ABSTRACT
COVID-19, a respiratory infectious disease, occurs due to Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Millions of individuals around the world have been impacted by the illness, which has gravely threatened human health. The development and active involvement of varied vaccines against the COVID-19 have played a great and relieving role in controlling the life-threatening disease. Both the conventional and advanced vaccine platforms are available now to develop vaccines against COVID-19. Therefore, the present systematic review focuses on the global landscape of the COVID-19 vaccines and their current status. Among COVID-19 vaccines, virus like particles (VLPs), subunit vaccines, DNA, RNA-based vaccines, viral vector-based vaccines, inactivated and live-attenuated vaccines are the major contenders and are currently in various phase of clinical trials. Protein subunit, RNA-based and non-replicating viral vector-based platforms have been used majorly. Nevertheless, inactivated virus vaccine has been utilized clinically around the world. The clinical trials revealed that most of the vaccines have local or systemic effects after vaccination and varied efficacy against SARS-CoV-2 and its variants. However, further studies are necessary to refine the technology to minimize adverse effects and improve the safety and efficacy.
Abbreviations
COVID-19: Coronavirus disease 2019; SARS-CoV-2: Severe acute respiratory syndrome coronavirus-2; VLPs: Virus like particles; WHO: World Health Organization; E: Envelope; M: Membrane; S: Spike; N: Nucleocapsid; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; FDA: Food and Drug Administration; LNP: lipid-nanoparticle; AZD1222: ChAdOx1 nCoV-19; BNT162b2: Pfizer-BioNTech mRNA vaccine; mRNA-1273: Moderna vaccine; Ad26.COV2.S: Johnson and Johnson – Janssen’s vaccine; Gam-COVID-Vac: Sputnik Vaccine; NVX-CoV2373: Novavax vaccine with Matrix-M™ adjuvant.
Introduction
Coronavirus disease 2019 (COVID-19), caused by Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has affected a huge population worldwide. During March 2020, the World Health Organization (WHO) specified it as a pandemic, since then it has caused extensive morbidity and mortality around the globe. As of now (December 2022), the disease has caused approximately 6.6 million deaths and infection in more than 651 million people.Citation1 SARS-CoV-2, produces a variety of health problems, from mild instances to severe ones with significant mortality rates. Among the four most important structural proteins of the coronavirus, three are envelope (E), membrane (M) and spike (S) proteins found on the viral surface envelope, while the fourth one is nucleocapsid (N) protein, present in the ribonucleoprotein core. These are the primary targets for potential vaccines. SARS-CoV-2 like other RNA viruses may undergo mutation very often while passing from one host to another causing emergence of new variants that differ in characteristics with the original strain and responsible for multiple episodes of the pandemic in due time.Citation2–6
Many countries are still suffering the multiple waves of disease outbreaks though the disease transmission and death has declined due to several effective measures taken by health providers, communities and concerned governments.Citation7–9 However, the safe and effective prophylactic vaccines are of immediate necessity to curb the disaster led by pandemic that has already shattered the medical, economic, and social status of the society. Therefore, most of the countries now indulged in the development of effective COVID-19 vaccines through their assessment and manufacture.
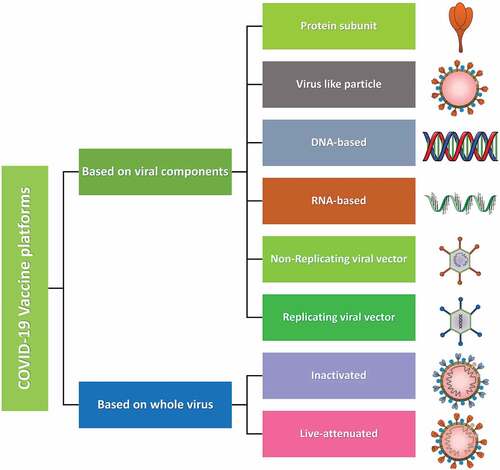
Vaccine is a crucial way to restrain the current pandemic and several research activities on COVID-19 therapeutics are going on with an exceptional speed. The vaccine platforms are of two categories one is based on viral components and another is based on the whole virus. Viral components include protein subunit, virus like particle, DNA-based, RNA-based, non-replicating viral vectors and replicating viral vectors. Whereas, the whole virus-based platform includes inactivated and live-attenuated vaccines (). Whatever may be the platform, a competent vaccine must be easy to develop, reproduce and administer, safe, thermostable and with low manufacturing cost. Although with several drawbacks presently over 200 vaccine research are in progress among which a large number of vaccines with a variety of platforms have been given approval for clinical trials.Citation1,Citation10 Pharmaceutical companies and their vaccine contenders like Pfizer-BioNTech’s BNT162, Oxford-AstraZeneca’s AZD1222, Sinovac’s CoronaVac, Moderna’s mRNA-1273, Johnson & Johnson’s Ad26.COV2.S, Sputnik-V, vector vaccines (Gamaleya National Research Centre for Epidemiology and Microbiology, and adjuvanted recombinant protein nanoparticles (Novavax) are currently leading with their excellent research and development on COVID-19.Citation11,Citation12
Figure 1. COVID-19 vaccines platforms. Types of various platforms used for the development of COVID-19 vaccines. Platforms based on viral components comprises protein-subunit vaccines which are isolated and purified viral proteins. Virus like particles (VLP) composed of viral proteins that mimic the structure of the virus, but no genetic material. DNA- and RNA-based vaccines are viral genetic material which encodes for viral proteins. Non-replicating viral vectors containing viral genetic material packaged inside viral vectors that cannot replicate. Replicating viral vectors containing viral genetic material packaged inside other viral vectors that can replicate. Whole virus-based vaccines are inactivated that contain copies of the virus that have been killed (inactivated) and live-attenuated which contains copies of the virus that have been weakened (attenuated).
