of mortality; cardiorespiratory instability and feeding difficulties are also common.Citation11,Citation13–15 The rates of prematurity complications decrease as the gestational age increases. Therefore, even if they constitute a minority of all preterm infants, extremely, very and moderate premature infants represent the most vulnerable categories of the preterm population.Citation12,Citation15 Levels of passively transferred maternal antibodies are generally low or even absent in very preterm infants who are born before antibody transfer starts (at around 28 weeks of gestation).Citation16 In addition, preterm infants have immature immune systems and are therefore highly vulnerable to infections, including VPDs such as pertussis.Citation14,Citation17
However, the same factors that make premature infants more susceptible to disease could also generate reluctance in vaccinating this population. Concerns are related to fear of AEs as well as to the ability of preterm infants to mount an adequate immune response to vaccination,Citation7 especially in those born extremely and very prematurely.Citation11 Often, these concerns lead to preterm infants being vaccinated with a delay, according to their corrected age and not their actual age, as recommended.Citation6,Citation16,Citation18–27
This systematic literature review aimed to gather and summarize data on high-risk infants vaccinated with diphtheria-tetanus-pertussis (DTP)-containing penta- and/or hexavalent vaccines. Among premature infants, extremely, very and moderate preterm infants born at <34 weeks were considered as high-risk; this group includes the most vulnerable categories of the preterm population.
Materials and methods
The systematic review was conducted in accordance with the Cochrane Collaboration guidelines for performing systematic reviewsCitation28 and reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines.Citation29
Objective and definitions
The objective of the systematic literature review was to compile data available on the immunogenicity, efficacy, effectiveness, safety, impact, compliance and completion of penta- and hexavalent vaccination in infants with special conditions (chronic convulsive disease, chronic heart or lung diseases with risk of decompensation in the presence of fever, disabling chronic neurological diseases, newborns who remain hospitalized in the neonatal unit at the age of vaccination, and immature newborns, defined as very low birthweight [VLBW] infants, born at <1500 g and/or extremely, very and moderate preterm infants, born at <34 weeks of gestation). In addition, we aimed to assess the breadth of data available in these specific high-risk populations and identify any potential data gaps.
Search strategy
A systematic literature search was conducted in Medline (via the PubMed interface) and Embase databases on October 4, 2021.
A broad search strategy was applied using combinations of search strings created solely for the intervention, i.e., penta- and/or hexavalent DTP vaccination but not for the study population or outcome. This decision was based on the fact that the list of special conditions included might not be exhaustive, and that multiple impact outcomes may apply.
Three search strings were created: one with terms for hexavalent vaccines, one with hexavalent vaccine formulation names, and a search string for pentavalent vaccine terms, including formulation names (Appendix A).
No limits regarding year of publication, language, geographical scope, or publication type were applied.
Study selection
In the first step of the selection process, after duplicate removal, two independent researchers screened in parallel the titles and abstracts of the publications retrieved from the literature search. The results of the two researchers were compared, and deviations were discussed.
Publications that included data related to the use of penta- and hexavalent vaccines in infants with special conditions were retained. Publications that reported data on healthy infants, other types of vaccines, as well as those that contained no data related to penta- or hexavalent vaccination were excluded. In case of doubt, the article was selected for full-text screening. The results of the title and abstract selection were registered in an EndNote library.
Full-text screening was conducted by two researchers in close collaboration. At this step, narrative reviews, case reports and case series, posters and conference abstracts were excluded, along with other types of publications that did not contain data relevant for the review objective. The reference lists of relevant meta-analyses and good-quality systematic reviews were checked for possibly missed relevant publications. If this was the case, the missed original articles were included, and the systematic review or meta-analysis itself was excluded. The full-text selection was documented in an Excel worksheet, by indicating which articles were included and which articles were excluded, while documenting the reasons for exclusion.
Study quality assessment
The methodological quality of the publications was assessed using the Joanna Briggs Institute (JBI) Critical Appraisal tools for cross-sectional studies and quasi-experimental studies (Appendix B), by the same researchers who performed the study selection. For this review, the overall appraisal options provided by the JBI checklists were adapted into “sufficient,” “poor,” and “exclude.”
Data extraction
Only data for preterm infants born at <34 weeks or VLBW infants (born at <1500 g) were extracted; data for late preterm infants born at 34–36 weeks were omitted. Post-booster data were only extracted if both primary and booster data were available.
Data were extracted and compiled into evidence tables by three researchers. Extracted data included the name of the first author, year of publication, country of data collection, study design, study population, details regarding the vaccines administered and data regarding any of the outcomes indicated in the study objectives (immunogenicity, safety, efficacy, effectiveness, impact, completion, and compliance with penta- or hexavalent vaccination schedules). Relevant findings were reported using the terminology, numbers and percentages included in the publications. Results were analyzed qualitatively using summary tables and descriptive synthesis. No meta-analysis was performed. During the data extraction step, publications were further checked for duplicate data.
Gray literature
A gray literature search was also performed. A Google search was conducted on November 11, 2021 using the keywords “DTP” in combination with the medical conditions of interest “premature” or “neonatal” or “chronic convulsive” or “chronic heart disease” or “chronic lung disease” or “neurological disease” and “infant.” For each search, the first three pages of the Google search results were screened. European Society for Paediatric Infectious Diseases (ESPID) conference abstracts of the last five years (2017–2021) were screened.
Results
Search results
Study selection
The search strategy yielded 963 unique titles and abstracts, 123 of which were included in the full-text review. Another 17 records were identified from systematic reviews, of which 16 were included in the full-text review. A total of 15 studies contained data pertaining to the objectives of this systematic review (). No additional relevant studies were found in the gray literature.
Figure 1. PRISMA flow diagram of study selection.
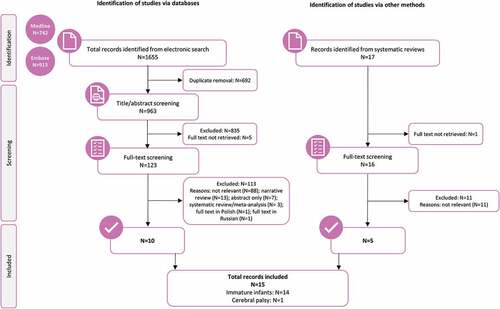
Among the 15 selected studies, 14 included immature infants meeting our inclusion criteria (infants born at <1500 g and/or preterm infants born at <34 weeks),Citation30–43 and only one study included another high-risk group, namely infants with cerebral palsy.Citation44 Therefore, it was decided to only assess data from studies on immature infants.
Study characteristics
Of the 14 studies on immature infants, six studies were on pentavalent vaccines,Citation30–35 four on hexavalent vaccines,Citation36–39 and four on penta- and hexavalent vaccines.Citation40–43 Six studies reported co-administration of other vaccines,Citation30,Citation31,Citation35,Citation40–42 and in four studies, at least part of the population received HBV birth doses.Citation30,Citation32,Citation35,Citation43
Eight of the 14 identified studies on immature infants were conducted in European countries including Germany, Greece, Italy, Poland, Spain, and Switzerland. One study was conducted in India, two in the United States/Canada, one in Peru, one in Israel and in one study, the country was not reported. Details of the studies included in this review are summarized in .
Table 1. Characteristics of studies included in the review.
None of the selected studies reported data on efficacy, effectiveness, or impact of penta- or hexavalent vaccinations.
Quality assessment
Of the 14 included studies, 11 were assessed as of “sufficient” quality and three were rated as “poor” quality due to poorly described methodology, missing baseline characteristics of the compared groups and differences in outcome measurement between the groups.
In one of the 14 studies,Citation33 the unvaccinated control group also included already vaccinated infants in an unknown proportion. Results from this control group were not included in this review due to poor description and because infants could be entered in the study once as a control and once as an immunized infant.
Immunogenicity
Six publications reported data on immunogenicity of penta- or hexavalent vaccines.Citation31,Citation32,Citation35,Citation38,Citation39,Citation42 In all studies, the primary vaccination consisted of three doses. Data are described separately per type of vaccine (penta- and hexavalent) and timing. Several studies reported seroprotection rates in preterm infants following vaccination, which were determined using thresholds demonstrated to confer protection in full-term infants. However, since the protective effect of these thresholds was not confirmed in preterm infants, such results are presented as seroconversion rates in this review. Data on seropositivity/seroconversion rates are presented in , and data on geometric mean antibody concentrations/titers (GMCs/GMTs) and overall immunogenicity conclusions are presented in .
