ABSTRACT
Immunization is one of the most cost-effective measures to prevent morbidity and mortality in children. Therefore, the purpose of this systematic review and meta-analysis was to determine the pooled prevalence of incomplete immunization among children in Africa as well as its determinants. PubMed, Google Scholar, Scopus, Science Direct, and online institutional repository homes were searched. Studies published within English language, with full text available for searching, and studies conducted in Africa were included in this meta-analysis. A pooled prevalence, Sub-group analysis, sensitivity analysis and meta-regression were conducted. Out of 1305 studies assessed, 26 met our criteria and were included in this study. The pooled prevalence of incomplete immunization was 35.5% (95% CI: 24.4, 42.7), I2 = 92.1%). Home birth (AOR=2.7; 95% CI: 1.5–4.9), rural residence (AOR = 4.6; 95% CI: 1.1–20.1), lack of antenatal care visit (AOR = 2.6; 95% CI: 1.4–5.1), lack of knowledge of immunizations (AOR=2.4; 95% CI: 1.3–4.6), and maternal illiteracy (AOR = 1.7: 95%CI: 1.3–2.0) were associated with incomplete immunization. In Africa, the prevalence of incomplete immunization is high. It is important to promote urban residency, knowledge of immunization and antenatal follow up care.
Introduction
Immunization involves the administration of a vaccine to reach immunity against an infectious agent, and especially for preventing the disease caused by the agent.Citation1,Citation2 It is one of the most cost-effective public health measures to reduce child morbidity and mortality.Citation3 Measles vaccination has been estimated to have prevented~23 million deaths during 2010–8.Citation4 More than half of infant and young child deaths are related to illnesses that could be readily avoided by taking simple, inexpensive measures, such as receiving vaccines.Citation5 Due to inadequate and low vaccination coverage, vaccine-preventable diseases continue to cause a public health risk in South-East Asia and sub-Saharan Africa.Citation6
A child is considered to have an incomplete immunization status if they failed to receive at least one of the recommended childhood vaccinations, which include BCG, three doses of the pentavalent vaccine, three doses of the PCV vaccine, two doses of the Rotavirus vaccine, and three doses of the polio and measles vaccines by the age of 12 months.Citation7 Globally, it has been estimated that 9 million children under the age of five die annually from vaccine-preventable diseases.Citation8 Infectious childhood diseases like measles, pertussis, diphtheria, tetanus, TB, meningitis, and tuberculosis in children can be prevented most effectively with vaccination.Citation9 Two-thirds of children under five now have a lower mortality risk because to vaccinations against infectious diseases.Citation10 An estimated 1–2 million under-five child deaths are prevented each year by routine childhood vaccinations (BCG, pentavalent, polio, and measles).Citation11,Citation12
The incidence of mortality among children under the age of five substantially decreased from 12.6 million in 1990 to 5.3 million in 2018Citation13,Citation14 when the EPI program was introduced. Despite a significant drops in the prevalence of vaccine-preventable deaths, a significant portion of children are not fully vaccinated, which results in substantial regional and international variation in vaccination coverage.Citation15,Citation16 For example, in 2017, ~83K measles-related fatalities were tabulated worldwide out of an estimated 17 million cases.Citation17,Citation18 Worldwide~116 million newborns (86%) received full 3 doses of the diphtheria-tetanus-pertussis (DTP) vaccine in 2018.Citation19,Citation20 According to reports from the WHO and UNICEF, more than 20 million children globally have not completed a full course of basic vaccinations.Citation21 In Africa, almost 10 million children are still either partially or completely unvaccinated.Citation22,Citation23
In accordance with the new Sustainable Development Goals (SDGs), all nations must work toward lowering neonatal mortality to at least 12 per 1,000 live births and under-5 mortality to at least 25 per 1,000 live births by 2030.Citation13 By 2030, every child should have received all recommended vaccinations, regardless of location, age, socioeconomic position, or gender-related barriers, according to the WHO’s global strategy known as the Immunization Agenda 2030. As a result, the WHO launched the Expanded Program on Immunization (EPI) in 1974 to control diseases that can be prevented by vaccination.Citation24 Incidence of under-five deaths remained 5.3 million in 2018 after the EPI program’s inception.Citation13,Citation14
Evidence suggested that a number of variables contribute to the incomplete vaccination coverage in developing countries. Incomplete vaccination has been linked to a number of factors, including birth order,Citation7,Citation25 maternal age,Citation7,Citation25,Citation26 distance from a health facility,Citation25,Citation27 maternal educational status,Citation25–29 antenatal care visit,Citation25,Citation30 postnatal care (PNC) visitCitation30 and delivery site.Citation18,Citation30,Citation31
The percentage of children in Africa who did not obtain all of the required vaccines ranges greatly, from Sudan (5.7%) to Nigeria (76.3%).Citation32,Citation33 Despite multiple primary studies that demonstrate the prevalence of incomplete immunization across Africa, there are no pooled data at the regional level. The relevant factors that have been found differ between studies as well. Therefore, the goal of this systematic review and meta-analysis study was to identify the prevalence of incomplete immunization among children in Africa and its determinants. Clinicians and other stakeholders will be able to address gaps in immunization coverage by prioritizing and customizing immunization campaigns and operational plans based on the study’s findings, which will provide them with the fundamental knowledge they need to provide every child with a life-saving vaccination.
Methods
Reporting
This systematic review and meta-analysis study was conducted to determine incomplete immunization and its determinant among children in Africa using the standard PRISMA checklist guidelineCitation34 (Additional 1). It was carried out by conducting a thorough synthesis of the relevant primary studies on children’s incomplete immunizations in Africa. The protocol was registered at CRD42022348643.
Search strategy
International online databases (Pub Med, Science Direct, Scopus, and Google Scholar) were used to search articles on the prevalence of incomplete immunization and determinant’s in Africa. We also retrieved gray literature from Addis Ababa University, Bahir Dar University, Georgia State University, University of Ghana and University of Western Cape online research institutional repository. The search string was established using “AND” and “OR” Boolean operators. The following core search terms and phrases with Boolean operators were used to search related articles: ((((Incomplete) OR (“Incomplete” OR “partially”)) AND Immunization) OR (“Immunization” OR “Vaccine” OR “Vaccination” “Immunization”)) AND Children) OR (“Child” OR “Childhood” OR “Baby”) AND Africa. Articles published from May 16, 2008, to May 2, 2022, were included in the search period, which ran from September 1, 2022, to October 10, 2022.
