ABSTRACT
Group B Streptococcus (GBS) vaccines, designed to be given to pregnant women, are in clinical trials. There is an opportunity to conduct preparatory research now to understand the drivers of and barriers to GBS vaccine acceptance. This will enable targeted interventions so that delays in vaccine uptake might be avoided. A multicenter, mixed-methodology, cross-sectional study evaluated the acceptability of a hypothetical GBS vaccine among pregnant women in two countries with differing health systems. Pregnant women in Philadelphia, US, and Dublin, Ireland, completed an electronic survey and a Discrete Choice Experiment. Five hundred and two women were included in the final analysis. Fifty-three percent of US and 30% of Irish participants reported both awareness and understanding of GBS. The median likelihood score for vaccine receipt (measured on a 10-point scale) was 9 (US: 9 (IQR 7–10), IRL: 9 (IQR 6–10)). Among the US participants, identifying as Black or African American was associated with a lower likelihood of vaccine receipt. Possession of a college degree was associated with increased likelihood of vaccine receipt. Perceived infant benefit was the most important driver of GBS vaccine acceptance. Safety concerns about a novel vaccine was the most prominent barrier identified. Good GBS vaccine uptake is achievable through strong messaging that highlights vaccine safety and the potential infant benefits. Preparation for vaccine implementation should include efforts to increase awareness among pregnant women about GBS infection and a continued focus on improving acceptability of currently recommended maternal vaccines, particularly in population subgroups with low uptake of maternal immunizations.
Introduction
Group B Streptococcus (GBS), a bacterium found in the genitourinary or gastrointestinal tract of 10–30% of pregnant women, is an important cause of sepsis, meningitis, and death in infants less than 3 months of age worldwide.Citation1,Citation2 Researchers have estimated that a GBS vaccine that is 80% effective with 50% coverage could prevent 127,000 perinatal infections, 23000 stillbirths, and 37,000 deaths annually.Citation3 Currently, there are several vaccines in phase 1 and 2 clinical trials that are intended for pregnant women to prevent severe infection in their infants.Citation4–6 The success of any such vaccine will depend on its uptake in the target population. Experience with prior maternal vaccines suggests that acceptance of a novel vaccine for pregnant women may be challenging. Vaccines currently recommended in pregnancy, such as influenza, pertussis, and Coronavirus Disease 2019 (COVID-19) vaccines, were introduced as emergent or urgent responses to increases in disease prevalence. The introduction of a GBS vaccine will differ in this respect. There is time now, prior to licensure, to carry out preparatory groundwork in order to ensure successful implementation of a GBS vaccination program.
GBS disease in infants is categorized into early-onset GBS (EOGBS), which occurs before 7 days of life, and late-onset GBS (LOGBS), which presents between 7 and 89 days of life. Current antenatal prevention strategies aim to prevent EOGBS by identifying at-risk women and giving antibiotics in labor. Several approaches are used in different parts of the world, including universal antenatal screening of all pregnant women for GBS in late pregnancy, targeted polymerase chain reaction (PCR)-based testing in labor, and risk-factor-based identification of women who should receive intrapartum antibiotics.Citation7 The receipt of intrapartum antibiotics does not impact the risk of late-onset infection. Moreover, these approaches cannot be widely implemented in low-income settings. It is hoped that an effective GBS vaccine would prevent both early and late-onset infection, could reduce antibiotic use during delivery, and could be integrated into existing maternal immunization programs in low-income settings.Citation8,Citation9
Little is known about awareness of GBS infection and acceptability of the vaccine among pregnant women. It is not known how regional variations in prevention strategies may impact both knowledge and awareness of GBS infection and vaccine acceptance. Established factors that impact maternal immunization uptake such as provider recommendation, perceived infant risks and benefits, and sociodemographic factors are also likely to impact GBS vaccine uptake.Citation10 However, there may also be important vaccine-specific factors that affect coverage rates.Citation11,Citation12 GBS vaccine will be a new vaccine, trialed in pregnant women, aiming to prevent an infection that many women may not have heard of and for which there are already some partially successful preventative strategies in place. It is not known how these issues, unique to this vaccine, will impact uptake. Moreover, geographic variations in GBS epidemiology and testing practices may impact knowledge of the infection and perceptions about a new vaccine. This study aims to measure acceptability of a future GBS vaccine and identify which vaccine and patient factors might influence choice to receive it among pregnant women in two countries with different approaches to GBS prevention.
Methods
Study design
This was a mixed-method, cross-sectional study.
Study setting
Participants were recruited from antenatal clinics in Philadelphia, United States (US), and Dublin, Ireland. In Philadelphia, women were recruited from three obstetric practices within Penn Obstetrics which is affiliated with the University of Pennsylvania and provides care to women with private and government funded (Medicaid) medical insurance. At the time of recruitment, influenza and pertussis vaccines were routinely recommended by these practices as per US national guidelines. They were freely available onsite through insurance coverage. Universal screening for GBS is recommended and offered to all pregnant women attending these practices.