Table 2. Seroconversion/Seropositivity rates after penta- or hexavalent vaccination.
Table 3. Antibody GMCs/GMTs after penta- or hexavalent vaccination and overall immunogenicity conclusion by the authors.
Pentavalent vaccines
One month after the third dose of primary vaccination, seropositivity for HBV surface antibody (anti-HBs, ≥10 mIU/mL) ranged from 91.7% to 97.5% in preterm infants who received pentavalent vaccines (DTwP-HBV-Hib [brand not specified], and DTaP-HBV-inactivated poliovirus vaccine [IPV] [Infanrix penta]) and was 100% in full-term infants in both studies.Citation32,Citation35 Omeñaca et al.Citation35 reported no significant difference between the preterm and full-term groups regarding the percentage of infants reaching either the 10 mIU/mL or the 100 mIU/mL cutoffs for anti-HBs one month after the third dose of primary vaccination with DTaP-HBV-IPV (Infanrix penta). However, the post-dose 3 anti-HBs GMC was significantly lower in the preterm group compared to the full-term group (no significance level reported). In this study, the pentavalent vaccine was co-administered with a combined Hib-Neisseria meningitidis serogroup C tetanus toxoid conjugate vaccine (Hib-MenC-TT, Menitorix) and a 7-valent pneumococcal conjugate vaccine (PCV7, Prevenar).
Kulkarni-Munje et al.Citation32 concluded that all preterm infants developed adequate antibody responses against tetanus and diphtheria. Despite remaining lower compared to those of their full-term counterparts, antibody levels also reached seroconversion thresholds for Hib (anti-polyribosylribitol phosphate [PRP] antibody levels: 1.6 µg/mL vs. 7.7 µg/mL) and HBV (anti-HBs antibody levels: 253.8 mIU/mL vs. 306.8 mIU/mL). The difference in GMC/GMT between preterm and full-term infants reached statistical significance for anti-PRP antibodies (p < .05), while no statistical test was reported or anti-HBs GMC. A low rate of response to PT in all infant groups emerged as a major concern (75.0% in very preterm infants and 74.1% in full-term infants) with the DTwP-HBV-Hib vaccine (brand name not specified).Citation32 In one study, antibody responses of all preterm and full-term infants who received DTaP-IPV-HBV (Pediarix) vaccine (co-administered with pneumococcal [PCV7, Prevnar] and Hib [ActHIB] vaccines) reached the seroconversion threshold (≥ 1:8) to all three poliovirus serotypes one month after the third dose of primary vaccination.Citation31 However, the GMT of poliovirus type 1 antibodies was significantly lower in preterm infants (p = .03; no significant differences in GMTs of poliovirus types 2 and 3 antibodies between the two groups).
One study presented data on seropositivity/response rates one month after the booster dose with DTaP-IPV/Hib (Infanrix IPV/Hib) with co-administration of a pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV, Synflorix), after primary vaccination with DTaP-HBV-IPV/Hib (Infanrix hexa).Citation42 Seropositivity/seroconversion rates were 100% for anti-PRP, anti-diphtheria, anti-tetanus, anti-poliovirus types 1–3, anti-PT, anti-filamentous hemagglutinin (FHA) and anti-pertactin (PRN) for both preterm and full-term infants. Antibody GMCs/GMTs were within the same ranges in both groups.
Hexavalent vaccines
In all three studies reporting on hexavalent vaccines, infants received DTaP-HBV-IPV/Hib (Infanrix hexa).Citation38,Citation39,Citation42 One month after the third dose of primary vaccination, one study found that all infants were seroconverted/seropositive for anti-HBs, anti-PRP, anti-diphtheria, anti-tetanus, anti-poliovirus types 1 and 2, anti-PT, anti-FHA and anti-PRN; one preterm infant was not seroconverted against poliovirus type 3 after primary vaccination.Citation42 In this study, DTaP-HBV-IPV/Hib (Infanrix hexa) was co-administered with PHiD-CV (Synflorix). Antibody GMCs/GMTs were within the same ranges in both groups and in line with previous experience with these DTaP-based combination vaccines when administered separately.
An earlier study by the same authors found seroconversion for anti-HBs one month after the third dose of primary vaccination in 83.3% of preterm infants with a gestational age of 31 to 33 weeks, in 100% of the preterm infants with a gestational age of ≤30 weeks and in 95.2% of full-term infants (gestational age ≥ 37 weeks).Citation38 One month after the booster dose, seroconversion for anti-HBs was found in 78.3% of preterm infants with a gestational age of 31–33 weeks, in 100% of the preterm infants with a gestational age of ≤30 weeks and in 98.5% of the full-term infants. The authors concluded that preterm babies mounted adequate immune responses, which were similar to those of children born at full term and that seroconversion rates were not influenced by either gestational age or birthweight.
In the study by Vázquez et al.Citation39 no significant differences were noted between the VLBW and LBW preterm groups (birthweight < 1500 g or between 1500 g and 2000 g, respectively) in terms of seroconversion or seropositivity rates one month after the third dose of primary vaccination and one month after the booster dose. The only exception was noted for anti-PRP antibody concentrations ≥ 1.0 µg/mL, which were observed in 65.4% of VLBW infants and 80.8% of LBW infants after primary vaccination (between-group difference 15.4%; 95% confidence interval [CI]: 1.5–28.9). In this study, preterm infants were not compared to full-term infants.
Safety
Eight publications reported data on the safety of penta- or hexavalent vaccines in immature infants born at <1500 g or <34 weeks of gestation ().Citation31,Citation33,Citation35–37,Citation39–41 Data are described separately per type of vaccine (penta- and hexavalent). More detailed data on specific local and systemic AEs can be found in Appendix C and Appendix D.
Table 4. Overall, serious, and cardiorespiratory adverse events after penta- and hexavalent vaccines.
Pentavalent vaccines
Infants received DTaP-IPV-HBV (Pediarix) in one study,Citation31 DTwP-IPV-Hib (brand not specified, until July 1, 1997) or DTaP-IPV-Hib (brand not specified, after July 1, 1997) in a second study,Citation33 and DTaP-HBV-IPV (Infanrix penta) in a third study.Citation35
In the first study, there were no medically attended events for fever, seizure or swelling for either preterm or full-term infants after any vaccine dose during primary vaccination.Citation31 No hospitalizations were recorded within 30 days in either group. In this study, the vaccine was co-administered with pneumococcal (PCV7, Prevnar) and Hib (ActHIB) vaccines.
The second study reported an increase in cardiorespiratory AEs (including apnea, bradycardia and desaturations) following the first dose of DTP-IPV-Hib (brand not specified): in 45.2% of the 124 preterm infants vaccinated prior to discharge from the neonatal intensive care, cardiorespiratory AEs increased in the 72 hours after vaccination compared to the 72 hours before vaccination.Citation33
In the third study, the authors did not draw any conclusion on the safety of the pentavalent vaccine in preterm infants.Citation35 Overall, unsolicited AEs were more frequent in full-term infants than in preterm infants (32.0% vs. 5.4%) after primary vaccination doses; the difference between groups was primarily due to a higher rate of injection-site reactions in the full-term group compared to the preterm group. Fatal events were rare in both groups (between 0.0% and 0.7% in preterm and full-term infants, respectively): one full-term infant died of meningococcal serogroup B sepsis 184 days after the third vaccine dose, and the event was considered to be unrelated to vaccination. One preterm infant (1.8%) with a history of bronchopulmonary dysplasia developed apnea. The frequency of local AEs related to the pentavalent vaccine (Appendix C) was largely comparable between preterm and full-term infants, based on overlapping 95% CIs. The only exception was redness, which occurred significantly more often in full-term infants (78.7%, 95% CI: 71.2–84.9) compared to preterm infants (50%, 95% CI: 36.3–63.7). The percentage of infants with systemic AEs (Appendix D) also appeared to be similar between the two groups based on overlapping CIs; however, no statistical tests were performed to compare the groups. In this study, the pentavalent vaccine was co-administered with Hib-MenC-TT (Menitorix) and PCV7 (Prevenar), and the systemic events could not be related to one vaccine or the other.
