?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The study evaluates the outcomes of including varicella vaccines (VarV) in the local expanded programme on immunization (EPI) on the seropositivity rates and corresponding protective effects for children aged 3–6 years in Suzhou. The study is observational. Varicella prevalence in children was assessed based on data from the China Information System for Disease Control and Prevention (CISDCP) and the Jiangsu Province Vaccination Integrated Service Management Information System (JPVISMIS). Seropositivity was determined using the enzyme-linked immunosorbent assay (ELISA). A total of 2,873 children aged 3–6 years were enrolled in this study. The seropositivity rates were 95.31% and 86.89% for children with and without the strategy, respectively. The difference in seropositivity rate in children using the different strategies was statistically significant (Trend χ2 = 0.397, P = .255). It is therefore suggested that Suzhou had a high rate of occult infection before the inclusion of varicella vaccine in the EPI. The difference in seroprevalence rate between children with no history of varicella vaccination and those with a history of varicella vaccination was statistically different (χ2 = 51.362, P < .001). The positive rates of antibodies increased with increasing doses of vaccination (χ2 = 56.252, P < .001). For the protective effect of one-dose and two-dose, it was found that the protection rates of one-dose were 72.98% and 100.00%, respectively. The varicella vaccine is an effective method to prevent varicella disease, which can increase serum seroprevalence levels and block the transmission of varicella disease.
Introduction
Varicella is a self-limiting disease caused by VZV.Citation1 Varicella is characterized by pruritic varicella rash and generalized papules, and is common in children during winter and spring. A research study reported that more than 90% of varicella cases in China occurred in children under 15 years of age, with preschool children being of particular concern.Citation2
Varicella vaccine, an effective method of preventing varicella disease, can reduce the incidence of varicella. One study showed that before the varicella vaccine was widely available, approximately 5.5 million cases of varicella in Europe were reported each year,Citation3 where 80% of unvaccinated children and adolescents in the region showed positive serum anti-VZV IgG.Citation4 Before the varicella vaccine was widely available in the United States, approximately 4 million cases of varicella were reported each year.Citation5 Since 1996, when one-dose of vaccinations was introduced for children 12–18 months of age, the incidence of varicella decreased by about 90% in 2005.Citation6
World Health Organization (WHO) statistics reveal that at least 140 million people worldwide are infected with VZVCitation7 each year, posing a severe social and economic burden.Citation8 WHO had also recommended that countries include varicella vaccines in routine childhood immunization strategies.Citation9
Suzhou was the first prefecture-level city in China to include varicella as a legally reported infectious disease on June 1, 2017. Jiangsu province included varicella in the management of a reported infectious disease in the following month of 2017. The suspected, clinically diagnosed cases and laboratory-confirmed cases of varicella found through the statutory reporting units of infectious disease and responsible epidemic reporters are reported concerning the requirements for reporting infectious disease epidemics in category C. The public health emergencies of varicella are reported concerning the relevant requirements for reporting public health emergencies. When confirmed as a varicella outbreak, the Center for Disease Control and Prevention (CDC) should collect blood specimens and pathogenic specimens from at least 5 cases in the early stage of the outbreak, and samples from all cases should be collected if the number of cases is less than 5.
Suzhou included varicella vaccination in the EPI on April 1, 2018, for one-dose and started January 1, 2020, for two-dose, which means children can get two-dose of varicella vaccines for free under the strategy. However, the varicella vaccine is not yet included in the Chinese national immunization program’s childhood immunization schedule.
Relevant conclusions were drawn by comparing differences in seropositivity rates between non-vaccinated and vaccinated groups and between different doses. The relationship between varicella seroprevalence rate and varicella vaccination in school-age children will be evaluated to discuss the significance of varicella vaccination and provide a scientific basis for the optimization and adjustment of varicella disease prevention and control strategies by relevant departments.
