ABSTRACT
We conducted a dose-finding phase 2 study of the HilleVax bivalent virus-like particle (VLP) vaccine candidate (HIL-214) in two cohorts of children, 6–≤12 months and 1–≤4 years of age (N = 120 per cohort), in Panama and Colombia (ClinicalTrials.gov, identifier NCT02153112). On Day 1, children randomized to one of the four equal groups received intramuscular injections of four different HIL-214 formulations containing 15/15, 15/50, 50/50, or 50/150 μg of GI.1/GII.4c genotype VLPs and 0.5 mg Al(OH)3. On Day 29, half the children in each group received a second vaccination (N = 60), while the other half received saline placebo injections to maintain the blind. VLP-specific ELISA Pan-Ig and histo-blood group binding antigen-blocking antibodies (HBGA) were measured on Days 1, 29, 57 and 210. On Day 29, after one dose, there were large Pan-Ig and HBGA responses in both age cohorts with some indication of dose-dependence, and higher geometric mean titers (GMT) in the older children. A further increase in titers was observed 28 days after a second dose in the 6–≤12-month-old groups, but less so in the 1–≤4-year-old groups; GMTs at Day 57 were broadly similar across doses and in both age groups. GMTs of Pan-Ig and HBGA persisted above baseline up to Day 210. All formulations were well tolerated with mostly mild-to-moderate transient solicited adverse events reported by parents/guardians, and no vaccine-related serious adverse events occurred. Further development of HIL-214 is warranted to protect the most susceptible young children against norovirus.
Introduction
Infection with norovirus typically results in severe but self-limiting morbidity due to acute gastroenteritis (AGE) with severe nausea, vomiting, and diarrhea. However, norovirus illness in the very young and the elderly can have more serious, and fatal, consequences.Citation1 Following the introduction of rotavirus vaccines, noroviruses have emerged as the single most significant cause of epidemic outbreaks of non-bacterial AGE worldwide.Citation1,Citation2 This is most apparent in high-income countries with national childhood rotavirus immunization campaigns, where a decrease in rotavirus disease means that norovirus is the major etiology for gastroenteritis hospitalizations in children up to 4 years of age,Citation2–6 with estimated 64,000 hospitalizations and 900,000 clinical visits per annum.Citation1 In low- and middle-income countries (LMIC) norovirus AGE may be responsible for 218,000 deaths in children up to 5 y of age each year.Citation1 There is no specific prophylaxis against norovirus infection other than maintaining strict conditions of sanitary hygiene nor any specific treatment for the illness available; therapy consists of ensuring adequate hydration. A study in infants in Nicaragua found AGE due to norovirus led to protection against subsequent episodes, supporting a potential role for immune protection through vaccination.Citation7
HilleVax is continuing the clinical development of a bivalent virus-like particle (VLP) vaccine candidate against norovirus (HIL-214) that was originally developed by Takeda Vaccines (as TAK-214). The vaccine consists of two VLPs representing the predominant circulating strains of norovirus, which are responsible for most human disease, GI.1 and GII.4, with aluminum hydroxide as adjuvant. The GII.4 component is a consensus sequence (GII.4c) of three different GII.4 genotype variants—2006a (Yerseke), 2006b (Den Haag) and 2002 (Houston)—designed to elicit a broad response against different GII.4 variants that occur due to antigenic drift.Citation8 The candidate has been shown to be safe and immunogenic in several clinical studies in adults.Citation9–12 In adults, one dose of HIL-214 was shown to elicit a rapid immune response suggestive of an amnestic response with no further increase following a second dose, indicating that most adults have previous immunologic experience of norovirus infection.Citation10,Citation11 Data from studies in adults showed GII.4c VLP is less immunogenic than GI.1 VLP and that high doses of GI.1 VLP interfered with the response to GII.4c VLP.Citation11 For that reason, an unbalanced formulation (15/50) was used in later adult studies and unbalanced formulations are also being investigated in pediatric studies.
To move the clinical development to the pediatric population, a large age de-escalation study covering a series of age groups in children has been performed to assess the safety, tolerability, and immunogenicity of HIL-214. Data using a clinical development formulation of the vaccine in two of the oldest age groups, 1–3 and 4–8 years of age, in Finland, Colombia, and Panama have been reported.Citation13 We now present the data from the first use of final manufacturing lot formulations with various dosages of GI.1 and GII.4c VLPs in children from 6 months to 4 years of age in Colombia and Panama, in whom we also assessed the optimal regimen of one or two doses of HIL-214.