Outcome measurement
Incomplete immunization
Receiving one dose of BCG, three doses of pentavalent, three doses of polio, and one shot of measles within the first year of life indicates complete immunization, according to the World Health Organization.Citation35 If a child does not receive all 8 doses of these vaccines before turning 12 months old, they were considered to be only incompletely vaccinated. However, we found that studies reporting the same results in children under the age of 5 were included in the analysis.
Inclusion and exclusion criteria
Only English language papers, including peer-reviewed (scientific) literature versus non peer-reviewed (gray) literature with full text available for search, adjusted odds ratio for factors and studies that took place in Africa were included in blind screening in this meta-analysis. This systematic review and meta-analysis excluded research that used duplicated sources, qualitative studies from countries outside Africa, and articles without full text.
Quality assessment
Two authors (NAT and KDG) independently appraised the standard of the studies using the Joanna Briggs Institute (JBI) standardized quality appraisal checklist.Citation36 The disagreement raised during the quality assessment was resolved through a discussion led by the third author (GAA). Finally, the argument was solved and reached with an agreement. The critical analysis checklist has eight parameters with yes, no, unclear, and not applicable options (Additional file 2).
Risk of bias assessment
Two authors (NAG and BAA) independently assessed included studies for risk of bias through the bias assessment tool developed by Hoy et al.,Citation37 consisting of ten items that assess four domains of bias and internal and external validity. Any disagreement raised during the risk of bias assessment was resolved through a discussion led by the third author (KAG). Finally, the argument was solved and reached with an agreement. The first four items (items 1–4) evaluate the presence of selection bias, non-response bias, and external validity. The other six items (items 5–10) assess the presence of measuring the bias, analysis-related bias, and internal validity. Therefore, studies that received ‘yes’ for eight or more of the ten questions were classified as ‘low risk of bias.’ If studies that received ‘yes’ for six to seven of the ten questions were classified as ’moderate risk’ whereas studies that received ‘yes’ for five or fewer of the ten questions were classified as ‘high risk’ as reported in a supplementary file (Additional file 3).
Data extraction
Microsoft Excel spreadsheet (2016) and STATA version 11 software were utilized for data extraction and analysis. Two authors (NAG and KDT) independently extracted all relevant data using a standardized Joanna Briggs Institute data extraction format. Any disagreement raised during data extraction was resolved through a discussion led by the third author (BAA). Finally, the argument was solved and reached with an agreement. The name of the first author, year of publication, age of target population, study region, study setting, study design, the prevalence of incomplete immunization, sample size, and quality of each paper was extracted.
Data analysis
After extracting all relevant findings in a micro-soft excel spreadsheet, the data were exported to STATA software version 14 for analysis. The pooled prevalence of incomplete immunization was computed using a 95% confidence interval. Publication bias was checked by funnel plot and more objectively through Begg and Egger’s regression tests, with P < .05 indicating potential publication bias. The presence of between-study heterogeneity was checked by using the Cochrane Q statistic. This heterogeneity between studies was quantified using I2, in which a value of 0, 25, 50, and 75% represented no, low, medium, and high heterogeneity, respectively. A forest plot was used to visually assess the presence of heterogeneity, which presented at a high-level random-effect model was used for analysis to estimate the pooled estimate of incomplete immunization. Subgroup analysis was done by sample size, publication, study sub-region and age of target population. Sensitivity analysis was executed to see the effect of a single study on the overall prevalence of the meta-analysis estimate. The findings of the study were presented in the form of text, tables, and figures
Results
Search findings and study characteristics
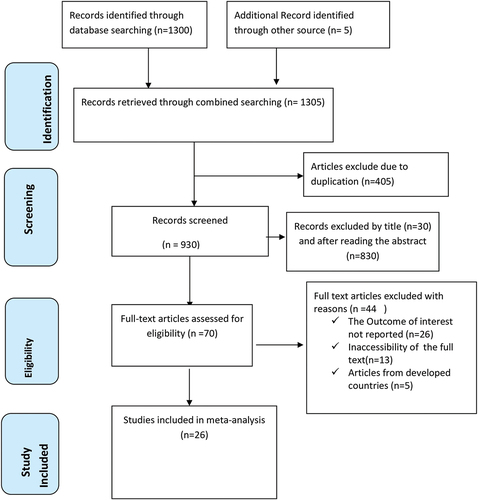
One thousand three hundred five articles were retrieved using a search strategy about incomplete immunization in Africa through online search engines. After duplicates were removed, 930 articles remained. Then, 860 studies were excluded after reviewing for full title and abstracts from the remaining 930 studies. Therefore, 70 full-text studies were assessed for eligibility criteria, which further excluded 44 studies due to reasons. Finally, 26 articlesCitation25,Citation27–29,Citation32,Citation33,Citation38–56 were included as criteria for this systematic review and meta-analysis study ().
Figure 1. A Prisma diagrammatic presentation used to show the selection of studies. The inclusion criteria were variation of the title and abstracts, place of study (Africa), presence of full abstract, and reporting different results. Studies were excluded if they criteria were duplicated source, studies from other countries and qualitative studies.
All included studies were employed by cross-sectional study design. Two of these were cross-sectional studies conducted at institutions, while the remaining 24 studies were community-based. Six studies conducted in Ethiopia,Citation38–43 five in Nigeria,Citation33,Citation44–47 three in Togo,Citation27,Citation29,Citation48 two in Malawi,Citation49,Citation50 one study in each of Uganda,Citation51 Gambia,Citation52 Cote d'ivoire,Citation53 Congo,Citation54 Mozambique,Citation28 Cameroon,Citation25 Mali,Citation55 Ghana,Citation56 South AfricaCitation57 and Sudan.Citation32 The sample sizes ranged from 213 to 14,593. The prevalence of incomplete vaccination ranged from 5.6% to 76.3%. All studies were assessed by using Joanna Briggs Institute (JBI) quality appraisal checklist and yielded low risk ().
Table 1. Characteristics of the included studies in the systematic review and meta-analysis for the prevalence of incomplete immunization in Africa.
