ABSTRACT
Hand, foot and mouth disease is a common acute viral infectious disease that poses a serious threat to the life and health of young children. With the development of an effective inactivated EV71 vaccine, CA16 has become the main pathogen causing HFMD. Effective and safe vaccines against this disease are urgently needed. In our previous study, a bivalent inactivated vaccine was shown to have good immunogenicity and to induce neutralizing antibodies in mice and monkeys. Repeated administration toxicity is a critical safety test in the preclinical evaluation of vaccines. In this study, BALB/c mice were used to evaluate the toxicity of the bivalent vaccine after multiple intradermal administrations. Clinical observation was performed daily, and body weight, food intake, hematological characteristics, serum biochemical parameters, antinuclear antibodies, CD4+/CD8a+ T-cell proportions, bone marrow smear results and pathology results were recorded. The results showed that there was no significant change at the injection site and no adverse reactions related to the vaccine. The bivalent inactivated EV71-CA16 vaccine exhibits good safety in mice, and these results provide a sufficient basis for further clinical trials.
Introduction
Hand, foot, and mouth disease (HFMD) is an acute infectious disease caused by an enterovirus. It is mainly characterized by fever and herpes or herpetic pharyngobuccal inflammation of the hands, feet and mouth.Citation1 It mostly occurs in infants under 5 years old.Citation2 The main pathogens causing HFMD are enterovirus 71 (EV71) and coxsackievirus A16 (CA16).Citation3 With the successful development of the inactivated EV71 vaccine, the number of cases of HFMD caused by EV71 has decreased each year, and CA16 has become the main pathogen causing HFMD.Citation4 Vaccines are among the most effective measures to prevent infectious diseases, so the need to develop a bivalent EV71-CA16 vaccine is particularly urgent. In a previous study, we developed a bivalent inactivated vaccine against EV71-CA16, which induced neutralizing antibodies in both mice and monkeys after intradermal immunization.Citation5,Citation6 A viral challenge study also showed that the bivalent inactivated vaccine successfully protected mice from wild-type EV71/CA16 infection.Citation5 Preclinical safety vaccine evaluation is an essential and critical step for clinical trials and vaccine marketing. Repeated administration toxicity is a safety test that describes the toxicity characteristics of animals after repeated administration of a vaccine.Citation7–9 BALB/c mice are widely used for drug safety evaluation, with clear genetic background data and sufficient domestic supply. These mice have also been used in previous immunogenicity studies of bivalent vaccines. Compared to muscle immunization, intradermal immunization has significant advantages, requiring a lower vaccine dose and stronger immune persistence. In a previous study, intradermal immunization addressed the poor protective effect of CA16 vaccines. In this study, the safety of the bivalent inactivated EV71-CA16 viral vaccine (human diploid cells), including the impact on immune organs and other toxicity target organs and the reversibility of toxicity, was evaluated in BALB/c mice after intradermal immunization. The results can be used to provide a sufficient experimental basis for clinical studies and predict possible adverse reactions when the vaccine is used in a large population.
Materials and methods
Vaccine production
The bivalent inactivated EV71-CA16 vaccine (human diploid cells) was generated by inactivation of EV71 (FY-23 strain, GenBank: EU812515.1)Citation10 and CA16 (KM/M08 strain, GenBank: MN046208).Citation11 Briefly, the EV71/CA16 viruses were inoculated into human embryonic lung diploid fibroblast cells (KMB17) for viral proliferation. The EV71- or CA16-harvested viral stock was concentrated and purified. The purified solution was inactivated by adding formaldehyde and then concentrated again to obtain EV71 or CA16 antigen solution. The inactivated EV71 and CA16 antigens were emulsified in 0.015 mg/ml Al(OH)3 adjuvant in equal amounts (1:1) and then mixed with glycine and PBS buffer.
Mice
Specific pathogen-free (SPF) BALB/c mice (6–9 weeks old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd; half of the mice were male (M) and half were female (F). The animal production license number was SCXK (Beijing) 2016–0006, and the weight range was M, 17.97–24.75 g, and F, 14.42–20.43 g. All the mice were fed in a barrier environment, with a temperature control of 20–26°C, humidity control of 40–70%, and free access to water and food. Quarantine and domestication were carried out before the formal test. The quarantine period was 3 days, and the domestication period was 5 days. The parameters monitored during the quarantine period included appearance, body shape, movement, breathing, hair, nose, mouth, eyes, ears, genitals, urine, and feces. The animal ethics committee (IACUC) of Shandong Xinbo Drug Research Co., Ltd. (IACUC approval No.: XB-IACUC-2019–0172) approved the experimental protocols.