Hexavalent vaccines
Infants received DTaP-HBV-IPV/Hib (Infanrix hexa) in two studiesCitation36,Citation39 and DTaP-IPV-Hib-HBV (Hexavac) in one study.Citation37
The first study concluded that infants born at < 30 weeks and < 1500 g vaccinated with DTaP-HBV-IPV/Hib (Infanrix hexa) in a hospital setting did not develop any cardiorespiratory AEs such as bradycardia, apnea, or drops in saturation during primary vaccination.Citation36 Neither subgroup (hospitalized or not hospitalized at the time of vaccination) experienced any serious AEs related to vaccination. Preterm infants were not compared to full-term infants in this study.
The second study concluded that DTaP-IPV-Hib-HBV (Hexavac, no co-administered vaccines) can cause AEs such as apnea, bradycardia or desaturation in very preterm infants < 31 weeks, with such events being recorded in 5/45 (11.1%) infants within three days after the first dose of primary vaccination.Citation37 Post-vaccination cardiorespiratory AEs were considered vaccine-related if such events had not occurred in the three days prior to immunization, or they doubled in frequency or increased significantly in severity after immunization. All events affected babies with chronic disease (bronchopulmonary dysplasia and/or gastroesophageal reflux) who were still in therapy. Hospitalized healthy preterm infants without chronic disease and not in therapy seemed to be less vulnerable to cardiorespiratory AEs, as no apnea, bradycardia or desaturation occurred in this group. This study did not include full-term infants.
In the third study, at least one serious AE was reported in 34/169 (20.1%) preterm infants with a birthweight <2000 g during primary immunization with DTaP-HBV-IPV/Hib (Infanrix hexa), the majority of which were infections.Citation39 Two infants (1.2%) died; one death was deemed as possibly related to the vaccination. After the booster dose, serious AEs (not considered related to the study vaccine) occurred in 3.2% and 4.9% of the preterm infants with birthweights of <1500 g and 1500 to 2000 g, respectively. The frequency of local AEs (Appendix C) was generally similar (based on overlapping CIs) between the two groups for all doses, except for pain, which occurred more often in preterm LBW infants than in VLBW infants after the first dose of primary vaccination (51.8%, 95% CI: 40.7–62.7, vs. 29.8%, 95% CI: 20.3–40.7). Systemic AEs (Appendix D) also occurred with relatively similar frequencies in the two groups, based on overlapping 95% CIs; the only exception was irritability, which occurred more often among LBW infants (51.8%, 95% CI: 40.7–62.7) than in VLBW infants (26.2%, 95% CI: 17.2–36.9).
Penta- and hexavalent vaccines
In the studies that investigated both penta- and hexavalent vaccines, the infants received either DTaP-IPV/Hib (Infanrix IPV/Hib) or DTaP-HBV-IPV/Hib (Infanrix hexa).Citation40,Citation41
Flatz-Jequier et al.Citation40 reported no overall conclusion on safety. In this study, 34/135 (25.2%) of the preterm infants who were hospitalized at the time of the first dose of primary vaccination experienced cardiorespiratory AEs, compared to none of the 308 preterm infants who were not hospitalized. The study vaccine was either given alone, or together with a pneumococcal vaccine (PCV7, Prevenar) or respiratory syncytial virus monoclonal antibodies. Following the second vaccine dose, 6/135 (4.4%) of the hospitalized preterm infants (representing 17.6% of those with a cardiorespiratory AE after the first dose) had cardiorespiratory AEs that needed medical intervention. The third dose of the vaccination was tolerated well by all infants. Preterm infants were not compared to full-term infants in this study, and the statistical significance of the difference between the groups was not tested.
Furck et al.Citation41 reported AEs in preterm VLBW infants following immunization with different vaccines/vaccine combinations: DTaP-IPV/Hib (Infanrix IPV/Hib) with an HBV vaccine (Gen H-B-Vax K pro infantibus), DTaP-HBV-IPV/Hib (Infanrix hexa) alone, or DTaP-HBV-IPV/Hib (Infanrix hexa) with PCV7 (Prevenar). Local AEs and fever occurred in 2.8% of infants (with no significant differences between the vaccine groups); cardiorespiratory AEs occurred more often, with 10.8% of the preterm infants experiencing apnea and bradycardia, two events that were interrelated. Apnea and bradycardia appeared significantly more often in the group of infants who received DTaP-HBV-IPV/Hib with PCV7 compared to the other two groups (DTP-IPV/Hib with Gen H-B-Vax K pro infantibus or DTaP-HBV-IPV/Hib alone). Of the 47 preterm infants who also received the second dose during their primary hospital stay, 4.3% experienced a cardiorespiratory AE after the second dose. No infant was readmitted by the community pediatrician for apnea or for an acute life-threatening event after a second or third vaccine dose.
Compliance
Five articles reported data on compliance.Citation30,Citation34,Citation36,Citation37,Citation43 Data are described separately per type of vaccine (penta- or hexavalent) ().
Table 5. Compliance with penta- and hexavalent vaccine schedules.
Pentavalent vaccines
Both studies that investigated compliance in immature infants who received pentavalent vaccinations concluded that VLBW infants (<1500 g) are likely to receive the first dose and all subsequent doses with delay.Citation30,Citation34 In a national birth cohort study from Israel that included over 180,000 infants, Bary-Weisberg et al.Citation30 showed that only half of the infants with a birthweight of <1000 g received the first vaccine dose on time. Infants with a birthweight of 1000–1499 g as well as those <1000 g were at a significantly higher risk of receiving the first dose of pentavalent vaccination with a delay (hazard ratios of 0.67, 95% CI: 0.63–0.72, and 0.45, 95% CI: 0.40–0.50, respectively) compared to infants with a normal birthweight. Albeit lower than for the first dose, the risk of delay remained significantly higher in VLBW infants for each subsequent dose.
The second study also showed longer delays for infants with lower birthweights. The mean age for the first pentavalent administration was 4.3 ± 1.4 months in infants with a birthweight of <1000 g vs. 3.1 ± 1.0 in infants with a birthweight of 1000–1500 g (p < .001).Citation34
Hexavalent vaccines
One of the two studies that assessed compliance in immature infants who received hexavalent vaccinations also concluded that the majority received their first vaccinations after the recommended period.Citation36 The mean age at first vaccination was significantly higher in infants who were not hospitalized. The second study did not draw any overall conclusions, but 48.8% of the preterm infants (<31 weeks) received their first vaccination on time. About 37.7% were immunized even earlier than recommended and only 13.3% later than recommended. Timing of the first vaccine dose was not significantly different for infants with or without chronic conditions.Citation37 Reasons for earlier vaccination included allowing immunization under medical monitoring before hospital discharge, while reasons for late vaccination included surgical interventions, femoral rupture, severe chronic lung disease and late-onset sepsis.
Penta- and hexavalent vaccines
One study in infants who received either penta- or hexavalent vaccines concluded that vaccination was clearly delayed in the studied population of preterm infants with a birthweight of <1500 g, for all primary doses and the booster.Citation43
Three studies reported the mean age of the infants at immunization,Citation34,Citation36,Citation43 which was 1.1 to 1.4 months older than recommended for the first dose, 1.0 to 1.5 months older than recommended for the second dose, 1.7 to 2.3 months older for the third dose and 1.7 months older for the fourth (booster) dose. The statistical significance of these differences was not assessed.
Completion
Two publications reported data on completion of pentavalent vaccine schedules and penta- and hexavalent vaccine schedules.Citation34,Citation43
For the first dose, completion was 100% in both studies. For the second dose, completion was 97.5% in the study with the pentavalent vaccine and 100% in the study with penta- and hexavalent vaccines. For the third dose, completion was 93.4% in the study with the pentavalent vaccine and 97.5% in the study with penta- and hexavalent vaccines. Finally, completion of the booster dose was 89.0% in the study with penta- and hexavalent vaccines.
Ochoa et al.Citation34 also stratified the data for preterm infants with <1000 g birthweight and those with a birthweight between 1000 and 1500 g. Completion of the second and the third dose was significantly lower in preterm infants with lower birthweight compared to those with a higher birthweight (92.7% vs. 98.7% and 80.5% vs. 96.8%, respectively).
