ABSTRACT
Patients received kidney transplantation (KTR) have a low seroconversion rate after vaccination. Our objective was to compare the seroconversion rates and adverse effects of additional different vaccinations in KTR patients in existing studies. Databases such as PubMed, Cochrane Library, Web of Science, Embase, ClinicalTrials.gov and others. Three high-quality RCT were included and showed no statistical difference in seroconversion rates between the two vaccines (RR = 0.93[0.76,1.13]). There was no statistical difference in seroconversion rates between the sexes, for men (RR = 0.93[0.69,1.25]) and women (RR = 0.91[0.62,1.33]). Among the adverse effects there was no statistically significant difference in fever (RR = 1.06[0.44,2.57]), while for injection site pain there was a statistically significant difference (RR = 1.14[1.18,1.84]). There was no significant difference in seroconversion rates in patients with KTR who received the two additional vaccines. Patients injected with the viral vector vaccine were less painful than those injected with the mRNA vaccine.
Introduction
Since the first announcement of the COVID-19 pandemic by the World Health Organization in March 2020 and up to November 2021, 250 million people worldwide have confirmed COVID-19, of which more than 4 million have died.Citation1 The mortality rate and the risks associated with the complications of COVID-19 have driven the development of COVID-19 vaccines around the world. The following vaccines are used worldwide against SARS-CoV-2: DNA vaccines, mRNA vaccines, viral vector vaccines, inactivated vaccines, live attenuated vaccines, subunit vaccines and other viral vaccines such as BCG, which induce antibodies to the new coronavirus in humans.Citation2 Among these vaccines, those approved by the World Health Organization are mRNA vaccines, viral vector vaccines and inactivated vaccines.Citation3
With clinical trials of the COVID-19 vaccine, researchers have conducted numerous clinical trials to demonstrate the efficacy of the vaccine against SARS-CoV-2 and the adverse effects of vaccination on humans. mRNA vaccines offer more than 50% protection in humans aged 16 years and older after the first dose of vaccine.Citation4 In people already infected with novel coronavirus, the vaccine was 66.9% effective in moderate cases within 2 weeks and 66.1% effective after 4 weeks, and 76.7% effective in severe cases at 2 weeks and 85.4% after 4 weeks.Citation5 These clinical data indicate that the mRNA vaccine and the viral vector vaccine have provided excellent protection in humans.
Although both vaccines have shown excellent results against SARS-CoV-2, specific populations, such as solid organ transplant patients, have been excluded from most clinical trials. In short the clinical data for the vaccines are still lacking in specific populations. These specific populations are not overrepresented among SARS-CoV-2 infected patients, and the protective effect of the new crown vaccine in them is different compared to that in healthy populations.
With the improvement of human medicine and technology in the field of solid organ transplantation, many diseases have been alleviated by organ transplantation, and among the many organ transplants, kidney transplantation is undoubtedly the most successful and common, accounting for 50% of solid organ transplants in the world, and kidney transplantation is also recognized by patients with end-stage renal disease.Citation6
The pandemic of novel coronary pneumonia poses a serious risk to KTR patients, with the first KTR combined with COVID-19 patient being published with 2020.Citation7 Kidney transplant patients are subject to intense rejection due to the transplantation of allogenic organs and therefore choose to take long-term immunosuppressive drugs to counteract their body’s rejection. Common immunosuppressive drugs include corticosteroids (prednisone), antimetabolites (mycophenolic acid), calcium-regulated phosphatase inhibitors (tacrolimus), mTOR inhibitors (sirolimus), and biologics including IL-2 inhibitors, and IL-6 inhibitors.Citation8–12 The use of these immunosuppressive agents exacerbates the damage caused by the SARS-CoV-2 virus and its complications in KTR patients.