Study design
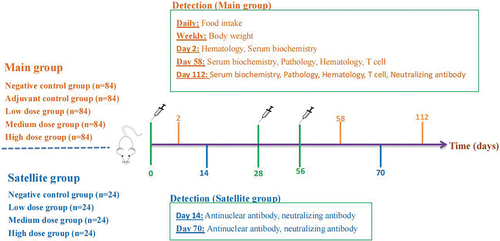
A total of 516 SPF mice were randomly divided into two groups; half of the mice in each group were male and half were female. The main experimental group was divided into five groups: the negative control group; the adjuvant control group; and the bivalent vaccine low-dose group (50 U), medium-dose group (100 U) and high-dose group (200 U). There were 84 mice in each group (M 42/F 42). The adjuvant control group was given 0.015 mg/ml Al(OH)3, and the negative control group was given 0.9% sodium chloride. Four satellite groups were used: the negative control group and the bivalent vaccine low-dose group (50 U), medium-dose group (100 U) and high-dose group (200 U), with 24 mice in each group (M 12/F 12) (). In a previous immunogenicity study, the vaccine was administered twice with an interval of 28 days. According to the guiding principles for safety evaluation, the vaccine should be administered at least once more, on days 0, 28 and 56, for a total of 3 times (except for animals planned to be used for autopsy). Before inoculation, the hair on the backs of the mice was removed, and the exposed area was as large as possible. The vaccine was injected intradermally at 4 points, with 50 µl at each injection site. After inoculation, all mice were observed once in the morning and once in the afternoon each day for half an hour each time. Body weight and 24 h food intake were measured once per week for the main experimental group. Two days after the first administration in the main group (F 6/M 6 in each group), hematological and serum biochemical analyses were performed. Two days after the last administration and 56 days after the last administration, hematological features, serum biochemical parameters, CD4+/CD8a+ T cells and pathological characteristics were investigated in the main group (F 9/M 9 in each group). Neutralizing antibody tests were performed only on day 56 in the main group. Antinuclear antibody and neutralizing antibody tests were performed on days 14 and 70 in the satellite group (F 6/M 6 in each group) ().
Figure 1. Schematic depicting the experimental process.
Tested indicators
Clinical observation
On administration days, the animals were monitored 2–3 times a day. The animals were observed before administration in the morning, 0.25–0.5 h after administration and once in the afternoon. When the vaccine was administered in the afternoon, the animals were observed once before administration and 0.25–0.5 h after administration. The animals were observed twice a day, in the morning and in the afternoon, on nonadministration days, and any abnormal symptoms were recorded during the observation.
Body weight and food intake
All surviving animals in the main group were measured once a week, and the 24 h food intake was measured once a week. During the determination, each animal was given sufficient feed, and the amount of remaining feed was measured after 24 ± 0.5 h; the rest of the time, food was freely available. The formula for calculating the food intake of each animal was as follows: food intake = (given amount – remaining amount)/number of animals.
Hematological examination
On days 2, 58 and 112, the main groups of mice fasted overnight and were anesthetized by subcutaneous injection of tiletamine hydrochloride and zolazepam hydrochloride 50 mg/kg into the back of the neck. Blood (0.3–0.5 ml) was collected from the abdominal vein and treated with EDTA for anticoagulation. The number of red blood cells (RBCs), hematocrit (HCT) level, hemoglobin content (HGB), mean hemoglobin content (MCH), mean hemoglobin concentration (MCHC), leukocyte count (WBC), platelet count (PLT), leukocyte classification count (LYM, NEU, BASO, EOS, MONO), and reticulocyte percentage (RETIC%) were determined with a blood analyzer.
Serum biochemistry
The group, collection time point and collection method were the same as those used for the hematological examination. Blood (0.7–0.9 ml) was collected from the abdominal vein and put into an anticoagulant-free plastic tube. It was stored at room temperature and centrifuged at 3000 rpm for 10 min. The serum was collected and tested at the clinical laboratory. The detection indexes included aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin (TBIL), total protein (TP), albumin (ALB), urea nitrogen (BUN), creatinine (CREA), total cholesterol (CHOL), triglyceride (TGL), glucose (GLU), creatine kinase (CK), potassium (K) and sodium (NA).
CD3+CD4+ and CD3+CD8a+ T-cell detection
On days 2 and 112 in the main group, the mice were anesthetized using the same method as described above. Blood (0.2–0.3 ml) was collected from the abdominal vein and added to an anticoagulant tube with heparin. The anticoagulant was fully shaken. According to the reagent instructions, the number of CD3+CD4+ cells and CD3+CD8a+ cells was detected with a Guava EasyCyte flow cytometer. The proportion of CD3+CD4+/CD3+CD8a+ cells among lymphocytes was analyzed with Guavasoft software.
