ABSTRACT
COVID-19 vaccine–related adverse events are mostly minor to moderate, and serious events are rare. Single cases of Raynaud’s phenomenon (RP) in temporal proximity to COVID-19 vaccination have been reported. Demographic data, medical history, and detailed information regarding vaccination status and RP characteristics were obtained from patients with confirmed RP after COVID-19 vaccination. Fifteen participants reported the initial manifestation of RP, which occurred in 40% after the first, in 33% after the second, and in 27% after the third vaccination. RP development and occurrence of episodes were not linked to any specific vaccine type. New onset of disease was observed in 40% of the vaccinees after BNT162b2, in 33% after mRNA-1273, and in 27% after ChAdOx1 vaccination. Three out of four participants with preexisting RP prior to COVID-19 vaccination reported aggravation in frequency and intensity after immunization. Although COVID-19 vaccination is pivotal in controlling the pandemic, the observed temporal association between vaccine administration and RP occurrence warrants global activities to support pharmacovigilance for the detection of adverse reactions, one of which may include RP.
Introduction
As of March 8, 2023, 13.33 billion COVID-19 vaccine doses have been administered globally, allowing for 69.7% of the world’s population to have received at least one vaccination.Citation1,Citation2 Active drug safety surveillance programs have led to numerous reports on vaccine-induced adverse effects of mostly mild-to-moderate severity. Rarely, severe adverse events including anaphylaxis, thrombosis with thrombocytopenia syndrome, myocarditis, and Guillain–Barré syndrome have been reported.Citation3 In 2021, we reported the case of a healthy woman who developed Raynaud’s phenomenon (RP) shortly after COVID-19 vaccination.Citation4 RP, an episodic, vasospastic disorder of the skin’s small muscular arteries, usually manifests with a triphasic attack of pallor, cyanosis, and rubor, particularly on fingers and toes.Citation5 The most common primary form is idiopathic, and the secondary form is associated with underlying autoimmune, inflammatory, hematopoietic, or vascular diseases, certain medications, or vibratory trauma.Citation5,Citation6
Methods
This study was instigated by several patients from different countries who had read the case reportCitation4 and reported the new onset or aggravation of RP after COVID-19 vaccination. Approval was obtained from the ethics committee of the Medical University of Vienna, Austria (EK 1152/2022). All participants gave written informed consent, and the study was conducted at the Department of Dermatology, Medical University of Vienna, Austria, in accordance with the principles stated in the Declaration of Helsinki, between January 1 and June 30, 2022.
A total of 19 adults with confirmed RP after COVID-19 vaccination were included in this study. Twelve participants resided in countries outside Austria, 6 participants were recruited from the Department of Angiology, Medical University of Vienna, Austria, and follow-up data from the initial patientCitation4 were obtained. The investigated vaccines included the mRNA-based vaccines BNT162b2 (C) (“Comirnaty;” Pfizer-BioNTech, USA/Germany) and mRNA-1273 (S) (“Spikevax;” Moderna Inc., USA) and the adenovirus vector-based vaccine ChAdOx1 (V) (“Vaxzevria;” AstraZeneca, UK/Sweden). Demographic parameters (age, sex, and country of residence), medical history (comorbidities, previous COVID-19 infection and surgeries, body mass index), and detailed information regarding COVID-19 vaccination status and RP characteristics (symptoms, localization, time between vaccination and occurrence of RP, number of attacks) were obtained via questionnaire. Additionally, 10 participants provided information on hematologic, biochemical, coagulation, and immunological blood parameters, and capillary microscopy and optical pulse oscillography results were available for five and six participants, respectively.
Results
Demographic characteristics of the study participants
A total of 19 patients with RP () were included, of whom 79% (15/19) reported the development of RP after and 21% (4/19) a history of preexisting RP prior to COVID-19 vaccination. The demographic data are summarized in .