There is also evidence that factors such as the underlying disease carried by the KTR patient and the type of immunosuppressant used can affect the immune efficacy of the vaccine against itself. A follow-up study from Barcelona showed that the vaccine was less protective in kidney transplant patients with diabetes.Citation13 A cohort study from France showed that although the type of immunosuppressant used varied, the combination still resulted in a strong suppression of the humoral immune response to vaccination.Citation14
In this systematic review and meta-analysis we compare the effects of mRNA vaccine and viral vector vaccine on seroconversion rates in KTR patients and the strength of the adverse effects of both vaccines, with the aim of providing a reference for patients and healthcare professionals to choose the type of vaccine to be administered.
This protocol is registered with the International Prospective Register of Systematic Reviews (PROSPERO). Registration number CRD (42022362759).
Method
This systematic review and meta-analysis study was carried out in strict accordance with the Preferred Report for Systematic Review and Integrated Analysis (PRISMA) guidelines.Citation15
Search strategy
PubMed, Cochrane Library, Web of Science, Embase, ClinicalTrials.gov, Zhiwang, Wanfang, Wipu and Chinese biomedical literature databases were searched by computer with unlimited search time and no language restrictions. The search terms were COVID-19 mRNA vaccine, 2019 nCoV Vaccine mRNA 1273, mRNA 1273, Moderna COVID-19 Vaccine RNA, Elasomeran, M1273, mRNA 1273.211, Vaccine, BNT162, and COVID-19 Vaccine Pfizer-BioNTech, Pfizer-BioNTech, COVID-19 Vaccine, kidney transplant, Renal Transplantation, Grafting, Kidney Transplantation, Kidney, random, randomized controlled trial.
Inclusion criteria
(1) Study population: renal transplant patients. (2) Study type: randomized controlled trial, RCT. (3) Primary outcome indicators: seroconversion rate in kidney transplant recipient patients, incidence of adverse events. (4) Secondary outcome indicators: seroconversion rate between genders in kidney transplant recipient patients.
Exclusion criteria
(1) Non-randomized controlled trials. (2) Review, meta-analysis. (3) Randomized controlled trials without published outcome indicators. (4) Non-KTR patients, non-COVID-19 vaccinated patients.
Literature selection and data extraction
Two independent researchers searched and screened the literature to exclude irrelevant literature, and the remaining literature was read in full to further exclude literature that did not meet the inclusion criteria. After screening, the two independent researchers each extracted relevant data, including basic information about the included studies, baseline characteristics of each study, interventions and outcome indicators, and cross-checked the results. In the event of disagreement during data extraction, the final data was agreed with the other independent researcher.
Risk of bias analysis of included studies
Quality was assessed using the RCT risk of bias assessment tool recommended by the Cochrane Handbook.Citation16 If all the issues mentioned in the Cochrane Handbook are low risk in a clinical trial, the article is judged to be low risk and conversely if one issue is high risk, the article is judged to be high risk and the work is carried out independently by two investigators, with a third investigator consulted and agreed in case of disagreement.
Statistical methods
A meta-analysis was conducted using ReviewManager version 5.3 software. Heterogeneity was analyzed using the I2 test, with a fixed-effects model for studies with little heterogeneity (P > .1, I2 ≤ 50%) and a random-effects model for studies with greater heterogeneity (p ≤ .1, I2 > 50%). The dichotomous variables were expressed as combined effect sizes using Risk Ratio (RR). Effect intervals were estimated using 95% confidence interval (CI), with p < .05 indicating a statistically significant difference.