Meta-analysis
Prevalence of incomplete immunization among children in Africa
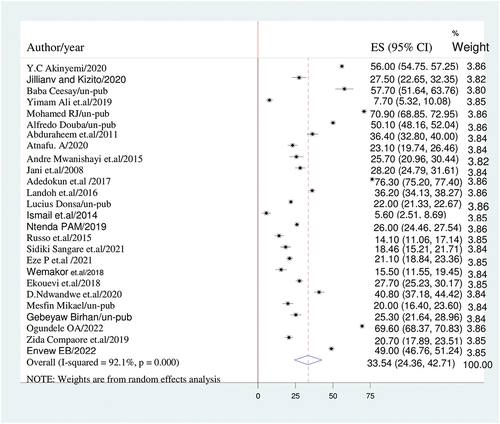
A DerSimonian and Laird random-effects model was used to determine the overall estimate of incomplete immunization. Accordingly, the regional pooled prevalence of incomplete vaccination coverage among children with a random-effects model was 35.5% (95% CI: 24.4, 42.7) with a heterogeneity index (I2) of 92.1% (p < .001) ().
Figure 2. The forest plot of incomplete immunization with the diamond represents the summary point estimate (35.5%) and the horizontal extremity of the diamond is the confidence interval at 95% (24.4–42.7). the standard error is plotted at the y-axis and the effect size plotted at x-axis. The squares represent the effect estimate of the individual studies and the horizontal lines indicate the confidence interval; the dimension of the square reflects the weight of each study.
Subgroup analysis
Since this meta-analysis showed a marked heterogeneity, subgroup analysis was done using sub-region where the studies were conducted, sample size, publication and age of children. Based on this, the highest (42.5%; 95% CI: 28.2, 52.8), I2 = 84.2%) and the lowest (19.8%; 95% CI: 8.4, 31.1), I2 = 93.9%) prevalence of incomplete immunization among children was observed in Western Africa region and Central Africa region, respectively The pooled prevalence for published research was (41%; 95% CI: 21.6–60.3); I2 = 79.3; while for unpublished studies, it was (31.3; 95% CI: 20.5, 42.1); I2 = 80.9%.
Regarding children’s ages, the highest prevalence of incomplete immunization was reported in children under the age of five (43.6; 95% CI: 23.6, 63.5); I2 = 68.8%; and the lowest prevalence was reported in children under the age of two (26.2; 95% CI: 18.5, 33.9; I2 = 95.7%). While the prevalence for samples with a sample size of less than 1000 was (24.3; 95%CI: 18.4, 30.2), I2 = 93.2%, the prevalence for samples with a sample size of more than 1000 was (45.9%; 95% CI: 31.5, 60.4), I2 = 91.7% ().
Table 2. The overall estimated of incomplete immunization in Africa, 95%CI and heterogeneity estimate with a p-value for sub-group analysis.
Heterogeneity and publication bias
To adjust the reported heterogeneity of this study (I2 = 92.1%), we computed a Sub-group analysis based on the sub-region of Africa, sample size, age of target population and publication. Univar ate meta-regression was also done to identify the source of heterogeneity using sample size and age of children as a covariate. It showed that there was an effect of sample size and age of children on heterogeneity between studies ().
Table 3. Meta-regression analysis of factors affecting between-study heterogeneity.
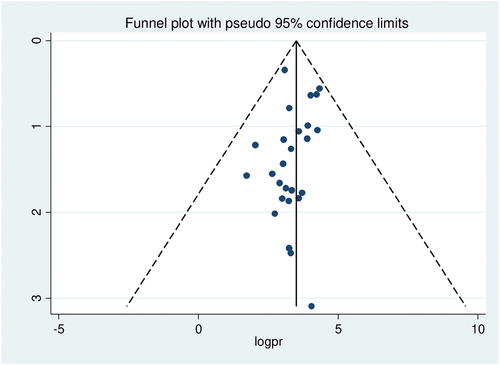
The presence of publication bias was assessed by funnel plot visually and by Egger’s test and Begg’s test objectively. The funnel plot shows asymmetrical distribution of studies by visual inspection (). Therefore, the presence of publication bias was also assessed by Egger’s regression test (p = .4) and Begg’s rank correlation test (p = .8) with no evidence of publication bias.
Figure 3. Funnel plot showing symmetrical distribution of studies indicating absence of publication bias. The Y-axis is the standard error and the X-axis is the study result or effect size. The dotted diagonal line of the funnel is the 95% confidence interval and the vertical. The vertical line denotes the no effect. The square represents the effect size of each study and the line across the square is confidence interval of each study.
Leave – one-out-sensitivity analysis
A leave-one-out sensitivity analysis was carried out to detect the effect of each study on the overall prevalence of incomplete immunization by excluding one study at a time. As a result, studies omitted at a time did not show a significant change on the overall prevalence of incomplete immunization ().
Table 4. The pooled prevalence of incomplete immunization in Africa when one study omitted from the analysis a step at a time.
Factors associated with incomplete immunization in Africa
In this meta-analysis, lack of knowledge, place of delivery, no antenatal care visit, rural residency and maternal illiteracy were significantly associated with incomplete vaccination. However, not attending postnatal care visit was not significantly associated with incomplete immunization.
Postnatal care
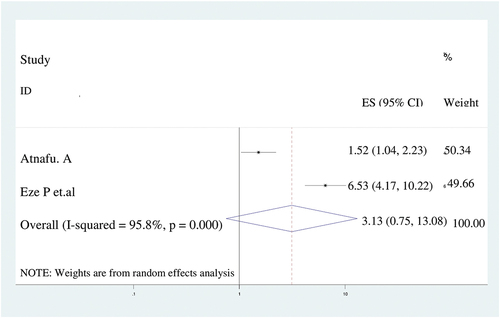
As evidenced in this study, postnatal care was not significantly associated with incomplete immunization. A random effect model was used because of the presence of significant heterogeneity (I2 = 95.8%) ().
Figure 4. The forest plot of pooled odds ratios showed no association between postnatal care visit and incomplete immunization with the height of the diamond is the overall effect size (3.1) while the width is the confidence interval at 95% (0.8–13.1). The y-axis shows the standard error of each study while the x-axis the estimate of effect size of the each study. The vertical line denotes the no effect. The square represents the effect size of each study and the line across the square is confidence interval of each study.