Methods
Study design and population
This phase 2 randomized, double-blind, trial was performed in one center each in Colombia and Panama. The study was registered on ClinicalTrial.gov, identifier NCT02153112, and the protocol was approved by the IRBs at each study center and the relevant national authorities. The study was conducted in accordance with the Declaration of Helsinki and ICH Good Clinical Practice guidelines. Parents/guardians supplied written informed consent for their child to be enrolled. The study's objective was to assess the safety, tolerability, and immunogenicity of one or two doses of different formulations of the bivalent norovirus VLP vaccine candidate (HIL-214) in two cohorts of children aged 6–≤12 months and 1–≤4 years.
Eligible participants were 6 months to <4 years of age and in good health based on medical history, physical examination at enrollment and the investigator’s judgment, and were available for the duration of the study. Major exclusion criteria were any infection at the time of enrollment or receipt of medications within 24 h of study start, any known or suspected allergy to vaccine components or any medical condition or treatment likely to lead to alteration of immune function, participation in any other clinical trial, or recent receipt of any inactivated vaccines within 14 days or live vaccines within 28 days of study initiation.
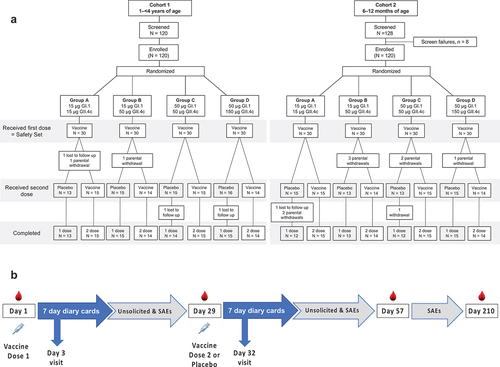
After screening, 120 children aged from 1 to ≤4 years were enrolled in the first age group (Cohort 1). Children were randomly allocated (1:1:1:1) using a sponsor-supplied schedule with an interactive web response system (IWRS) to four equal groups, which were then further randomized into two equal subsets (). On Day 1 each participant received a first vaccination with the group-assigned HIL-214 formulation, and on Day 29 one subset in each group received a second vaccination with the same dose, while the other subset received a saline placebo injection to maintain the blind. The study design is shown in . Following an assessment of the reactogenicity and safety by an independent data safety monitoring board (DSMB) in this older age group to confirm there were no safety signals, the procedure from enrollment through vaccination was repeated with Cohort 2 which comprised 120 children aged from 6 to ≤12 months.
Vaccines
The study vaccine HIL-214 (batches 3-FIN-2444, 3-FIN-2504, or 3-FIN-2505; Althea Technologies Inc., San Diego, California, USA) is composed of four different mixtures as indicated of the two VLP antigens with 500 μg Al(OH)3 in each 0.5 mL dose. The four formulations were 15 μg GI.1 and 15 μg GII.4c (15/15), 15 μg GI.1 and 50 μg GII.4c (15/50), 50 μg GI.1 and 50 μg GII.4c (50/50), and 50 μg GI.1 and 150 μg GII.4c (50/150). In the one dose group, the second vaccination was replaced by a saline placebo injection (Westward Pharmaceuticals, Eatontown, New Jersey, USA). All injections were administered in the deltoid muscle or anterolateral thigh according to national guidelines.
Safety and immunogenicity assessments
Children were monitored for 30 min after each vaccination, and a follow-up telephone call was made on Day 3. For 7 days after each vaccination, parents completed diary cards, which solicited local reactions (injection site pain, erythema, induration, swelling), and systemic adverse events (irritability/fussiness, drowsiness, loss of appetite, vomiting, and diarrhea). Parents also recorded their child’s body temperature each day for 7 days, as well as any unsolicited adverse events (AEs) until the next study visit (28 days after each vaccination). These AEs were graded for severity (mild, moderate, or severe) and relationship of to the vaccination by the study investigators (Supplementary table S1) and subsequently coded by the study sponsor using Medical Dictionary for Regulatory Activities (MedDRA) definitions. Throughout the duration of the study up to Day 210, any serious adverse event (SAE) defined according to MedDRA was to be reported immediately to the investigator, who would then notify the study sponsor.