Results
Results of the literature screening
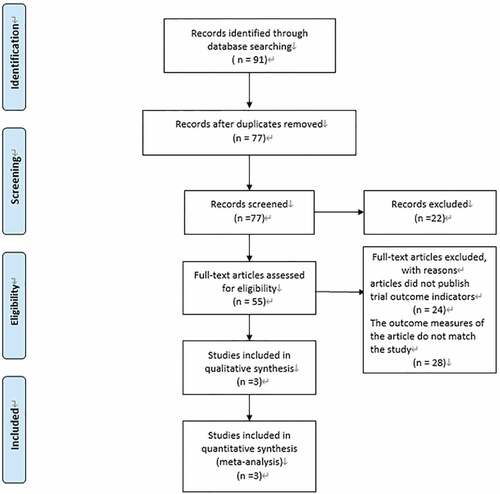
A total of 91 articles were retrieved according to the previously developed search strategy, of which 14 were duplicates. 77 articles remained after elimination of duplicates. Of the 55 articles, 24 articles with no published outcomes and 28 articles reporting outcome indicators that were not consistent with the current study were excluded, resulting in the inclusion of 3 RCT studies.Citation17–19 A flow chart of the literature screening is shown in .
Basic characteristics and quality assessment of the included literature
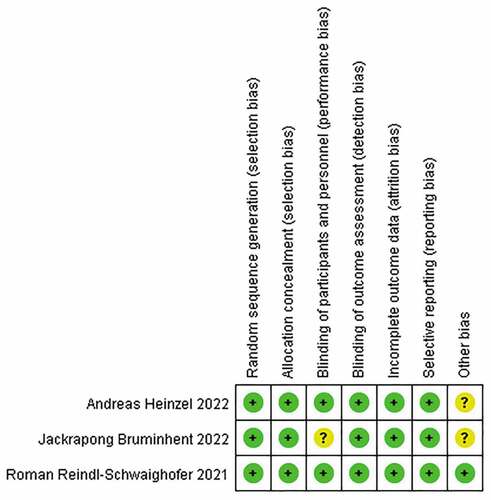
Of the three high-quality RCTs included, the total sample was 443 KTR patients, including 224 patients injected with COVID-19 mRNA vaccine and 219 patients injected with COVID-19 viral vector vaccine, all aged 50–61 years, with the specific characteristics shown in . The risk of bias analysis quality assessment table is shown in . One of the three studies had one article with a low risk of bias in the Seven of the articles in the Cochrane Handbook’s recommended risk of bias assessment tool for RCTs showed a low risk, one article did not specify whether blinding was chosen to randomize KTR patients, and two articles did not specify whether there were other sources of bias.
Table 1. Basic characteristics of the included literature.
Results of meta-analysis
Comparison of seroconversion rates between injected mRNA vaccine and viral vector vaccine in patients with KTR
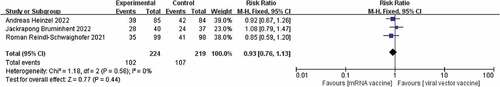
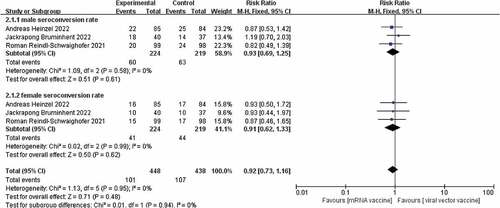
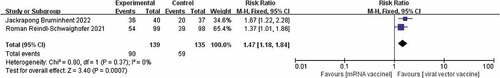
Of the three studies included.Citation17–19 There was no heterogeneity between the studies (I2 = 0%, p = .56) and therefore a fixed effects model was chosen. Although studies showed higher seroconversion rates for the viral vector vaccine than for the mRNA vaccine after administration of both vaccines, a combined analysis showed no significant difference in seroconversion rates between the two [RR = 0.93, p = .44]. The results are shown in . It has been suggested that there is a difference in immunological performance between male and female patients during SARS-CoV-2 infection, with female patients having a higher T-cell response than male patients, and therefore women achieving a higher antibody response than men after vaccination.Citation20 Subgroup analysis of the seroconversion rates of male and female KTR patients given the two vaccines separately,Citation17–19 showed that there was also no significant difference in the seroconversion rates of male and female KTR patients after the two vaccinations, with risk ratios of [RR = 0.93,95%, p = .61] [RR = 0.91, p = .62]. The results are shown in .