In Dublin, women were recruited from the Rotunda Hospital. This, large academic maternity hospital is the busiest maternity center in Ireland (>8000 deliveries per year) and among the busiest in Europe. Women attending the Rotunda Hospital can receive private, semi-private, or public care. At the time of recruitment, influenza and pertussis vaccines were routinely recommended as per Irish national guidelines. These vaccines are (for the most part) not available on site but are delivered in the primary care setting in line with the national approach to maternal immunization in Ireland. Rotunda Hospital practices intrapartum PCR-based screening for GBS colonization for women with term spontaneous rupture of the membranes who are not in labor, or for women who are having term elective inductions. GBS PCR screening is not offered to women in active term labor.Citation13
Questionnaire design
A 34-question electronic survey instrument was designed and piloted, first among a convenience sample of women of childbearing age from both countries, and then among 25 pregnant women attending for care in Philadelphia. Literature review informed the questionnaire design. The first part of the questionnaire collected sociodemographic data, obstetric history, and information about prior experience with vaccines in pregnancy. Women were then shown a small paragraph describing the purpose and benefits of a future GBS vaccine (). They were asked to respond to their likelihood of receipt of a GBS vaccine using a 10-point scale (1–10) where 1 indicated very unlikely and 10 indicated very likely. An open-ended question “Why would you be likely or unlikely to receive this vaccine?” accompanied the scale question. A sample survey instrument is included as supplemental data.
Figure 1. Text provided to all participants.

The final part of the questionnaire included a Discrete Choice Experiment (DCE) used to measure respondent preferences.Citation14 Participants completed 12 tasks. Each task asked participants to choose between two hypothetical vaccines in which attributes were varied (). The list of attributes and levels used is shown in . A literature review informed attribute and level selection.Citation15 Attributes were chosen with the aim of investigating vaccine-specific factors that could impact uptake. Chosen levels aimed to reflect real-life scenarios. Where there was no existing data to inform level definitions, only plausible levels were included. For example, for vaccine efficacy, only levels of efficacy that could be potentially approved for market were included. A forced choice model was used for this experiment, meaning that women were forced to choose one of two vaccines on each task and there was no “neither” option. The survey was designed in SawtoothTM software.
Table 1. List of attributes and levels.
Participant recruitment
Recruitment initially commenced in person in Philadelphia on March 12, 2020 and was then paused on March 19, 2020 due to COVID-19-related restrictions and recommenced using e-mail on November 11, 2020. To mimic in-person recruitment, clinic lists were accessed, and women were emailed during the week they attended clinic. All women who listed an e-mail address in the electronic health record and were currently pregnant were contacted via e-mail. One reminder e-mail was sent. Recruitment finished on March 15, 2021. In the US, recruitment stopped early due to logistical challenges, however at cessation sample size was considered adequate for analysis of the DCE.Citation16 In Ireland, recruitment took place between December 4, 2020 and January 14, 2021. Due to COVID-19 related restrictions a mixture of online and in-person recruitment was also used. Obstetricians and midwives distributed study information leaflets in antenatal clinics which contained a link to the online survey. Women completed the survey at home on their own device as restricted waiting times (due to COVID-19 era measures) did not permit ‘in the moment’ completion of the survey. Additionally, a short video which explained the purpose of the research and provided a link to the survey appeared on the hospital’s Instagram and Twitter accounts. Women were also recruited in-person during oral glucose tolerance testing. In this setting, women completed the survey on study tablets while awaiting blood testing.
Sample size
Sample size was calculated based on minimum requirements for a Discrete Choice Experiment in which groups are compared. Experts recommend a minimum of 200 in each group.Citation16 We aimed to recruit 300 in each group.
Data analysis
Analysis of survey data
Descriptive statistics were used to report sociodemographic data and vaccine history. Median values and interquartile ranges were reported for continuous variables. Frequencies were reported for categorical variables. To test for differences in distributions for continuous variables between groups, we used two sample t-test for normally distributed data and Wilcoxon Rank Sum for non-normally distributed data. We used chi-square tests and fisher’s exact tests to test the relationship between pairs of categorical variables.
Multivariable logistic regression was used to measure the association between sociodemographic factors and likelihood of receipt of a GBS vaccine. Participants were stratified according to country. An outcome variable ‘likely to receive a GBS vaccine’ was created by dichotomizing scale responses. A cutoff of 9, which was the median score in each country, was used. The covariates used in the multivariable models were the set of variables found to have some univariable association with outcome (i.e., p-values of less than 0.2). Adjusted odds ratios (aOR) and 95% Confidence intervals are reported from the multivariable logistic regressions. STATA version 16 was used for statistical analyses.