Antinuclear antibody detection
On days 14 and 70, the satellite group animals were anesthetized using the same method as described above. Blood (0.5–0.8 ml) was collected from the abdominal vein, placed into anticoagulant-free plastic tubes at room temperature, and centrifuged at 3000 rpm for 10 min. The serum was separated and analyzed by the indirect immunofluorescence method, and the remaining serum was used for neutralizing antibody detection.
Neutralizing antibody detection
On days 14 and 70 in the satellite group and on day 112 in the main group, 0.3 ml of blood was collected, placed into a nonanticoagulant plastic tube, centrifuged at 3000 rpm for 10 min, and separated from the serum for antibody detection. Briefly, EV71 or CA16 virus was diluted to 2 × 103 CCID50/ml and added to a 96-well plate (50 μl/well). Serial dilutions of mouse serum (1:4, 1:8, 1:16, 1:32, 1:64… 1:024) were added to 96-well plates (50 μl/well) containing EV71 or CA16 virus. After incubation at 37°C for 2 h, Vero cell suspension (2.5 × 105/ml) was added to the well. The plates were incubated in a 5% CO2 incubator at 37°C for 7 days to observe pathological changes. The neutralizing antibody titer was calculated according to the Reed Muench method.Citation12
Bone marrow smear examination
On days 58 and 112 in the main group, the assessment of bone marrow smears was performed by Wright staining. Bone marrow smear cytology is a commonly used examination method in clinical hematology. It usually involves extracting the bone marrow and then smearing it on a slide, mainly to determine the proliferation of nuclear cells in the body.
Pathological examination
On days 58 and 112 in the main group, all mice were killed by bleeding after anesthesia, and visual observations and materials were collected during autopsy. (1) The following organs were weighed wet: brain, lung, thymus, heart, liver (including gallbladder), spleen, kidney, adrenal gland, testis, epididymis, ovary, and uterus. Tumors were also weighed wet when found. The organ coefficient was calculated as organ wet weight g/body weight 10 g. The left and right sides of the same organ were measured. (2) Pathological examination was performed to evaluate the following: all weighed organs, spinal cord (neck, chest and waist), pituitary, trachea, esophagus, submandibular gland, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, pancreas, aorta, skin (abdomen), mammary glands (female), vagina, sciatic nerve, bladder, optic nerve, sternum (including bone marrow), femur (including bone marrow), muscle (skeletal muscle), submaxillary lymph node, mesenteric lymph node, inguinal lymph node, fallopian tube, seminal vesicle gland, eyeball, Harderian gland (left), thyroid (including parathyroid gland), prostate, tongue, local administration site and other organs and tissues with changes. The testis, epididymis and eyeball were fixed with Davidson’s solution. After 24 h of fixation, these organs were moved to 10% formalin solution for fixation. Other organs were fixed with 10% formalin solution. Then, they were embedded in paraffin, sectioned, stained with H-E and evaluated by microscopy.
Statistical analysis
Body weight and body weight growth rate of animals (body weight growth rate = (body weight -body weight before medication)/body weight before medication × 100%)), food intake, hematology, serum biochemical indicators, CD3+CD4+/CD3+CD8a+ T cells, organ coefficients and other measurement data as well as histopathological results and other count data were recorded with Excel software (2010). Compared with the negative or adjuvant control group, the administration groups were statistically analyzed with multiple group tests in ToxStat 2006, * P < .05, ** P < .01. The statistical methods used for measurement data were as follows. (1) First, the Bartlett test method was used to test the data uniformity. If the data were uniform (P > .05), analysis of variance (F test) was carried out; if the result of the Bartlett test was significant (P ≤ .05), the Kruskal – Wallis test was performed. (2) If the test result of analysis of variance was significant (P ≤ .05), the Dunnett parameter test method was further used for multiple comparison tests; if the analysis of variance was not significant (P > .05), the statistical analysis was complete. (3) If the result of the Kruskal – Wallis test was significant (P ≤ .05), Dunnett’s nonparametric test was further used as a multiple comparison test; if the result of the Kruskal – Wallis test was not significant (P > .05), the statistical analysis was complete.
