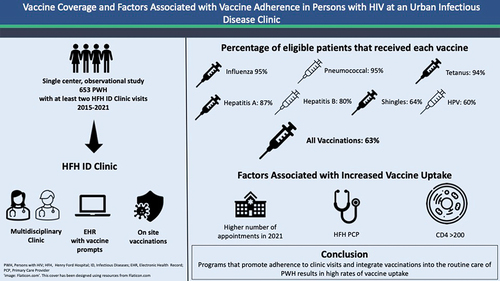
ABSTRACT
Information on vaccination rates and factors associated with adherence in persons with HIV (PWH) is limited. We report vaccine adherence in 653 adult PWH attending an urban Infectious Disease Clinic from January 2015 to December 2021. Vaccines evaluated included influenza, pneumococcal, tetanus, hepatitis A virus (HAV) and hepatitis B virus (HBV), human papillomavirus (HPV), and zoster vaccines. Vaccine reminders were triggered at every visit, and all vaccines were accessible in the clinic. The mean age was 50 y (±SD 13), male gender was 78.6%, and black race was 74.3%. The overall adherence to all recommended vaccines was 63.6%. Vaccine adherence was >90% for influenza, pneumococcal, and tetanus, >80% for HAV and HBV, and ≥60% for HPV and zoster vaccines. The main predictor of adherence to all vaccines was ≥2 annual clinic visits (odds ratio [OR] 3.45; 95% confidence interval [CI] 2.36–5.05; p < .001). Other predictors included an assigned primary care provider within the system (OR 2.89 [95% CI 1.71–5.00, p < .001]) and CD4 >200 cell/mm3 at entry into care (OR 1.91 [95% CI 1.24–2.94, p = .0003]). Retention in care combined with vaccine reminders and accessibility of vaccines in the clinic can achieve high vaccine uptake in PWH.
Introduction
Persons with HIV (PWH) are at increased morbidity and mortality risk from vaccine-preventable diseases. Yet, vaccination opportunities are often overlooked in PWH. This occurs for several reasons including patients’ access to care, vaccine hesitancy, providers’ lack of knowledge of appropriate vaccine regimens in PWH, and burden of other health issues often taking precedence in office visits over vaccinations.Citation1–3 There is limited data on vaccination coverage and factors associated with vaccine adherence in PWH.
A large population of PWH receive care in the Henry Ford Hospital (HFH) Infectious Diseases Clinic (ID Clinic). HFH is in Detroit located in Wayne County, one of the counties in Michigan with the highest number of new HIV diagnoses. We evaluated the rates of uptake of all recommended vaccines per Centers for Disease Control (CDC) guidelinesCitation4 among PWH at HFH ID Clinic and the factors associated with higher rates of vaccination.
Materials and methods
This was a single-center, retrospective observational study conducted at the HFH ID Clinic. Inclusion criteria were as follows: PWH who receive care at the clinic, were >18 y of age, and had at least two visits within the 7-y study period from January 1, 2015, to December 31, 2021. Patients who did not have at least two clinic visits within the study period were excluded. All consecutive patients who met the inclusion criteria were enrolled and followed until December 31, 2021. The electronic medical records (EMRs) were reviewed at the end of the study period for demographic data (age, sex, race, education, income, and city of residence). The EMR was implemented in 2013 and contained quality care measures and care gaps including eligible vaccines and vaccination reminders. The vaccine reminder system was present for the duration of the study (2015–2021), and no EMR modifications were made during the study. US 2010 Census block-level data based on the geocoded information from the residential addresses were utilized as a proxy for the patients’ socioeconomic status (SES), which included the proportion of adults with at least a high school diploma (educational level), and median household income in that patient’s zip code.Citation5 Data related to HIV infection and treatment were collected and included age at diagnosis, men who have sex with men (MSM) status, intravenous drug use (IDU), CD4 count and HIV viral load at entry into care, and number of ID clinic visits per year since the entry into care.