Sera were collected on Days 1, 29, 57 and 210 for assessment of immune responses (PPD/BioA, Richmond, VA, USA). ELISA Pan-Ig antibodies against the two vaccine antigens, GI.1 and GII.4c, and 50% blocking titers (BT50) of histoblood group antigen-blocking (HBGA) antibodies were measured as described previously.Citation9,Citation14 Briefly, 96-well microtiter plates are coated with pig gastric mucin (PGM). A separate 96-well polypropylene microtitter plate was prepared with 10 two-fold serial dilutions of sera from a starting 15-fold dilution in dPBS with 5% nonfat dried milk. An equal volume of VLPs (at 0.200 μg/ml for GI.1, 0.150 μg/ml for GII.4) was added to each well and allowed to react. The diluted serum samples and VLPs were transferred to the 96-well microtiter plate previously coated with PGM and incubated at room temperature. Bound VLPs were detected with rabbit anti-VLP antibodies, followed by goat anti-rabbit IgG and 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) substrate. Optical density was plotted against the dilution for each sample, and the dilution profile was fit to an unweighted, four-parameter, constrained logistic regression model. The titer was defined as the reciprocal of the interpolated dilution that is equal to 50% of the median OD of the eight ligand control wells (cut point). For each sample, the geometric mean titer (GMT) was determined across the two replicates that were performed within a given run. The limit of detection was a titer of 15, and values <15 were assigned a value of 7.5 for calculation of GMTs.
Statistics
This trial was designed to be descriptive and was not based on testing formal null hypotheses, and therefore the sample size was not determined based on formal statistical power calculations but was considered adequate to assess the primary and secondary endpoints to make decisions for further clinical development.
The primary descriptive safety endpoints were proportions of each study group with solicited local reactions and systemic AEs for 7 days post-vaccination, unsolicited AEs for 28 days post-vaccination, and SAEs throughout the study duration. These were analyzed in the Safety Analysis Set (SAS), which comprised all participants who received at least one dose of HIL-214 vaccine or placebo and provided post-vaccination data.
The primary immunogenicity analyses were done in the Per-Protocol Analysis Set (PPS), which comprised all participants who received their planned vaccinations and had no major protocol violations. The primary immunogenicity endpoint was the seroresponse rate (SRR; group proportions with 4-fold or greater increases in titer over baseline) measured as GI.1 and GII.4c ELISA Pan-Ig antibodies at Day 57 after one or two doses of HIL-214. Secondary immunogenicity endpoints included GI.1 and GII.4c Pan-Ig geometric mean titers (GMTs) and geometric mean-fold rises over baseline (GMFR) at each timepoint, and GMTs and SRR for GI.1 and GII.4c histoblood group antigen (HBGA) 50% blocking titers (BT50) which were analyzed in the Full Analysis Set (FAS) comprising all randomized participants who received at least one study injection. All statistical analyses were performed with SAS Version 9.2 or higher.
Results
We screened 120 1–<4-year-olds who were all enrolled into study Cohort 1, and 128 6–≤12-month-old infants, of whom 8 had one or more exclusion criteria, leaving 120 to be enrolled into study Cohort 2. All enrolled children in both cohorts received their first vaccination and are included in the Safety Set for analyses of reactogenicity; three children from Cohort 1 did not receive their second assigned injection, and six infants from Cohort 2 were withdrawn by their parents before receiving their second injection (). In Cohort 1, two single-dose children were lost to follow-up, one single-dose infant was lost to follow-up, and three single-dose infants were withdrawn by their parents from Cohort 2. Demographics of the study populations were similar across one or two dose groups in terms of sex and ethnicity (Supplementary table S2).
Safety
HIL-214 in children from 6 months up to 4 years of age was generally well tolerated, with no safety signals. For Cohort 1 (1–≤4 years), after the first vaccination, solicited local reactions (see Supplementary Table S1 for adverse event criteria) were the most common with an incidence rate of 26.1%, 31 of a total of 119 children having any local AE (). The incidence rates of any local reaction of 13.3%, 16.7%, 40%, and 33.3% for the 15/15, 15/50, 50/50, and 50/150 dose groups, respectively, show some association between increased incidence rate and higher VLP dosage. These local reactions consisted almost entirely of transient mild (25/120) or moderate (5/120) pain at the injection site. There were single cases of mild erythema and swelling (). After the second injection, the incidence rates of local reactions were lower, ranging from 14.3% to 18.8% for the second dose of HIL-214 and from 7.1% to 25% for the placebo group. For the groups receiving the second HIL-214 vaccination, there was no observed dose-dependent difference in incidence rate. For the HIL-214 and placebo groups, 6 of 7 children with a solicited local reaction experienced only mild pain ().
Table 1. Incidences of solicited local reactions and systemic adverse events in the 7 days and unsolicited adverse events in the 28 days after the first and second doses of HIL-214 or placebo, and serious adverse events (SAEs) throughput the study duration.
For Cohort 2 (6–<12 months), after the first vaccination 14 (11.7%) of the 120 infants had a solicited local reaction (). Unlike the older children in Cohort 1, there was no detectable correlation between local reactions and increased HIL-214 dosage. The highest local reaction incidence rates, 23.3% for 15/15 and 13.3% for 15/50, occurred in the two groups, which received the lowest dosage. As observed for Cohort 1, the most frequent local reaction for Cohort 2 was local pain with 12 (10%) of 120 infants presenting (). Like Cohort 1, the Cohort 2 local reactions were mostly (10/12) mild pain, but unlike Cohort 1, there was one case of severe local injection site pain in the 15/15 dose group.