Material and methods
Subjects and sample sampling
In June 2020, 2,873 kindergarten students aged 3–6 years in Suzhou Industrial Park and Wuzhong district (Jiangsu, China) were invited to participate in the current study when they received an annual physical examination. The blood samples were collected for anti-VZV IgG testing. Informed consent was obtained from the parents of the children after explanation of the aims of the study.
By checking the varicella vaccination status and morbidity in children, we found that, among the studied 2,873 children, 1,009 children had not yet received a varicella vaccine, 974 children had received only one-dose, and 890 children had received two-dose. Among children with varicella disease, twenty-three of the children had not received varicella vaccine and six of the children had received one-dose.
This study used multi-stage sampling. According to the administrative division, Suzhou was divided into urban and rural areas. Participants were randomly selected from kindergartens in urban and rural areas, and a total of 2,873 cases were included in this study.
The study protocol was approved by the ethics committees of Suzhou CDC, per the ethical guidelines of the 1975 Declaration of Helsinki.
Varicella vaccination strategy in Suzhou
Suzhou included one-dose in the EPI on April 1, 2018, and two-dose on January 1, 2020. The children within the strategy were born on or after January 1, 2016. Children need to be 12 months old and less than 7 years old on the date of one-dose of vaccination. To receive two-dose of vaccination, children must be 4 years old and must have received one-dose more than three months prior.
Children outside and within the strategy
The target population for the implementation of free varicella vaccination in Suzhou is children born after January 1, 2016, and according to this strategy, we divided the children into outside and within strategy vaccination groups in this study.
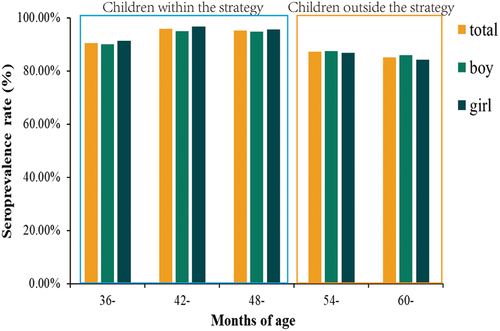
Up to the sampling time, the strategy primarily benefited children younger than 54 months of age. Therefore, we consider children younger than 54 months of age (children 36–53 months of age) to be in the outside strategy vaccination group, and children older than or equal to 54 months of age as part of the within strategy vaccination group.
Assays to detect anti-VZV IgG antibody
ELISA
The samples were centrifuged, and serum was extracted and stored frozen. Samples were tested using the anti-VZV IgG antibody test kit (Beier Biotechnology, Beijing, China), and the presence or absence of anti-VZV IgG in the samples was determined using absorbance (ΔA) of the ELISA test according to the manufacturer’s instructions. The results of each test were independent, and the results were judged using threshold values. Specimens with an ΔA value less than the threshold value were considered negative, and vice versa.
Vaccine protection rate
Children in the study were divided into control and vaccinated groups according to whether or not they had a history of varicella vaccination. Vaccine protection rates were calculated based on varicella prevalence in 2,873 children.
Public health emergency event
The occurrence of public health emergency events (PHEEs) may cause serious damage to public health. The current standard for PHEE is ≥10 cases of varicella (including clinically diagnosed cases) occurring in a kindergarten and other collective units within 1 week.
Statistical analysis
This study used Microsoft Excel 2016 and R 4.1.3 for data cleaning and statistical analysis. χ2 tests or trends χ2 were used to compare the differences in seropositivity rates among children of different ages. It is also used to compare the differences in their seroprevalence rates and incidence following one-dose and two-dose vaccination. In this study, P < .05 was considered statistically significant.
Results
Demographic characteristics
Of the 2,873 studied children aged 3–6 years, 1,442 were boys and 1,431 were girls. A total of 909 (63.04%) boys and 955 (66.73%) girls within the cohort had received varicella vaccines. The difference is not statistically significant (χ2 = 0.1043, P = .7467). The relevant information is shown in .
Table 1. Gender, age and number of morbidities in the study population.