Results
Clinical symptoms
During the study, the negative control group was generally in good condition. The animals in the adjuvant control group and each vaccine group began to exhibit nodules at the site of injection on day 30. On day 38 after administration, the proportions of nodules in the adjuvant group, low-dose group, medium-dose group and high-dose group were 68% (41/60), 85% (51/60), 92% (55/60) and 95% (57/60), respectively (). During the recovery period in the administration interval, the mice gradually recovered. On day 49, the proportion of nodules decreased gradually to 42% (25/60), 58% (35/60), 55% (33/60) and 50% (30/60) in the adjuvant group, low-dose group, medium-dose group and high-dose group, respectively (). On day 56, the proportion of nodules had decreased to 20% (12/60), 13% (8/60), 15% (9/60) and 8% (5/60) in the same groups (). Some mice still did not recover by autopsy on day 112. The nodules of the mice were not significantly different between the adjuvant group and the vaccine group. The nodules at the administration site may be caused by the adjuvant aluminum hydroxide, which had nothing to do with the vaccine itself.
Table 1. Number of nodules at the injection site of mice.
Body weight and food intake
Compared with that in the negative control group, the body weight growth rate of male animals in the low-dose group and medium-dose group was high in the fourth week (P < .01), and the body weight growth rate of female animals was high in the sixth week (P < .05). In the high-dose group, only the body weight growth rate of male animals was higher in the fourth week (P < .01). The body weight growth rate of animals exhibited a statistically significant difference during this period, but this difference did not appear continuously (). This may have been caused by physiological fluctuations in the mice.
Table 2. Statistical data of weight growth rate of mice.
Compared with that in the negative control group, the food intake of male animals in the low-dose group was higher in the first and seventh weeks (P < .01), and the food intake of female animals was low in the 17th week (P < .05). In the medium-dose group, the food intake of male animals was higher in the first and seventh weeks and the 9th week (P < .01), while that of female animals was lower in the eighth week (P < .05). In the high-dose group, the food intake of male animals in the first and seventh weeks (P < .01) and the 9th week was high (P < .05), and the food intake of female animals in the 17th week was low (P < .05) (). None of the above significant differences appeared continuously, and there were no time or dose correlations. These differences may have been caused by physiological fluctuations in the mice that were not related to the vaccine.
Table 3. Statistical data of food intake of experimental animals.
Hematological examination
Compared with those of the negative control group, in the adjuvant control group, the RBC and HGB values of male animals were low (P < .05) on day 2, and the RETIC% values of female animals were high (P < .05). In the low-dose group, the WBC value of male animals was high (P < .01), and the RETIC% value of female animals was high (P < .05). In the medium-dose group, the WBC (P < .05) and NEU values (P < .01) of male animals were high. In the high-dose group, the WBC, NEU and LYM values of male animals were high (P < .01). On day 58, the NEU value of male animals in the adjuvant control group was high (P < .05); in the low-dose group, the EOS value of male animals was high (P < .01), and in the high-dose group, the RBC and HCT values of male animals were high (P < .05). The above differences were statistically significant. On day 112, no abnormalities were found in any of the hematological examination indexes in each group ().
Table 4. Statistical data of hematological examination of mice.
All of the above results show that there was no time correlation between the values on day 2 and day 58. There was no time correlation between each administration group and no vaccine dose correlation. It is speculated that this may be due to the large fluctuations in the data for animals in the negative control group, which revealed no toxicity and was of physical significance.
Biochemical index detection
On day 2, compared with the negative control group, in the adjuvant control group, the GLU and K values of male animals were high (P < .05). In the medium-dose group, the K value of male animals was high (P < .01). In the high-dose group, the TP and K values of male animals were high (P < .01), and the GLU values of female animals were low (P < .01), with a statistically significant difference. On day 58, in the medium-dose group, the ALP and ALB values of male animals were low (P < .01), and the ALP values of female animals were low (P < .05). In the high-dose group, the ALB value of male animals was low (P < .05). Two days after the first administration, the GLU and K values of animals in the administration group were abnormal, but no changes were found in animals in each group two days after the last administration, which may be due to the stress response of animals and not related to the vaccine (). Combined with the data obtained after the first and last administration, it was found that the value fluctuated greatly, and the trends of the dose groups were inconsistent, so these differences were not related to the vaccine.
Table 5. Serum biochemical statistics of mice.
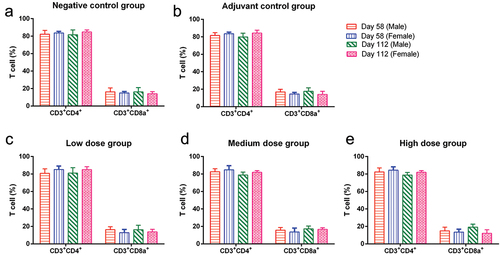
CD3+CD4+ and CD3+CD8a+ T-cell examination
Generally, the CD4/CD8 ratio fluctuates within a certain range, but when the body’s immune function is abnormal, this ratio will exceed the normal range. After vaccination, testing this indicator can directly reflect whether the immune function of mice is normal. On days 58 and 112, the ratio of CD3+CD4+/CD3+CD8a+ T cells in each group was normal ().