Solicited systemic AEs were reported after the first vaccination in 20–50% and 26.7–50% of the older and younger age cohorts, respectively, with no consistent trend to increased incidence with higher doses of VLPs either between the cohorts or within a cohort (). The most frequent events were irritability/fussiness and drowsiness in the 1–≤4-year-olds and irritability/fussiness and diarrhea in the 6–≤12-month-old infants (). In both cohorts, the frequency of most systemic AEs decreased after the second injection, with similar rates after a second vaccination or a placebo dose.
Unsolicited adverse events, most of which were mild in severity, were reported at similar frequencies across dose groups (). Two unsolicited AEs, diarrhea and gastroenteritis, were considered by the investigator to be related or possibly related to the first vaccination in Cohort 1. Two unsolicited AEs, diarrhea and gastroenteritis, were considered to be related to the first dose of HIL-214 and one unsolicited AE, diarrhea, after placebo injection.
In Cohort 1, SAEs were reported for seven children, five of which were after placebo injections, but none were considered to be related to the study treatments. In Cohort 2, SAEs were reported in 11 infants, three after the first dose of HIL-214, four after a second dose, and four after a placebo injection. Because most of these SAEs were typical childhood infections, such as bronchiolitis, gastroenteritis, or pneumonia (Supplementary table S3), none of these SAEs were considered to be related to the study treatment. Since the remaining SAEs included a testicular torsion and asthmatic crises that occurred after saline placebo injection, they were not considered to be related to the study treatment.
Immunogenicity – Pan-Ig
The primary measure of immune response was the seroresponse rate (SRR) at Day 57, expressed as the proportions of each group displaying a fourfold or greater increase in Pan-Ig antibody titers against GI.1 and GII.4c VLPs from baseline, four weeks after the second vaccination or placebo injection. The results for individual groups in show that there was no trend to increased responses with VLP dosage against either VLP in either cohort, with small increases after a second dose. In the older Cohort 1 the SRR against GI.1 and GII.4c were 79% (95% CI: 64–89) and 64% (95% CI: 49–77) of all groups combined after one dose, the rates ranging from 58–92% to 50–75% across the groups, respectively (). In the younger Cohort 2, the combined SRR across all four formulation groups against GI.1 and GII.4c were 96% (95% CI: 86–100) and 76% (95% CI: 61–87) after one dose, and 100% (95% CI: 94–100) and 93% (95% CI: 82–98) after two doses. SRR across groups in Cohort 2 were 92–100% and 100% against GI.1 after one or two doses, and 50–92% and 86–100% against GII.4c after one and two doses, respectively.
Table 2. Seroresponse ratesa (95% CI) at Day 57, 28 days after the last dose of the study vaccine or placebo in group participants (Per protocol set).
When expressed as geometric mean titers (GMT) of Pan-Ig antibodies groups, there was an overall consistency in responses to the same dosage of VLP (). For example, the GI.1 responses were similar in the two groups that received 15 μg GI.1 VLP (15/15 and 15/50 groups), and also in the two groups that received 50 μg GI.1 VLP (50/50 and 50/150 groups), with a general trend to higher titers with the higher dosage of VLP. The kinetics of the response were similar across all four dosage groups (). When assessing responses against the GI.1 VLP, it was evident that the older children in Cohort 1 had higher titers at baseline than the infants in Cohort 2; baseline GMTs were 319 (95% CI: 216–470) for 108 children in Cohort 1 and 22.3 (16.4–30.1) for 108 children in Cohort 2. After the first dose, there were marked increases in titers in both age groups, but the difference between the two age cohorts persisted (overall GMTs across groups at Day 29 were 5537 (4119–7442) and 1084 (887–1325) in Cohorts 1 and 2, respectively). After the second dose of HIL-214, there were further increases in GMTs that were greater in Cohort 2 than in Cohort 1. GMTs at Day 57 fell in a similar range across both cohorts, with overall GMTs across the dosage groups of 12,624 (10074–15819) and 7139 (5864–8692) in the 56 children in each cohort who received two doses. Titers waned to a similar extent five months later at Day 210. In the one dose group, titers waned more in the older children than in the infants. In infants, levels appeared to plateau except for the lowest (15/15) HIL-214 dose group. Final GI.1 GMTs overall at Day 210, irrespective of number of doses or dosage, were in a similar range in both cohorts: 2890 (2732–3518) in Cohort 1 and 1750 (1430–2142) in Cohort 2.