Place of delivery
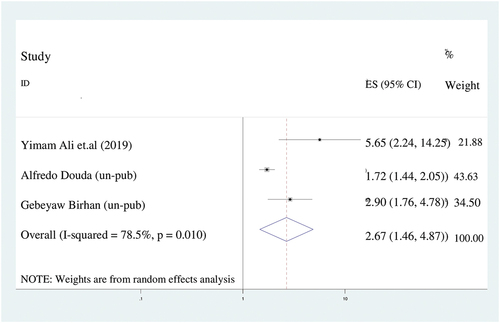
In this meta-analysis, women who gave birth at home were 2.7 times more likely to have incompletely immunized children (AOR = 2.7; 95%CI: 1.5–4.9) than women who delivered at health facility. We utilized random effect model for meta-analysis due to I2 was 78.5% ().
Figure 5. The forest plot of pooled odds ratios showed the association between place of delivery and incomplete immunization with the height of the diamond is the overall effect size (2.7) while the width is the confidence interval at 95% (1.5–4.9). The y-axis shows the standard error of each study while the x-axis the estimate of effect size of the each study. The vertical line denotes the no effect. The square represents the effect size of each study and the line across the square is confidence interval of each study.
Antenatal care
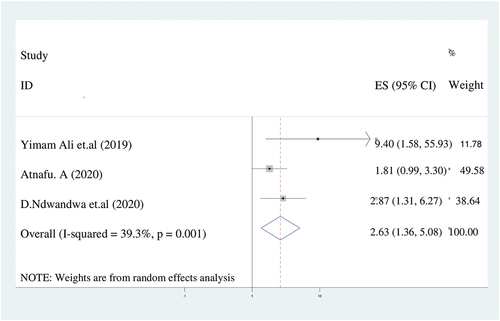
According to this meta-analysis, women who had no antenatal care visit were 2.6 times more likely to have incompletely immunized children (AOR = 2.6; 95%CI: 1.4–5.1) than their counterparts. We analyzed by using random effect model because the value of I2 was 39.3% ().
Figure 6. The forest plot of pooled odds ratios showed the association between lack of antenatal care and incomplete immunization with the height of the diamond is the overall effect size (2.6) while the width is the confidence interval at 95% (1.4–5.1). The y-axis shows the standard error of each study while the x-axis the estimate of effect size of the each study. The vertical line denotes the no effect. The square represents the effect size of each study and the line across the square is confidence interval of each study.
Rural residency
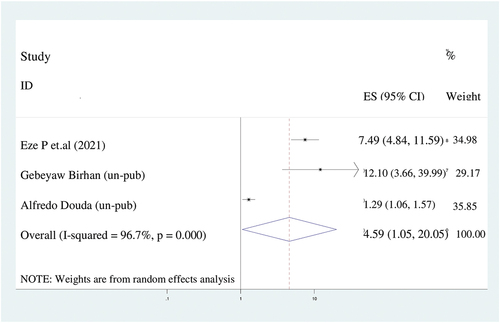
There were a significant association between place of residency and incomplete immunization. The odds of having incompletely immunized children among rural women was 4.6 times higher (AOR = 4.6; 95%CI: 1.5–20.1) than urban women. In spite of the presence of significant heterogeneity (I2 = 96.7%), We used random effect model ().
Figure 7. The forest plot of pooled odds ratios showed the association between rural residence and incomplete immunization with the height of the diamond is the overall effect size (4.6) while the width is the confidence interval at 95% (1.1–20.1). The y-axis shows the standard error of each study while the x-axis the estimate of effect size of the each study. The vertical line denotes the no effect. The square represents the effect size of each study and the line across the square is confidence interval of each study.
Maternal illiteracy
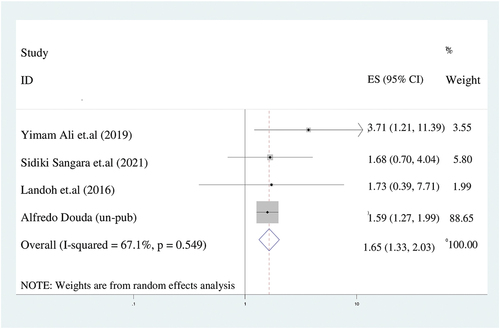
In our study, maternal educational profile affects the incomplete immunization. Mothers who had no formal education were 1.6 times more likely to have incompletely immunized children (AOR = 1.7:95%CI: 1.3–2.0) compared to mothers who had high school and above education. Since there was substantial heterogeneity (I2 = 67.1%), We used the random effect model ().
Figure 8. The forest plot of pooled odds ratios showed the association between maternal illiteracy and incomplete immunization with the height of the diamond is the overall effect size (1.7) while the width is the confidence interval at 95% (1.3–2). The y-axis shows the standard error of each study while the x-axis the estimate of effect size of the each study. The vertical line denotes the no effect. The square represents the effect size of each study and the line across the square is confidence interval of each study.
Maternal knowledge
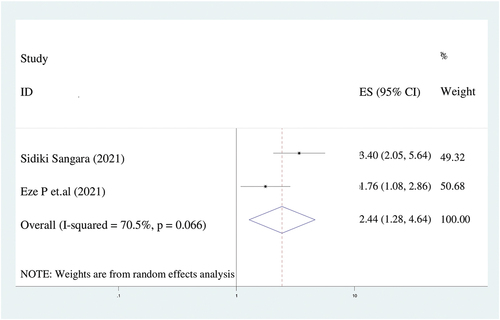
In this analysis, women who had poor knowledge of child vaccination were 2.4 times more likely to have incompletely immunized children (AOR = 2.4; 95%CI: 1.3–4.6) than their counterparts (). We assumed a random effect model for the analysis due to the presence of heterogeneity (I2 = 70.5%).
Figure 9. The forest plot of pooled odds ratios showed the association between lack of knowledge of immunization and incomplete immunization with the height of the diamond is the overall effect size (2.4) while the width is the confidence interval at 95% (1.3–4.6). The y-axis shows the standard error of each study while the x-axis the estimate of effect size of the each study. The vertical line denotes the no effect. The square represents the effect size of each study and the line across the square is confidence interval of each study.