Conjoint analysis
Conjoint analysis was used to quantify which attributes are most important to respondents in deciding whether to get a vaccine. Both the design of the conjoint experiment and the quantitative analysis were done using Sawtooth’s Lighthouse Studio software version 9.8.0. The software uses Hierarchical Bayes (HB) estimation to assign a utility, which is a relative measure of preference, to each level of the vaccine attribute.Citation17 The sum of the utilities for all the levels of each attribute sums to 0, known as “zero centered differences.” A higher utility score indicates a greater preference for that level. Negative utility scores indicate lower preferences for those levels, but not necessarily that those levels are completely unattractive options.Citation18 We report average attribute importance. This is ratio data that describes the relative impact of each attribute on participant choice. To calculate importance, first the range of utility scores across each attribute is calculated by subtracting the lowest utility score of a level within an attribute from the highest score. These ranges are then expressed as a percentage of the total utility range (sum of all the ranges of all the attributes). HB analysis calculates attribute importance at the individual level and then averages this across the sample.
Qualitative data analysis
Two authors independently coded all survey free text responses using NVivo software. Thematic analysis was performed using a predefined list of codes based on the SAGE working group determinants of vaccine hesitancy.Citation19 In the initial stages, three authors independently coded 20 open-ended questions at a time. Coders met weekly to discuss interpretation of codes, and to make refinements to the codebook. Assessment of inter-coder reliability used Cohen’s kappa coefficient at the level of each code. A coefficient of greater than or equal to 0.9 was acceptable. If the coefficient was lower than this, coding discrepancies were reviewed, and codes were redefined or new codes were added.
Ethics
The Research Ethics Committee of the Rotunda Hospital, Dublin, Ireland, approved this study. Informed Consent was obtained from all Irish participants before completing the survey. The Institutional Review Board at the Children’s Hospital of Philadelphia reviewed the study and considered it exempt. Participants were provided with study information which stated that completion of the survey implied consent.
Results
Participant recruitment and characteristics of study participants
Six hundred and twenty-two pregnant women started the survey, and after exclusion of incomplete surveys 502 women, 202 from the US and 300 from Ireland were included in the final analysis. In the US, 55 were recruited in person and 147 via e-mail recruitment. The response rate for completed surveys by in-person recruitment was 55/70 (79%). The response rate for completed surveys by e-mail was 147/880 (17%). In Ireland, response rate was 80/94 (85%) for those recruited in person, and the response rate for those recruited online could not be calculated as the denominator is not known due to social media recruitment. In both countries, participants recruited online or via e-mail tended to report higher levels of formal education and higher household income and appeared more inclined toward vaccination than those recruited in person. These differences are presented as supplemental data (Supplemental Tables S1 and S2). The sociodemographic and obstetric history of participants from the US and Ireland are shown in .
Table 2. Characteristics of participants.
Knowledge, awareness, and experience of GBS infection
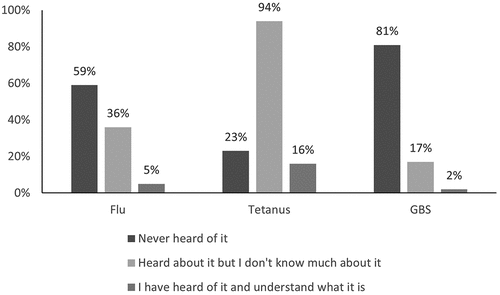
Eighty-three percent of US participants and 78% of Irish participants reported having heard of GBS; however, only 52% (US) and 30% (Irish) reported understanding what it is. Knowledge of GBS infection was lower than that of pertussis and influenza in both countries (). A prior history of GBS testing was reported in 53% of US women and in 18% of Irish women. Of all women who recalled being tested, 25% reported a positive result. Overall, 8% of all study participants reported a history of a positive GBS test during pregnancy.
Attitudes toward currently available vaccines
Twenty-one (4%) participants reported deciding not to vaccinate a child, 7/184 (3.8%) in Ireland, 14/125 (11.2%) in the US. The median score for the question “how likely are you to receive recommended vaccines in pregnancy” was 9 in the US (Inter-quartile Range (IQR), 7–10) and also in Ireland (IQR 8–10). Receipt or intention to receive a flu and pertussis vaccine was reported by 429/502 (85%) and 407/502 (81%) women, respectively. Two hundred and four (41%) women were in their first pregnancy. Of the remaining 298 women who had completed a previous pregnancy 229 (76.8%) reported receiving at least one vaccine during that pregnancy ().
Table 3. Vaccine receipt and acceptability.