Figure 2. GMTs (with 95% CI) for Pan-Ig antibodies against GI.1 and GII.4c VLP antigens in the four study groups in the two age cohorts (Cohort 1, 1–≤4-year-olds; Cohort 2, 6–≤12 month-olds) after one or two doses of the four HIL-214 formulations.
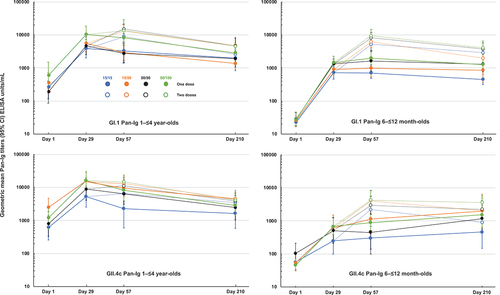
When measured against GII.4c VLP the lower baseline GMT in the infant groups was also evident compared with the older children, 62.6 (47.7–82.1) vs. 1109 (751–1638). Both age cohorts mounted immune responses after one dose of HIL-214, to 10,203 (7228–14402) and 491 (334–722) in Cohorts 1 and 2, respectively. In the older children, there was little further increase in GMT after a second dose of HIL-214 to 10,897 (8452–14048), and titers waned in all groups to a range of similar GMTs at Day 210, with an overall GMT of 3328 (2666–4155). In the infant groups in Cohort 2, there was a further increase in titers after a second dose of HIL-214; the Day 57 GMT was 3252 (2403–4401) with some evidence of a dosage-dependent response. Interestingly, in the one-dose groups GMTs appeared to continue to increase to Day 210, while they wanted in the two-dose groups, again with the exception of the lowest dose group. By Day 210 GMTs fell in a narrow range with overlapping confidence intervals, with an overall GMT for all four groups of 1564 (1179–2077).
Immunogenicity -HBGA
Histo-blood group antigen contains oligosaccharides that serve as receptors for norovirus on the surface of intestinal mucosal epithelial cells, so blocking this antigen can play a role in preventing norovirus infection.Citation14 When HBGA blocking activity was measured against the GI.1 and GII.4c VLPs, higher baseline titers were observed in the older children; GI.1 geometric mean blocking titers (GMBT50) were 26.3 (95% CI: 21.4–32.4) and 16.7 (15.2–18.5) in 114 children in Cohort 1 and 116 infants in Cohort 2, respectively. Against this background there were clear increases in GI.1 HBGA activity after one dose in both age cohorts when expressed as the SRR () or GMBTs (). There was no clear trend to increased response with higher dosage except for the observation that the lowest responses were with the lowest dosage formulation (15/15). A second dose elicited higher responses in both age groups, such that 98% of Cohort 1 and 96% of Cohort 2 had responded by Day 57; titers then waned but remained higher in the two-dose groups than in the one-dose groups. At Day 210 overall GMBT50 values against GI.1 were 74.6 (55.9–99.6) vs. 160 (126–203) in the combined one- vs. two-dose groups in Cohort 1, and 52.6 (40.3–68.7) vs. 149 (123–179) in combined one- vs. two-dose groups of Cohort 2.
Figure 3. GMTs (with 95% CI) for HBGA antibodies against GI.1 and GII.4c VLP antigens in the four study groups in the two age cohorts (Cohort 1, 1–≤4-year-olds; Cohort 2, 6–≤12 month-olds) after one or two doses of the four HIL-214 formulations.
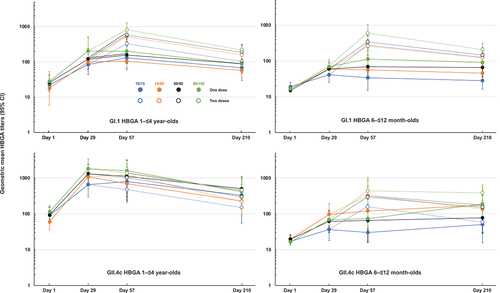
For GII.4c HBGA activity the difference between baseline titers in the two age cohorts was more evident, with GMBT50 values of 55.3 (43.2–70.8) and 18.2 (16.3–20.4) in Cohorts 1 (n = 117) and 2 (n = 107), respectively. In the older children of Cohort 1, the marked response to the first dose was not dosage sensitive, with SRR ranging from 58% to 75% across groups, which increased to 83% to 100% after the second dose (). This enhancement of the response following a second dose was not evident in the GMTs (), and the second dose had little impact on the persistence of the response. At Day 210, overall GMBT50 values against GII.4c were 237 (151–373) and 364 (2618–507) in the combined one- and two-dose groups of Cohort 1. In contrast, there was an obvious impact of a second dose in the infants in Cohort 2, which increased the overall SRR at Day 57 from 47.4% after one dose to 87.0% after a second dose () and also increased the GMBT values above those in the one-dose group for all VLP dosages (). A combination of waning titers in the two-dose groups and an apparent increase in titers in the one-dose groups as noted with the Pan-Ig values, meant that by Day 210 the second dose had a less apparent beneficial effect on GMBT values, which were 119 (72–197) and 153 (104–223) in one- and two-dose groups, respectively.