The median age of children with no history of varicella vaccination was 53 months (Quartile: 47–59 months), while the median age of children with one-dose was 48 months (Quartile: 42–54 months), and the median age of children with two-dose was 51 months (Quartile: 48–54 months).
Comparison differences in seropositivity rates between children within and outside the strategy
We collected information on children within and outside the strategy and found that there were 1,050 boys and 1,083 girls within the strategy and 392 boys and 348 girls outside the strategy. The seroprevalence rate was 95.31% (2,033/2,133) within the strategy and 86.89% (643/740) outside the strategy. The relevant information is shown in .
Table 2. Basic information about the children within and outside the strategy.
From shows that the overall seropositivity rate was higher within the strategy group than outside the strategy group. It is probably because the children are the target group within the strategy in Suzhou, and the number of vaccinated children is higher. Analysis of the overall seroprevalence rates of children of all ages using Trend χ2 revealed that the difference in seropositivity rates among children in different age groups was not statistically significant (Trend χ2 = 0.397, P = .255). We suggest that before VarV was included in the EPI in Suzhou, there was a high rate of occult varicella infection in children.
Relationship between varicella vaccination history and seroprevalence rate
shows that 95.65% (1783/1864) of children with a history of varicella vaccination had varicella antibodies present in blood samples, while 88.50% (893/1009) of children without a history of varicella vaccination had varicella antibodies present. We analyzed children with different varicella vaccination histories. Firstly, we roughly divided children into vaccinated and unvaccinated groups based on whether they received VarV or not. The difference in seroprevalence rate between children with no history of varicella vaccination and those with a history of varicella vaccination is statistically significant (χ2 = 51.362, P < .001).
Table 3. Relationship between the presence or absence of varicella vaccination history and seroprevalence rate.
Because different doses of varicella vaccination produce different antibody levels, we grouped children for varicella vaccination and compared the differences in varicella antibody positivity between groups. In , the number of positive blood samples in children with no history of varicella immunization, children with one-dose immunization, and children with two-dose immunization were 893, 921, and 862, respectively. And the seroprevalence rates were 88.5%, 94.56%, and 96.85%, respectively. The difference in antibody positivity between the three strategies was compared using the χ2 test and was found to be statistically significant (χ2 = 56.252, P < .001), indicating that seropositivity rates gradually increased with increasing doses of vaccination.
Table 4. Seroprevalence rate at different doses of VarV.
Incidence of varicella in children, vaccine protection rate
Twenty-nine of the 2,873 children in this study had varicella disease, of which 23 were without varicella vaccination and 6 with varicella vaccination. compares the difference in incidence between vaccinated and unvaccinated children. The incidence of children who received one-dose or two-dose was compared with those of children who did not receive VarV, and the differences were found to be statistically significant.
Table 5. Comparison of case and control groups with varicella vaccination, varicella vaccine protective effect.
We evaluated the protection rate of different doses of VarV and found that one-dose had a protection rate of 72.98%, while the protection of two-dose was 100%. Two-dose vaccination was more effective than one-dose vaccination.
Impact of vaccine strategies on varicella PHEEs
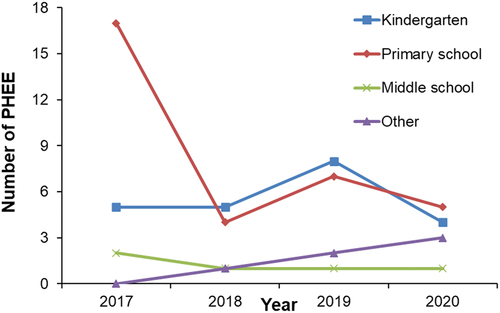
The changes in varicella outbreak PHEEs under different vaccine strategies were analyzed to guide the government in optimizing vaccination procedures. As shown in , the overall number of varicella PHEEs decreased since the beginning of free one-dose of vaccinations in 2018, and the total number of varicella PHEEs in Suzhou was lowest after two-dose of vaccinations became freely available in 2020, especially in kindergarten and primary school children. It should be noted that the numbers of varicella PHEEs in other places continued to increase.