Comparison of adverse reactions to mRNA vaccine versus viral vector vaccine in KTR patients
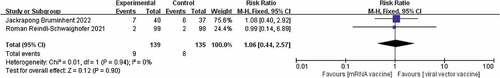
Studies have shown that COVID-19 vaccine can cause adverse reactions after injection, including pain at the injection site, fever, fatigue, headache, muscle pain, nausea, vomiting, itching, and joint pain, and possibly anaphylaxis.Citation21 The only two adverse reactions reported in the two included papersCitation17,Citation19 were injection site pain and fever,These two adverse reactions were therefore included in this study. There was no heterogeneity between the two included studies in the adverse effect of injection site pain (I2 = 0%, p = .37) and a fixed effects model was chosen. The results showed that injection site pain was much lower with the COVID-19 virus vector vaccine than with the New Coronavirus mRNA vaccine [RR = 1.47, p = .0007] The results are shown in .
There was also no heterogeneity in the two included papers in the adverse effect of fever (I2 = 0%, p = .94), and a fixed effects model was chosen. The results showed no difference in fever caused by the two vaccines in KTR patients [RR = 1.06, p = .90]. The results are shown in .
Conclusions
KTR patients require long-term immunosuppressive drugs and therefore have low levels of antibodies in their bodies. The COVID-19 vaccine does not achieve the seroconversion rates of the non-KT transplant population, and it is important to find a vaccine that can improve the seroconversion rates of KTR patients among the available vaccines. In this study, there was no difference in seroconversion rates between the COVID-19 mRNA vaccine and the COVID-19 viral vector vaccine, nor was there any difference in gender. However, in terms of post-injection adverse effects, one of the adverse effects of the viral vector vaccine, injection site pain, was less than that of the mRNA vaccine, which reduced the distress of KTR patients in the fight against COVID-19. This meta-analysis may help in the selection of vaccines for KTR patients and may help healthcare policy makers to provide more detailed policies on vaccination and personal protection for KTR patients.
Discussion
Vaccines are biological agents that have been developed to build or enhance the body’s immunity to a specific type of disease. With the introduction of vaccines, the global morbidity and mortality rates of diseases that have wreaked havoc on human history, such as smallpox and polio, have been significantly reduced.Citation22–24 Most vaccines activate active immunity by promoting the production of antibodies in the body, in part by an increase in B-cells, antibody responses and an increase in T-cell sensitivity. The remaining part is through passive immunity, which directly increases the body’s antibody levels and achieves a state of instantaneous immunity.Citation25,Citation26
Patients who have received solid organ transplants have low levels of antibodies due to immunosuppressive drugs and do not have high seroconversion rates after vaccination to produce high levels of immunity to the respective disease.Citation27,Citation28 In a study on the analysis of antibody responses to COVID-19 vaccination in liver transplant recipients and patients with chronic liver disease, the results showed that the use of two or more immunosuppressive drugs resulted in lower antibody levels and poorer immune responses in patients.Citation29
Although there are fewer clinical studies of KTR patients receiving the COVID-19 vaccine, it is still clear from the available studies that KTR patients are generally less likely to produce an immune effect after receiving the vaccine. A study from Germany on the impaired immune response to SARS-CoV-2 vaccination in KTR patients and renal dialysis patients showed that after two doses of vaccine, KTR patients had the lowest levels of SARS-CoV-2-specific IgG and neutralization levels. From the data reported in the study, the SARS-CoV-2 peak S1-specific IgG level was (10 ± 24 BAU/ml) in KTR patients compared to (81 ± 125 BAU/ml; p < .001) in the control group, with only 2 of 28 KTR patients (7%) having IgG levels above the threshold of 35.2 BAU/ml. In contrast, IgG levels above the threshold were detected in 33 (42%) of 78 controls The peak SARS-CoV-2 S1-specific IgG level in KTR patients after the second vaccination was (54 ± 93 BAU/ml) compared with (1922 ± 2485 BAU/ml; p < .001) in controls, and in addition 73 of 78 controls (94%) compared with 10 of 28 KTRs (36%) had IgG levels above the critical value of 35.2 BAU/ml.Citation30 Meanwhile another study on IgG antibodies in hemodialysis, peritoneal dialysis and renal transplant recipients immunized with BNT162b2 Pfizer-Biotech SARS-CoV-2 vaccine showed that KTR patients had the lowest antibody concentrations and the lowest seroconversion rates after two doses of the vaccine.Citation31 The above two clinical trials have shown that KTR patients do not achieve the same antibody levels and seroconversion rates as normal subjects after COVID-19 vaccination. It is not only the COVID-19 vaccine but also other vaccine types that do not show promising antibody levels and seroconversion rates in KTR or solid organ recipients.