PWH received care at HFH ID Clinic, a specialty clinic compromised of nurses, pharmacists, social workers, and physicians knowledgeable in the care of PWH. The EMR homepage for each patient indicates missing care gaps, including incomplete vaccinations, and automatically generates vaccine reminders for providers at each clinic visit as EMR alerts. During the influenza season, an automatic order for the administration of the influenza vaccine is placed in all patient charts. Orders for future doses of serial vaccines are placed at the time of administration of the first dose. All adult vaccines are accessible in the clinic allowing for the administration of recommended vaccines during the clinic visit. COVID-19 (Pfizer-BioNTech) vaccine was available in the clinic in January 2020.
If patients meet certain criteria for our Ryan White Funding program,Citation6 all vaccinations are covered regardless of insurance status. If the patient does not have a payer source (i.e. insurance) and does not meet Ryan White funding criteria, we have a philanthropic grant that will cover the cost of the vaccination as long as it is provided within our clinic. If patients have insurance and the vaccinations have a co-pay cost, the Michigan Drug Assistance ProgramCitation7 is utilized for our HIV-positive patients to reimburse the vaccination cost.
Vaccination data were obtained from the EMR and from the Michigan Care Improvement Registry (MCIR) that include the receipt of vaccines administered in the state of Michigan. The vaccines evaluated based on the 2021 CDC guidelines were Influenza, Pneumococcal conjugate vaccine (PCV13), Pneumococcal Polysaccharide (PCV23), Hepatitis A virus (HAV), Hepatitis B virus (HBV), Human Papillomavirus (HPV), Tetanus-Diphtheria-acellular Pertussis (Tdap and Td), Zoster live (ZVL), and Zoster recombinant (ZVR).Citation4 In addition, COVID-19 mRNA (Pfizer-BioNTech, Moderna) and COVID-19 viral vector (Janssen) were also evaluated.Citation4 For HAV and HBV vaccines, patients were considered eligible if they were seronegative upon entry into HFH-ID clinic. Patients were considered eligible for the HPV vaccine through 26 y of age and for zoster vaccines if 50 years of age or older.
The primary outcome was the rate of adherence to all recommended vaccines. Secondary outcomes were as follows: (a) rates of adherence to individual vaccines and (b) factors associated with adherence to vaccination. Patients were classified as adherent if they were eligible and received at least one dose of the recommended vaccine and non-adherent if they were eligible and did not receive a single dose of recommended vaccine during the study period. For age-based vaccines, patients were considered eligible for HPV vaccine through 26 y of age and for zoster vaccines if 50 y of age or older. If patients did not meet the age criteria for age-related vaccination (Zoster and HPV), they were excluded from the calculation.
Continuous variables were reported as the median and interquartile range (IQR) and compared using the Wilcoxon rank-sum test for non-parametric data or reported as mean and standard deviation (SD) and compared using t-test if normally distributed. Categorical data were reported as the number and percentage and compared using the chi-square test or Fisher’s exact test for small samples. Associations between patient characteristics and vaccine adherence (adherence vs. non-adherence) were examined by multivariate unconditional logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs). An acceptable type I error rate for all analyses was α = 0.05. All analyses were performed using Stata (version 17; College Station, TX). The study was approved by the institutional IRB (IRB Project 15330).
Results
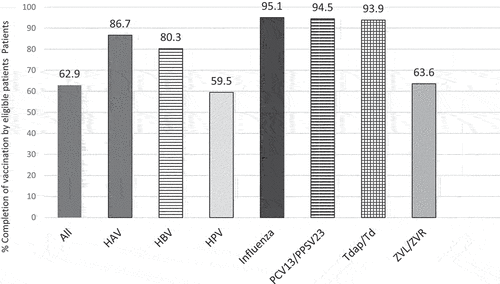
A total of 653 PWH met the inclusion criteria and were enrolled in the study. The overall rate of adherence to all eligible recommended vaccines was 62.9%, and the exact adherence rates for each individual vaccine are shown in . Rates of adherence to influenza, PCV-13/PPSV23, and Tdap/Td vaccinations were over 90% and over 80% for HAV and HBV vaccinations. Adherence to age-specific HPV and zoster vaccinations were 59.5% and 63.6%, respectively. COVID-19 vaccination rate was 42.1%.