Acceptability of a GBS vaccine
The likelihood of receiving a GBS vaccine was similar across both countries. The median self-reported likelihood score in the US and Ireland was 9 {US: 9 (IQR 7–10, Ireland: 9 (IQR 6–10)}. One hundred and twelve (55%) US and 169 (56%) Irish participants responded with a score of 9 or greater. The reported likelihood of receiving recommended vaccines correlated moderately with the likelihood of receiving a GBS vaccine in each country group (Kendall’s tau B: 0.54 (US), 0.64 (IRE), p < .001). Our multivariable logistic regression model examining the association of sociodemographic patient factors on GBS vaccine receipt found that identifying as Black or African American (adjusted OR 0.20, 95% CI (0.08–0.50)) and being aged 25–30 years (adjusted OR 0.34, 95% CI 0.15–0.74) were associated with a lower likelihood of vaccine receipt among US participants. Having a college degree was associated with increased likelihood of vaccine receipt (adjusted OR 4.40, 95% CI 1.21–15.87) (). Among Irish women, being aged 25–30 (adjusted OR 0.50, 95% CI 0.28–0.92) was associated with a lower likelihood of vaccine receipt compared to those aged 31 years or older (Supplemental Table S3)
Table 4. Likelihood of GBS vaccine receipt: Multivariable analysis (US participants).
Qualitative analysis of survey data
Responses to the open-ended question “Why would you be unlikely or likely to receive this vaccine?” were analyzed for themes. The most important themes to emerge were.
protection of the infant from harm,
the importance of vaccine safety data,
trust in healthcare providers and science,
concerns about “new vaccines” and,
the impact of available alternatives for GBS prevention on perceptions about GBS and a vaccine.
Among those who were likely to accept the vaccine, perceived infant benefit was the most prominent motivating factor. For some women, this was the primary driver for vaccination, while others were motivated by a combination of factors, as one woman described; “I would do anything to protect my baby and I believe in the science behind vaccines. If my providers recommended it, I would not hesitate to get it.” (US, Likelihood Score: 10) Safety concerns, particularly around “new vaccines” and potential for unknown “long term” side effects on the infant were common among those less likely to receive a vaccine.
Supportive quotations are shown below in and .
Table 5. Facilitators to GBS vaccination.
Table 6. Barriers to GBS vaccination.
Reasons for lower likelihood of vaccine receipt among Black women
Given the significant association between Black race and lower reported likelihood of GBS vaccine among the US cohort, an exploratory analysis was performed post-hoc to investigate the reasons behind this difference. A lower proportion (39%) of Black US women reported having ever had a GBS test compared with white US women (59%), and a higher proportion (31%) reported having never heard of the bacteria when compared with white US women (6.7%). History of vaccine receipt during pregnancy, intention to receive a vaccine during pregnancy, and self-reported overall likelihood of receipt of a vaccine during pregnancy were also lower among Black women than White women, and history of childhood vaccine refusal was higher among Black women. (Supplemental Table S4)
On qualitative analysis of free text responses among Black women with lower scores, the responses were diverse and included safety concerns and a desire for more information on vaccine safety and efficacy. One woman expressed general concerns about taking too many vaccines during pregnancy.
Discrete choice experiment
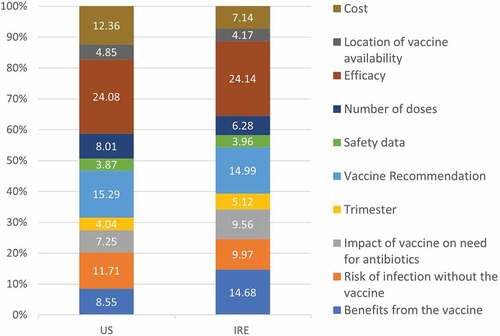
Attribute importance was similar for each country (). Vaccine efficacy and potential benefits of the vaccine for the baby were the most important attributes. Within the potential benefits of the vaccine, women placed the most importance on a vaccine that could prevent against severe infection in their infants. A healthcare provider recommendation was also important to women in both countries, with women placing more importance on healthcare provider recommendations than those of friends and family. In both cohorts, there was a marginal preference toward a vaccine recommended by an obstetrician or midwife compared to that recommended by a primary care physician reflected by higher utility scores for the option ‘vaccine recommended by your obstetrician or midwife’ (Supplemental Tables S5 and S6)
Figure 4. Relative attribute importance. These percentages reflect the relative impact an attribute had on choice compared with the other attributes in the experiment.
Subgroup analysis
We conducted an exploratory analysis of subgroups that had significant associations with decreased likelihood of GBS vaccine receipt using multivariable logistic regression models. There were no differences in attribute preferences according to race (US) or age group. Additionally, no differences were seen among those who reported a low likelihood (≤4) of GBS vaccine acceptability compared with the rest of the population. These results are included as supplemental data (Tables S7–S10).