In a meta-analysis of seroconversion rates after vaccination of solid organs against various infectious diseases, tetanus, diphtheria, poliomyelitis, hepatitis A and B, and influenza vaccines did not achieve the seroconversion rates of the normal population after vaccination of solid organ transplant patients. Although seroconversion rates for the same vaccines varied between organ transplant patients, overall the vaccines were generally less effective in protecting solid organ transplant patients.Citation32
COVID-19 mRNA vaccines and viral vector vaccines have shown very high efficacy in clinical trials, with both types of vaccines inducing anti-SARS-CoV-2 spike (S) protein or antibody receptor binding domain (anti-RBD) binding levels at or above those of recovered patients. The difference is that the vector virus may produce multifunctional antibodies after injection, which may help to combat the development of the disease, and it has also been shown that vector virus vaccines can increase phagocytosis by monocytes as well as neutrophil-mediated phagocytes.Citation33,Citation34 The above conclusions are based on the results of COVID-19 vaccination in general subjects (healthy people without underlying disease), but in patients with KTR it has been shown that the COVID-19 mRNA vaccine has a higher seroconversion rate than the viral vector vaccine and a higher concentration of anti-spike immunoglobulin.Citation35 There is also evidence of the effectiveness of combination vaccination, with higher seroconversion rates in patients who received the viral vector vaccine followed by the mRNA vaccine.Citation36 The above evidence suggests that the COVID-19 mRNA vaccine may be the first choice for KTR patients when selecting a vaccine for KTR patients.The types of adverse reactions collected globally include: myocarditis, immune thrombocytopenia, occasional facial nerve palsy, venous embolism of the brain and internal organs, in addition to a small number of more serious adverse reactions, a large number of mild adverse reactions including skin reactions, injection site pain, fever, malaise, vomiting, etc.Citation37–40 A cross-sectional study of adverse events associated with the administration of both types of vaccine from Poland showed that the COVID-19 mRNA vaccine produced more adverse reactions in the general population than the COVID-19 viral vector vaccine, including injection site pain, fatigue and headache.Citation41 This finding is consistent with our analysis of adverse reactions to both vaccine injections in KT patients. The adverse effects of COVID-19 vaccination are not known, but it has been suggested that the adverse effects of the vaccine may be due to the excipients added to the vaccine, which bind to IgE receptors on mast cells and basophils and produce histamine, prostaglandins, leukotrienes and 5-hydroxytryptamine mediators. The development of adverse reactions. It is interesting to note that in the case of both mRNA and BNT162b2 vaccines, the number of allergic reactions in humans was much higher in females than in males, although the raw data show that the number of vaccinations in females was higher than in males, but this result clearly does not account for the higher rate of allergy in females than in males.Citation42 The vaccines currently available on the market are still effective against SARS-CoV-2 and its variants. With the prevalence of COVID-19, the development of vaccines and the frequent mutations of the virus, it is important to keep up with the development of vaccines to deal with possible future outbreaks.