Figure 1. The vaccination rate among eligible patients.
The characteristics of the study population and patient groups adherent and non-adherent to all vaccines are shown in . Overall, the mean age was 50 y, 78.6% were male and 74.3% were black. Demographics including age, race, socioeconomic status (education and income), residence in the city of Detroit, and distance from the clinic were comparable in both groups. Patients who had received all recommended vaccines were more likely to be male (p = .017), younger age at the time of HIV diagnosis (p = .032), men who have sex with men (MSM) status (p < .001), higher CD4 count on entry into care (p < .001), receiving primary care in the HFH system (p < .001), and more ID clinic visits each year (p < .001).
Table 1. Characteristics of patients adherent and non-adherent to all vaccines.
The logistic regression of variables associated with adherence to all vaccines is shown in . The main factor independently associated with vaccine adherence were two or more clinic visits within a calendar year (OR 5.85 [95% CI 0.36–0.72], p < .001). An initial CD4 count above 200 cell/mm3 (OR 2.72 [95% CI 0.18–1.11], p = .01) and receiving primary care within the HFH system (OR 2.89 [95% CI 0.27–1.39], p = .004) were also associated with adherence.
Table 2. Odds ratio of factors associated with adherence to all vaccines.
Discussion
Our study demonstrated that more than 60% of 653 PWH receiving care in the ID Clinic were adherent to all recommended vaccines. Predictors of adherence were frequent clinic attendance, CD4 count over 200 cell/mm3, younger age, and receipt of primary care within the system.
In our study, rates of adherence were over 80% for influenza, pneumococcal, tetanus, and hepatitis A and B vaccines. Vaccine uptake was lower for HPV and zoster vaccines. No study has reported vaccination rates in PWH for all age- and condition-related vaccines including COVID-19 vaccines. A retrospective single-center study of 502 PWH followed in an infectious diseases clinic reported that 41% had received all eligible vaccines, and poor uptake correlated with non-adherence with clinic appointments, MSM status, and CD4 counts <200 cells/mm3.Citation1 These findings are in line with our strongest predictor of adherence being two or more annual clinic visits, suggesting engagement in care improves opportunities for vaccine completion. We also demonstrated that a CD4 >200 cells/mm3 at entry into the study may indirectly reflect engagement in care as a predictor of adherence. This finding is consistent with the prior study demonstrating lower vaccine adherence in patients with CD4 <200 cells/mm3.Citation1 Although CD4 counts at entry into care was associated with higher CD4 counts, we did not examine the effects of time-updated CD4 counts and viral loads and adherence over the course of the study. Our study also showed that having a PCP within HFH health system was associated with improved vaccine uptake and may be due to familiarity of providers within the health system with the care of PWH as well as a common EMR. Notably, age below 50 y was associated with increased uptake and a finding that has not been previously reported. Uptake of herpes zoster and HPV vaccines were low in our study. Studies have demonstrated low provider knowledge related to herpes zoster and HPV vaccines, and uptake of these vaccines in PWH may benefit from programs that strengthen provider knowledge and patient education regarding current recommendations for these vaccines.Citation2,Citation8,Citation9
Other studies have examined the uptake of specific vaccines such as influenza, pneumococcal, HAV and HBV, zoster, and HPV vaccines. Uptake of these vaccines ranged from 2% to 80% and varied by type of vaccine, specific population characteristics and clinical care setting.Citation10–14 Factors related to poor vaccine adherence included low education level, financial stressors, lack of patient knowledge related to the need for vaccination and the benefits and risks of vaccination,Citation10–13 and lack of routine screening of patient vaccination records.Citation11
The uptake of COVID-19 vaccine in our study was low (42%). COVID-19 vaccines were incorporated into routine care and was made available in the ID clinic in late January 2020. This together with the transition to telemedicine during the pandemic may have contributed to the low vaccination rate. In a recent study, almost all of 101 black American PWH endorsed at least COVID-19 belief mistrust and greater mistrust correlated with vaccine hesitancy.Citation14 COVID-19 vaccine adherence in PWH was lowest among non-Hispanic blacks, those not virally suppressed, and those who did not receive HIV care in 2020.Citation15 Retention and re-linkage to HIV care and incorporating COVID-19 vaccination into existing HIV care programs may improve uptake.Citation16
Although minority populations have lower general vaccine uptake,Citation15,Citation17 our study demonstrates a high rate of vaccine adherence in a predominately black minority population. The high uptake of vaccine among PWH in our study may be the result of several factors, especially the role of retention in care to establish a trusting relationship between PWH and provider. Furthermore, our specialty clinic had incorporated several strategies recommended by the CDC to optimize vaccine uptake in adults.Citation16 These included clinic providers knowledgeable in the care of PWH and vaccine recommendations, assessment of vaccine completion, vaccine reminders automatically generated by EMR, standing orders for influenza and serial vaccines, and accessibility of all vaccines in the clinic.