Figure 2. CD3+CD4+ and CD3+CD8a+ T-cell counts and ratios in mice.
Antinuclear antibodies
To check whether mice develop autoimmune diseases after vaccination, we conducted antinuclear antibody testing. In the study, the results of the antinuclear antibody test in each group were negative (data not shown).
Neutralizing antibody
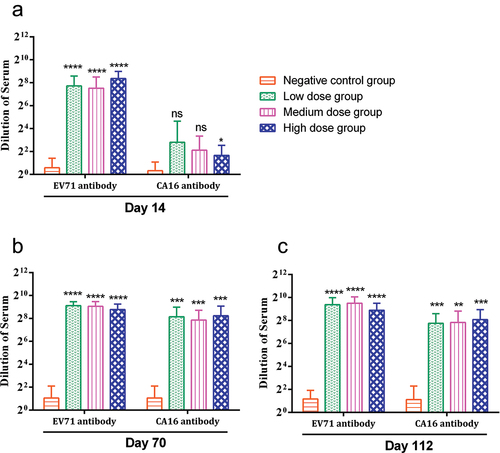
During the study, EV71 and CA16 antibodies were negative in the negative control group. Regarding the EV71 antibody in the vaccine group, animals in each group generated obvious antibodies on day 14, and the antibody titer range was 1:184–1:331, which was significantly different from that of the negative control group (P < .0001). With the increase in administration times, the antibody titers of animals in each group increased on days 70 and 112, with values of 1:439–1:555 and 1:476–1:727, respectively, which were significantly different from those in the negative control group (P < .0001). No obvious CA16 antibody was produced in any group 14 days after the first administration, and the antibody titer ranged from 1:3–1:7 (high-dose group, P < .5). With the increase in administration day, the antibody titer of animals in each group increased on day 70, ranging from 1:231–1:301, which was significantly different from that of the negative control group (P < .001); on day 112, the antibody titer decreased slightly, and the titer range was 1:216–1:270, which was significantly different from that of the negative control group (P < .001) (). The bivalent vaccine produced neutralizing antibody levels in mice after two immunizations.
Figure 3. Neutralizing antibody in mice at different time points.
Pathological examination
On day 58, the organ coefficients of all tissues of the animals in each group were not significantly abnormal. However, on day 112, only the body weight coefficients of the lung and thymus of female animals in the adjuvant control group were low, which was significantly different from those of the negative control group and had no correlation with the vaccine.
The results of gross autopsy showed that the proportion of gray‒white nodules in the adjuvant control group and in the low-, medium- and high-dose groups was 17% (3/17), 72% (13/18), 56% (10/18) and 67% (12/18), respectively. During the recovery period, except for that in the adjuvant control group, the proportion of gray‒white nodules in the vaccine group gradually decreased, and the proportions in the low-, medium- and high-dose groups were 58% (7/12), 33% (4/12) and 50% (6/12), respectively ().
Table 6. Number of gray white nodules in mice on days 58 and 112.
On day 58, the results obtained for the local irritant response showed that, except for those of the negative group, the local manifestations of animals in each group were degeneration and necrosis of muscle fibers in the subcutaneous muscle layer of the back, infiltration of inflammatory cells, basophilic particles with cell fragments, proliferation of macrophages, phagocytosis of basophilic particles and proliferation of fibrous tissue. In the negative control group, blood vessel wall necrosis, bleeding, inflammatory cell infiltration and histopathological changes related to the injection were observed, and the severity of lesions was very mild ().
Figure 4. Pathological results of inoculation site skin and inguinal lymph nodes.
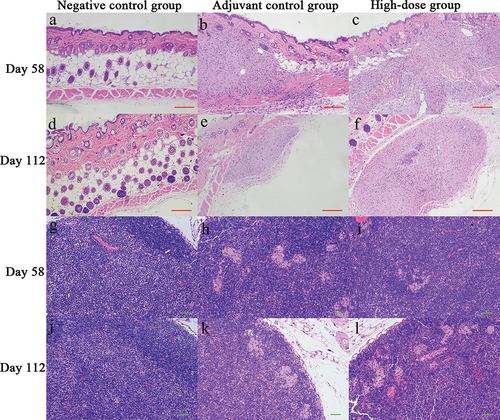
Compared to the observations made on day 58, the adjuvant control group and each vaccine group had major local changes, and the severity of lesions decreased on day 112, suggesting that the above changes can be gradually recovered after withdrawal. However, the recovery of inguinal lymph nodes was relatively slow. On days 58 and 112, macrophages phagocytizing basophilic particles were observed in the inguinal lymph nodes of animals in the adjuvant control group and each vaccine group, and the severity of lesions was mild (). These changes were considered to be related to the administration of the adjuvant and vaccine in the mice.