Figure 1. Representative female participant with new onset of Raynaud´s phenomenon after COVID-19 vaccination. a. Well-demarcated, white-pale, cold fourth toe of the right foot. b. Affected index and third finger of the right hand with sharp demarcation of skin pallor. c. Nailfold capillaroscopic image of the affected third finger of the right hand, showing dilatation, torsion and reduced capillary density, but lack of megacapillaries. d and e. Optical pulse oscillograms of the index (d) and the third finger (e) of the affected right hand (red) and the unaffected left hand (blue-green) revealing regular oscillations.
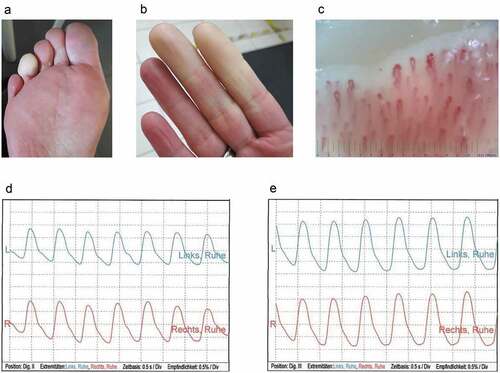
Table 1. Demographic data of 19 individuals with Raynaud’s phenomenon.
Initial manifestation of RP after COVID-19 vaccination
The new onset of RP after COVID-19 vaccination occurred in 15 participants (Patients 1–15). Sixty percent (9/15) were female and 40% (6/15) were male, with a median (range) age of 42 (31–63) years. In total, 42 vaccine doses were administered consisting of 23 doses of C, 11 doses of S, and 8 of V (). 27% (4/15) of the participants received two, 67% (10/15) three, and 7% (1/15) four immunization doses.
Table 2. Main characteristics of 15 individuals with new-onset Raynaud’s phenomenon.
Disease development did not depend upon the number of vaccine doses received, as the initial manifestation of RP was observed in 40% (6/15) after the first, in 33% (5/15) after the second, and in 27% (4/15) after the third immunization. RP attacks continued to be present after subsequent immunizations in the majority of the afflicted participants. However, Patients 7 and 8 reported RP attacks after the first and third, but not after the second vaccination, and in Patients 4 and 6, RP was not observed after booster vaccinations. The typical RP symptoms occurred after 3–21 (median 12) days after the first, 3–180 (median 16) days after the second and 0–112 (median 7) days after the third vaccination dose, and the reported numbers of attacks ranged from 1 to 15 after the first, from 31 to more than 50 after the second, and from 1 to 30 episodes after the third immunization. New onset of disease was observed in 40% (6/15) of the participants after C, in 33% (5/15) after S, and in 27% (4/15) after V vaccine administration. Evaluation of all, primary and booster, vaccinations administered to the participants revealed that RP episodes had been reported after 46% (10/22) of C, after 73% (8/11) of S, and after 88% (7/8) of V immunizations. In most of the participants (67%, 10/15), predisposing factors for RPCitation6 were identified, such as detectable antinuclear (20%, 3/15) or anti-cardiolipin antibodies (7%, 1/15), thyroid disorders (20%, 3/15), smoking (13%, 2/15), concomitant use of beta-blockers (13%, 2/15), or antecedent frostbites (13%, 2/15). Otherwise, timely matching blood results did not reveal grossly abnormal values.
Exacerbation of preexisting RP following COVID-19 vaccination
Preexisting RP was reported by four out of the 19 participants (Patients 16–19). All (4/4) were females with a median age of 52 years, range 25–60 years. In 75% (3/4) of them, predisposing factors for RP were present and comprised smoking, thyroid disorders, a low body mass index, and the use of certain medications (). Notably, none suffered from a concurrent autoimmune or connective tissue disease linked to RP, such as systemic lupus erythematosus or scleroderma. Aggravation in frequency and intensity of the RP attacks after COVID-19 vaccination, including the involvement of previously unaffected fingers (Patient 16), was reported by 75% (3/4) after homologous and heterologous vaccinations with C, S, and V vaccines.