Our study is limited by the inherent biases of a retrospective study. The single-center design may not permit the generalizability of the findings. We relied on the MCIR for verification of vaccination status; however, this system only provides data on vaccinations administered in Michigan, and out-of-state vaccination history may be missing. Other limitations in our study include the characterization of adherence as the uptake of at least one vaccine in a vaccine series. We excluded the meningococcal vaccine from the study as the provision of meningococcal vaccination to PWH was not present in 2015 at the onset of the study. Our study has several strengths, including the large sample size and is the first study to evaluate rates of all vaccinations including COVID-19.
We conclude that even in a highly vulnerable population of PWH, retention in care and adherence to routine clinic visits can result in high vaccination rates.
Author contributions
N.B., I.B., and G.A. conceived and designed the study. N.B., L.M., T.S., M.C., M.H., B.H., and A.C. collected the data. N.B., L.M. I.B., and G.A. analyzed and interpreted the data. N.B. and G.A. drafted the manuscript. N.B., L.M., I.B. and G.A. critically reviewed and revised the manuscript.
All authors have reviewed and agree to the submitted manuscript.
Patient consent statement
Our study did not require patient consent.
Acknowledgments
We would like to thank the Richard Krajenta for evaluation and analysis of census data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Johnson TM, Klepser DG, Bares SH, Scarsi KK. Predictors of vaccination rates in people living with HIV followed at a specialty care clinic. Hum Vaccin Immunother. 2021 Mar 4;17(3):1–5. doi:10.1080/21645515.2020.1802163.
- Aziz M, Kessler H, Huhn G. Providers’ lack of knowledge about herpes zoster in HIV-infected patients is among barriers to herpes zoster vaccination. Int J STD & AIDS. 2013 Jun;24(6):433–9. doi:10.1177/0956462412472461. PMID: 23970744.
- Morales Rodriguez K, Khalili J, Trevillyan J, Currier J. What is the best model for HIV primary care? Assessing the influence of provider type on outcomes of chronic comorbidities in HIV infection. J Infect Dis. 2018 July 15;218(2):337–9. doi:10.1093/infdis/jiy101.
- CDC- Adult Immunization Schedule. Recommendations for ages 19 years or older. United States; 2022 Aug 20. https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html.
- Darrat I, Tam S, Boulis M, Williams AM. Socioeconomic disparities in patient use of telehealth during the coronavirus disease 2019 surge. JAMA Otolaryngol Head Neck Surg. 2021;147(3):287–95. doi:10.1001/jamaoto.2020.5161.
- Ryan White HIV/AIDS Program (including CAREWare). [accessed 2023 Feb 24]. https://www.michigan.gov/mdhhs/keep-mi-healthy/chronicdiseases/hivsti/resources/ryan-white-hiv-aids-program-including-careware.