Discussion
Norovirus infection is a global health concern and is not specifically associated with low standards of hygiene or sanitation.Citation15 In countries where routine rotavirus vaccination has been introduced, norovirus has become the major cause of pediatric acute gastroenteritis, mostly affecting very young children.Citation2–6 The predominant circulating norovirus strains belong to the GII.4 genotype, which have been identified as causing the most severe and persistent illness in children.Citation16,Citation17 The GII.4c VLP, consisting of a consensus sequence of three different GII.4 strains,Citation8 has been developed to meet the medical need for a broadly effective vaccine against different GII.4 variants. This GI.1 VLP has been included in HIL-214 to cover the other major genogroup of circulating noroviruses. This study was intended to determine the optimal formulation of these two VLPS for a pediatric vaccine targeting the most vulnerable age group.
In this assessment of the clinical lot specification of the bivalent VLP norovirus vaccine candidate HIL-214, we found that one or two doses, with a range of total VLP concentrations from 30 μg to 200 μg, were well tolerated by children in two age cohorts from 6 months up to <4 years of age. The reported reactogenicity consisted of mainly mild local reactions and systemic AEs, which resolved rapidly within the surveillance period. The incidence rates were lower after a second dose, with no consistently higher incidence of AEs with the higher doses of VLP evaluated. Importantly, there were no vaccine-related serious adverse events (SAE) reported. Immune responses measured as Pan-Ig antibody seroresponse rates against the vaccine antigens GI.1 and GII.4c were evident when measured four weeks after the first vaccination, and these were generally enhanced by administration of a second dose. Responses were highest against the GI.1 VLP with two doses eliciting seroresponses in 85–100% of Cohort 1 groups and 100% of the Cohort 2 groups after two doses. Responses against the GII.4c VLP were lower and did not achieve the high levels observed against GI.1 even after two doses; SRR ranged from 67–77% to 86–100% in Cohorts 1 and 2, respectively. A similar pattern of responses was observed when assessed as HBGA-blocking antibody titers, which has been suggested to be a correlate of protection against norovirus illness,Citation14,Citation18 and there was an overall trend for HBGA responses to increase with higher vaccine doses.
In a study in Peruvian infants, Chhabra et al. found that 80% had experienced at least one norovirus infection by their first birthday.Citation19 Further, the authors noted that there was a higher likelihood of experiencing a GII infection by one year of age than for GI genotype, which may explain why the older children were more likely to display a response to the GI.1 component than to the GII.4c VLP after a second dose. Chhabra et al.Citation19 also showed that immunity against GII.4 following natural infection confers protection against other norovirus strains, including GI genotypes, whereas prior exposure to some to other genotypes can result in increased susceptibility to further infections. Vielot et al.Citation7 have confirmed this observation in a study in a birth cohort in Nicaragua with two episodes of GII infection providing protection against further homologous infections. This indicates that an early vaccination against norovirus will be of most benefit.
A notable observation from our data is the higher baseline immunity against both VLP antigens in the older cohort of children when compared with the infants, suggesting that the 1–≤4-year-olds in Cohort 1 had already been exposed to this ubiquitous virus and so had already developed some immunity. In this situation, the first vaccination was likely acting as a booster dose to the natural-acquired immunity and may explain why there was little or no effect of a second vaccination in many cases in these older children. This is consistent with observations in studies in adultsCitation11,Citation12 and in older children up to 8 years of age.Citation13 In contrast, the less exposed or unexposed infants in Cohort 2 displayed responses to the first vaccination that were more consistently enhanced by a second dose.
A purpose of this study was to select an appropriate formulation of HIL-214 for pediatric studies. The 15/50 formulation has been previously selected for adult use.Citation11,Citation12 It is clear in this pediatric study that the immune responses to a two-dose regimen of the four different formulations of HIL-214 are superior to a single-dose regimen, and it is notable that no apparent increases in reactogenicity or safety concerns with the highest dosages were observed. In selecting a candidate for further development for pediatric use, we must take into consideration the apparent background immunity of the older children and the probability that a norovirus vaccine would be of greatest benefit in the younger age group. It has also been recommended to focus in the GII.4 component of any vaccine.Citation19 With the previously observed interference of the response to GII.4 by GI.1 in adultsCitation11 an unbalanced formulation (15/50 or 50/150) would be recommend as we do not know whether the older children have all been previously infected and that the younger ones may not have been infected.