The discussion of the two vaccines and the clinical data support that both vaccines provide good protection against SARS-CoV-2 infection and the complications that arise after infection. However, the low efficiency of the vaccines against the virus in specific populations such as KTR patients suggests the development of targeted vaccines to help achieve better immunity in these populations in the future.
Limitations of the study
There are several limitations to this study, the first being the small number of articles included. Although the three RCTs included are of high quality and reflect the immune effects and adverse effects of different vaccines in patients with KTR, they can to some extent allow patients to select the right vaccine. However, the prevalence of COVID-19 and the fact that the vaccine has not been developed for a long time and that most of the clinical trials on the vaccine have excluded patients with KTR and other such patients, has resulted in too few original studies. Secondly, due to regional and methodological differences in the studies included, there were inconsistencies in the outcome indicators of improved human antibody production to SARS-CoV-2 due to the vaccine, which resulted in fewer outcome indicators being included in this study. Finally, although seroconversion rate is an indicator of immune response to the vaccine, it is only one indicator of the impact of the vaccine on the prevalence and severity of COVID-19 infection, and there is a lack of data on clinical efficacy endpoints in the included studies.
Acknowledgments
SKC completed the data extraction and writing of the essay, WX W and FY H checked the data in the essay, JW, XYL, ZXG, FG and TWD helped with the writing of the essay, PFW, XBY and FM revised the essay and guided the logic of the essay.
Disclosure statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Lee A, Wong SY, Chai LYA, Lee SC, Lee MX, Muthiah MD, Tay SH, Teo CB, Tan BK, Chan YH, et al. Efficacy of COVID-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376:e068632. doi:10.1136/bmj-2021-068632.
- Han X, Xu P, Ye Q. Analysis of COVID-19 vaccines: types, thoughts, and application. J Clin Lab Anal. 2021;35(9):e23937. doi:10.1002/jcla.23937.
- Park H, Park MS, Seok JH, You J, Kim J, Kim J, Park M-S. Insights into the immune responses of SARS-CoV-2 in relation to COVID-19 vaccines. J Microbiol. 2022;60(3):308–8. doi:10.1007/s12275-022-1598-x.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi:10.1056/NEJMoa2034577.
- Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med. 2021;384(23):2187–201. doi:10.1056/NEJMoa2101544.
- Glebova K, Reznik ON, Reznik AO, Mehta R, Galkin A, Baranova A, Skoblov M. siRNA technology in kidney transplantation: current status and future potential. BioDrugs. 2014;28(4):345–61. doi:10.1007/s40259-014-0087-0.
- Zhu L, Xu X, Ma K, Yang J, Guan H, Chen S, Chen Z, Chen G. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. 2020;20(7):1859–63. doi:10.1111/ajt.15869.
- Strehl C, Ehlers L, Gaber T, Buttgereit F. Glucocorticoids-all-rounders tackling the versatile players of the immune system. Front Immunol. 2019;10:1744. doi:10.3389/fimmu.2019.01744.
- Zeng H. mTOR signaling in immune cells and its implications for cancer immunotherapy. Cancer Lett. 2017;408:182–9. doi:10.1016/j.canlet.2017.08.038.
- Maeda T, Obata R, Rizk DD, Kuno T. The association of interleukin-6 value, interleukin inhibitors, and outcomes of patients with COVID-19 in New York City. J Med Virol. 2021;93(1):463–71. doi:10.1002/jmv.26365.
- Hage R, Steinack C, Gautschi F, Schuurmans M. Transplant drugs against SARS, MERS and COVID-19. Transplantology. 2020;1(2):71–84. doi:10.3390/transplantology1020007.
- Ahmadian E, Zununi Vahed S, Mammadova S, Abediazar S. Immunosuppressant management in renal transplant patients with COVID-19. Biomed Res Int. 2021;2021:9318725. doi:10.1155/2021/9318725.