Discussion
Incomplete immunization and its determinants among children in Africa were the targets of this systematic review and meta-analysis. The key findings were that the national pooled estimate of incomplete immunization and incomplete immunization were significantly associated with lack of knowledge, place of delivery, no antenatal care visit, rural residency and maternal illiteracy. Consequently, the pooled prevalence of incomplete immunization in this study was 35.5% (95% CI: 24.4, 42.7), which is consistent with research from India (32%),Citation58 Aurangabad (37%),Citation59 the global coverage of vaccinations in 2017 (30%),Citation60 and Ethiopia’s meta-analysis study (30%).Citation61
The pooled prevalence of incomplete immunization found in the current study is higher than those studies done in Brazil (10.9%),Citation62 Australia (20%)Citation63 and Myanmar (25.8%).Citation64 In contrast, the result of the current study is lower than research conducted in Indonesia (40%)Citation65 and Pakistan (46%).Citation66 The disparity may result from differences in the time gaps between the studies, infrastructure of the health system, and sociocultural factors. For example, countries with huge financial power and better health system structure have lowest rate of incomplete immunization.
The age of the target population, sample size, publication, and study sub-region were taken into account when performing sub-group analysis. As a result, West Africa and Central Africa had the highest (42.5%) and lowest (19.8%) prevalence of incomplete immunization among children, respectively. Children under the age of 5 had the highest prevalence of incomplete immunization (43.6%) while children under the age of 2 had the lowest prevalence (26.2%). The variation might be because of the difference in sample size and number of included studies in this meta-analysis. For example, twelve studies were included in West Africa whereas two studies were used in Central Africa. For published studies and unpublished studies, the prevalence of incomplete immunization was 41% and 31.3%, respectively. This might be because studies with statistically significant results aren’t inflated and effect size estimates are less precise than when gray literature is included in the meta-analysis.
In this meta-analysis, place of delivery, no antenatal care visit, poor maternal knowledge of immunization, rural residency and maternal illiteracy were factors significantly associated with incomplete vaccination. Compared to mothers who gave birth in a healthcare facility, mothers who gave birth at home were 2.7 times more likely to have children who had not received all recommended vaccinations. This result is congruent with research from the Ethiopia,Citation61 Pakistan,Citation67 Philippines,Citation68 a global systematic review,Citation69 as well as studies from low- and middle-income nations.Citation70 The cause may be that women who gave birth at home did not receive enough child health education and counseling, which has a negative impact on the immunization travel schedule completion
The current study found a positive association between place of residency and incomplete immunization. Incomplete immunization rates were 4.6 times higher among mothers who resided in rural areas. This is in line with a study conducted in Ethiopia that involved a systematic review and meta-analysis.Citation61 The explanation might come from the fact that rural women lack better knowledge of the value of immunization. However, studies conducted in Sub-Saharan Africa and India found that urban women were more likely than rural women to have children who had only had a partial immunization. This result is in contradiction to those findings.Citation58,Citation71 The difference could be caused by the existence of impoverished children who live in urban slums with little access to vaccination programs.
Mothers who did not receive antenatal care were 2.6 times more likely than women who did to have children who were just partially vaccinated. This outcome is consistent with research carried out in Ethiopia.Citation61 This is because prenatal care gives opportunities for mother and healthcare professional to communicate about the advantages of childhood immunization, which motivates parents to thoroughly immunize their children.
Women with no education had a 1.6 times greater chance of their children receiving only a partial immunization than those with secondary education or higher. The finding is in agreement with research from India,Citation58 Ethiopia,Citation61 Indonesia,Citation65 Pakistan,Citation66,Citation67 a systematic review of low- and middle-income countries,Citation70 and studies in Sub-Saharan AfricaCitation71 This might be due to the fact that uneducated women are not aware of the value of immunization, are resistant to change and stiff to new ideas, and find it difficult to make decisions on their health, including immunization.
Women who were less knowledgeable about child immunization were 4.6 times less likely than their counterparts to have provided their children the vaccines. This is supported by the fact that vaccinations and understanding about children’s health have gained widespread acceptance.Citation72
To handle a large variance that occurred in between-study heterogeneity, a random-effect model was used in this research. We conducted leave-one-out sensitivity, and the results reveal that no single study had a substantial effect on the overall prevalence of incomplete immunization. We assessed the possible variability source via sub-group analysis using the study sub-region, sample size, publication and age of children. The high heterogeneity might be due to differences in the sample populations, paper qualities, or socio-cultural, ethnic, and regional differences.
Conclusion
In conclusion, there was a high rate of incomplete immunization in Africa (35.5%). Additionally, the prevalence of incomplete immunization differed by study sub-region, sample size, publication, and child age. Home birth, no antenatal visits, lack of immunization knowledge, maternal illiteracy, and rural residency were factors for incomplete immunization. The prevalence of incomplete immunization was highest in the Western region of Africa. It has been recommended that women have access to maternal education and maternal health services, such as prenatal care visits and institutional delivery services. To reach rural areas, it’s also a good idea to organize a sizable campaign and engage the local population.
Strength and limitation of study
This study has some limitations. First, articles were restricted to only being published in the English language. Second, all of the included studies were cross-sectional, which might affect the outcome variable because of other confounding factors.
This research has some strength. First, compressive electronic online international searching engines were used. Second, our review incorporated gray literature as part of the primary studies.
Third, prevalence incomplete immunization predictors were discovered.
Authors’ contributions
NAG conceptualized the study: NAG and KDT contributed during data extraction and analysis: NAG and KAG wrote result interpretation: NAG and KDT, Prepared the first draft: NAG, KDT, GAA, MMG and BAA contributed during the conceptualization and interpretation of results and substantial revision: NAG, KAG, KDT, GAA, MMG and BAA. Revised and finalized the final draft manuscript. All the authors read and approved the final version of the manuscript.
Authors’ information
NAG: School of Midwifery, College of Medicine and Health Sciences, Wolaita Sodo University, Sodo, Ethiopia. KAG: School of Midwifery, College of Medicine and Health Sciences, Wolaita Sodo University, Sodo, Ethiopia. KDT: Department of Comprehensive Nursing, College of Medicine and Health Science, Wollo University, Dessie, Ethiopia. GAA: Department of Reproductive Healths, College of Medicine and Health Science, Wolaita Sodo University, Sodo, Ethiopia. BAA: Department of Anesthesia, College of Medicine and Health Science, Gondar University, Gondar, Ethiopia. MMG: Department of Midwifery, College of Medicine and Health Science, Wolaita Sodo University, Sodo, Ethiopia.