Discussion
Overall, preterm infants developed adequate antibody responses in terms of seroconversion/seropositivity to all vaccine antigens following primary and booster vaccination, regardless of the type of vaccine used. Pneumococcal and other vaccines were not included in the analysis. The only exception was a low percentage of responders to PT (≤ 75.0%) among both preterm and full-term infants who received primary vaccination with a DTwP-HBV-Hib pentavalent vaccine (brand not specified);Citation32 the cause of this observation and whether it is an isolated finding have not been elucidated. In all studies that included a control group, seroconversion/seropositivity rates in preterm (including VLBW) infants were similar to those in full-term infants.Citation31,Citation32,Citation35,Citation38,Citation42 Similar findings were observed when VLBW infants (<1500 g) were compared to preterm infants with a higher birthweight (1500–2000 g), except for a significantly lower percentage of VLBW infants with anti-PRP antibody concentrations ≥1.0 µg/mL after primary vaccination.Citation39 In some of the included studies, GMCs/GMTs in preterm infants were lower than those in full-term infants, but most infants still reached the threshold for seroconversion.Citation30,Citation31 These results further support the widely established consensus that preterm infants should be vaccinated according to their actual age and not to their corrected age. Several guidelines issued by national authorities and professional societies from the European Union,Citation6,Citation18–21,Citation45 the United Kingdom,Citation22 the United States,Citation23,Citation24 Canada,Citation16 Israel,Citation25 Vietnam,Citation26 and AustraliaCitation27 reflect this consensus, although in some cases, differences exist. For example, in Poland, an aP combination vaccine should be used in premature (<37 weeks) and LBW (<2500 g) infants for primary vaccination and booster instead of the standard vaccine with a wP component included in the national immunization program.Citation46 In Vietnam, the Ministry of Health recommends vaccinating children with a pentavalent vaccine according to the schedule established for full-term infants, regardless of weight. However, children weighing <2000 g should be transferred to a hospital for screening and vaccination.Citation26,Citation47 In Belgium, the fourth (booster) dose of hexavalent vaccine should be administered early to premature infants, at 13 months of age instead of the 15-month mark included in the national vaccination schedule.Citation21 In Sweden, a first, additional dose of hexavalent vaccine is to be administered at six weeks of uncorrected age to preterm infants born at ≤32 weeks; however, infants born ≤26 weeks should only be vaccinated after they reach a corrected gestational age of at least 34 weeks.Citation20
However, it must be emphasized that the immunogenicity results that stood at the base of this consensus (similarly to those included in this review) were all obtained using a 3+1 vaccination schedule. In some countries, such as Denmark, Finland, France, Germany, Italy, Spain and Sweden, the number of primary vaccinations with penta-and hexavalent vaccines only includes two doses.Citation48 As it is yet unclear whether the optimal immunogenicity elicited by the 3+1 schedule will also be reached with the 2+1 schedule, professional societies like the Pediatric Infectious Diseases Group (GPIP) in France, the Ständige Impfkommission (STIKO) at the Robert Koch Institute in Germany, and the Public Health Agency of Sweden, recommend using a 3+1 schedule in preterm infants.Citation19,Citation20,Citation49 More studies are needed on the immunogenicity of the 2+1 vaccination schedule in premature infants, especially in those <28 weeks.Citation6
The reactogenicity profile of penta- and hexavalent vaccines in preterm infants appears to be similar or even more favorable compared to full-term infants,Citation35 possibly due to a lower ability of preterm infants to mount an inflammatory response.Citation6 Serious and fatal AEs were generally low in the studies included. Nevertheless, there is some evidence that penta- and hexavalent vaccination could increase cardiorespiratory AEs such as apnea, bradycardia and desaturation in preterm infants,Citation33,Citation37 although these findings were not consistent across the studies included in this review.Citation36
In one study, apnea and bradycardia appeared significantly more often in infants vaccinated with a hexavalent vaccine (DTaP-HBV-IPV/Hib, Infanrix hexa) co-administered with a pneumococcal vaccine (PCV7, Prevenar) compared to infants who received a pentavalent vaccine (DTaP-IPV/Hib, Infanrix IPV/Hib) co-administered with a HBV vaccine (Gen H-B-Vax K pro infantibus) or DTaP-HBV-IPV/Hib (Infanrix hexa) alone.Citation41 Cardiorespiratory AEs have been reported mainly after the first dose of penta- or hexavalent vaccine; however, such events might reoccur at a lower frequency after the second and subsequent doses of the vaccination schedule.Citation40,Citation41,Citation50,Citation51
In two studies, the reported cardiorespiratory AEs appeared only in infants with bronchopulmonary dysplasia or gastroesophageal reflux;Citation35,Citation37 information regarding any comorbidities in infants who experienced cardiorespiratory AEs following penta- or hexavalent vaccination was not available in the other studies. In a review summarizing data from clinical studies and post-marketing safety surveillance of DTaP-HBV-IPV/Hib (Infanrix hexa), the authors suggested that the observed post-immunization cardiorespiratory AEs might be influenced by the presence and severity of various underlying prematurity-related comorbidities, including preexisting apnea-bradycardia requiring respiratory support, bronchopulmonary dysplasia, severe infections, necrotizing enterocolitis, peri- or intraventricular hemorrhage and cystic periventricular leukomalacia.Citation52 However, they also highlight that even in these populations of clinically unstable preterm infants, most cardiorespiratory AEs either resolved spontaneously or following minimal intervention.Citation52 Preterm infants in more critical clinical conditions appear to be more predisposed to experience apnea episodes after penta- or hexavalent vaccination. Flatz-Jequier et al.Citation40 reported that 25% of preterm infants who were hospitalized (for reasons that were not specified) at the time of vaccination had cardiorespiratory AEs, compared to none of the children vaccinated outside of a hospital setting. However, no information was given regarding any variations in underlying conditions or gestational age at birth that could potentially explain this difference. On the other hand, Czajka et al.Citation36 did not observe cardiorespiratory AEs in either group (hospitalized and non-hospitalized), despite the lower gestational age of the included infants. Similarly, lower birthweight and lower gestational age might contribute to a higher risk of cardiorespiratory AEs following vaccination.Citation7,Citation52–54
Furthermore, a causal relationship between the vaccine and the appearance of cardiorespiratory AEs is difficult to confirm, as the evidence supporting it often comes from retrospective and/or uncontrolled studies with a small sample size. In addition, distinguishing between apnea episodes caused by vaccination and prematurity-associated comorbidities might be challenging.Citation6 To date, only one prospective randomized controlled trial explored the association of cardiorespiratory AEs and hexavalent vaccination in preterm infants; results of this study indicated that the frequency of prolonged episodes of apnea and bradycardia was not significantly different between vaccinated and unvaccinated infants.Citation55
To correctly interpret any results regarding the frequency of cardiorespiratory AEs after vaccination, additional factors that might influence this frequency must be considered. However, the marked heterogeneity of the preterm infant population, in terms of gestational age, birthweight and the presence of prematurity-associated comorbidities, poses a considerable challenge in the way of defining the risk of cardiorespiratory AEs after penta- or hexavalent vaccination.Citation52 To reach a definitive conclusion, prospective studies are needed in all categories of preterm infants. This includes the extremely preterm, in whom the immunogenicity and safety of penta- and hexavalent vaccines are most likely to differ from those described in full-term infants, as well as the moderate to late preterm, who might react more similarly to full-term infants and constitute the largest part of the preterm population.Citation11
However, given the potential risk of apnea reported in several studies, monitoring preterm infants (especially very and extremely preterm infants), VLBW infants and premature infants still hospitalized at the time of the first penta- or hexavalent vaccination for 48–72 hours is recommended by professional societies in several countries.Citation7,Citation16,Citation18,Citation24,Citation27,Citation45,Citation56 In addition, vaccinating preterm infants who are considered at high risk of cardiorespiratory AEs in a hospital setting, as well as administering the second dose of penta- or hexavalent vaccine under cardiorespiratory monitoring to premature infants who experienced cardiorespiratory AEs following the first dose might be warranted.Citation6,Citation7,Citation18,Citation21,Citation27,Citation45,Citation56 On the downside, such recommendations could elicit serious concerns in healthcare providers responsible for vaccination outside of neonatal wards when they are confronted with a young child who was born preterm.