Discussion
To date, only isolated cases of RP in temporal proximity to COVID-19 vaccination have been published.Citation4,Citation7,Citation8 In this case series, 19 individuals with RP occurring after COVID-19 vaccination, of whom 15 negated previous RP attacks, are presented. This case series does not infer a causal relationship between vaccination and the occurrence of RP, however, the temporal relationship seems unsettling. The occurrence of RP was additionally reported after human papillomavirus, hepatitis B, and diphtheria-tetanus vaccination.Citation9–13
The current COVID-19 vaccines were designed to direct human cells to produce the SARS-CoV-2 spike protein, which mediates entry of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into the host cells via its receptor, angiotensin-converting enzyme 2 (ACE2). The introduction of the viral protein into the host's organism in turn stimulates production of neutralizing antibodies and T-cell-mediated immune responses in vaccinated subjects. Despite the indisputable, beneficial effects of COVID-19 vaccination, the presence of the spike protein could nevertheless account for some undesired adverse effects, including RP, in selected individuals. In this regard, circulating spike protein was detected in the blood, and deposition of the spike protein was shown in the microvasculature of the skin of COVID-19 vaccine recipients.Citation14,Citation15 Different scenarios are possible how the vaccine’s spike protein might promote RP. First, the spike protein has been shown to be able to induce a hyperinflammatory state including the release of a pro-inflammatory cytokine storm, subsequently causing immune cells to target and damage endothelium, neurons, perivascular cells, or other parts of the skin's neurovascular system.Citation16,Citation17 Second, the spike protein per se was shown to be able to impair endothelial cell function, which might favor vasoconstrictory events, as observed in RP.Citation18–20 Third, activation of the renin angiotensin system (RAS), caused by the interaction of the spike protein with the ACE2 receptor, could lead to an increase in the sympathetic nervous system tone and subsequent dysregulation of acral perfusion.Citation21 Fourth, the spike protein was shown to influence hemostasis by supporting a procoagulant state.Citation20 Finally, the spike protein might induce cross-reactive anti-viral antibodies via molecular mimicryCitation16,Citation22 that in turn could activate autoimmunological processes against endothelial and nerve cells or favor platelet activation. Although the exact pathogenic mechanisms are not yet known, each aforementioned scenario or a combination thereof raises the possibility that the spike protein may act as an additional trigger in the development of RP.
Primary RP, however, is not a rare disease. An overall pooled prevalence of primary RP in the general population of 4.85% with a mean annual incidence of 0.25% was reported in a meta-analysis comprising more than 33,000 participants, and female gender, a family history of primary RP, migraine, and smoking were associated with disease development.Citation23 Given the high incidence of predisposing factors in our cohort, it is conceivable that some of our participants with new-onset RP could have acquired disease at some point during their lifetime, regardless of COVID-19 vaccination and therefore disease development after vaccination could merely be coincidental. Future, particularly mechanistic as well as large scale, studies are needed to elucidate a potential association between COVID-19 vaccination and RP and to investigate whether COVID-19 vaccination might provide an additional stimulus for an earlier manifestation of RP in cases with predisposition.
The therapeutic options in patients with primary RP do not differ between COVID-19 vaccinated and unvaccinated individuals and include lifestyle changes, such as avoidance of cold exposure and cessation of smoking, and pharmacologic management with calcium channel blockers. COVID-19 vaccination should still be performed to obtain protection against COVID-19 disease, including the risk of severe illness and death.
This study is limited by the small sample size, lack of a control group, and its questionnaire-based design, the latter of which was necessary due to the transnational inclusion of participants. Furthermore, a selection bias may exist as the majority of the study participants consisted of individuals who had read the initial case report.Citation4 However, this case series was not intended to compare the effect of different vaccine types or brands, as valid statistical analyses were hampered by the limited sample size and the binary nature of the findings.