A recombinant COVID-19 vaccine consists of an antigenic part of the virus that not only minimizes the secondary complications associated with live or attenuated vaccines but also has shown improved efficacy and safety. Till now the performance of the recombinant vaccines has been very promising and going through much detailed evaluation. Several vaccine candidates are under clinical trials and some of them have crossed phase III trials successfully and got administrative approval for further processes. Vaccine studies are mostly designed as individually randomized, placebo-controlled clinical trials (RCTs) and will certainly help to gather the essential information very fast and in an efficient way complying with desired ethical and scientific standards. We have earlier presented the advances in recombinant COVID-19 vaccine research and development and associated issues.Citation12 Therefore, the present systematic review focuses on the global landscape of the COVID-19 vaccines and their current status.
Methods
Literature search strategy and selection criteria
According to the WHO’s most recent update, “DRAFT landscape of COVID-19 potential vaccines (December 2022),”Citation1 was retrieved to ascertain the prospective vaccine contenders. This study was designed according to PRISMA 2020 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) protocol, a reconstructed guideline for reporting systematic review statements.Citation13 The concerned literature was searched in PubMed, Google Scholar, Cochrane databases, WHO databases and COVID-19 vaccine tracker2 till December 30, 2022. The literature available in English language was only considered with search terms such as “COVID-19 Vaccines,” “Coronavirus Vaccines,” “SARS-CoV-2 vaccine,” and individual vaccine names. The literature with vaccines against COVID-19 in phase I to phase IV trials were selected for the study. Only the interventional investigations that evaluated the effectiveness and safety aspects of COVID-19 vaccines in phase III/IV trials among healthy people of all age-groups in both sexes were included and the study that showed statistical information on all types of secondary complications. Based on the title and abstract of the articles, those studies and reviews that were not related to the current study or in duplication were rejected. The studies irrelevant to the inclusion criteria were rejected after reading the full text.
Results and discussion
COVID-19 vaccines and their status
Number of COVID-19 vaccines and their status have been summarized in . As per the data exhibited in a total of 242 vaccines against COVID-19 are in clinical studies.Citation1,Citation2 Among which 46 are in phase III clinical trials while 46 in phase IV which have been approved in various countries as on 30 December 2022. Among the phase IV/approved vaccines, 17 are protein subunit vaccines, 2 are VLP vaccines, 1 is DNA vaccine, 8 are RNA vaccines, 7 are non-replicating viral vector vaccines, 1 is replicating viral vector, 9 are inactivated vaccines and 1 is live attenuated vaccine. Whereas, 20 protein subunit vaccines, 1 is VLP vaccine, 2 are DNA vaccines, 12 are RNA vaccines, 3 are non-replicating viral vector vaccines, and 8 inactivated vaccines are in phase III trials (). In addition, 72 vaccine candidates are in phase II trials among which 25 are protein subunit-based vaccines, 4 VLP-based vaccines, 6 DNA-based vaccines, 23 are RNA vaccines, 7 are non-replicating viral vector vaccines, 3 are replicating viral vectors, and 4 are inactivated vaccines. Importantly, few of the phase II trials related vaccines have been approved in some countries (). Moreover, 66 vaccine candidates are in phase I trial among which 18 are protein subunit vaccines, 2 are VLP vaccines, 6 are DNA based vaccines, 21 are RNA vaccines, 12 are non-replicating viral vector vaccines, 3 are replicating viral vector vaccines, and 4 are inactivated vaccines (). Importantly, few of the vaccines have been discontinued even at the stage of phase III trials ().Citation2 There are a huge number of publications available on COVID-19 vaccine clinical trials and research, but this still requires a comprehensive analysis on the efficacy and safety. This article reviews and explores the efficacy and various secondary complications observed with COVID-19 vaccines currently in phase III and phase IV clinical trials that may offer assistance to further associated clinical research.
Figure 2. Status of COVID-19 vaccines. Bar graph showing the number of COVID-19 vaccines in different stages of clinical trials. Among the phase IV trials (n = 46), 17 are protein subunit vaccines, 2 are VLP vaccines, 1 is DNA vaccine, 8 are RNA vaccines, 7 are non-replicating viral vector vaccines, 1 is replicating viral vector, 9 are inactivated vaccines and 1 is live attenuated vaccine. In phase III trials (n = 46), 20 protein subunit vaccines, 1 is VLP vaccine, 2 are DNA vaccines, 12 are RNA vaccines, 3 are non-replicating viral vector vaccines, and 8 inactivated vaccines. In phase II trials (n = 72), 25 are protein subunit-based vaccines, 4 VLP based vaccines, 6 DNA based vaccines, 23 are RNA vaccines, 7 are non-replicating viral vector vaccines, 3 are replicating viral vectors, and 4 are inactivated vaccines. In phase I trial (n = 66), 18 are protein subunit vaccines, 2 are VLP vaccines, 6 are DNA based vaccines, 21 are RNA vaccines, 12 are non-replicating viral vector vaccines, 3 are replicating viral vector vaccines, and 4 are inactivated vaccines.
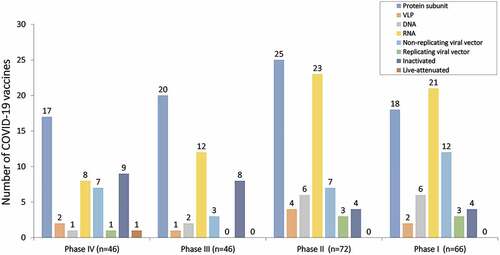
Table 1. Status of COVID-19 vaccines under phase III, and phase IV trials/approved.