Discussion
Acceptability of a future GBS vaccine was high in this cohort of pregnant women receiving antenatal care in academic tertiary centers in Philadelphia, US, and Dublin, Ireland. This is consistent with prior reports from the US, Australia, and the UK where greater than 70% of pregnant women reported they would be likely to receive a GBS vaccine after receiving information about the vaccine.Citation20–22 The strongest motivator for vaccine uptake among pregnant women in both Philadelphia and Dublin was a desire to protect their unborn infant from severe infection. This was demonstrated through quantitative and qualitative data in this study. In the DCE, efficacy of the vaccine and potential infant benefits were important drivers of vaccine receipt. In the free-text responses, protection of their infant was the most cited reason for accepting a novel vaccine against GBS. Women will likely balance the efficacy of the vaccine against safety concerns when choosing a new vaccine and will be more likely to take a vaccine that is highly effective at preventing illness in their infant. A novel GBS vaccine may achieve licensure using correlates of protection as end points in a phase III trial,Citation23 and thus efficacy data for infant disease may not be available until phase IV studies. In this scenario, it will be important that available efficacy data is clearly explained to pregnant women and their maternity care providers. Sociodemographic factors impacting vaccine uptake differed between the US and Irish cohorts. In Philadelphia, Black race was associated with a decreased reported likelihood of GBS vaccine uptake, independent of age, income, and education level. Surrogate markers of attitudes toward vaccines in pregnancy, such as prior vaccine receipt and intent to receive a recommended vaccine, were lower among Black women compared with White women. This is consistent with national US reports of decreased uptake of influenza and pertussis vaccines among Non-Hispanic Black women.Citation24–26 The reasons for this remain under-researched and are likely multifactorial. A qualitative study in Atlanta, Georgia, examined messaging strategies that might increase influenza vaccine uptake. Positive messaging focused on benefits to infants was found to be important for motivating behavior change. Most women interviewed placed greatest importance on receiving a recommendation from their providers.Citation27 In a survey of 1862 pregnant women in Atlanta and Colorado, Dudley et al. demonstrated that compared with White women, Black Non-Hispanic and Hispanic women had lower confidence in vaccine safety and efficacy, had lower perceptions of susceptibility to infection, and were less likely to report having sufficient knowledge about vaccines.Citation25 Maternal colonization and of invasive GBS disease rates in the US are highest among Black woman and infants.Citation28–30 Moreover, recent data suggest that Black women may be at higher risk of GBS conversion in late pregnancy. ‘GBS conversion’ refers to women who screen negative for GBS in the third trimester but test positive during delivery. In a recent study of 737 pregnant women, non-Hispanic black women were more likely to convert to GBS positive than non-Hispanic white women, 9.2% as compared to 5.3% (RR: 2.0; 95% CI: 1.02–3.8)Citation31 If a GBS vaccine is made available it will be important that specific efforts are made to ensure good uptake in this high-risk population.Citation30 There are likely other important factors influencing the responses of Black women in this study that could not be captured by the measurement tools in this research. It is known that distrust, systematic racism and bias in the healthcare system, perceived discrimination, and reduced access to reproductive services can all impact how black women experience maternity care in the United States and thus may also impact the uptake of maternal vaccines.Citation32–37
Healthcare provider recommendation was important to pregnant women in Ireland and the US, consistent with previous studies.Citation10 In Ireland, where maternal vaccines are administered by general practitioners rather than obstetricians or midwives, a recommendation from an obstetrician or midwife was ranked more important than that of their GP. Obstetrician and midwife recommendation was frequently mentioned as a reason for vaccination in free text survey responses. There is debate about the optimal location for vaccine delivery in countries, such as Ireland, where antenatal care is shared between primary and secondary care. However, the findings in this study reinforce the importance of strong recommendations from obstetricians and midwives regardless of where vaccines are administered. This is likely to be particularly important for GBS vaccine, given the obstetric-specific nature of GBS infection.
Seventeen percent of US participants and 22% of Irish participants had never heard of GBS, and a further 31% (US) and 58% (Irish) had heard of GBS but reported knowing little about it. This lack of knowledge is not surprising in the Irish cohort as women are not routinely screened for GBS in Ireland. However, even in the US cohort, where universal screening is performed, knowledge was poor among pregnant women regardless of parity. Fifty-two percent of US participants reported understanding what GBS infection is, this is compared with 90% for influenza infection and 75% for pertussis infection. Lack of awareness of GBS has been demonstrated in other countries that practice universal screening. In a 2018 survey of Australian women, 63% of pregnant women had never heard of GBS infection.Citation22 Knowledge and awareness of GBS infection is likely to be important in achieving adequate uptake and may also contribute to the speed of uptake. Conceptualizing the additional benefits of a GBS vaccine may be difficult for women due to the availability of alternative prevention strategies for early onset disease. The limitations of screening such as the differential impact of antibiotics on early and late-onset disease will need to be clearly communicated. There is now an opportunity to prepare for vaccine implementation. Efforts should include education of pregnant women and their care providers regarding the threat of GBS infection and the limitations of current prevention strategies.