Discussion
The current study reports a statistically significant difference in seroprevalence between children with and without a history of varicella vaccination (χ2 = 51.362, P < .001). Notably, seroprevalence rate had a statistically significant difference (χ2 = 56.252, P < .001) among three immunization strategies (no varicella vaccination, one-dose vaccination, and two-dose vaccination), suggesting that varicella vaccination produces higher seropositivity rates than natural infection and that varicella vaccination. Children can achieve a higher seroprevalence rate with two-dose, therefore two-dose can be an effective means of preventing varicella disease and protecting children.
The change in the incidence of varicella in Suzhou reflects the effect of vaccination. Zhang et al.’s study support the conclusion that the incidence of varicella decreased significantly after the inclusion of two-dose in the EPI in Suzhou compared with the one-dose vaccination.Citation10
When the overall seroprevalence rates for children in different age groups were analyzed, no statistically significant difference was found (Trend χ2 = 0.397, P = .255). This suggests a high rate of occult varicella infection before the inclusion of VarV in the Suzhou EPI. The difference in seroprevalence between the outside and within the strategy groups of children (χ2 = 0.389, P = .533) supports this outcome.
There are similar results in other related studies.Citation11
In a survey of the seropositivity rates of children aged 4–6 years in Suzhou in 2017, it was found that among unvaccinated children, there was no difference in the seropositivity rate for children aged 4–6 years (Trend χ2 = 8.681, P = .070).Citation12 In contrast, among children who had completed varicella vaccination, the detection of seropositivity rates increased with age. A cross-sectional survey conducted by the Jiangsu Provincial CDC in 2018 on the seron-epidemiological characteristics in children aged 1–9 yearsCitation10 reported that the varicella vaccination rate was only 43.1%. The seroprevalence rate of children vaccinated with one-dose was 57.1%. Antibody levels in children gradually decrease over time, and the decline in antibody levels may result in the desired protective effect. Therefore, timely vaccination with two-dose can provide better protection for children.
We check the incidence of varicella in these children through the CISDCP and found that 29 children had varicella. Of these children who had varicella, 23 had not been vaccinated against varicella and 6 had received one-dose of VarV. None of the children who had received two-dose of VarV had varicella disease. Six children with varicella disease following one-dose of vaccination were varicella breakthrough cases. In contrast, no breakthrough cases occurred with two-dose of vaccination, suggesting that children who received only one-dose were more likely to develop breakthrough cases than those who received two-dose.Citation13 Regarding breakthrough cases in children who received one-dose and two-dose, two-dose provided better protection and reduced the number of breakthrough cases to some extent.Citation14–16 One-dose significantly reduced the incidence of varicella but did not prevent local viral circulation and outbreaks, whereas two-dose of vaccination further reduced the number of cases and outbreaks of varicella disease.Citation9,Citation17,Citation18
Depending on the number of doses of VarV given to children, we divide them into children who receive one-dose and children who receive two-dose. We calculated the VPR for both groups. It was found that one-dose was 72.98% protective, while the protectiveness of two-dose was up to 100.00%.
At the same time, we could observe the changes in the number of varicella PHEEs under different immunization strategies in Suzhou. The occurrence of varicella PHEEs can reflect the effect of incorporating VarV into Suzhou’s EPI. We analyzed the distribution of places, where PHEEs occurred in Suzhou from 2017–2020. By looking at the graph, we see a significant decrease in the number of varicella PHEEs in primary school, which may be due to the establishment of an immunization barrier after a significant increase in the vaccination rate of children around 6 years of age in Suzhou and their successful entry into primary school within 1–2 years, reducing the incidence of PHEEs. In other areas where varicella vaccination is free, the number of varicella outbreaks also declined.Citation19–21
The anti-VZV IgG assay used in this study is not the gold standard for detecting anti-VZV IgG, the gold standard is the fluorescent antibody to membrane antigen (FAMA).Citation22 The lower specificity of ELISA compared with FAMACitation22 may cause some bias in the results. Secondly, the relatively short time since including varicella vaccines in the Suzhou EPI, and the small proportion of the total population benefiting from this strategy, may cause a relatively limited protective effect on the overall population. Finally, the purpose of this study is to investigate the seroprevalence survey of varicella vaccine in 3–6 -year-olds, which not only includes the positive rate, but also more importantly includes the antibody level.