Table 2. Status of COVID-19 vaccines under phase II trials/approved.
Table 3. Status of COVID-19 vaccines under phase I trials.
Table 4. Discontinued COVID-19 vaccines.
COVID-19 Vaccines in phase III and IV clinical trial
DNA based vaccines
DNA vaccines rely on the in-situ production of antigenic proteins. Plasmid containing DNA sequence encoding the antigenic viral protein is injected in the host body against which the host body elicits an immune response that protects from future infections.Citation14 During August 2021, the Government of India approved Emergency Use Authorization to a DNA-based vaccine against COVID-19.
A pharmaceutical company Zydus Cadila, in association with the Department of Biotechnology, India, came with a 3-dose intradermal vaccine, ZyCov-D, intended to administer in people of age twelve years and above. This was the first case of clinical usage of a DNA-based vaccine among humans. The company claims lack of any severity and mortality in phase I trial and about 1000 youngsters have already registered for phase III trial.Citation15 Another study on ZyCov-D was conducted.Citation16 It was a multicenter, double-blind, randomized, placebo-controlled phase III clinical study conducted at 49 sites in India among subjects of age twelve years and above predominantly males. A needle-free vaccine shot was used to inoculate 3 doses of vaccine or placebo intradermally keeping the distance of 28 days in-between each dose. The ZyCoV-D vaccine was able to promote neutralizing antibodies and cellular immune responses in the recipients making the vaccine about 66·6% efficient with some cases of mild adverse events. The safety profile of ZyCoV-D was equivalent to other DNA-based vaccines in development. Overall, the analysis demonstrates the immunogenicity, efficacy and the safety aspects of ZyCoV-D vaccine in phase III clinical trials. Since the ZyCoV-D vaccine has been constructed on a plasmid DNA platform, the development of new constructs could be fast and therefore will be easy to handle the upcoming new mutant strains.Citation16,Citation17
Pennsylvania-based INOVIO has recently got FDA approval for the phase III trial of INO-4800. It is a SARS-CoV-2 Spike DNA-based vaccine administered intradermally with subsequent electroporation with the help of CELLECTRA® 2000. Andrade et al. (2021)Citation18 evaluated post-vaccination functional antibodies and T cell responses in hosts against SARS-CoV-2 B.1.1.7, B.1.351, and P.1 variants. In agreement to the previous trial results, INO-4800 was found to provoke a satisfactory immune response characterized by the appearance of counteracting antibodies as well as T cell responses. Further, the vaccine was well tolerated and safe for young and elderly people.Citation14–Citation18–20
There are few other DNA-based vaccines that are in phase II/III trial. One is AG0302-COVID19 [NCT04655625], a two-dose DNA plasmid vaccine that codes for SARS-CoV-2 S protein administered via intramuscular inoculation designed by AnGes in association with Osaka University and Japan Agency for Medical Research and Development. Another in pipeline is GX-19N [NCT05067946], a two-dose DNA plasmid vaccine expressing SARS-CoV-2 antigenic S protein together with the antigenic nucleocapsid protein (N) developed by Genexine.Citation21 Even with few limitations, the recent development in DNA vaccine technology and the availability of molecular adjuvants has improved the immunogenicity of these vaccines. The cost-effective production and ease to store are some characteristics that will make them popular especially in third-world countries.
mRNA based vaccines
This platform uses genetically engineered mRNA to train the host cells to express antigenic viral proteins against which the immune system produces antibodies that protect the host body in case of later infections with the same virus. The use of mRNA in the development of a vaccine against COVID-19 is a novel approach. The major advantage of mRNA vaccine platform is its flexibility and effectiveness. Therefore, several RNA vaccine contenders in different developmental stages are under evaluation for their effectiveness against COVID-19. The Food and Drug Administration (FDA) currently endorsed the three mRNA-based vaccinations against COVID-19, these are Pfizer – BioNTech’s BNT162b2, Moderna’ mRNA-1273 and Johnson and Johnson – Janssen’s Ad26.COV2.S. These vaccines have shown high efficacy of about 72% to 95% in trials against moderate-to-severe COVID-19 among adults.Citation22 The WHO Strategic Advisory Group of Experts on Immunization (SAGE) has recommended the immunization with Pfizer BioNTech (BNT162b2) vaccine against COVID-19. BNT162b2 is a lipid nanoparticle-based nucleoside-modified RNA vaccine encoding a prefusion stabilized, membrane-bound SARS-CoV-2 complete S protein. Polack et al.Citation23 have investigated the safe use and effectiveness of the BNT162b2 vaccine. The mRNA vaccine was found to cause a brief, mild-to-moderate pain at the inoculation site, tiredness, and headache with minimal cases of severity. The effects were alike in the vaccinated and placebo groups. The two-dose regime of the vaccine offered about 95% safety against the COVID-19 in individuals aged 16 years or above. The study outcomes strengthened the potential of RNA-based vaccines as an efficient protective measure against the disease.