Among women who were less likely to receive a GBS vaccine, the concept of a “new vaccine” was an important deterrent. Women expressed concerns about “long-term” effects on their infants. There is an understandable reluctance to automatically recommend or receive a novel vaccine. However, it is important that these concerns be addressed rationally through clear explanations of the scientific process that leads to vaccine development, so that women can make informed decisions.
There was a correlation between the reported likelihood of receiving a recommended vaccine in pregnancy and the likelihood of receiving a novel GBS vaccine in this study. Attitudes toward current maternal vaccines will influence acceptance of novel vaccines, and thus efforts to build population confidence in vaccines administered during pregnancy are important in preparing for new maternal vaccine.
In most industrialized countries, routine vaccination during pregnancy is a relatively new concept that has evolved from public health emergencies such as the H1N1 pandemic and the surge in neonatal pertussis infections.Citation2 It has taken close to a decade to achieve what is still regarded as suboptimal coverage in most high-income countries. Group B Streptococcus vaccination will differ from prior maternal immunization programs as this vaccine is not part of an emergent public health response, and it will have undergone clinical trials on pregnant women. The onus will be on healthcare professionals, and public health authorities to effectively communicate this difference to the public so that the concept of ‘newness’ does not lead to unnecessary delays in vaccine uptake.
With GBS vaccines, there is a unique opportunity to prepare, to work with healthcare providers to ensure there is a strong endorsement of the vaccine and an acceptable implementation plan, and to prepare the population by increasing understanding of GBS disease. In this way, a situation where women are being asked to take a new vaccine for a disease that they have never heard of can be avoided.
This study has important strengths. Recruitment of participants from two countries with different approaches to GBS prevention, universal screening in the US and risk-based screening and intrapartum screening in Ireland, enabled comparison of knowledge, awareness, and acceptability of GBS vaccines across two settings. Mixed methodology strengthened the validity of the findings and the use of a DCE permitted examination of the importance of vaccine-specific attributes.
This study has limitations. This is not population-based sampling. While centers in both countries serve a diverse community, COVID-19 related restrictions necessitated online recruitment, and different recruitment methods were used at different times and sites. The use of online recruitment may have introduced a selection bias toward a higher educated group who were more inclined toward vaccination. Low survey completion rate and high GBS vaccine acceptability in those recruited online in the US may also reflect a social desirability bias. The development, approval, and media coverage of COVID-19 vaccines may have led to differences in attitudes toward novel vaccines among those recruited in late 2020 and early 2021 compared with those recruited in March 2020 prior to the onset of the pandemic. Health literacy was not assessed. The reported likelihood of acceptance of a new GBS vaccine may have been impacted by differences in health literacy. Additionally, the survey was administered only through English, which meant that foreign-born women and other women whose first language was not English were likely underrepresented. This is particularly important in Ireland where decreased uptake in women from Eastern European countries has previously been demonstrated.Citation38 These limitations may have led to a falsely elevated acceptability of GBS vaccine. Vaccine receipt in this study was self-reported and thus is subject to recall bias.
Conclusion
Good GBS vaccine uptake among pregnant women is achievable through strong provider recommendations which highlight the potential benefits of this vaccine for infants. Preparation for GBS vaccine implementation should include maternity care provider engagement, efforts to increase awareness among pregnant women about GBS infection, and a continued focus on improving overall uptake and acceptability of currently recommended maternal vaccines, particularly in population subgroups with low uptake of maternal immunizations.
Supplemental Material
Download MS Word (49.2 KB)Acknowledgments
Thank you to Dr Stephen Ralston for his help with facilitating recruitment in Philadelphia and to all the doctors and midwives at the Rotunda Hospital who facilitated recruitment. Special thanks also to Elisa Belmonte and Cormac McAdam for their help in preparing the content for social media. Finally, thanks to all the women who participated in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2195331
Additional information
Funding
References
- Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, Harrison LH, Farley MM, Reingold A, Bennett NM, et al. Bacterial meningitis in the United States, 1998–2007. N Engl J Med. 2011;364(21):2016–12. doi:10.1056/NEJMoa1005384.
- Baker CJ. The spectrum of perinatal Group B Streptococcal disease. Vaccine. 2013;31(Suppl 4):D3–6. doi:10.1016/j.vaccine.2013.02.030.
- Seale AC, Bianchi-Jassir F, Russell NJ, Kohli-Lynch M, Tann CJ, Hall J, Madrid L, Blencowe H, Cousens S, Baker CJ, et al. Estimates of the burden of Group B Streptococcal disease worldwide for pregnant women, stillbirths, and children. Clin Infect Dis. 2017;65(suppl_2):S200–s219. doi:10.1093/cid/cix664.
- Carreras-Abad C, Ramkhelawon L, Heath PT, Le Doare K. A vaccine against Group B Streptococcus: recent advances. Infect Drug Resist. 2020;13:1263–72. doi:10.2147/IDR.S203454.