In summary, the study outcome shows that including varicella vaccination in the Suzhou EPI resulted in 72.98% protection of the population by one-dose and 100.00% protection of the population by two-dose. Compared to the one-dose vaccination, two-dose vaccination is better in reducing breakthrough cases and protecting the population. China should consider including varicella vaccines in the national immunization program to minimize, moderate, and prevent varicella disease.
Authors’ contribution
Zhuoyu Zhang and Yunyan Zhang contributed equally as joint senior authors. Lin Luan, Jun Zhang, and Na Liu conceived and designed the study. Chihua Qian provided biological samples. Chen Dong performed experiments. Zhuoyu Zhang and Jiangtao Yu analyzed the data. Zhuoyu Zhang wrote the manuscript. Lin Luan and Yunyan Zhang reviewed all versions of the article before submission and during the version. All authors reviewed and approved the final manuscript.
Acknowledgments
We wanted to thank the technicians from the Suzhou Center for Disease Control and Prevention, China National GeneBank, Suzhou Biobank, and School of Public Health, Medical College of Soochow University, who assisted with data collection and blood sample collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Freer G, Pistello M. Varicella-zoster virus infection: natural history, clinical manifestations, immunity and current and future vaccination strategies. New Microbiol. 2018;41:1–6.
- Yang Y, Geng X, Liu X, Wang W, Zhang J. Association between the incidence of varicella and meteorological conditions in Jinan, Eastern China, 2012–2014. BMC Infect Dis. 2016;16(1):179. doi:10.1186/s12879-016-1507-1.
- Riera-Montes M, Bollaerts K, Heininger U, Hens N, Gabutti G, Gil A, Nozad B, Mirinaviciute G, Flem E, Souverain A, et al. Estimation of the burden of varicella in Europe before the introduction of universal childhood immunization. BMC Infect Dis. 2017;17(1):353. doi:10.1186/s12879-017-2445-2.
- Wiese-Posselt M, Siedler A, Mankertz A, Sauerbrei A, Hengel H, Wichmann O, Poethko-Müller C. Varicella-zoster virus seroprevalence in children and adolescents in the pre-varicella vaccine era, Germany. BMC Infect Dis. 2017;17(1):356. doi:10.1186/s12879-017-2461-2.
- Seward JF. Varicella disease after introduction of varicella vaccine in the United States, 1995-2000. JAMA. 2002;287(5):606. doi:10.1001/jama.287.5.606.
- Guris D, Jumaan AO, Mascola L, Watson BM, Zhang JX, Chaves SS, Gargiullo P, Perella D, Civen R, Seward J. Changing varicella epidemiology in active surveillance sites—United States, 1995–2005. J Infect Dis. 2008;197(s2):S71–5. doi:10.1086/522156.
- Hu P, Yang F, Li X, Wang Y, Xiao T, Li H, Wang W, Guan J, Li S. Effectiveness of one-dose versus two-dose varicella vaccine in children in Qingdao, China: a matched case-control study. Hum Vaccin Immunother. 2021;17(12):5311–5. doi:10.1080/21645515.2021.1982281.
- Xu Y, Liu S, Che X, Liu Y, Zhang X, Du J, Zhang X, Wang J, Xu E. Seroepidemiology of varicella in Hangzhou, China in the vaccine era. Hum Vaccin Immunother. 2018;14(10):2464–71. doi:10.1080/21645515.2018.1477909.
- Varicella and herpes zoster vaccines: WHO position paper, June 2014 – recommendations. PLoS One. 2014;34(2):198–9. doi:10.1016/j.vaccine.2014.07.068.