In United States, a phase III trial [NCT04470427] was carried out at various centers for mRNA-1273 vaccine, a lipid-nanoparticle (LNP)–encapsulated mRNA vaccine that expresses the prefusion-stabilized antigenic S protein of SARS-CoV-2, developed by Moderna in collaboration with Vaccine Research Center at the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). The vaccine was about 94.1% effective against COVID-19 along with better ability to prevent the severe conditions too. Further, the minimal secondary complications make the vaccine safe and appealing for the mass application.Citation24 Stamatatos et al. (2021)Citation25 collected pre- and post-immunization sera from individuals recovered from COVID-19 and from those who were naive and investigated whether the vaccination with BNT162b2 and mRNA-1273 may effective against Wuhan-Hu-1 and B.1.351 strains. The neutralization of Wuhan-Hu-1 strain with pre-immunization sera from recovered individuals were reported while it also counteracted B.1.351 up to certain extent. Moreover, a single shot of vaccines heightened the antibody titer upto 1000 times against both the variants although the second dose of vaccination did not cause any considerable enhancement of neutralization reaction. Upon vaccination the neutralization reaction was also observed in naive individuals, but the magnitude was comparatively low. This study justifies the significance of mRNA vaccines in both COVID-19 affected and naive populations. In one studyCitation22 evaluated the efficacy of three RNA vaccines, Ad26.COV2.S, BNT162b2, and mRNA-1273 in COVID-19 infected patients. They found that the appearance and predominance of delta variants in circulation reduced the efficacy of these vaccines. However, the patients of age 65 years or above showed good response with BNT162b2 and mRNA-1273.
World Health OrganizationCitation1 has standardized the doses of BNT162b2 mRNA vaccine that has to be administered 3 to 4 weeks apart. A study was outlined to check the effectiveness of the vaccine if the distance between two doses increases.Citation26 Worked with the longitudinal data on generation of humoral immunity against the D614G strain and other SARS-CoV-2 variants in a group of previously affected people with SARS-CoV-2 and naive ones who were vaccinated with two doses 16 weeks apart. The distantly administered doses were found to produce much stronger responses especially among naive individuals suggesting that this strategy not only improved the efficacy of doses but also makes the vaccination regime flexible. However, the recognition of all variants and Omicron was found to decline speedily among naive individuals as compared to the previously infected individuals.Citation26 A similar investigation was led by Chatterjee et al, Citation27who have evaluated the host immune response against the Omicron variant in a cohort of previously infected and naive individuals who underwent BNT162b2 mRNA vaccination with a gap of 16 weeks. As compared to the D614G, Alpha, Beta, Gamma, and Delta Spikes the Omicron Spike antigen was not recognized much proficiently. Moreover, those who received two vaccination doses with larger gaps had better antigen recognition and neutralization than the individuals whose two doses were four weeks apart. Most of the studies suggest that the immune system of previously infected individuals was able to recognize and respond more well against all Spike antigens than the naive individuals as observed after 3 weeks and 16 weeks of second dose.Citation25–27
Narowski et al.Citation6 investigated longitudinal immunogenic activity induced by Pfizer-BioNTech mRNA vaccine (BNT162b2), and Moderna mRNA-1273 vaccine against the reference Wuhan strain (WIV04), Delta variant, seven other variants, and four endemic corona viruses in 168 health workers among which 20 were previously infected while 148 were naive at three different stages, prior to vaccination, post first vaccination, and post second vaccination. Following full vaccine doses both the recipients developed a prominent immune response against SARS-CoV-2 spike and varied magnitude of cross-reactive antibodies to seasonal variants. Though the intensity and rate of SARS-CoV-2 counterbalancing antibody response in naive were observed lesser as compared to the previously infected participants. The study also found weaker immune reactions against the Alpha and Delta variants as compared to the reference strain in all participants. The results recommend the development of custom-made vaccines against evolving SARSCoV-2 variants since the current mRNA-vaccine induces inconstant neutralizing antibodies among individuals.
A study was conducted to test the sera obtained from 51 participants inoculated with 2 or 3 doses of mRNA vaccine BNT162b2 against Wuhan-Hu-1 reference strain, Beta (B.1.351), Delta (B.1.617.2), or Omicron pseudo-viruses. Next to the second dose the Omicron neutralizing titers were found to decline by more than 22-fold in comparison to those concentrations found with Wuhan reference strain. Although, a month afterward the third dose, immune response against Omicron showed 23-fold amplification in comparison to their level after the second dose and was equivalent to the concentrations of reference strain neutralizing titers in the second dose afterward. The necessity of a third vaccine dose for effective neutralization of Omicron was defined with sera obtained from a population employing live SARS-CoV-2. The outcomes support the use of the mRNA vaccine in a three-dosage regimen to counteract Omicron infection.Citation28,Citation29 BNT162b2 mRNA vaccination has been found to be effective against Omicron subvariants including BA.4 and BA.5.Citation30 In addition, two doses of BNT162b2 have been found to provide 54.9% efficacy in children and adolescents against SARS-CoV-2 subvariant BA.2 infection in Hong Kong over the past 6 months.Citation31
Anti-nucleocapsid antibody (anti-NAb) seropositivity in mRNA-1273 (Moderna) vaccinated individuals have been evaluated who have had severe COVID-19. Out of 700 PCR-confirmed COVID-19 positive participants, 52 were vaccinated with mRNA-1273 in which 21 (40%) showed seroconversion to anti-N Abs. On the other hand, 605 out of 648 placebo recipients exhibited the seroconversion which was about 93%. Each 1-log rise in SARS-CoV-2 copy number at the time of analysis was correlated with 90% higher odds of anti-NAb seroconversion (odds ratio, 1.90). mRNA-1273 vaccination has been found to provide moderate and short-lived (~90 days) protection against several of the Omicron subvariants including BA.1, BA.2, BA.2.12.1, BA.4 and BA.5.Citation32 Currently three candidate mRNA vaccines including mRNA-1273 and BNT162 (3 LNP-mRNAs) are in phase IV clinical trials although the final data is yet to come.Citation1,Citation12 Overall, the mRNA vaccination programme appears to be quite effective at reducing the number of COVID-19 variations in circulation, although more study is required to improve its specificity and efficacy.