The current study confirms the overall safety and acceptable tolerability of HIL-214 formulations containing up to 200 μg of VLPs against GI.1 and GII.4c norovirus strains in children as young as 6 months of age. While quantitative differences in immune responses between different formulations were small, the trend is that the highest dosage tested provided the best overall response without impacting the reactogenicity. Thus, the formulation with 50 μg GI.1 and 150 μg GII.4c and 0.5 mg Al(OH)3 will be taken further forward in clinical assessment.
Noting the apparent exposure in those aged over 1 year evidenced by the high baseline immunity in Cohort 1 the target age may be younger than the 6 months of age tested here. For that reason, a further extension of this study has been performed with the same formulations in younger infants from 6 weeks to 6 months of age, with a modification of the schedule to assess whether two or three doses are necessary in very young infants. The results of this later study will be presented separately.
This study is limited most notably by the small numbers in each group, especially after splitting the four different dosage groups to assess one or two doses. However, the results do allow the identification of trends for the responses, and the lack of meaningful differences in reactogenicity between the lowest and highest dosages of VLPS administered. A further limitation is the age range of the children studied, as the data on baseline immunity suggest that immunization will be most effective in younger children before they are exposed to the virus. This will be addressed in the extension of this study as mentioned above.
In conclusion, this study demonstrates that two doses of the HilleVax norovirus vaccine candidate, HIL-214, are well tolerated by children from 6 months to 4 years of age. HIL-214 was immunogenic in this age groups, eliciting Pan-Ig and HBGA-blocking antibodies. These data support the further investigation of the administration schedule and dosage of HIL-214 in younger infants.
Supplemental Material
Download PDF (193.4 KB)Acknowledgments
We are grateful to all the participants and their parents, and to all the staff at each of the study centers at CEVAXIN Plaza Carolina, Panama, and the Centro de Estudios en Infectología Pediátrica S.A.S., Colombia. We thank Keith Veitch (Keithveitch Communications, Amsterdam, the Netherlands) for preparing a first draft and editorial management of the manuscript funded by HilleVax.
(1) This work is supported by the US Army Medical Research and Materiel Command under Contract No. W81×WH-15-C-0063
(2) The views, opinions, and/or findings contained in this report are those of the author(s) and should not be construed as an official Department of the Army position, policy, or decision unless so designated by other documentation.
(3) The investigator(s) adhered to the policies regarding the protection of human subjects as prescribed by Code of Federal Regulations (CFR) Title 45, Volume 1, Part 46; Title 32, Chapter 1, Part 219; and Title 21, Chapter 1, Part 50 (Protection of Human Subjects).
Disclosure statement
TM, PMM, JS, FB and AB were full-time employees of Takeda Vaccines at the initiation and running of the study; JS and AB were full-time employees of HilleVax Inc. at the time of this report. Other authors have no conflicts to declare.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2204787
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinjé J, Parashar UD. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008;14(8):1–9. doi:10.3201/eid1408.071114.
- Hoa Tran TN, Trainor E, Nakagomi T, Cunliffe NA, Nakagomi O. Molecular epidemiology of noroviruses associated with acute sporadic gastroenteritis in children: global distribution of genogroups, genotypes and GII.4 variants. J Clin Virol. 2013;56(3):269–77. doi:10.1016/j.jcv.2012.11.011.
- Payne DC, Vinje J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, Hall CB, Chappell J, Bernstein DI, Curns AT, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med. 2013;368(12):1121–30. doi:10.1056/NEJMsa1206589.
- Kõivumägi K, Toompere K, Soeorg H, Kallas E, Jõgeda EL, Huik K, Lutsar I. Acute gastroenteritis hospitalizations after implementation of universal mass vaccination against rotavirus. Vaccine. 2020;38(13):2879–86. doi:10.1016/j.vaccine.2020.01.098.
- Halasa N, Piya B, Stewart LS, Rahman H, Payne DC, Woron A, Thomas L, Constantine-Renna L, Garman K, McHenry R, et al. The changing landscape of pediatric viral enteropathogens in the post–rotavirus vaccine era. Clin Infect Dis. 2020 Feb 3;72(4):ciaa100. doi:10.1093/cid/ciaa100.
- Quee FA, de Hoog MLA, Schuurman R, Bruijning-Verhagen P. Community burden and transmission of acute gastroenteritis caused by norovirus and rotavirus in the Netherlands (RotaFam): a prospective household-based cohort study. Lancet Infect Dis. 2020;20(5):508–606. doi:10.1016/S1473-3099(20)30058-X.