Discussion
In recent years, HFMD, a common acute viral infectious disease in children, has been spreading in the Asia Pacific Rim region and increasing year by year, posing a serious threat to the life and health of infants and young children.Citation1,Citation13,Citation14 With the development of a successful inactivated EV71 vaccine,Citation15 although the proportion of severe cases and mortality have decreased each year, the infection rate remains at a relatively high level and presents some new characteristics, such as the continuous emergence of severe cases caused by CA16 and patients with repeated infections.Citation16,Citation17 Vaccines have always been one of the most effective means to prevent infectious diseases. Given this serious situation, it is particularly important to develop a bivalent inactivated EV71-CA16 vaccine.Citation18 In our previous study, the bivalent inactivated EV71-CA16 vaccine had good immunogenicity in animals and produced neutralizing antibodies.Citation5,Citation6 According to guiding principles, the evaluation of nonclinical safety must be carried out before vaccines are applied in clinical trials, and repeated administration toxicity testing is one of the key studies.Citation19 Through repeated administration toxicity tests, the possible clinical adverse reactions caused by the vaccine can be predicted, including the dose-effect and time-effect relationship.Citation20 Second, this test can also reveal the toxicity target organ or target tissue after repeated administration of the vaccine.Citation21 Moreover, it can also determine the initial dose for the first clinical trial and provide a safe dose range for clinical trials.Citation22
According to current guidelines, the repeated administration toxicity test should include no less than three dose groups (low, medium and high) and one vehicle (0.9% NaCl in this study) control group. In addition, the high dose should cause obvious toxicity in animals. In principle, the low dose is equivalent to or higher than the equivalent dose for animal efficacy or the clinical dosage. The medium dose should be set between the high dose and the low dose in combination with the characteristics of toxicity to investigate the toxicity dose – response relationship.Citation23 According to the characteristics of the vaccine and the previous immunogenicity data, three doses of 200 U, 150 U and 50 U were selected,Citation5,Citation6 and the 0.9% NaCl negative group and aluminum hydroxide adjuvant group were included as controls.
In this study, BALB/c mice were injected intradermally 3 times. Fifty-six days after the last injection, the possible toxic reactions and severity of the intradermal bivalent inactivated EV71-CA16 viral vaccine (human diploid KMB17 cells) and whether there was delayed toxicity or reversibility of toxicity were observed. Through the above experimental study, a large amount of research data was obtained. Some of these data were measurement data, such as body weight, food intake, hematological indexes and serum biochemical indexes, and some were count data, such as the number of animals with toxic reactions and abnormal pathological examination after administration. Each data point is important. The purpose of the preclinical safety evaluation is to identify possible drug-related toxicity. Due to the length limitations of this article, it is impossible to describe each index in detail. This study only analyzed and discussed the key data.
BALB/c mice are widely used in drug safety evaluations, and they have clear background data and sufficient availability.Citation24 In addition, BALB/c mice were used in the preliminary immunogenicity evaluation of the bivalent inactivated EV71-CA16 vaccine in our previous study.Citation5 When using mice as experimental animals, the administration volume should be considered. According to the available guidelines, the vaccination dose should achieve the best immune response, or a high dose should be used directly in clinical trials for long-term toxicity testing. However, due to the limited administration volume, it is difficult to reach the clinical dose requirements with the inoculation dose used for some small animals. In such cases, the test can be carried out by multipoint administration. Therefore, multipoint administration on the backs of the mice was adopted. Each animal was administered the vaccine at 4 points, and the volume administered at each point did not exceed 50 μl. The cumulative administration volume was 0.2 ml/animal. Although the multipoint administration method can solve the problem of the limited administration volume in mice, there are 12 administration points for each mouse. Due to individual differences in mice and other factors, intradermal administration can cause nodules at the administration site of individual mice, and it is difficult to recover from these nodules. Multipoint administration further increases the probability of nodules, which may be the reason why nearly 50% of the nodules at the administration site of animals had not recovered 55 days after the last administration. The gross anatomical examination showed that there were gray‒white nodules under the skin at the administration sites. As nodules occurred in both the adjuvant control group and the administration group, the probability of occurrence was roughly the same, and the aluminum hydroxide adjuvant may have stimulated the formation of skin nodules, which is unrelated to the vaccine itself.Citation25
To collect and analyze the data, a main group and a satellite group were used in our study. The main group was mainly used for toxicity observation, physiological indexes and pathological examination, and the satellite group was used for antinuclear antibody and neutralizing antibody evaluation.Citation26 The two groups had their own research focus, which makes the research data more comprehensive and detailed. During the hematological examination, the EOS and MONO values of the animals in the main test group were abnormal, and the trend of the changes between different doses was inconsistent, with opposite changes occurring in different stages, which may have been due to physiological fluctuations in the animals and not related to the vaccine. In addition, the animals in the administration group at each time point showed increases or decreases in individual indexes. The numerical changes between different doses were inconsistent, and there was no correlation between time and dose. For example, high WBC and NEU values were observed in the male animals of the administration group 2 days after the first administration and the adjuvant control group 2 days after the last administration, which may be caused by the adjuvant or vaccine treatment. No obvious abnormality was found in any of the hematological indexes 56 days after the last administration. The above analysis showed that repeated intradermal inoculation of the bivalent inactivated EV71-CA16 vaccine caused a local irritant reaction, and some hematological and serum biochemical indexes were abnormal, which may be caused by the adjuvant or vaccine.