Vector-based vaccines
A viral vector-based vaccine utilizes a viral vector to carry genetic material that codes for a sought-after antigenic protein inside the recipient’s host cells. The adenoviral vector-based vaccines have long been studied for their safe and effective use. These vaccines have the ability to stimulate cellular as well as humoral immune response.Citation11 Ramasamy et al.Citation33 reported the immunogenicity of ChAdOx1 nCoV-19 (AZD1222), a chimpanzee adenovirus-vectored vaccine with defective replication that express the full-length SARS-CoV-2 antigenic S protein gene. The safety and efficacy of this vaccine were tested on a widespread population including young adults as well as those with age 70 years or more. This single-blind, randomized, controlled, phase II/III trial (COV002) recruited healthy adults of age 18 years and above in two clinical research facilities in the United Kingdom (UK). The study was conducted in a hierarchical manner by grouping participants into various subgroups; first with age between 18 to 55 years, second with age 56 to 69 years, and third subgroup with 70 years and above. ChAdOx1 nCoV-19 was found to be tolerated well among old adult subgroups as compared to the younger adult subgroup with equivalent immune response following a booster dose. Further evaluation of the vaccine among all age groups and people with comorbidities is necessary regarding its safe and effective application. AZD1222 vaccine with hybrid immunity has been found effective after 6 months of the vaccination (COV005) against Omicron BA.1 and BA.4 variants.Citation34
Another phase III clinical trial of rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine Gam-COVID-Vac (Sputnik V) developed by Gamaleya Research Institute of Epidemiology and Microbiology was conducted at 25 clinical setups in Moscow, Russia. The vaccine was reported as a very safe and potent immune stimulator during phase I/II clinical trials. The vaccine exhibited about 91.6% efficacy against COVID-19 and was well tolerated among a large group as exhibited in the preliminary analysis of phase III trial.Citation11 As of June 2021, the Sputnik V vaccine has been approved in 68 countries, though the efficacy of the vaccine needs more analysis on large samples.Citation35
The phase III clinical trial of Ad26.COV2.S, a non-replicating adenovirus vector-based vaccine revealed that even one dose (5 × 1010 viral particles) have capability to induce 73.1% and 81.7% efficacy in critically infected subjects on 14 and 28 days, respectively, presenting its safer side.Citation36 Another phase III clinical trial [NCT04516746] funded by AstraZeneca and others was conducted by Falsey et al..Citation37 They inspected the safety, effectiveness, and immunogenic potential of two dose regimes of AZD1222 vaccine in blocking the commencement of symptomatic and severe COVID-19 within 15 days or more following second dose in young and older adults, in the United States, Chile, and Peru. There were few local and systemic effects but largely mild or moderate was reported in both cohorts although the vaccine was found about 74.0% effective. The first dose of vaccine escalated the neutralizing antibodies that further increased on 28th day following the second dose. So far AZD1222 was found safe and effective against symptomatic and severe COVID-19 cases among different populations including older adults. Viral vector-based vaccines are among the most studied platforms and currently 4 candidate vaccines are in phase IV clinical trials including ChAdOx1-S (AZD1222), Ad5-nCoV and Ad26.COV2.S.Citation1,Citation12
Subunit vaccines
Heath et al.Citation38 recently completed a Phase III clinical trial for the comparison of immunogenic potential and safety of 3 distinct sets of Novavax vaccine with Matrix-M™ adjuvant (NVX-CoV2373). It is a recombinant nanoparticle-based vaccine containing complete S glycoprotein of the prototype SARSCoV-2 strain with Matrix-M adjuvant. The NVX-CoV2373 vaccine imparted about 89.7% protection to the adult participants against SARS-CoV-2 infection who received two doses. The vaccine had substantial ability to restrain the B.1.1.7 variant. The trial was sponsored by Novavax and was carried out at 33 locations in the UK [EudraCT number 2020–004123–16]. One more observer-blinded phase III trial was conducted in the United States and Mexico during the initial phase of the year 2021 to assess the safety and effectiveness of NVX-CoV2373 in grown-ups. The clinical study involved 29,949 participants and was funded by Novavax and others [NCT04611802]. The study reported mild to moderate immune response after the first dose that became more prominent after the second dose. The vaccine was found about 92.6% (95% CI, 83.6 to 96.7) efficient against the variant under examination. Further analysis is underway to prove the NVX-CoV2373 vaccine safer and effective against COVID-19.Citation39 Recently, Novavax has been found to provide neutralization of Omicron subvariants including BA.1, BA.4, and BA.5 after three doses of NVX-CoV2373 vaccine.Citation40
COVID-19 vaccines and emerging SARS-CoV-2 variants
Since the past few years COVID-19 has imposed a huge issue not only regarding human health but also affected the social and economic status of the society. Even after several strategies made mandatory by the administrative bodies and practicing personal hygiene and maintaining social distance the endemic has caused a huge morbidity and mortality worldwide. Even the administration of antiviral drugs couldn’t be able to curb the dissemination of COVID-19 and prevention of endemic. Vaccines were found as an indispensable tool to fight against the hazardous disease. Currently varied platforms are available for development of vaccines against COVID-19, each with their associated pros and cons. Since the last two years numerous vaccines have been given approval to curtail morbidity and mortality linked to COVID-19, regardless of the potential risks accompanying newly permitted vaccines. The quick licensing of COVID-19 vaccinations could potentially be very important in managing the dangerous disease.Citation41 Several of the crucial factors which may determine efficacy of vaccines are route of vaccination, its dose and frequency. Most of the current vaccines are designed to induced immune response via targeting systemic immune system and generating weak mucosal immunity. Importantly, mucosal immunity is crucial to achieve for robust immune response due to its microenvironment comprising of integrated network of lymphoid, nonlymphoid, tissue, and effector molecules. In case of COVID-19, vaccines targeting mucosal immunity is crucial to achieve since it may prevent the spread of SARS-CoV-2 in the lower airways and lungs and therefore important as a preventive measures to reduce the transmission. AZD1222 has been shown to induce durable nasal anti-spike IgG response.Citation42 Importantly, BNT162b2 has been shown to induce robust systemic immune response. However, it has failed to provide mucosal immunity.Citation43,Citation44
Systemic mRNA vaccination has been shown to induce weak mucosal immunity. Interestingly, combination of mucosal adenovirus-S along with mRNA immunization has been found to induce robust neutralizing antibody response not only toward previous variants but also against Omicron subvariant BA.1.1.Citation45 Importantly, recurrent emerging variants of SARS-CoV-2 are continuously challenging the efficacy of COVID-19 vaccines. Recent emergence of Omicron and its subvariants has transmitted throughout the globe as a VOCs. Therefore, it is crucial to address the efficacy of the vaccines against emerging variants. Interestingly, BNT162b2 mRNA vaccine has been found to be effective against Omicron subvariants including BA.4 and BA.5.Citation30 In addition, it has been found to provide 54.9% efficacy after two doses of BNT162b2.Citation31 Importantly, mRNA 1273 has been found to be effective against several of the Omicron subvariants including BA.1, BA.2, and BA.4/BA.5.Citation32 AZD1222 has been recently found to provide hybrid immunity against Omicron subvariants including BA.1 and BA.4.Citation34 Recently, Novavax has been found to provide neutralization of Omicron subvariants including BA.1, BA.4/BA.5 after three doses of NVX-CoV2373 vaccine.Citation40 Several of the vaccines have been administered as a booster dose to combat the newly emerging SARS-CoV-2 variants.