Given the high number of administered COVID-19 vaccines and the recent authorization of adapted vaccine formulations targeting new SARS-CoV-2 subvariants,Citation24 immunization as part of managing the COVID-19 pandemic will remain of pivotal importance. Thus, physicians and patients need to stay alert to adverse effects, one of which may include RP. Global collaborations and support of the pharmacovigilance systems need to be continued and strengthened to ensure the rapid identification and efficient reduction of potential safety risks in order to protect public health and to keep the public’s trust in vaccination.
Acknowledgments
We sincerely thank the patients for their enthusiasm and their substantial contribution to this study. We thank Kim Purkhauser, BSc, for the excellent technical help.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- World Health Organization. WHO coronavirus (COVID-19) dashboard. [accessed 2023 Mar 15]. https://covid19.who.int/.
- Mathieu E, Ritchie H, Ortiz-Ospina E, Roser M, Hasell J, Appel C, Giattino C, Rodés-Guirao L. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5(7):947–5. doi:10.1038/s41562-021-01122-8.
- Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28(2):202–21. doi:10.1016/j.cmi.2021.10.005.
- Urban N, Weber B, Deinsberger J, Gschwandtner M, Bauer W, Handisurya A. Raynaud’s phenomenon after COVID-19 vaccination: causative association, temporal connection, or mere bystander? Case Rep Dermatol. 2021;13(3):450–6. doi:10.1159/000519147.
- Herrick AL, Wigley FM. Raynaud’s phenomenon. Best Pract Res Clin Rheumatol. 2020;34(1):101474. doi:10.1016/j.berh.2019.101474.
- Belch J, Carlizza A, Carpentier PH, Constans J, Khan F, Wautrecht JC, Visona A, Heiss C, Brodeman M, Pécsvárady Z, et al. ESVM guidelines – the diagnosis and management of Raynaud’s phenomenon. Vasa. 2017;46(6):413–23. doi:10.1024/0301-1526/a000661.
- Freeman EE, Sun Q, McMahon DE, Singh R, Fathy R, Tyagi A, Blumenthal K, Hruza GJ, French LE, Fox LP. Skin reactions to COVID-19 vaccines: an American Academy of Dermatology/International League of Dermatological Societies Registry update on reaction location and COVID vaccine type. J Am Acad Dermatol. 2022;86(4):e165–7. doi:10.1016/j.jaad.2021.11.016.
- Pham-Viet T, Thai-Phuong P, Bui-Viet T, Nguyen-Thi-Ngoc A, Tran-Quoc H, Tran-Phuc L, Duong-Quy S. Subacute Raynaud's syndrome associated with a rare adverse drug reaction of Vaxzevia - AstraZeneca COVID‐19 vaccine: a case report. J Func Vent Pulm. 2021;37(12):1–84. doi:10.12699/jfvpulm.12.37.2021.80.
- Tomljenovic L, Colafrancesco S, Perricone C, Shoenfeld Y. Postural orthostatic tachycardia with chronic fatigue after HPV vaccination as part of the “Autoimmune/auto-inflammatory syndrome induced by adjuvants”: case report and literature review. J Investig Med High Impact Case Rep. 2014;2(1):2324709614527812. doi:10.1177/2324709614527812.
- Bouquet É, Urbanski G, Lavigne C, Lainé-Cessac P. Unexpected drug-induced Raynaud phenomenon: analysis from the French national pharmacovigilance database. Therapie. 2017;72(5):547–54. doi:10.1016/j.therap.2017.01.008.
- Hviid A, Svanström H, Scheller NM, Grönlund O, Pasternak B, Arnheim-Dahlström L. Human papillomavirus vaccination of adult women and risk of autoimmune and neurological diseases. J Intern Med. 2018;283(2):154–65. doi:10.1111/joim.12694.