Despite the widespread recommendation of vaccinating preterm infants according to their actual age, and the relatively high completion rate of the primary immunization schedule, vaccination is often delayed in this population, as highlighted by results of the studies included in this review.Citation30,Citation34,Citation36,Citation37,Citation43 One of the studies stratified the data per birthweight and found that completion of the second and third dose was significantly lower in preterm infants with lower birthweight (<1000 g vs. 1000–1500 g).Citation34 In addition, the results indicated that completion of penta- and/or hexavalent vaccines might be lower in the extremely LBW preterm infants (<1000 g). This phenomenon was also reported by Tozzi et al.,Citation57 who found that over 30% of premature infants were not vaccinated with a hexavalent vaccine at the end of the recommended period, with delay in vaccination being associated with LBW. The wider window of vulnerability caused by postponing vaccination represents an even bigger concern in preterm infants, considering their higher susceptibility to VPDs like pertussis, compared to their full-term counterparts.Citation58
Preterm infants who were still hospitalized at the time of vaccination (but were clinically stable) were more likely to receive their first vaccine dose on time, as one study found,Citation36 suggesting that implementation of vaccination programs in neonatal wards could be beneficial for increasing penta- and hexavalent vaccine compliance.Citation16 Unfortunately, opportunities to promote timely vaccination in neonatal units are not always adequately exploited.Citation59 Parents might have concerns about vaccinating their children born prematurely or with LBW due to concerns of adverse effects. Their concerns might also be shared by the healthcare providers working outside a neonatal unit, as they cannot provide the same level of monitoring to minimize the risk of cardiorespiratory AEs.
The reasons for delaying vaccination of preterm infants are multifactorial, and include limited knowledge of healthcare providers and parents or caregivers about vaccine effectiveness and fear of AEs;Citation17 overall, preterm birth is commonly misperceived as a contraindication for vaccination.Citation60 A recent cross-sectional survey conducted in 2021 among 428 Italian healthcare providers (including primary care providers as well as nurses and pediatricians working in neonatal intensive care units) revealed that only 70% of them recommend timely vaccination of preterm infants.Citation59 Those who were aware that preterm infants should be vaccinated according to the same schedule as full-term infants and strongly agreed with this recommendation, as well as those who considered vaccines as very effective in preterm infants, were less likely to delay vaccinations. This finding suggests that educating healthcare providers might favorably impact timely penta- and hexavalent vaccine uptake in preterm infants.
Although the objective of this systematic literature review was to compile data available on the immunogenicity, efficacy, effectiveness, safety, impact, compliance, and completion of penta- and hexavalent vaccination in infants with special conditions (including immature newborns born at <1500 g or <34 weeks of gestation), no data was found on the efficacy, effectiveness, or impact of penta- and hexavalent vaccination in any of these categories. Furthermore, for the immunogenicity, safety, compliance, and completion outcomes, with the exception of one article on infants with cerebral palsy, only articles on immature infants were found, highlighting a major knowledge gap related to vaccination of infants with other high-risk profiles. In addition, a large part of the immunogenicity data retrieved came from preterm infants vaccinated with one hexavalent vaccine, DTaP-HBV-IPV/Hib (Infanrix hexa). This highlights the need for all vaccine manufacturers to conduct prospective clinical studies in a wide range of special populations, in order to generate robust data on different vaccine types that would support timely vaccination of high-risk infants. Despite the broad search strategy applied, it is possible that articles were not retrieved if they only used very specific terms for the vaccine in their titles and abstracts, which were not covered by our search strings. Indeed, at least four publications were missed by the original search and were subsequently identified during writing; these publications would have provided additional data on immunogenicity, compliance and completion.Citation57,Citation61–63 Rouers et al.Citation61 investigated the immunogenicity of the Dutch routine national immunization program in the first year of life in a large cohort of preterm infants of different gestational age groups; vaccine antigen responses were compared with a historical cohort of full-term infants. This study found that the proportion of preterm infants with antibody levels reaching seroconversion against PT (using an arbitrary threshold of ≥20 IU/mL), diphtheria toxoid and tetanus toxin (using thresholds of ≥0.1 IU/mL) was greater than 90% across all gestational age groups following three primary doses of DTaP-HBV-IPV/Hib (Infanrix hexa) and did not significantly differ from the full-term control group. These results are in line with the results of the studies included in this review.Citation39,Citation42 However, Rouers et al.Citation61 also found that significantly fewer preterm infants with gestational ages of <28 and 28–<32 weeks had anti-PRP levels reaching the threshold for seroconversion (and thus potentially seroprotection) for Hib, compared to their full-term counterparts (34.7% and 40.4% vs. 83.6%), a result which was in contrast with the results of the studies included in this review, except for those reported by Kulkarni-Munje et al.Citation32 Slack et al.Citation62 assessed immune responses in preterm (<32 weeks) infants in the UK vaccinated with three doses of DT5aP-Hib-IPV (Pediacel), and compared the results with those in full-term infants. Following an accelerated vaccination schedule (2, 3, 4 months), all infants achieved titers corresponding to seroconversion against diphtheria, tetanus, and the three poliovirus types, with>80% achieving rises in immunoglobulin G (IgG) against the five pertussis antigens. Regarding the immune response to Hib, higher antibody levels were generated in preterm infants vaccinated with Pediacel in comparison to historical data following Infanrix hexa vaccination. Nevertheless, 20% of infants who received DT5aP-Hib-IPV (Pediacel) still had IgG concentrations <0.15 μg/mg and 40% had concentrations of <0.1 μg/mg after completing the primary vaccination series. This prompted authors to suggest that in such cases, a fourth dose of Hib conjugate vaccine could be warranted in this population. Tozzi et al.Citation57 conducted a follow-up study of vaccination compliance and completion in preterm infants (22–31 weeks) in Italy. The results revealed that the start of immunization with a hexavalent vaccine against diphtheria, tetanus, pertussis, poliomyelitis, HBV and Hib (DTP-Pol-HBV-Hib, brand not specified) was considerably delayed, even though hexavalent vaccination was eventually initiated in 95.9% of infants before the age of 24 months. Pinquier et al.Citation63 found that immunization of preterm infants<33 weeks with a pentavalent vaccine against diphtheria, tetanus, pertussis, poliomyelitis and Hib (DTPCoqHib, brand not specified) in France usually began after the fourth month of life, with 45% of infants having received three doses by six months of age. At two years of age, pentavalent vaccine coverage was 99% for three doses and 83% for four doses. The latter two studies reinforce the finding that penta- and hexavalent vaccination is often delayed in premature infants, contrary to the current national and international recommendations.
Our review had further limitations. Apart from the exclusion of case studies and case series, no limits were applied regarding sample size; therefore, three of the included studies had relatively small sample sizes (between 30 and 50 infants), especially in the subgroups of preterm infants. For some immunogenicity sub-analyses, the sample sizes were very small (<20 or even<10 infants). Second, results of preterm or LBW infants were not compared to results of full-term or normal birthweight infants in six studies; this may be less important for the interpretation of the compliance and completion outcomes but may hamper the interpretability of safety outcomes. None of these studies reported immunogenicity outcomes. Some studies used preterm infants with a higher birthweight as comparator or compared hospitalized to non-hospitalized infants or different vaccines/co-administrations. While we acknowledge that these comparisons are important, this heterogeneity in comparators, in combination with the scarcity of data in the preterm infant population makes it difficult to draw conclusions. Studies with no control group were not downgraded during quality assessment; this limitation must be kept in mind when interpreting the results. Finally, data from late preterm infants born at ≥34 weeks were not included in this report, as we aimed to focus on the more vulnerable population of extremely and very-to-moderate preterm infants. Although infants born at <32 weeks represent only around 16% of the preterm population, they are more dependent on specialized neonatal care to survive than infants born after 32 weeks of gestation, and, along with moderately preterm infants born at <34 weeks, are more likely to require prolonged hospitalization.Citation11
Overall, studies were quite heterogeneous, including differences in study population (hospitalized vs. not hospitalized, presence of chronic diseases), type (penta- or hexavalent) and brand of vaccine, co-administrations, and application of HBV birth doses. All these factors may influence immunogenicity, safety, compliance, and completion results.