- Michigan Drug Assistance Program (MIDAP). [accessed 2023 Feb 24]. https://www.michigan.gov/mdhhs/keep-mihealthy/chronicdiseases/hivsti/michigan-drug-assistance-program/michigan-drug-assistance-program.
- Erlandson KM, Streifel A, Novin AR, Hawkins KL, Foster C, Langness J, Bessesen M, Falutz J, Moanna A, Looney D, et al. Low rates of vaccination for herpes zoster in older people living with HIV. AIDS Res Hum Retrovir. 2018 7;34(7):603–6. doi:10.1089/aid.2017.0315.
- Oliver SE, Hoots BE, Paz-Bailey G, Markowitz LE, Meites E, Study Group NHBS. Increasing human papillomavirus vaccine coverage among men who have sex with men—National HIV behavioral surveillance, United States, 2014. J Acquir Immune Defic Syndr. 2017 July 1;Suppl 75(Suppl 3):S370–4. doi:10.1097/QAI.0000000000001413. PMID: 28604441; PMCID: PMC5761322.
- Tsachouridou O, Georgiou A, Naoum S, Vasdeki D, Papagianni M, Kotoreni G, Forozidou E, Tsoukra P, Gogou C, Chatzidimitriou D, et al. Factors associated with poor adherence to vaccination against hepatitis viruses, streptococcus pneumoniae and seasonal influenza in HIV-infected adults. Hum Vaccin Immunother. 2019;15(2):295–304. doi:10.1080/21645515.2018.1509644.
- Harrison N, Poeppl W, Herkner H, Tillhof KD, Grabmeier-Pfistershammer K, Rieger A, Forstner C, Burgmann H, Lagler H. Predictors for and coverage of influenza vaccination among HIV-positive patients: a cross-sectional survey. HIV Med. 2017 08;18(7):500–6. doi:10.1111/hiv.12483.
- Hoover KW, Butler M, Workowski KA, Follansbee S, Gratzer B, Hare CB, Johnston B, Theodore JL, Tao G, Smith BD, et al. Low rates of hepatitis screening and vaccination of HIV-infected MSM in HIV clinics. Sex Transm Dis. 2012 May;39(5):349–53. doi:10.1097/OLQ.0b013e318244a923.
- McClung N, Burnett J, Wejnert C, Markowitz LE, Meites E, Study Group NHBS. Human papillomavirus vaccination coverage among men who have sex with men-National HIV behavioral surveillance, United States, 2017. Vaccine. 2020;38(47):7417–2111 03. doi:10.1016/j.vaccine.2020.08.040.
- Bogart LM, Ojikutu BO, Tyagi K, Klein DJ, Mutchler MG, Dong L, Lawrence SJ, Thomas DR, Kellman S. COVID-19 related medical mistrust, health impacts, and potential vaccine hesitancy among Black Americans living with HIV. J Acquir Immune Defic Syndr. 2021;86(2):200–7. doi:10.1097/QAI.0000000000002570.
- Tesoriero JM, Patterson W, Daskalakid D, Chicoine J, Morne J, Braunstein S, Rajulu DT, Rosenberg E. Notes from the field: COVID-19 vaccination among persons living with diagnosed HIV infection—New York, October 2021. MMWR Morb Mortal Wkly Rep. 2022;71(5):182–4. doi:10.15585/mmwr.mm7105a4.
- Strategies for increasing adult vaccination rates | CDC. [accessed 2022 Oct 25]. https://www.cdc.gov/vaccines/hcp/adults/for-practice/increasing-vacc-rates.html.
- Black CL, O’Jalloran A, Hung M, Srivastav A, Lu P-J, Garg S, Jhung M, Fry A, Jatlaoui TC, Davenport E, et al. Vital signs: influenza hospitalizations and vaccination coverage by race and ethnicity—United States, 2009–10 through 2021–22 influenza seasons. MMWR Morb Mortal Wkly Rep. ePub. 2022 October 18;71(43):1366–73. http://dx.doi.org/10/15585/mmwr.mm7143e1.