- Study of a Group B Streptococcus vaccine in pregnant women living with HIV and in pregnant women who do not have HIV. https://clinicaltrials.gov/ct2/show/NCT04596878.
- Trial to evaluate the safety, tolerability, and immunogenicity of a multivalent Group B Streptococcus vaccine in healthy nonpregnant women and pregnant women and their infants. https://www.clinicaltrials.gov/ct2/show/NCT03765073?cond=group+b+strep+vaccine&draw=2&rank=3.
- Le Doare K, O’Driscoll M, Turner K, Seedat F, Russell NJ, Seale AC, Heath PT, Lawn JE, Baker CJ, Bartlett L, et al. Intrapartum antibiotic chemoprophylaxis policies for the prevention of Group B Streptococcal disease worldwide: systematic review. Clin Infect Dis. 2017;65(suppl_2):S143–51. doi:10.1093/cid/cix654.
- Edwards MS, Gonik B. Preventing the broad spectrum of perinatal morbidity and mortality through Group B streptococcal vaccination. Vaccine. 2013;31(Suppl 4):D66–71. doi:10.1016/j.vaccine.2012.11.046.
- Schrag SJ. Group B Streptococcal vaccine for resource-poor countries. Lancet. 2011;378(9785):11–12. doi:10.1016/S0140-6736(10)61932-0.
- Wilson RJ, Paterson P, Jarrett C, Larson HJ. Understanding factors influencing vaccination acceptance during pregnancy globally: a literature review. Vaccine. 2015;33(47):6420–9. doi:10.1016/j.vaccine.2015.08.046.
- Larson HJ, Jarrett C, Eckersberger E, Smith DMD, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine. 2014;32(19):2150–9. doi:10.1016/j.vaccine.2014.01.081.
- Larson HJ, Jarrett C, Schulz WS, Chaudhuri M, Zhou Y, Dube E, Schuster M, MacDonald NE, Wilson R. Measuring vaccine hesitancy: the development of a survey tool. Vaccine. 2015;33(34):4165–75. doi:10.1016/j.vaccine.2015.04.037.
- Ramesh Babu S, McDermott R, Farooq I, Le Blanc D, Ferguson W, McCallion N, Drew R, Eogan M. Screening for Group B Streptococcus (GBS) at labour onset using PCR: accuracy and potential impact – a pilot study. J Obstet Gynaecol. 2018;38(1):49–54. doi:10.1080/01443615.2017.1328490.
- Wang Y, Wang Z, Wang Z, Li X, Pang X, Wang S. Application of discrete choice experiment in health care: a bibliometric analysis. Front Pub Heal. 2021;9. doi:10.3389/fpubh.2021.673698.
- Geoghegan S, Shuster S, Butler KM, Feemster KA. Understanding barriers and facilitators to maternal immunization: a systematic narrative synthesis of the published literature. Matern Child Health J. 2022;26(11):2198–209. doi:10.1007/s10995-022-03508-0.
- Sawtooth. Sample size issues for conjoint analysis studies. [accessed 2021 Mar 3]. https://sawtoothsoftware.com/resources/technical-papers/sample-size-issues-for-conjoint-analysis-studies.
- Lenk PJ, DeSarbo WS, Green PE, Young MR. Hierarchical Bayes conjoint analysis: recovery of partworth heterogeneity from reduced experimental designs. Mark Sci. 1996;15(2):173–91. doi:10.1287/mksc.15.2.173.
- Sawtoothsoftware. CBC/HB v5 manual. [accessed 2021 Sep 2]. https://content.sawtoothsoftware.com/assets/276545e9-0445-474c-b01c-f5b24c3eba6d.
- MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161–4. doi:10.1016/j.vaccine.2015.04.036.
- Dempsey AF, Pyrzanowski J, Donnelly M, Brewer S, Barnard J, Beaty BL, Mazzoni S, O’Leary ST. Acceptability of a hypothetical group B strep vaccine among pregnant and recently delivered women. Vaccine. 2014;32(21):2463–8. doi:10.1016/j.vaccine.2014.02.089.
- McQuaid F, Jones C, Stevens Z, Plumb J, Hughes R, Bedford H, Heath PT, Snape MD. Attitudes towards vaccination against Group B Streptococcus in pregnancy. Arch Dis Child. 2014;99(7):700–U104. doi:10.1136/archdischild-2013-305716.
- Giles ML, Buttery J, Davey M-A, Wallace E. Pregnant women’s knowledge and attitude to maternal vaccination including Group B Streptococcus and respiratory syncytial virus vaccines. Vaccine. 2019;37(44):6743–9. doi:10.1016/j.vaccine.2019.08.084.