Supplemental Material
Download Zip (627.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability statement
All relevant data are within the Manuscript and its Supporting Information files.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2202125.
Additional information
Funding
References
- World Health Organization. Immunization coverage. Fact sheets; 2020. https://wwwS.who.int/news-room/fact-sheets/detail/immunization-coverage.
- United Nations Children Fund (UNICEF). Immunization. UNICEF data: monitoring the situation of children and women. 2020. https://data.unicef.org/topic/child-health/immunization/.
- Subaiya SDL, Lydon P, Gacic-Dobo M, Eggers R, Conklin L. Global routine vaccination coverage. Morb Mortal Wkly Rep. 2014;64:1–12. doi:10.15585/mmwr.mm6444a5.
- Patel MK, Dumolard L, Nedelec Y, Sodha SV, Steulet C, Gacic-Dobo M, Kretsinger K, McFarland J, Rota PA, Goodson JL. Progress toward regional measles elimination — worldwide, 2000–2018. Morb Mortal Wkly Rep. 2019;68:1105–11. doi:10.15585/mmwr.mm6848a1.
- World Health Organization (WHO). National immunization coverage score cards estimates for 2018 [internet]. World Health Organization; 2018 [accessed 2020 May 2]. http://www.who.int/immunization/monitoring_surveillance/data/en
- Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–87. doi:10.1016/S0140-6736(10)60549-1.
- Negussie Abel A, Sahilu W. Factors associated with incomplete childhood immunization in Arbegona district, southern Ethiopia: a case – control study. BMC Public Health. 2016;16. doi:10.1186/s12889-015-2678-1.
- Services USDoHaH. The state of the national vaccine plan 2013 annual report, protecting the nation’s health through immunization. 2013.
- Greenwood B. The contribution of vaccination to global health: past, present and future. Phil Trans Royal Soc B. 2014;369(1645):20130433. doi:10.1098/rstb.2013.0433.
- Olusegun OL, Ibe RT, Micheal IM. Curbing maternal and child mortality: the Nigerian experience. Int J Nurs Midwifery. 2012;4(3):33–9. doi:10.5897/IJNM11.030.
- Oliwa JN, Marais BJ. Vaccines to prevent pneumonia in children–a developing country perspective. Paediatr Respir Rev. 2017;22:23–30. doi:10.1016/j.prrv.2015.08.004.
- Whitney CG, Zhou F, Singleton J, Schuchat A. Benefits from immunization during the vaccines for children program era—United States, 1994–2013. Morb Mortal Wkly Rep. 2014;63:352.
- Neonatal, infant, and under-5 mortality and morbidity burden in the Eastern Mediterranean region: findings from the global burden of disease 2015 study. Int J Public Health. 2018;63: 63–77. doi:10.1007/s00038-017-0998-x.
- Organization WH. Levels and trends in child mortality: report 2019. The World Bank; 2019.
- Clark A, Sanderson C. Timing of children’s vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet. 2009;373:1543–9. doi:10.1016/S0140-6736(09)60317-2.
- Rainey JJ, Watkins M, Ryman TK, Sandhu P, Bo A, Banerjee K. Reasons related to non-vaccination and under-vaccination of children in low and middle-income countries: findings from a systematic review of the published literature, 1999–2009. Vaccine. 2011;29:8215–21. doi:10.1016/j.vaccine.2011.08.096.
- Larson HJ, de Figueiredo A, Xiahong Z, Schulz WS, Verger P, Johnston IG, Cook AR, Jones NS. The state of vaccine confidence 2016: global insights through a 67-country survey. EBioMedicine. 2016;12:295–301. doi:10.1016/j.ebiom.2016.08.042.
- Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1736–88. doi:10.1016/S0140-6736(18)32203-7.
- World Health Organization (WHO). Immunization coverage. Fact sheet. WHO; 2019.1.
- World Health Organization (WHO). Annex to the global vaccine action plan review and lessons learned report [Internet]. World Health Organization; 2018. [accessed 2020 May 18]. http://apps.who.int/bookorders.
- World Health Organization (WHO). Immunization agenda 2030: a global strategy to leave no one behind. WHO. 2019:1–29. https://www.who.int/publications/m/item/immunization-agenda-2030-a-globalstrategy-to-leave-no-one-behind.
- Madhi SA, Rees H. Special focus on challenges and opportunities for the development and use of vaccines in Africa. Hum Vaccines Immunother. 2018;14:2335–9. doi:10.1080/21645515.2018.1522921.
- World Health Organization (WHO). Meeting of the strategic advisory group of experts on immunization, October 2009 - conclusions and recommendations. Biological. 2010;38:170–7. doi:10.1016/j.biologicals.2009.12.007.
- Machingaidze S, Wiysonge CS, Hussey GD. Strengthening the expanded program on immunization in Africa: looking beyond 2015. PLoS Med. 2013;10:e1001405. doi:10.1371/journal.pmed.1001405.
- Russo G, Miglietta A, Pezzotti P, Biguioh RM, Bouting Mayaka G, Sobze MS, Stefanelli P, Vullo V, Rezza G. Vaccine coverage and determinants of incomplete vaccination in children aged 12–23 months in Dschang, West Region, Cameroon: a cross-sectional survey during a polio outbreak. BMC Public Health. 2015;15(1). doi:10.1186/s12889-015-2000-2.
- Sheikh N, Sultana M, Ali N, Akram R, Mahumud R, Asaduzzaman M, Sarker A. Coverage, timelines, and determinants of incomplete immunization in Bangladesh. Trop Med Infect Dis. 2018;3(3):72. doi:10.3390/tropicalmed3030072.
- Ekouevi DK, Gbeasor-Komlanvi FA, Yaya I, Zida-Compaore WI, Boko A, Sewu E, Lacle A, Ndibu N, Toke Y, Landoh DE. Incomplete immunization among children aged 12–23 months in Togo: a multilevel analysis of individual and contextual factors. BMC Public Health. 2018;18(1):952. doi:10.1186/s12889-018-5881-z.