- Cucchiari D, Egri N, Bodro M, Herrera S, Del Risco-Zevallos J, Casals-Urquiza J, Cofan F, Moreno A, Rovira J, Banon-Maneus E, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21(8):2727–39. doi:10.1111/ajt.16701.
- Danthu C, Hantz S, Dahlem A, Duval M, Ba B, Guibbert M, El Ouafi Z, Ponsard S, Berrahal I, Achard J-M, et al. Humoral response after SARS-CoV-2 mRNA vaccination in a cohort of hemodialysis patients and kidney transplant recipients. J Am Soc Nephrol. 2021;32(9):2153–8. doi:10.1681/ASN.2021040490.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71.
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi:10.1136/bmj.l4898.
- Bruminhent J, Setthaudom C, Phornkittikorn P, Chaumdee P, Prasongtanakij S, Srisala S, Malathum K, Boongird S, Nongnuch A, Assanatham M, et al. An additional dose of viral vector COVID-19 vaccine and mRNA COVID-19 vaccine in kidney transplant recipients: a randomized controlled trial (CVIM 4 study). Am J Transplant. 2022;22(11):2651–60.
- Heinzel A, Schretzenmeier E, Regele F, Hu K, Raab L, Eder M, Aigner C, Jabbour R, Aschauer C, Stefanski A-L, et al. Three-month follow-up of heterologous vs. homologous third SARS-CoV-2 vaccination in kidney transplant recipients: secondary analysis of a randomized controlled trial. Front Med (Lausanne). 2022;9:936126. doi:10.3389/fmed.2022.936126.
- Reindl-Schwaighofer R, Heinzel A, Mayrdorfer M, Jabbour R, Hofbauer TM, Merrelaar A, Eder M, Regele F, Doberer K, Spechtl P, et al. Comparison of SARS-CoV-2 antibody response 4 weeks after homologous vs heterologous third vaccine dose in kidney transplant recipients: a randomized clinical trial. JAMA Intern Med. 2022;182(2):165–71. doi:10.1001/jamainternmed.2021.7372.
- Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, Silva J, Mao T, Oh JE, Tokuyama M, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588(7837):315–20. doi:10.1038/s41586-020-2700-3.
- Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of pfizer/BioNTech and moderna vaccines. Eur Rev Med Pharmacol Sci. 2021;25(3):1663–9. doi:10.26355/eurrev_202102_24877.
- Bartlett BL, Pellicane AJ, Tyring SK. Vaccine immunology. Dermatol Ther. 2009;22(2):104–9. doi:10.1111/j.1529-8019.2009.01223.x.
- Centers for Disease Control and Prevention (CDC). Impact of vaccines universally recommended for children—United States, 1990–1998. MMWR Morb Mortal Wkly Rep. 1999;48(12):243–8.
- Centers for Disease Control and Prevention (CDC). Ten Great public health achievements—United States, 1900–1999. MMWR Morb Mortal Wkly Rep. 1999;48(12):241–3.
- Gardner P, Eickhoff T, Poland GA, Gross P, Griffin M, LaForce FM, Schaffner W, Strikas R. Adult immunizations. Ann Intern Med. 1996;124:35–40. doi:10.7326/0003-4819-124-1_Part_1-199601010-00007.
- Casadevall A, Scharff MD. Serum therapy revisited: animal models of infection and development of passive antibody therapy. Antimicrob Agents Chemother. 1994;38(8):1695–702. doi:10.1128/AAC.38.8.1695.
- Manuel O, Pascual M, Hoschler K, Giulieri S, Alves D, Ellefsen K, Bart P-A, Venetz J-P, Calandra T, Cavassini M. Humoral response to the influenza a H1N1/09 monovalent AS03-adjuvanted vaccine in immunocompromised patients. Clin Infect Dis. 2011;52(2):248–56. doi:10.1093/cid/ciq104.