Conclusions
The results of the repeated administration toxicity test in BALB/c mice injected intradermally with a vaccine for the prevention of HFMD showed that there were no obvious abnormalities in indexes except for hematological changes caused by the adjuvant, the local irritant reaction caused by the adjuvant and the phagocytosis of basophilic particles by inguinal lymph node macrophages in the adjuvant control and administration groups. The bivalent inactivated EV71-CA16 vaccine showed good safety in mice.
Author contributions
S.F. and Q.L. designed the studies and wrote the original paper. H.Z., G.J. and Y.Z. performed the neutralization assays. J.Y., Q. J, C.L., M.S., B.L. and L.S. performed the animal studies. Q.W., T.N. and N.P. analyzed the data. X.H., Y.L. and C.Z. conducted the HE experiments. Y.L., L.W. and L.Y. performed the immunological assays. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Esposito S, Principi N. Hand, foot and mouth disease: current knowledge on clinical manifestations, epidemiology, aetiology and prevention. Eur J Clin Microbiol Infect Dis. 2018;37(3):391–12. doi:10.1007/s10096-018-3206-x.
- Li D, Su M, Sun P-P, Guo W-P, Wang C-Y, Wang J-L, Wang H, Zhang Q, Du L-Y, Xie G-C. Global profiling of the alternative splicing landscape reveals transcriptomic diversity during the early phase of enterovirus 71 infection. Virology. 2020;548:213–25. doi:10.1016/j.virol.2020.06.011.
- Liu SL, Pan H, Liu P, Amer S, Chan T-C, Zhan J, Huo X, Liu Y, Teng Z, Wang L, et al. Comparative epidemiology and virology of fatal and nonfatal cases of hand, foot and mouth disease in mainland China from 2008 to 2014. Rev Med Virol. 2015;25(2):115–28. doi:10.1002/rmv.1827.
- Yu S, Liao Q, Zhou Y, Hu S, Chen Q, Luo K, Chen Z, Luo L, Huang W, Dai B, et al. Population based hospitalization burden of laboratory-confirmed hand, foot and mouth disease caused by multiple enterovirus serotypes in Southern China. PLoS One. 2018;13(12):e0203792. doi:10.1371/journal.pone.0203792.
- Fan S, Liao Y, Jiang G, Wang L, Zhao H, Yu L, Xu X, Li D, Zhang Y, Li Q. Efficacy of an inactivated bivalent vaccine for enterovirus 71 and coxsackievirus A16 in mice immunized intradermally. Vaccine. 2021;39(3):596–604. doi:10.1016/j.vaccine.2020.11.070.
- Fan S, Liao Y, Jiang G, Jiang L, Wang L, Xu X, Feng M, Yang E, Zhang Y, Cui W, et al. Study of integrated protective immunity induced in rhesus macaques by the intradermal administration of a bivalent EV71-CA16 inactivated vaccine. Vaccine. 2020;38(8):2034–44. doi:10.1016/j.vaccine.2019.12.057.
- Forster R. Study designs for the nonclinical safety testing of new vaccine products. J Pharmacol Toxicol Methods. 2012;66(1):1–7. doi:10.1016/j.vascn.2012.04.003.
- Sun Y, Gruber M, Matsumoto M. Overview of global regulatory toxicology requirements for vaccines and adjuvants. J Pharmacol Toxicol Methods. 2012;65(2):49–57. doi:10.1016/j.vascn.2012.01.002.