- Jani JV, De Schacht C, Jani IV, Bjune G. Risk factors for incomplete vaccination and missed opportunity for immunization in rural Mozambique. BMC Public Health. 2008;8(1). doi:10.1186/1471-2458-8-161.
- Zida-Compaore WI, Ekouevi DK, Gbeasor-Komlanvi FA, Sewu EK, Blatome T, Gbadoe AD, Agbèrè DA, Atakouma Y. Immunization coverage and factors associated with incomplete vaccination in children aged 12 to 59 months in health structures in Lomé. BMC Res Notes. 2019;12(1):84. doi:10.1186/s13104-019-4115-5.
- Yenit MK, Assegid S, Abrha H. Factors associated with incomplete childhood vaccination among children 12-23 months of age in Machakel Woreda, East Gojjam zone: a case control study. J Preg Child Health. 2015;2(180):1–6.
- Shrestha S, Shrestha M, Wagle RR, Bhandari G. Predictors of incompletion of immunization among children residing in the slums of Kathmandu valley, Nepal: a case-control study. BMC Public Health. 2016;16(1). doi:10.1186/s12889-016-3651-3.
- Ismail ITA, El-Tayeb EM, Omer MDFA, Eltahir YM, El-Sayed ETA, Deribe K. Assessment of routine immunization coverage in Nyala locality, reasons behind incomplete immunization in South Darfur State, Sudan. Asian J Med Sci. 2015;6:1–8. February 25. Author manuscript; available in PMC. doi:10.19026/ajms.6.5348.
- Adedokun ST, Uthman OA, Adekanmbi VT, Wiysonge CS. Incomplete childhood immunization in Nigeria: a multilevel analysis of individual and contextual factors. BMC Public Health. 2017;17:236. doi:10.1186/s12889-017-4137-7.
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA group. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed1000097.
- Etana B, Deressa W. Factors associated with complete immunization coverage in children aged 12–23 months in ambo Woreda, Central Ethiopia. BMC Public Health. 2012;12(1):566. doi:10.1186/1471-2458-12-566.
- Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy K, Mu PF. Checklist for analytical cross-sectional studies. Joanna Briggs Institute Reviewer’s Manual. 2017. Available from: JBI critical appraisal-checklist for analytical cross-sectional studies 2017.
- Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of inter-rater agreement. J Clin Epidemiol. 2012;65(9):934–9. doi:10.1016/j.jclinepi.2011.11.014.
- Ali Y, Mekonnen FA, Molla Lakew A, Wolde HF. Poor maternal health service utilization associated with incomplete vaccination among children aged 12-23 months in Ethiopia. Hum Vaccines Immunother. 2019. doi:10.1080/21645515.2019.1670124.
- Mohammed RT. Assessment of factors associated with incomplete immunization among children aged 12-23 months in Ethiopia. 2015.
- Atnafu A, Andargie G, Yitayal M, Ayele TA, Alemu K, Demissie GD, Wolde HF, Dellie E, Azale T, Geremew BM, et al. Prevalence and determinants of incomplete or not at all vaccination among children aged 12–36 months in Dabat and Gondar districts, northwest of Ethiopia: findings from the primary health care project. BMJ Open. 2020;10:e041163. doi:10.1136/bmjopen-2020-041163.
- Mesfin M. Incomplete vaccination and associated factors among children aged 12-23 months in Yirgalem Town, South Ethiopia [ Masters thesis]. Addis Ababa University; 2015.
- Gebeyaw B. Incomplete vaccination and associated factors among children aged 12–23 months in Bahir Dar Zuriya District, Northwest Ethiopia. Community Based Cross Sectional Study; 2020. http://ir.bdu.edu.et/handle/123456789/13545.Date:2020-07.
- Enyew EB, Tareke AA. Vaccination status and factors associated among children age 12–23 months in Ethiopia, based on 2016 EDHS: logit based multinomial logistic regression analysis. Plos One. 2022;17(2):e0264004. doi:10.1371/journal.pone.0264004.
- Akinyemi YC. Geospatial analysis of vaccination coverage and predictors of non- and under-vaccination of children aged 12–23 months in Nigeria, (2020): papers in applied geography. 64:1183–92. doi:10.1080/23754931.2020.1809009.
- Abdulraheem IS, Onajole AT, Jimoh AA, Oladipo AR Reasons for incomplete vaccination and factors for missed opportunities among rural Nigerian children. J Public Health Epidemiol. April 2011;3(4):194–203. ISSN 2141-2316 ©2011 Academic Journals. http://www.academicjournals.org/jphe.
- Eze P, Agu UJ, Aniebo CL, Agu SA, Lawani LO, Acharya Y. Factors associated with incomplete immunisation in children aged 12–23 months at subnational level, Nigeria: a cross-sectional study. BMJ Open. 2021;11:e047445. doi:10.1136/bmjopen-2020-047445.
- Ogundele OA, Ogundele T, Fehintola FO, Fagbemi AT, Beloved OO, Osunmakinwa OO Determinants of incomplete vaccination among children 12-23 months in Nigeria: an analysis of a national sample. Tzu Chi Med J. [Epub ahead of print] [accessed 2022 Jun 4]. https://www.tcmjmed.com/preprintarticle.asp?id=344479.
- Landoh DE, Ouro-Kavalah F, Yaya I, Kahn A-L, Wasswa P, Lacle A, Nassoury DI, Gitta SN, Soura AB. Predictors of incomplete immunization coverage among one to five years old children in Togo. BMC Public Health. 2016;16:968. doi:10.1186/s12889-016-3625-5.
- Donsa LD. An examination of mothers’ socio-demographic factors associated with incomplete vaccination status among under-five populations in Malawi [ Thesis]. Georgia State University; 2013. doi: 10.57709/4116987.
- Ntenda PAM. Factors associated with non- and under-vaccination among children aged 12–23 months in Malawi. A multinomial analysis of the population-based sample. Pediatr Neonatol. 60;623–33. doi:10.1016/j.pedneo.2019.03.005.
- Jillian O, Kizito O. Socio-cultural factors associated with incomplete routine immunization of children _ Amach Sub-County, Uganda. Cogent Med. 2020;7(1):1848755. doi:10.1080/2331205X.2020.1848755.