- Vielot NA, Reyes Y, Blette B, González F, Toval-Ruiz C, Gutiérrez L, Vílchez S, Diez-Valcarce M, Vinjé J, Becker-Dreps S, et al. First episodes of norovirus and sapovirus gastroenteritis protect against subsequent episodes in a Nicaraguan birth cohort. Epidemiology. 2022;33(5):650–3. doi:10.1097/EDE.0000000000001500.
- Parra GI, Bok K, Taylor R, Haynes JR, Sosnovtsev SV, Richardson C, Green KY. Immunogenicity and specificity of norovirus consensus GII.4 virus-like particles in monovalent and bivalent vaccine formulations. Vaccine. 2012;30(24):3580–6. doi:10.1016/j.vaccine.2012.03.050.
- Treanor JJ, Atmar RL, Frey SE, Gormley R, Chen WH, Ferreira J, Goodwin R, Borkowski A, Clemens R, Mendelman PM. A novel intramuscular bivalent norovirus virus-like particle vaccine candidate—Reactogenicity, safety, and immunogenicity in a phase 1 trial in healthy adults. J Infect Dis. 2014;210(11):1763–71. doi:10.1093/infdis/jiu337.
- Atmar RL, Baehner F, Cramer JP, Song E, Borkowski A, Mendelman PM, NOR-201 Study Group. Rapid responses to 2 virus-like particle norovirus vaccine candidate formulations in healthy adults: a randomized controlled trial. J Infect Dis. 2016;214(6):845–53. doi:10.1093/infdis/jiw259.
- Leroux-Roels G, Cramer JP, Mendelman PM, Sherwood J, Clemens R, Aerssens A, De Coster I, Borkowski A, Baehner F, Van Damme P. Safety and immunogenicity of different formulations of norovirus vaccine candidate in healthy adults: a randomized, controlled, double-blind clinical trial. J Infect Dis. 2018;217(4):597–607. doi:10.1093/infdis/jix572.
- Atmar RL, Baehner F, Cramer JP, Lloyd E, Sherwood J, Borkowski A, Mendelman PM, Al-Ibrahim MS, Bernstein DL, Brandon DM, et al. Persistence of antibodies to 2 virus-like particle norovirus vaccine candidate formulations in healthy adults: 1-year follow-up with memory probe vaccination. J Infect Dis. 2019;220(4):603–14. doi:10.1093/infdis/jiz170.
- Vesikari T, Saez-Llorens X, Blazevic V, Lopez P, Lopez E, Masuda T, Mendelman PM, Liu M, Sherwood J, Baehner F, et al. Immunogenicity of a bivalent virus-like particle norovirus vaccine in children from 1 to 8 years of age: a phase 2 randomized, double-blind study. Vaccine. 2022;40(26):3588–96. doi:10.1016/j.vaccine.2022.04.089.
- Reeck A, Kavanagh O, Estes MK, Opekun AR, Gilger MA, Graham DY, Atmar RL. Serological correlate of protection against Norovirus-induced gastroenteritis. J Infect Dis. 2010;202(8):1212–18. doi:10.1086/656364.
- Ahmed SM, Hall AJ, Robinson AE, Verhoef L, Premkumar P, Parashar UD, Koopmans M, Lopman BA. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(8):725–30. doi:10.1016/S1473-3099(14)70767-4.
- Bhavanam S, Freedman SB, Lee BE, Zhuo R, Qiu Y, Chui L, Xie J, Ali S, Vanderkooi OG, Pang XL, et al. Differences in illness severity among circulating norovirus genotypes in a large pediatric cohort with acute gastroenteritis. Microorganisms. 2020;8(12):1873. doi:10.3390/microorganisms8121873.
- Saito M, Goel-Apza S, Espetia S, Velasquez D, Cabrera L, Loli S, Crabtree JE, Black RE, Kosek M, Checkley W, et al. Multiple norovirus infections in a birth cohort in a Peruvian periurban community. Clin Infect Dis. 2014;58(4):483–91. doi:10.1093/cid/cit763.
- Atmar RL, Bernstein DI, Lyon GM, Treanor JJ, Al-Ibrahim MS, Graham DY, Vinjé J, Jiang X, Gregoricus N, Frenck RW, et al. Serological correlates of protection against a GII.4 norovirus. Clin Vaccine Immunol. 2015;22(8):923–9. doi:10.1128/CVI.00196-15.
- Chhabra P, Rouhani S, Browne H, Yoir PP, Sala MS, Olortegui MPO, Moulton LH, Kosek MN, Vinje J. Homotypic and heterotypic protection and risk of reinfection following natural norovirus infection in a highly endemic setting. Clin Infect Dis. 2021;72(2):222–9. doi:10.1093/cid/ciaa019.