- Birdwell KA, Ikizler MR, Sannella EC, Wang L, Byrne DW, Ikizler TA, Wright PF. Decreased antibody response to influenza vaccination in kidney transplant recipients: a prospective cohort study. Am J Kidney Dis. 2009;54(1):112–21. doi:10.1053/j.ajkd.2008.09.023.
- Thuluvath PJ, Robarts P, Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol. 2021;75(6):1434–9. doi:10.1016/j.jhep.2021.08.008.
- Kolb T, Fischer S, Müller L, Lübke N, Hillebrandt J, Andrée M, Schmitz M, Schmidt C, Küçükköylü S, Koster L, et al. Impaired immune response to SARS-CoV-2 vaccination in dialysis patients and in kidney transplant recipients. Kidney360. 2021;2(9):1491–8. doi:10.34067/KID.0003512021.
- Piotrowska M, Zieliński M, Tylicki L, Biedunkiewicz B, Kubanek A, Ślizień Z, Polewska K, Tylicki P, Muchlado M, Sakowska J, et al. Local and systemic immunity are impaired in end-stage-renal-disease patients treated with hemodialysis, peritoneal dialysis and kidney transplant recipients immunized with BNT162b2 Pfizer-BioNTech SARS-CoV-2 vaccine. Front Immunol. 2022;13:832924. doi:10.3389/fimmu.2022.832924.
- Eckerle I, Rosenberger KD, Zwahlen M, Junghanss T. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS One. 2013;8(2):e56974. doi:10.1371/journal.pone.0056974.
- Barrett JR, Belij-Rammerstorfer S, Dold C, Ewer KJ, Folegatti PM, Gilbride C, Halkerston R, Hill J, Jenkin D, Stockdale L, et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2021;27(2):279–88. doi:10.1038/s41591-020-01179-4.
- Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21(8):475–84. doi:10.1038/s41577-021-00578-z.
- Correia AL, Leal R, Pimenta AC, Fernandes M, Guedes Marques M, Rodrigues L, Santos L, Romãozinho C, Sá H, Pratas J, et al. The type of SARS-CoV-2 vaccine influences serological response in kidney transplant recipients. Clin Transplant. 2022;36(4):e14585. doi:10.1111/ctr.14585.
- Borobia AM, Carcas AJ, Pérez-Olmeda M, Castaño L, Bertran MJ, García-Pérez J, Campins M, Portolés A, González-Pérez M, García Morales MT, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacs): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398(10295):121–30. doi:10.1016/S0140-6736(21)01420-3.
- Kerneis M, Bihan K, Salem JE. COVID-19 vaccines and myocarditis. Arch Cardiovasc Dis. 2021;114(6–7):515–7. doi:10.1016/j.acvd.2021.06.001.
- Welsh KJ, Baumblatt J, Chege W, Goud R, Nair N. Thrombocytopenia including immune thrombocytopenia after receipt of mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2021;39:3329–32. doi:10.1016/j.vaccine.2021.04.054.
- Sato K, Mano T, Niimi Y, Toda T, Iwata A, Iwatsubo T. Facial nerve palsy following the administration of COVID-19 mRNA vaccines: analysis of a self-reporting database. Int J Infect Dis. 2021;111:310–2. doi:10.1016/j.ijid.2021.08.071.
- Bellinato F, Maurelli M, Gisondi P, Girolomoni G. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10(22):10. doi:10.3390/jcm10225344.
- Dziedzic A, Riad A, Attia S, Klugar M, Tanasiewicz M. Self-reported adverse events of COVID-19 vaccines in polish healthcare workers and medical students. Cross-sectional study and pooled analysis of CoVaST project results in central Europe. J Clin Med. 2021;10(22):10. doi:10.3390/jcm10225338.
- Cabanillas B, Novak N. Allergy to COVID-19 vaccines: a current update. Allergol Int. 2021;70(3):313–8. doi:10.1016/j.alit.2021.04.003.