In conclusion, the studied penta- and hexavalent vaccines administered according to a 3+1 schedule appear to be immunogenic in extremely and very-to-moderate preterm infants and infants with VLBW, with an acceptable safety profile. Cardiorespiratory AEs (apnea, bradycardia, desaturation) were commonly reported following vaccination in the population of immature infants, mainly after the first dose; therefore, cardiorespiratory surveillance during and after vaccination is warranted and recommended in several countries. Despite the existing recommendations, delays in immunization are frequent in preterm infants, prolonging the window of susceptibility to VPDs in this already vulnerable population. National guidelines on vaccinating preterm infants do not exist in all countries, and those that are available sometimes include special indications for preterm infants, potentially increasing the reluctance of healthcare providers in vaccinating a child born prematurely. Misconceptions and concerns regarding the vaccination of preterm infants that lead to delayed immunization should be further explored and addressed in order to promote vaccinating this susceptible population on time. In addition, robust data should be generated in a prospective way for other vaccine types besides DTaP-HBV-IPV/Hib (Infanrix hexa) in preterm infants, as well as for high-risk groups other than immature infants.
Trademark statement
ActHIB, Hexavac and Pediacel are trademarks of Sanofi Pasteur. Gen H-B-Vax K pro infantibus is a trademark of Aventis Pasteur. Prevenar/Prevnar are trademarks of Wyeth LLC. Infanrix hexa, Infanrix penta, Infanrix IPV/Hib, Menitorix, Pediarix and Synflorix are trademarks owned by or licensed to the GSK group of companies.
Acknowledgments
Authors thank the Akkodis Belgium platform for editorial assistance, manuscript coordination, and design support on behalf of GSK. Timea Kiss provided medical writing support.
Disclosure statement
MD and VB are employees of GSK, own restricted shares in the company and declare financial/non-financial relationships and activities. LM and PK’s institution received financial support from GSK during the conduct of the research. MK, ISC, PNTN and MLC have nothing to disclose.
Additional information
Funding
References
- Skibinski DA, Baudner BC, Singh M, O’Hagan DT. Combination vaccines. J Glob Infect Dis. 2011;3(1):63–29. doi:10.4103/0974-777X.77298.
- Vidor E, Soubeyrand B. Manufacturing DTaP-based combination vaccines: industrial challenges around essential public health tools. Expert Rev Vaccines. 2016;15(12):1575–82. doi:10.1080/14760584.2016.1205492.
- Obando-Pacheco P, Rivero-Calle I, Gómez-Rial J, Rodríguez-Tenreiro Sánchez C, Martinón-Torres F. New perspectives for hexavalent vaccines. Vaccine. 2018;36(36):5485–94. doi:10.1016/j.vaccine.2017.06.063.
- World Health Organization. DT-based combined vaccines. World Health Organization. [Accessed 2022 Jul 22]. https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/dt-based-combined-vaccines.
- Orsi A, Azzari C, Bozzola E, Chiamenti G, Chirico G, Esposito S, Francia F, Lopalco P, Prato R, Russo R, et al. Hexavalent vaccines: characteristics of available products and practical considerations from a panel of Italian experts. J Prev Med Hyg. 2018;59(2):E107–19.
- Chiappini E, Petrolini C, Caffarelli C, Calvani M, Cardinale F, Duse M, Licari A, Manti S, Martelli A, Minasi D, et al. Hexavalent vaccines in preterm infants: an update by Italian Society of Pediatric Allergy and Immunology jointly with the Italian Society of Neonatology. Ital J Pediat. 2019;45():145. doi:10.1186/s13052-019-0742-7.
- Aronsson B, Florén CC, Greve-Isdahl M, Lindstrand A, Nøkleby H, Riise Ø, Storsæter J, Vestrheim DF, Watle SV. Vaccination of preterm infants against pertussis and pneumococci. Immunogenicity, effectiveness and safety. Oslo (Norway): Norwegian Institute of Public Health; 2018 [accessed 2022 Jul 20]. https://www.fhi.no/globalassets/dokumenterfiler/rapporter/vaksine/vaccination-of-preterm-infants-against-pertussis-and-pneumococci_web.pdf.
- World Health Organization. Preterm birth; 2018 [accessed 2022 Sep 7]. https://www.who.int/news-room/fact-sheets/detail/preterm-birth.
- World Health Organization. Global nutrition targets 2025: low birth weight policy brief. Geneva (Swizerland): World Health Organization; 2014 [accessed 2022 Jul 22]. https://www.who.int/publications/i/item/WHO-NMH-NHD-14.5.
- Bruijning-Verhagen P, Mangen MJJ, Felderhof M, Hartwig NG, van Houten M, Winkel L, de Waal WJ, Bonten MJM. Targeted rotavirus vaccination of high-risk infants; a low cost and highly cost-effective alternative to universal vaccination. BMC Med. 2013;11():112. doi:10.1186/1741-7015-11-112.
- March of Dimes. The partnership for maternal, newborn and child health, save the children, World Health Organization. Born too soon: the global action report on preterm birth. Geneva: World Health Organization; 2012.
- Walsh MC, Bell EF, Kandefer S, Saha S, Carlo WA, D’Angio CT, Laptook AR, Sanchez PJ, Stoll BJ, Shankaran S, et al. Neonatal outcomes of moderately preterm infants compared to extremely preterm infants. Pediatr Res. 2017;82(2):297–304. doi:10.1038/pr.2017.46.
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–40. doi:10.1016/S0140-6736(14)61698-6.
- Baxter D. Impaired functioning of immune defenses to infection in premature and term infants and their implications for vaccination. Hum Vaccin. 2010;6(6):494–505. doi:10.4161/hv.6.6.12008.
- Chapter 10. Mortality and acute complications in preterm infants. In: Behrman RE, Butler AS, editors. Preterm birth: causes, consequences, and prevention. Washington (DC): National Academies Press (US); 2007. p. 313–45.
- Government of Canada. Immunization of infants born prematurely: Canadian immunization guide; 2015 [accessed 2022 Jul 18]. https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-3-vaccination-specific-populations/page-5-immunization-infants-born-prematurely.html.
- Gagneur A, Pinquier D, Quach C. Immunization of preterm infants. Hum Vaccin Immunother. 2015;11(11):2556–63. doi:10.1080/21645515.2015.1074358.
- Ministère de la Santé et de la Prévention. Calendrier des vaccinations et recommandations vaccinales 2022 [Vaccination schedule and vaccine recommendations 2022]. Paris (France): Ministère de la Santé et de la Prévention; 2022 [accessed 2022 Jul 20]. https://solidarites-sante.gouv.fr/IMG/pdf/calendrier_vaccinal_2022_mis_a_jour_juin_2022_v2.pdf.
- Robert Koch Institute. Immunization schedule. Recommendations of the standing committee on vaccination (STIKO), 2022. Berlin (Germany): Robert Koch Institute; 2022 [accessed 2022 Jul 20]. https://www.rki.de/DE/Content/Infekt/Impfen/Materialien/Downloads-Impfkalender/Impfkalender_Englisch.pdf?__blob=publicationFile.
- Public Health Agency of Sweden. Vaccination av för tidigt födda barn [Vaccination of premature babies]. Solna (Sweden): Public Health Agency of Sweden. [Accessed 2022 Sep 8]. https://www.folkhalsomyndigheten.se/smittskydd-beredskap/vaccinationer/rekommendationer-for-vaccination/vaccination-av-prematura-barn/#:~:text=F%C3%B6rsta%20dosen%20vaccin%20mot%20rotavirusinfektion,vaccin%20mot%20rotavirusinfektion%20p%C3%A5%20neonatalavdelningen.
- Conseil Supérieur de la Santé de Belgique. Prématurés: aspects spécifiques de la vaccination [Premature infants: specific aspects of vaccination]; 2014 [accessed 2022 Sep 8]. https://www.vaxinfopro.be/spip.php?article1066&lang=fr&retour=1.