- Zhang L, Ma W, Liu Y, Wang Y, Sun X, Hu Y, Deng X, Lu P, Tang F, Wang Z, et al. Analysis of sero-epidemiological characteristics of varicella in healthy children in Jiangsu Province, China. BMC Infect Dis. 2018;18(1):563. doi:10.1186/s12879-018-3496-8.
- Kurukulasooriya GMPCP, Thevanesam V, Agampodi SB, Abeykoon AMSB, Amarasiri SP, Goonasekera KPC. Susceptibility of new entrant university students in Sri Lanka to varicella zoster infection. Asia Pac J Public Health. 2010;22(2):219–24. doi:10.1177/1010539509334625.
- Luan L, Shen X, Qiu J, Jing Y, Zhang J, Wang J, Zhang J, Dong C. Seroprevalence and molecular characteristics of varicella-zoster virus infection in Chinese children. BMC Infect Dis. 2019;19(1):643. doi:10.1186/s12879-019-4233-7.
- Yasui Y, Mitsui T, Arima F, Uchida K, Inokuchi M, Tokumura M, Nakayama T. Changes in epidemiological characteristics and sero-prevalence against the varicella zoster virus in school-age children after the introduction of a national immunization program in Japan. Hum Vaccin Immunother. 2021;17(8):2494–500. doi:10.1080/21645515.2021.1890968.
- Sun X, Zhu Y, Sun H, Xu Y, Zhang L, Wang Z. Comparison of varicella outbreaks in schools in China during different vaccination periods. Hum Vaccin Immunother. 2022;18(6):2114255. doi:10.1080/21645515.2022.2114255.
- Andrade AL, da Silva Vieira MA, Minamisava R, Toscano CM, de Lima Souza MB, Fiaccadori F, Figueiredo CA, Curti SP, Nerger MLBR, Bierrenbach AL, et al. Single-dose varicella vaccine effectiveness in Brazil: a case-control study. Vaccine. 2018;36(4):479–83. doi:10.1016/j.vaccine.2017.12.011.
- Suo L, Lu L, Wang Q, Yang F, Wang X, Pang X, Marin M, Wang C. Varicella outbreak in a highly-vaccinated school population in Beijing, China during the voluntary two-dose era. Vaccine. 2017;35(34):4368–73. doi:10.1016/j.vaccine.2017.06.065.
- Quinn HE, Gidding HF, Marshall HS, Booy R, Elliott EJ, Richmond P, Crawford N, McIntyre PB, Macartney KK. Varicella vaccine effectiveness over 10 years in Australia; moderate protection from 1-dose program. J Infect. 2019;78(3):220–5. doi:10.1016/j.jinf.2018.11.009.
- Pan X, Shu M, Ma R, Fang T, Dong H, Sun Y, Xu G. Varicella breakthrough infection and effectiveness of 2-dose varicella vaccine in China. Vaccine. 2018;36(37):5665–70. doi:10.1016/j.vaccine.2018.05.025.
- Suo L, Li J, Zhao D, Yang F, Liu W, Wu J, Pang X, Deng Y, Lu L. Effect evaluation of a 2 dose varicella vaccine immunization strategy implemented to control outbreaks in school and kindergarten settings. Chin J Prev Med. 2015;49(6):485–9. doi:10.3760/cma.j.issn.0253-9624.2015.06.006.
- Wang J, Xu Z, Gao Q. Varicella outbreaks in schools and kindergartens in Shanghai, China from 2011 to 2020. PLoS One. 2022;17(6):e0270630. doi:10.1371/journal.pone.0270630.
- Leung J, Lopez AS, Marin M. Changing epidemiology of varicella outbreaks in the United States during the varicella vaccination program, 1995–2019. J Infect Dis. 2022;226(Supplement_4):S400–6. doi:10.1093/infdis/jiac214.
- Plotkin SA, Orenstein WA, Offit PA, editors. Plotkin’s vaccines. 7th ed. Philadelphia (PA): Elsevier; 2018.