Conclusions
However, the vaccines developed in rush without suitable time-taking clinical trials may generate some long-lasting or life-threatening health hazards as most of these vaccines have mild to moderate local and systemic adverse effects. Studies have reported pain in the injection site, erythema, headache, fatigue, malaise, etc.Citation24–48 One major threat is Vaccine-associated enhanced disease (VAED) or antibody-dependent enhancement (ADE) although an uncommon secondary complication, it may give rise to some life-threatening adverse consequences developed due to pathogen-specific antibodies generated either by vaccination or primary infection.Citation41 The current phase III and phase IV vaccines seem promising, and the study will help to find a better option and strategy to manage the disease. To halt the pandemic spread it is essential to speed up the current vaccination drives. The earlier studies on COVID-19 have generated a large quantity of data on virus as well as vaccine mediated immune responses that will certainly improve the vaccine development strategy. We have recently discussed the progress and challenges toward generation and maintenance of long-lived memory T lymphocyte responses during COVID-19 in detail.Citation49
Future perspectives
The phase III and phase IV clinical trial will pave a path to clear substantial uncertainties about the developed COVID-19 vaccines and their application. It is crucial to thoroughly assess the COVID-19 vaccines’ safety and effectiveness in a sizable population. Waning immunity following COVID-19 vaccination is a big concern, and millions of vaccinated individuals have opted out of booster vaccination. There is an absolute need for a long-term follow-up strategy to uncover any underlying adverse events post vaccination. However, the novel vaccines may contribute a lot in minimizing the devastating effect of the COVID-19 pandemic. Most of the vaccines are designed to induce an antibody mediated protection against SARS-CoV-2 infection. However, due to vaccine breakthrough cases and antibody escape mechanisms attained by the newer emerging strains of SARS-CoV-2, it is imperative to look for the robust T memory cell response for long-term protection against COVID-19.
Author contributions statement
SKS conceived the idea and planned the study. TY, SK, and SKS collected the data, devised the initial draft, reviewed the final draft, and contributed equally to this study as the first author. TY, SK, GM, and SKS finalized the draft for submission. All authors read and approved the final version of the manuscript.
Acknowledgments
The authors are grateful to the Vice Chancellor, King George’s Medical University (KGMU), Lucknow, India for the encouragement for this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- WHO. World Health Organization. COVID-19 vaccine tracker and landscape. 2022. [accessed 2022 Dec 30]. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- Basta NE, Moodie EMM on behalf of the VIPER (Vaccines, infectious disease prevention, and epidemiology research) Group COVID-19 vaccine development and approvals tracker team. COVID-19 vaccine development and approvals tracker. 2020. [accessed 2022 Dec 30]. https://covid19.trackvaccines.org/.
- Srinivasan S, Cui H, Gao Z, Liu M, Lu S, Mkandawire W, Narykov O, Sun M, Korkin D. Structural genomics of SARS-CoV-2 indicates evolutionary conserved functional regions of viral proteins. Viruses. 2020;12(4):360. doi:10.3390/v12040360.
- Saxena SK, Kumar S, Ansari S, Paweska JT, Maurya VK, Tripathi AK, Abdel-Moneim AS. Transmission dynamics and mutational prevalence of the novel severe acute respiratory syndrome coronavirus-2 omicron variant of concern. J Med Virol. 2022;94(5):2160–15. doi:10.1002/jmv.27611.
- Dai L, Gao GF. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021;21(2):73–82. doi:10.1038/s41577-020-00480-0.
- Narowski TM, Raphel K, Adams LE, Huang J, Vielot NA, Jadi R, de Silva AM, Baric RS, Lafleur JE, Premkumar L. SARS-CoV-2 mRNA vaccine induces robust specific and cross-reactive IgG and unequal neutralizing antibodies in naive and previously infected people. Cell Rep. 2022;38:110336. doi:10.1016/j.celrep.2022.110336.
- Kim JH, Marks F, Clemens JD. Looking beyond COVID-19 vaccine phase 3 trials. Nat Med. 2021;27(2):205–11. doi:10.1038/s41591-021-01230-y.