- Public Health England. Updated schedule for 2020. A quick guide to childhood immunisations for the parents of premature babies born on or after 1 January 2020; 2019 [accessed 2022 Jul 21]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/849306/PHE_11490_premature_quickguide_Jan2020.pdf.
- Centers for Disease Control and Prevention. Special Situations. General best practice guidelines for immunization: best practices guidance of the Advisory Committee on Immunization Practices (ACIP). Atlanta (GA, USA): National Center for Immunization and Respiratory Diseases; 2022 [accessed 2022 Jul 19]. https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/special-situations.html.
- Saari TN. American Academy of Pediatrics Committee on Infectious Diseases. Immunization of preterm and low birth weight infants. Pediatrics. 2003;112(1):193–8. doi:10.1542/peds.112.1.193.
- State of Israel. Ministry of health. Vaccines for premature babies. Israel: Ministry of Health. [Accessed 2022 Jul 19]. https://www.health.gov.il/English/Topics/Pregnancy/Vaccination_of_infants/Pages/vaccine_premature_baby.aspx.
- Health Strategy and Policy Institute. Quyết định 2470/QĐ-BYT hướng dẫn khám sàng lọc trước tiêm chủng đối với trẻ em [Decision 2470/QD-BYT guiding pre-vaccination screening for children]. Hanoi (Vietnam): Institute of Health Strategy and Policy; 2019 [accessed 2022 Sep 6]. http://www.hspi.org.vn/vcl/Decision%202470%20/%20QD-BYT%20Vietnamese%20Ministry%20of%20Health%20guiding%20pre-vaccination%20screening%20checks%20for%20children.%20em-t98-8951.html.
- Australian Government. Department of health and aged care. Vaccination for preterm infants; 2020 [accessed 2022 Jul 18]. https://immunisationhandbook.health.gov.au/contents/vaccination-for-special-risk-groups/vaccination-for-preterm-infants.
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions. Chichester (UK): Wiley Blackwell; 2019.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71.
- Bary-Weisberg D, Stein-Zamir C. Vaccination timeliness and completeness among preterm and low birthweight infants: a national cohort study. Hum Vaccin Immunother. 2021;17(6):1666–74. doi:10.1080/21645515.2020.1840255.
- Klein NP, Gans HA, Sung P, Yasukawa LL, Johnson J, Sarafanov A, Chumakov K, Hansen J, Black S, Dekker CL. Preterm infants’ T cell responses to inactivated poliovirus vaccine. J Infect Dis. 2010;201(2):214–22. doi:10.1086/649590.
- Kulkarni-Munje A, Malshe N, Palkar S, Amlekar A, Lalwani S, Mishra AC, Arankalle V. Immune response of Indian preterm infants to pentavalent vaccine varies with component antigens and gestational age. Front Immunol. 2021;12:592731. doi:10.3389/fimmu.2021.592731.
- Lee J, Robinson JL, Spady DW. Frequency of apnea, bradycardia, and desaturations following first diphtheria-tetanus-pertussis-inactivated polio-Haemophilus influenzae type B immunization in hospitalized preterm infants. BMC Pediatr. 2006;6():20. doi:10.1186/1471-2431-6-20.
- Ochoa TJ, Zea-Vera A, Bautista R, Davila C, Salazar JA, Bazán C, López L, Ecker L. Vaccine schedule compliance among very low birth weight infants in Lima, Peru. Vaccine. 2015;33(2):354–8. doi:10.1016/j.vaccine.2014.11.014.
- Omeñaca F, Arístegui J, Tejedor JC, Moreno-Perez D, Ruiz-Contreras J, Merino JM, Muro Brussi M, Sánchez-Tamayo T, Castro Fernandez J, Cabanillas L, et al. Combined Haemophilus Influenzae type B-Neisseria meningitidis serogroup C vaccine is immunogenic and well tolerated in preterm infants when coadministered with other routinely recommended vaccines. Pediatr Infect Dis J. 2011;30(11):e216–24. doi:10.1097/INF.0b013e3182293a82.
- Czajka H, Lauterbach R, Pawlik D, Radziszewska R. Implementation of mandatory vaccinations against diphtheria, tetanus and pertussis in preterm infants as part of the Polish Immunization Programme. Pediatr Pol. 2017;92(5):485–93. doi:10.1016/j.pepo.2017.05.008.
- Faldella G, Galletti S, Corvaglia L, Ancora G, Alessandroni R. Safety of DTaP–IPV–Hib–HBV hexavalent vaccine in very premature infants. Vaccine. 2007;25(6):1036–42. doi:10.1016/j.vaccine.2006.09.065.
- Omeñaca F, Garcia-Sicilia J, Boceta R, García-Corbeira P. Hepatitis B response of premature infants after primary and booster immunisation with a diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus/Haemophilus influenzae type B vaccine. Infect Dis Obstet Gynecol. 2010;2010:802503. doi:10.1155/2010/802503.
- Vázquez L, Garcia F, Rüttimann R, Coconier G, Jacquet JM, Schuerman L. Immunogenicity and reactogenicity of DTPa-HBV-IPV/Hib vaccine as primary and booster vaccination in low-birth-weight premature infants. Acta Paediatr. 2008;97(9):1243–9. doi:10.1111/j.1651-2227.2008.00884.x.
- Flatz-Jequier A, Posfay-Barbe KM, Pfister RE, Siegrist CA. Recurrence of cardiorespiratory events following repeat DTaP-based combined immunization in very low birth weight premature infants. J Pediatr. 2008;153(3):429–31. doi:10.1016/j.jpeds.2008.03.043.
- Furck AK, Richter JW, Kattner E. Very low birth weight infants have only few adverse events after timely immunization. J Perinatol. 2010;30(2):118–21. doi:10.1038/jp.2009.112.
- Omeñaca F, Merino JM, Tejedor JC, Constantopoulos A, Papaevangelou V, Kafetzis D, Tsirka A, Athanassiadou F, Anagnostakou M, François N, et al. Immunization of preterm infants with 10-valent pneumococcal conjugate vaccine. Pediatrics. 2011;128(2):e290–8. doi:10.1542/peds.2010-1184.
- Ziegler B, Strassburg HM. Impfstatus bei Frühgeborenen mit einem Geburtsgewicht unter 1500 g im Alter von 2 Jahren – eine deutschlandweite Piloterhebung. Klin Padiatr. 2010;222(4):243–7. doi:10.1055/s-0030-1247586.
- Bozkaya-Yilmaz S, Karadag-Oncel E, Olgac-Dundar N, Gencpinar P, Sarioglu B, Arican P, Ersen A, Yilmaz-Ciftdoğan D, Yuksel MF, Bektas O, et al. Evaluation of immunization status in patients with cerebral palsy: a multicenter CP-VACC study. Eur J Pediatr. 2022;181(1):383–91. doi:10.1007/s00431-021-04219-4.
- Grupo de trabajo vacunación en prematuros de la Ponencia de Programa y Registro de Vacunaciones. Vacunación en prematuros [Vaccination of preterm infants]; 2019 [accessed 2022 Nov 7]. https://www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/enfermedades/docs/Vacunacion_Prematuros.pdf.
- European Centre for Disease Prevention and Control. Vaccine scheduler. Poland: Recommended vaccinations. [Accessed 2022 Jul 21]. https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByCountry?SelectedCountryId=166&IncludeChildAgeGroup=true&IncludeChildAgeGroup=false&IncludeAdultAgeGroup=true&IncludeAdultAgeGroup=false.
- Ministry of Health Viet Nam. National expanded program on immunization. Comprehensive multi-year plan cMYP for extended program on immunization, 2016-2020. Hanoi (Vietnam): World Health Organization/Ministry of Health Viet Nam; 2015 [accessed 2022 Nov 10]. https://extranet.who.int/countryplanningcycles/sites/default/files/planning_cycle_repository/viet_nam/cmyp_vietnam_2016-2020.pdf.
- European Centre for Disease Prevention and Control. Vaccine scheduler. Vaccine schedules in all countries in the EU/EEA. [Accessed 2022 Jul 21]. https://vaccine-schedule.ecdc.europa.eu/.
- Gaudelus J, Pinquier D, Romain O, Thiebault G, Vie le Sage F, Dommergues MA, Hau I, Bakhache P, Virey B, Dufour V, et al. Le nouveau calendrier vaccinal est-il adapté à l’ancien prématuré ? Arch Pediatr. 2014;21(9):1062–70. doi:10.1016/j.arcped.2014.06.020.