- Selvaraj V, Ogunneye O, Lagu T, Ryzewicz S. A rare case of Raynaud’s vasculitis secondary to hepatitis B vaccination: the induced auto-immune attack syndrome. Case Rep Int Med. 2014;1(1):17–20. doi:10.5430/crim.v1n1p17.
- Aksay A, Düzgöl M, Bayram N, Devrim I. A Raynaud phenomonic attack induced after an adult diphtheria-tetanus vaccine. Haydarpasa Numune Med J. 2019;59(3):296–8. doi:10.14744/hnhj.2018.04274.
- Ogata AF, Cheng CA, Desjardins M, Senussi Y, Sherman AC, Powell M, Novack L, Von S, Li X, Baden LR, et al. Circulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine antigen detected in the plasma of mRNA-1273 vaccine recipients. Clin Infect Dis. 2022;74(4):715–8. doi:10.1093/cid/ciab465.
- Magro C, Crowson AN, Franks L, Schaffer PR, Whelan P, Nuovo G. The histologic and molecular correlates of COVID-19 vaccine-induced changes in the skin. Clin Dermatol. 2021;39(6):966–84. doi:10.1016/j.clindermatol.2021.07.011.
- Trougakos IP, Terpos E, Alexopoulos H, Politou M, Paraskevis D, Scorilas A, Kastritis E, Andreakos E, Dimopoulos MA. Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Trends Mol Med. 2022;28(7):542–54. doi:10.1016/j.molmed.2022.04.007.
- Ostrowski SR, Søgaard OS, Tolstrup M, Stærke NB, Lundgren J, Østergaard L, Hvas AM. Inflammation and platelet activation after COVID-19 vaccines - possible mechanisms behind vaccine-induced immune thrombocytopenia and thrombosis. Front Immunol. 2021;12:779453. doi:10.3389/fimmu.2021.779453.
- Lei Y, Zhang J, Schiavon CR, He M, Chen L, Shen H, Zhang Y, Yin Q, Cho Y, Andrade L, et al. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2. Circ Res. 2021;128(9):1323–6. doi:10.1161/CIRCRESAHA.121.318902.
- Terentes-Printzios D, Gardikioti V, Solomou E, Emmanouil E, Gourgouli I, Xydis P, Christopoulou G, Georgakopoulos C, Dima I, Miliou A, et al. The effect of an mRNA vaccine against COVID-19 on endothelial function and arterial stiffness. Hypertens Res. 2022;45(5):846–55. doi:10.1038/s41440-022-00876-6.
- de Laat B, Stragier H, de Laat-Kremers R, Ninivaggi M, Mesotten D, Thiessen S, Van Pelt K, Roest M, Penders J, Vanelderen P, et al. Population-wide persistent hemostatic changes after vaccination with ChAdOx1-S. Front Cardiovasc Med. 2022;9:966028. doi:10.3389/fcvm.2022.966028.
- Angeli F, Reboldi G, Trapasso M, Zappa M, Spanevello A, Verdecchia P. COVID-19, vaccines and deficiency of ACE2 and other angiotensinases. Closing the loop on the “spike effect”. Eur J Intern Med. 2022;103:23–8. doi:10.1016/j.ejim.2022.06.015.
- Nunez-Castilla J, Stebliankin V, Baral P, Balbin CA, Sobhan M, Cickovski T, Mondal AM, Narasimhan G, Chapagain P, Mathee K, et al. Potential autoimmunity resulting from molecular mimicry between SARS-CoV-2 spike and human proteins. Viruses. 2022;14(7):1415. doi:10.3390/v14071415.
- Garner R, Kumari R, Lanyon P, Doherty M, Zhang W. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: systematic review and meta-analysis of observational studies. BMJ Open. 2015;5(3):e006389. doi:10.1136/bmjopen-2014-006389.
- U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes Moderna, Pfizer-bioNTech bivalent COVID-19 vaccines for use as a booster dose. [accessed 2023 Feb 8]. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use.