- Mathieu E, Ritchie H, Ortiz-Ospina E, Roser M, Hasell J, Appel C, Giattino C, Rodés-Guirao L. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5(7):947–53. doi:10.1038/s41562-021-01122-8.
- Aleem A, Samad ABA, Slenker AK Emerging variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19). StatPearls [Internet]: StatPearls Publishing; 2022.
- Peng F, Yuan H, Wu S, Zhou Y. Recent advances on drugs and vaccines for COVID-19. Inquiry. 2021;58:469580211055630. doi:10.1177/00469580211055630.
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–81. doi:10.1016/S0140-6736(21)00234-8.
- Yadav T, Srivastava N, Mishra G, Dhama K, Kumar S, Puri B, Saxena SK. Recombinant vaccines for COVID-19. Hum Vaccin Immunother. 2020;16(12):2905–12. doi:10.1080/21645515.2020.1820808.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71.
- Shafaati M, Saidijam M, Soleimani M, Hazrati F, Mirzaei R, Amirheidari B, Tanzadehpanah H, Karampoor S, Kazemi S, Yavari B, et al. A brief review on DNA vaccines in the era of COVID-19. Future Virol. 2022;17(1):49–66. doi:10.2217/fvl-2021-0170.
- Abbasi J. India’s new COVID-19 DNA vaccine for adolescents and adults is a first. JAMA. 2021;326(14):1365. doi:10.1001/jama.2021.16625.
- Khobragade A, Bhate S, Ramaiah V, Deshpande S, Giri K, Phophle H, Supe P, Godara I, Revanna R, Nagarkar R, et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): the interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet. 2022;399(10332):1313–21. doi:10.1016/S0140-6736(22)00151-9.
- Blakney AK, Bekker L-G. DNA vaccines join the fight against COVID-19. The Lancet. 2022;399(10332):1281–82. doi:10.1016/S0140-6736(22)00524-4.
- Andrade VM, Christensen-Quick A, Agnes J, Tur J, Reed C, Kalia R, Marrero I, Elwood D, Schultheis K, Purwar M, et al. INO-4800 DNA vaccine induces neutralizing antibodies and T cell activity against global SARS-CoV-2 variants. NPJ Vaccines. 2021;6(1):1–4. doi:10.1038/s41541-021-00384-7.
- Tebas P, Yang S, Boyer JD, Reuschel EL, Patel A, Christensen-Quick A, Andrade VM, Morrow MP, Kraynyak K, Agnes J, et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine. 2021;31:100689. doi:10.1016/j.eclinm.2020.100689.
- Kraynyak KA, Blackwood E, Agnes J, Tebas P, Giffear M, Amante D, Reuschel EL, Purwar M, Christensen-Quick A, Liu N, et al. SARS-CoV-2 DNA vaccine INO-4800 induces durable immune responses capable of being boosted in a Phase I open-label trial. J Infect Dis. 2022;225(11):1923–32. doi:10.1093/infdis/jiac016.
- Sheridan C. First COVID-19 DNA vaccine approved, others in hot pursuit. Nat Biotechnol. 2021;39(12):1479–82. doi:10.1038/d41587-021-00023-5.
- Rosenberg ES, Dorabawila V, Easton D, Bauer UE, Kumar J, Hoen R, Hoefer D, Wu M, Lutterloh E, Conroy MB, et al. Covid-19 vaccine effectiveness in New York state. N Engl J Med. 2022;386(2):116–27. doi:10.1056/NEJMoa2116063.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi:10.1056/NEJMoa2034577.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. doi:10.1056/NEJMoa2035389.
- Stamatatos L, Czartoski J, Wan Y-H, Homad LJ, Rubin V, Glantz H, Neradilek M, Seydoux E, Jennewein MF, MacCamy AJ, et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021;372(6549):1413–18. doi:10.1126/science.abg9175.
- Tauzin A, Gong SY, Beaudoin-Bussières G, Vézina D, Gasser R, Nault L, Marchitto L, Benlarbi M, Chatterjee D, Nayrac M, et al. Strong humoral immune responses against SARS-CoV-2 spike after BNT162b2 mRNA vaccination with a 16-week interval between doses. Cell Host Microbe. 2022;30(1):97–109. e5. doi:10.1016/j.chom.2021.12.004.
- Chatterjee D, Tauzin A, Marchitto L, Gong SY, Boutin M, Bourassa C, Beaudoin-Bussières G, Bo Y, Ding S, Laumaea A, et al. SARS-CoV-2 omicron spike recognition by plasma from individuals receiving BNT162b2 mRNA vaccination with a 16-week interval between doses. C Cell Rep. 2022;38(9):110429. doi:10.1016/j.celrep.2022.110429.
- Muik A, Lui BG, Wallisch A-K, Bacher M, Mühl J, Reinholz J, Ozhelvaci O, Beckmann N, Güimil Garcia RDLC, Poran A, et al. Neutralization of SARS-CoV-2 omicron by BNT162b2 mRNA vaccine–elicited human sera. Science. 2022;375(6581):678–80. doi:10.1126/science.abn7591.
- Follmann D, Janes HE, Buhule OD, Zhou H, Girard B, Marks K, Kotloff K, Desjardins M, Corey L, Neuzil KM, et al. Antinucleocapsid antibodies after SARS-CoV-2 infection in the blinded phase of the randomized, placebo-controlled mRNA-1273 COVID-19 vaccine efficacy clinical trial. Ann Intern Med. 2022;175(9):1258–65. doi:10.7326/M22-1300.
- Tartof SY, Slezak JM, Puzniak L, Hong V, Frankland TB, Ackerson BK, Takhar H, Ogun OA, Simmons S, Zamparo JM, et al. Bnt162b2 vaccine effectiveness against SARS-CoV-2 omicron BA.4 and BA.5. Lancet Infect Dis. 2022;22(12):1663–65. doi:10.1016/S1473-3099(22)00692-2.