- Anderson J, Noori K, Morris SA. Apnoea after the 2-month immunisation in extremely preterm infants: what happens with the 4-month immunisation? J Paediatr Child Health. 2013;49(3):E217–20. doi:10.1111/jpc.12110.
- Clifford V, Crawford NW, Royle J, Lazzaro T, Danchin M, Perrett KP, Lee KJ, Buttery JP. Recurrent apnoea post immunisation: informing re-immunisation policy. Vaccine. 2011;29(34):5681–7. doi:10.1016/j.vaccine.2011.06.005.
- Omeñaca F, Vázquez L, Garcia-Corbeira P, Mesaros N, Hanssens L, Dolhain J, Gómez IP, Liese J, Knuf M. Immunization of preterm infants with GSK’s hexavalent combined diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus-Haemophilus influenzae type b conjugate vaccine: A review of safety and immunogenicity. Vaccine. 2018;36(7):986–96. doi:10.1016/j.vaccine.2018.01.005.
- Pfister RE, Aeschbach V, Niksic-Stuber V, Martin BC, Siegrist CA. Safety of DTaP-based combined immunization in very-low-birth-weight premature infants: frequent but mostly benign cardiorespiratory events. J Pediatr. 2004;145(1):58–66. doi:10.1016/j.jpeds.2004.04.006.
- Buijs SC, Boersma B. Cardiorespiratoire verstoringen na 1e vaccinatie bij prematuur geboren kinderen: een prospectief cohortonderzoek [Cardiorespiratory events after first immunization in premature infants: a prospective cohort study]. Ned Tijdschr Geneeskd. 2012;156 3 :A3797.
- Carbone T, McEntire B, Kissin D, Kelly D, Steinschneider A, Violaris K, Karamchandani N. Absence of an increase in cardiorespiratory events after diphtheria-tetanus-acellular pertussis immunization in preterm infants: a randomized, multicenter study. Pediatrics. 2008;121(5):e1085–90. doi:10.1542/peds.2007-2059.
- Wiese-Posselt M, Tertilt C, Zepp F. Vaccination recommendations for Germany. Dtsch Arztebl Int. 2011;108 45 :771–80. doi:10.3238/arztebl.2011.0771.
- Tozzi AE, Piga S, Corchia C, Di Lallo D, Carnielli V, Chiandotto V, Fertz MC, Miniaci S, Rusconi F, Cuttini M. Timeliness of routine immunization in a population-based Italian cohort of very preterm infants: results of the ACTION follow-up project. Vaccine. 2014;32(7):793–9. doi:10.1016/j.vaccine.2013.12.044.
- Riise ØR, Laake I, Vestrheim D, Flem E, Moster D, Riise Bergsaker MA, Storsæter J. Risk of pertussis in relation to degree of prematurity in children less than 2 years of age. Pediatr Infect Dis J. 2017;36(5):e151–6. doi:10.1097/INF.0000000000001545.
- Napolitano F, Miraglia Del Giudice G, Pelullo CP, Di Giuseppe G, Pavia M. Do pediatricians and nurses recommend vaccines for preterm infants? A survey in Italy. J Pediatr. 2022;246:64–70.e2. doi:10.1016/j.jpeds.2022.04.026.
- Kroger A, Bahta L, Hunter P General best practice guidelines for immunization. Best practices guidance of the Advisory Committee on Immunization Practices (ACIP). [Accessed 2022 Sep 8]. https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf.
- Rouers EDM, Bruijning-Verhagen PCJ, van Gageldonk PGM, van Dongen JAP, Sanders EAM, Berbers GAM. Association of routine infant vaccinations with antibody levels among preterm infants. JAMA. 2020;324(11):1068–77. doi:10.1001/jama.2020.12316.
- Slack MH, Cade S, Schapira D, Thwaites RJ, Crowley-Luke A, Southern J, Borrow R, Miller E. DT5aP-Hib-IPV and MCC vaccines: preterm infants’ response to accelerated immunisation. Arch Dis Child. 2005;90(4):338–41. doi:10.1136/adc.2004.052720.
- Pinquier D, Adde-Michela C, Ploin D, Levêque C, Marret S. Couverture vaccinale des grands prématurés à 6 mois et à 2 ans : étude pilote. Arch Pediatr. 2009;16(12):1533–9. doi:10.1016/j.arcped.2009.09.009.
Appendices Appendix A.
Search strategy.
MEDLINE (via the PubMed interface): a combination of search strings #1 OR #2 OR #3 was used.
#1 Hexavalent DTP vaccines
“dtwp hib hepb ipv”[tiab] OR “dtpa hbv ipv hib”[tiab] OR DTwP-Hib/HepB-IPV[tiab] OR DTaP-IPV-Hib-HepB[tiab] OR DTaP-IPV-Hep B-Hib[tiab] OR DTaP-HB-IPV-Hib[tiab] OR DTaP/IPV/Hib/HepB[tiab] OR DTPa-HBV-IPV/Hib[tiab] OR hexavalent vaccin*[tiab] OR Hepatitis B, Diphtheria-Tetanus-acellular Pertussis-IPV-Haemophilus influenzae B[tiab]
#2 Infanrix hexa and other hexavalent formulation names
Vaxelis[tiab] OR Hexavac[tiab] OR hexaxim[tiab] OR hexyon[tiab] OR “Infanrix hexa”[tiab] OR hexacima[tiab] OR Easysix[tiab]
#3 Pentavalent DTP vaccines
DTaP-IPV-Hib[tiab] OR DTaPIPVHib[tiab] OR DTP-IPV/Hib[tiab] OR DTaP(5)-IPV-Hib[tiab] OR Pentacel[tiab] OR Pediacel[tiab] OR Infanrix IPV+Hib[tiab] OR Infanrix-IPV/Hib[tiab] OR pentavalent vaccin*[tiab] OR Quinvaxem[tiab] OR Pentabio[tiab] OR Pentavac[tiab] OR shan5[tiab] OR Easyfive[tiab]
Embase:
#1 Hexavalent DTP vaccines
‘dtwp hib hepb ipv’:ti,ab OR ‘dtpa hbv ipv hib’:ti,ab OR ‘DTwP-Hib/HepB-IPV’:ti,ab OR ‘DTaP-IPV-Hib-HepB’:ti,ab OR ‘DTaP-IPV-Hep B-Hib’:ti,ab OR ‘DTaP-HB-IPV-Hib’:ti,ab OR ‘DTaP/IPV/Hib/HepB’:ti,ab OR ‘DTPa-HBV-IPV/Hib’:ti,ab OR ‘hexavalent vaccin*’:ti,ab OR ‘Hepatitis B, Diphtheria-Tetanus-acellular Pertussis-IPV-Haemophilus influenzae B’:ti,ab
#2 Infanrix hexa and other hexavalent formulation names
‘Vaxelis’:ti,ab OR ‘Hexavac’:ti,ab OR ‘hexaxim’:ti,ab OR ‘hexyon’:ti,ab OR ‘nfanrix hexa’:ti,ab OR ‘hexacima’:ti,ab OR ‘Easysix’:ti,ab
#3 Pentavalent DTP vaccines
‘DTaP-IPV-Hib’:ti,ab OR ‘DTaPIPVHib’:ti,ab OR ‘DTP-IPV/Hib’:ti,ab OR ‘DTaP(5)-IPV-Hib’:ti,ab OR ‘Pentacel’:ti,ab OR ‘Pediacel’:ti,ab OR ‘Infanrix-IPV+Hib’:ti,ab OR ‘Infanrix-IPV/Hib’:ti,ab OR ‘pentavalent vaccin*’:ti,ab OR ‘Quinvaxem’:ti,ab OR ‘Pentabio’:ti,ab OR ‘Pentavac’:ti,ab OR ‘shan5’:ti,ab OR ‘Easyfive’:ti,ab
Appendix B.
JBI critical appraisal checklists.
JBI, The Joanna Briggs Institute
Appendix C.
Local adverse events after penta- and hexavalent vaccines.
Appendix D.
Systemic adverse events after penta- and hexavalent vaccines.