- Vekemans J, Crofts J, Baker CJ, Goldblatt D, Heath PT, Madhi SA, Le Doare K, Andrews N, Pollard AJ, Saha SK, et al. The role of immune correlates of protection on the pathway to licensure, policy decision and use of Group B Streptococcus vaccines for maternal immunization: considerations from World Health Organization consultations. Vaccine. 2019;37(24):3190–8. doi:10.1016/j.vaccine.2019.04.039.
- Ding H, Black CL, Ball S, Fink RV, Williams WW, Fiebelkorn AP, Lu P-J, Kahn KE, D’Angelo DV, Devlin R, et al. Influenza vaccination coverage among pregnant women — United States, 2016–17 influenza season. MMWR-Morbidity Mortality Weekly Rep. 2017;66(38):1016–22. doi:10.15585/mmwr.mm6638a2.
- Dudley MZ, Limaye RJ, Salmon DA, Omer SB, O’Leary ST, Ellingson MK, Spina CI, Brewer SE, Bednarczyk RA, Malik F, et al. Racial/Ethnic disparities in maternal vaccine knowledge, attitudes, and intentions. Public Health Rep (Washington, DC: 1974). 2021;136(6):699–709. doi:10.1177/0033354920974660.
- Arnold LD, Luong L, Rebmann T, Chang JJ. Racial disparities in U.S. maternal influenza vaccine uptake: results from analysis of Pregnancy Risk Assessment Monitoring System (PRAMS) data, 2012–2015. Vaccine. 2019;37(18):2520–6. doi:10.1016/j.vaccine.2019.02.014.
- Marsh HA, Malik F, Shapiro E, Omer SB, Frew PM. Message framing strategies to increase influenza immunization uptake among pregnant African American women. Matern Child Health J. 2014;18(7):1639–47. doi:10.1007/s10995-013-1404-9.
- Edwards JM, Watson N, Focht C, Wynn C, Todd CA, Walter EB, Heine RP, Swamy GK. Group B Streptococcus (GBS) colonization and disease among pregnant women: a historical cohort study. Infect Dis Obstet Gynecol. 2019;2019:5430493. doi:10.1155/2019/5430493.
- Nanduri SA, Petit S, Smelser C, Apostol M, Alden NB, Harrison LH, Lynfield R, Vagnone PS, Burzlaff K, Spina NL, et al. Epidemiology of invasive early-onset and late-onset Group B Streptococcal disease in the United States, 2006 to 2015: multistate laboratory and population-based surveillance. JAMA Pediatr. 2019;173(3):224–33. doi:10.1001/jamapediatrics.2018.4826.
- Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, Craig AS, Schaffner W, Zansky SM, Gershman K, et al. 2008. Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA. 299(17):2056–65. doi:10.1001/jama.299.17.2056.
- Spiel MH, Hacker MR, Haviland MJ, Mulla B, Roberts E, Dodge LE, Young BC. Racial disparities in intrapartum Group B Streptococcus colonization: a higher incidence of conversion in African American women. J Perinatol. 2019;39(3):433–8. doi:10.1038/s41372-018-0308-3.
- Giscombé CL, Lobel M. Explaining disproportionately high rates of adverse birth outcomes among African Americans: the impact of stress, racism, and related factors in pregnancy. Psychol Bull. 2005;131(5):662–83. doi:10.1037/0033-2909.131.5.662.
- Nuru-Jeter A, Dominguez TP, Hammond WP, Leu J, Skaff M, Egerter S, Jones CP, Braveman P. “It’s the skin you’re in”: African-American women talk about their experiences of racism. an exploratory study to develop measures of racism for birth outcome studies. Matern Child Health J. 2009;13(1):29–39. doi:10.1007/s10995-008-0357-x.
- Dominguez TP. Adverse birth outcomes in African American women: the social context of persistent reproductive disadvantage. Soc Work Public Health. 2011;26(1):3–16. doi:10.1080/10911350902986880.
- Rosenthal L, Lobel M. Explaining racial disparities in adverse birth outcomes: unique sources of stress for Black American women. Soc Sci Med (1982). 2011;72(6):977–83. doi:10.1016/j.socscimed.2011.01.013.
- Alhusen JL, Bower KM, Epstein E, Sharps P. Racial discrimination and adverse birth outcomes: an integrative review. J Midwifery Women’s Health. 2016;61(6):707–20. doi:10.1111/jmwh.12490.
- Njoku A, Evans M, Nimo-Sefah L, Bailey J. Listen to the whispers before they become screams: addressing black maternal morbidity and mortality in the United States. Healthcare (Basel). 2023;11(3):438. doi:10.3390/healthcare11030438.
- Cleary BJ, Rice Ú, Eogan M, Metwally N, McAuliffe F. 2009 A/H1N1 influenza vaccination in pregnancy: uptake and pregnancy outcomes – a historical cohort study. Eur J Obstet Gynecol Reprod Biol. 2014;178:163–8. doi:10.1016/j.ejogrb.2014.04.015.