- Leung D, Rosa Duque JS, Yip KM, So HK, Wong WHS, Lau YL. Effectiveness of BNT162b2 and CoronaVac in children and adolescents against SARS-CoV-2 infection during omicron BA.2 wave in Hong Kong. Commun Med (Lond). 2023;3(1):3. doi:10.1038/s43856-022-00233-1.
- Tseng HF, Ackerson BK, Bruxvoort KJ, Sy LS, Tubert JE, Lee GS, Ku JH, Florea A, Luo Y, Qiu S, et al. Effectiveness of mRNA-1273 vaccination against SARS-CoV-2 omicron subvariants BA.1, BA.2, BA.2.12.1, BA.4, and BA.5. Nat Commun. 2023;14(1):189. doi:10.1038/s41467-023-35815-7.
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, Voysey M, Aley PK, Angus B, Babbage G, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase II/3 trial. Lancet. 2020;396(10267):1979–93. doi:10.1016/S0140-6736(20)32466-1.
- Madhi SA, Kwatra G, Richardson SI, Koen AL, Baillie V, Cutland CL, Fairlie L, Padayachee SD, Dheda K, Barnabas SL, et al. Durability of ChAdOx1 nCoV-19 (AZD1222) vaccine and hybrid humoral immunity against variants including omicron BA.1 and BA.4 6 months after vaccination (COV005): a post-hoc analysis of a randomised, phase 1b–2a trial. Lancet Infect Dis. 2022;23(3):295–306. 00596-5. doi:10.1016/S1473-3099(22)00596-5.
- Vanaparthy R, Mohan G, Vasireddy D, Atluri P. Review of COVID-19 viral vector-based vaccines and COVID-19 variants. Infez Med. 2021;29(3):328–38. doi:10.53854/liim-2903-3.
- Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, et al. Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–201. doi:10.1056/NEJMoa2101544.
- Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, Neuzil KM, Hahn W, Hunt J, Mulligan MJ, et al. Phase 3 safety and efficacy of AZD1222 (ChAdox1 nCoV-19) Covid-19 vaccine. N Engl J Med. 2021;385(25):2348–60. doi:10.1056/NEJMoa2105290.
- Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, Chadwick DR, Clark R, Cosgrove C, Galloway J, et al. Safety and efficacy of NVX-Cov2373 Covid-19 vaccine. N Engl J Med. 2021;385(13):1172–83. doi:10.1056/NEJMoa2107659.
- Dunkle LM, Kotloff KL, Gay CL, Áñez G, Adelglass JM, Barrat Hernández AQ, Harper WL, Duncanson DM, McArthur MA, Florescu DF, et al. Efficacy and safety of NVX-Cov2373 in adults in the United States and Mexico. N Engl J Med. 2022;386:531–43. doi:10.1056/NEJMoa2116185.
- Bhiman JN, Richardson SI, Lambson BE, Kgagudi P, Mzindle N, Kaldine H, Crowther C, Gray G, Bekker L-G, Koen A, et al. Novavax NVX-COV2373 triggers neutralization of omicron sub-lineages. Sci Rep. 2023;13(1):1222. doi:10.1038/s41598-023-27698-x.
- Rahman MA, Islam MS. Early approval of COVID-19 vaccines: pros and cons. Hum Vaccin Immunother. 2021;17(10):3288–96. doi:10.1080/21645515.2021.1944742.
- Aksyuk AA, Bansal H, Wilkins D, Stanley AM, Sproule S, Maaske J, Sanikommui S, Hartman WR, Sobieszczyk ME, Falsey AR, et al. AZD1222-induced nasal antibody responses are shaped by prior SARS-CoV-2 infection and correlate with virologic outcomes in breakthrough infection. Cell Rep Med. 2023;4(1):100882. doi:10.1016/j.xcrm.2022.100882.
- Azzi L, Dalla Gasperina D, Veronesi G, Shallak M, Ietto G, Iovino D, Baj A, Gianfagna F, Maurino V, Focosi D, et al. Mucosal immune response in BNT162b2 COVID-19 vaccine recipients. EBioMedicine. 2022;75:103788. doi:10.1016/j.ebiom.2021.103788.
- Azzi L, Dalla Gasperina D, Veronesi G, Shallak M, Maurino V, Baj A, Gianfagna F, Cavallo P, Dentali F, Tettamanti L, et al. Mucosal immune response after the booster dose of the BNT162b2 COVID-19 vaccine. EBioMedicine. 2023;88:104435. doi:10.1016/j.ebiom.2022.104435.
- Tang J, Zeng C, Cox TM, Li C, Son YM, Cheon IS, Wu Y, Behl S, Taylor JJ, Chakaraborty R, et al. Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination. Sci Immunol. 2022;7(76):eadd4853. doi:10.1126/sciimmunol.add4853.
- Ali K, Berman G, Zhou H, Deng W, Faughnan V, Coronado-Voges M, Ding B, Dooley J, Girard B, Hillebrand W, et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N Engl J Med. 2021;385(24):2241–51. doi:10.1056/NEJMoa2109522.
- Frenck JR, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, Perez JL, Walter EB, Senders S, Bailey R, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239–50. doi:10.1056/NEJMoa2107456.
- Thomas SJ, Moreira JE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–73. doi:10.1056/NEJMoa2110345.
- Kumar S, Saxena SK, Maurya VK, Tripathi AK. Progress and challenges toward generation and maintenance of long-lived memory T lymphocyte responses during COVID-19. Front Immunol. 2022;12:804808. doi:10.3389/fimmu.2021.804808.