- Ceesay B. Factors associated with incomplete childhood vaccination among children aged 24-35 months In Kanifing Municipality. The Gambia. University of Ghana; 2020. http://ugspace.ug.edu.gh.
- Douba A. An analysis of risk factors for incomplete immunization for children in Côte d’Ivoire: examination of 1998-1999 and 2011-2012 demographic and health survey data [ Thesis]. Georgia State University; 2015. doi: 10.57709/6466450.
- Kabudi AM, Lutala PM, Kazadi JM, Bardella IJ. Prevalence and associated factors of partially/non-immunization of under-five in Goma city, Democratic Republic of Congo: a community-based cross-sectional survey. Pan Afr Med J. 2015;20. ISSN: 1937- 8688 (www.panafrican-med-journal.com) Published in partnership with the African Field Epidemiology Network (AFENET). (www.afenet.net). doi:10.11604/pamj.2015.20.38.4483.
- Sangaré S, Sangho O, Doumbia L, Marker H, Sadio Sarro YD, Dolo H, Tel-Ly N, Zakour IB, Ndiaye HM, Sanogo M, et al. Concordance of vaccination status and associated factors with incomplete vaccination: a household survey in the health district of Segou, Mali, 2019. Pan Afr Med J. 2021;40(102). doi:10.11604/pamj.2021.40.102.29976.
- Wemakor A, Helegbe GK, Abdul-Mumin A, Amedoe S, Zoku JA, Dufie AI. Prevalence and factors associated with incomplete immunization of children (12–23 months) in Kwabre East District, Ashanti Region, Ghana. Arch Public Health. 2018;76:67. doi:10.1186/s13690-018-0315-z.
- Ndwandwe D, Nnaji CA, Mashunye T, Uthman OA, Wiysonge CS. Incomplete vaccination and associated factors among children aged 12–23 months in South Africa: an analysis of the South African demographic and health survey 2016. Human Vaccines & Immunotherapeutics. 2020;17:247–54. doi:10.1080/21645515.2020.1791509.
- Francis MR, Nohynek H, Larson H, Balraj V, Mohan VR, Kang G, Nuorti JP. Factors associated with routine childhood vaccine uptake and reasons for non-vaccination in India: 1998–2008. Vaccine. 2018;36:6559–66. doi:10.1016/j.vaccine.2017.08.026.
- Ingale A, Dixit JV, Deshpande D. Reasons behind incomplete immunization: a cross-sectional study at Urban Health Centre of Government Medical College, Aurangabad. Natl J Community Med. 2013;4:353–6. http://njcmindia.org/uploads/4-2_353-356.pdf%0Ahttp://www.njcmindia.org/home/abstrct/438/Apr_June.
- Vanderende K, Gacic-Dobo M, Diallo MS, Conklin LM, Wallace AS. Global routine vaccination coverage — 2017. Global Pediatric Health Morb Mortal Wkly Rep. 2018;67:1261–4. doi:10.15585/MMWR.MM6745A2.
- Desalew A, Semahegn A, Birhanu S, Tesfaye G. Incomplete vaccination and its predictors among children in Ethiopia: a systematic review and meta-analysis. Global Pediatr Health. 7;1–20. doi:10.1177/2333794X20968681.
- Konstantyner T, Taddei JADAC, Rodrigues LC. Risk factors for incomplete vaccination in children less than 18 months of age attending the nurseries of day-care centres in Sao Paulo, Brazil. Vaccine. 2011;29:9298–302. doi:10.1016/j.vaccine.2011.10.020.
- Lim C, Currie GE, Waddington CS, Wu Y, Setijo S, Leask J., Marsh JA, Snelling TL. Identification of the determinants of incomplete vaccination in Australian children. Vaccine: X. 2019;1:100010. doi:10.1016/j.jvacx.2019.100010.
- Than SE, Mongkolchati A, Laosee O. Determinants of incomplete immunization among hill tribe children aged under two years in. Myanmar J Pub Health Dev. 2016;14:17–31.
- Hardhantyo M, Chuang YC. Urban-rural differences in factors associated with incomplete basic immunization among children in Indonesia: a nationwide multilevel study. Pediatr Neonatol. 2021;62:80–9. doi:https://doi.org/10.1016/j.pedneo.2020.09.004.
- Riaz A, Husain S, Yousafzai MT, Nisar I, Shaheen F, Mahesar W, Dal SM, Omer SB, Zaidi S, Ali A. Reasons for non-vaccination and incomplete vaccinations among children in Pakistan. Vaccine. 2018;36:5288–93. doi:10.1016/j.vaccine.2018.07.024.
- Noh JW, Kim YM, Akram N, Yoo K-B, Park J, Cheon J, Kwon YD, Stekelenburg J. Factors affecting complete and timely childhood immunization coverage in Sindh, Pakistan; a secondary analysis of cross-sectional survey data. Plos One. 2018;13:1–15. doi:10.1371/journal.pone.0206766.
- Malecosio SO, Celis MJLD, Delicana KB, Deopido AM, Bacale KMFK, Labasan VKL, Ladiao RMC, Militante KMD, Paguntalan JM, Sabusap VEP. Vaccination coverage and factors associated with incomplete childhood vaccination among children aged 12-59 months in Miagao, Iloilo, Philippines. Int J Community Med Public Health. 2020;7:2492. doi:10.18203/2394-6040.ijcmph20202971.
- Tauil MC, Sato APS, Waldman EA. Factors associated with incomplete or delayed vaccination across countries: a systematic review. Vaccine. 2016;34:2635–43. doi:10.1016/j.vaccine.2016.04.016.
- Hajizadeh M. Socioeconomic inequalities in child vaccination in low/middle-income countries: what accounts for the differences? J Epidemiol Community Health. 2018;72:719–25. doi:10.1136/jech-2017-210296.
- Wiysonge CS, Uthman OA, Ndumbe PM, Hussey GD. Individual and contextual factors associated with low childhood immunization coverage in Sub-Saharan Africa: multilevel analysis. Plos One. 2012;7:e37905. doi:10.1371/journal.pone.0037905.
- Jaca A, Mathebula L, Iweze A, Pienaar E, Wiysonge CS. A systematic review of strategies for reducing missed opportunities for vaccination. Vaccine. 2018;36:2921–7. doi:10.1016/j.vaccine.2018.04.0.