- Barrow P. Developmental and reproductive toxicity testing of vaccines. J Pharmacol Toxicol Methods. 2012;65(2):58–63. doi:10.1016/j.vascn.2011.12.001.
- Liu L, Zhang Y, Wang J, Zhao H, Jiang L, Che Y, Shi H, Li R, Mo Z, Huang T, et al. Study of the integrated immune response induced by an inactivated EV71 vaccine. PLoS One. 2013;8(1):e54451. doi:10.1371/journal.pone.0054451.
- Fan S, Liao Y, Lian Y, Jiang G, Jiang L, Dong C, Yang E, Wang L, Xu X, Feng M, et al. Role of innate lymphoid cells and dendritic cells in intradermal immunization of the enterovirus antigen. NPJ Vaccines. 2019;4(1):14. doi:10.1038/s41541-019-0108-6.
- Pizzi M. Sampling variation of the fifty percent end-point, determined by the Reed-Muench (Behrens) method. Hum Biol. 1950;22:151–90.
- Huang J, Liao Q, Ooi MH, Cowling BJ, Chang Z, Wu P, Liu F, Li Y, Luo L, Yu S, et al. Epidemiology of recurrent hand, foot and mouth disease, China, 2008–2015. Emerg Infect Dis. 2018;24(3)432. doi:10.3201/eid2403.171303.
- Zhuang ZC, Kou Z-Q, Bai Y-J, Cong X, Wang L-H, Li C, Zhao L, Yu X-J, Wang Z-Y, Wen H-L. Epidemiological research on hand, foot, and mouth disease in Mainland China. Viruses. 2015;7(12):6400–11. doi:10.3390/v7122947.
- Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z, Zhang Y, Li Y, Mao Q, Wang J, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med. 2014;370(9):829–37. doi:10.1056/NEJMoa1303224.
- Saguil A, Kane SF, Lauters R, Mercado MG. Hand-foot-and-mouth disease: rapid evidence review. Am Fam Physician. 2019;100:408–14.
- Jia J, Kong F, Xin X, Liang J, Xin H, Dong L, Jiang F. Epidemiological characteristics of hand, foot, and mouth disease outbreaks in Qingdao, 2009-2018. Iran J Public Health. 2021;50(5):999–1008. doi:10.18502/ijph.v50i5.6117.
- He X, Zhang M, Zhao C, Zheng P, Zhang X, Xu J. From monovalent to multivalent vaccines, the exploration for potential preventive strategies against hand, foot, and mouth disease (HFMD). Virol Sin. 2021;36(2):167–75. doi:10.1007/s12250-020-00294-3.
- CDE, 2008 (China). The general principles for the technical review of pre-clinical safety evaluation of biological products for prevention.
- Verdier F, Barrow PC, Burge J. Reproductive toxicity testing of vaccines. Toxicology. 2003;185(3):213–9. doi:10.1016/S0300-483X(02)00611-X.
- Lebron JA, Wolf JJ, Kaplanski CV, Ledwith BJ. Ensuring the quality, potency and safety of vaccines during preclinical development. Expert Rev Vaccines. 2005;4(6):855–66. doi:10.1586/14760584.4.6.855.
- Baldrick P. Dose site reactions and related findings after vaccine administration in safety studies. J Appl Toxicol. 2016;36(8):980–90. doi:10.1002/jat.3314.
- Wang Z, Zhou C, Gao F, Zhu Q, Jiang Y, Ma X, Hu Y, Shi L, Wang X, Zhang C, et al. Preclinical evaluation of recombinant HFMD vaccine based on enterovirus 71 (EV71) virus-like particles (VLP): immunogenicity, efficacy and toxicology. Vaccine. 2021;39(31):4296–305. doi:10.1016/j.vaccine.2021.06.031.
- Salari S, Sharifi I, Keyhani AR, Ghasemi Nejad Almani P. Evaluation of a new live recombinant vaccine against cutaneous leishmaniasis in BALB/c mice. Parasit Vectors. 2020;13(1):415. doi:10.1186/s13071-020-04289-7.
- Nakayama T. An inflammatory response is essential for the development of adaptive immunity-immunogenicity and immunotoxicity. Vaccine. 2016;34(47):5815–8. doi:10.1016/j.vaccine.2016.08.051.
- Huang Z, Jiang Q, Wang Y, Yang J, Du T, Yi H, Li C, Li Y, Wu Z, Fan S, et al. SARS-CoV-2 inactivated vaccine (Vero cells) shows good safety in repeated administration toxicity test of Sprague Dawley rats. Food Chem Toxicol. 2021;152:112239. doi:10.1016/j.fct.2021.112239.
