ABSTRACT
Since March 2020, the pandemic caused by SARS-CoV-2 has affected nearly all aspects of daily life. In this study, we investigated the age-stratified prevalence and genotype distribution of human papillomavirus (HPV) among females in Shandong province (eastern China) and aimed to provide guidance on HPV-based cervical cancer screening and vaccination. The distribution of HPV genotypes was analyzed using PCR-Reverse Dot Hybridization. The overall infection rate of HPV was 16.4%, which was dominated by high-risk genotypes. The most prevalent genotype was HPV16 (2.9%), followed by HPV52 (2.3%), HPV53 (1.8%), HPV58 (1.5%), and HPV51 (1.3%). Among the positive cases with HPV infection, single-genotype infection was significantly higher than that of multi-genotype infection. In subgroup analyses by age (≤25, 26–35, 36–45, 46–55, >55), HPV16, 52, and 53 were consistently the three most common hrHPV genotypes in all age groups. The infection rate of multi-genotypes in the ≤25 and >55 age groups was significantly higher than that in other age groups. A bimodal distribution of HPV infection rate was observed in different age groups. Among lrHPV genotypes, HPV6, HPV11, and HPV81 were the three most common types in the ≤25 age group, while in other age groups, HPV81, HPV42, and HPV43 are the three most common lrHPV genotypes. This study provides basic information on the distribution and genotypes of HPV in the female population in eastern China, which could improve the application of HPV diagnostic probes and vaccines.
Introduction
Cervical cancer is one of the important causes of cancer deaths in women worldwide. The global burden of cervical cancer is heavy, and the prevention and control situations remain to be a big challenge for public health and medical care. Cervical cancer affects millions of women worldwide, with an estimated incidence of 604,200 new cases and 310,000 deaths in 2020 worldwide and 109,700 new cases and 59,700 deaths in China.Citation1 Cervical cancer is the second malignant tumor threatening the life and health of women in China, and it is also the only malignant tumor that can be eliminated through tertiary preventive measures. Recently, the incidence of cervical cancer continued to increase with a trend of increasing occurrence in younger populations. At the same time, China has gradually entered an aging stage, and consequently, the burden of cervical cancer in the elderly population increases consequently.Citation2
Studies have shown that the occurrence of cervical cancer is closely associated with human papillomavirus (HPV), and persistent infection with high-risk human papillomavirus (hrHPV) is one of the important factors resulting in cervical cancer.Citation3 HPV is a closed circular double-stranded DNA virus that can lead to the proliferation of squamous epithelium of skin mucosal tissues. HPV has a variety of genotypes, which can be divided into low-risk and high-risk types according to their risk of developing cancer. Low-risk genotypes including HPV6, 11, 81 are generally not carcinogenic, but can cause low-grade squamous intraepithelial lesions (LSIL) and anogenital warts (condyloma cumulus). The genotypes of hrHPV include HPV16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, and infection with hrHPV is closely associated with cervical cancer and precancerous lesions.Citation4,Citation5
In November 2020, the “Global strategy to accelerate the elimination of cervical cancer” was officially launched, proposing to achieve the “90-70-90” target by 2030 (90% of girls fully vaccinated with HPV vaccine by age 15 years; 70% of women are screened with a high-performance test by 35 years of age and again by 45 years of age, 90% of women identified with cervical disease receive treatment) in order to eliminate cervical cancer (the incidence of cervical cancer is below 4/100,000) as a public health problem by the end of this century.Citation6,Citation7 From 2020 to 2021, Chinese scholars conducted a model study of strategy analysis based on the cervical cancer prevention and control situation in China, and further verified that the screening strategy using HPV DNA detection as the primary screening method combined with HPV vaccination is the optimal path to achieve cervical cancer elimination in China.Citation8–10 However, at present, China is still facing the practical problems of low coverage of cervical cancer screening, lack of advanced technology for large-scale screening, limited community service capacity, and poor accessibility of HPV screening.Citation11 HPV vaccination is an important part of the primary prevention of cervical cancer, and at present in China, HPV vaccine belongs to nonimmune program vaccines (the second type of vaccine). Vaccinations of nonimmune program vaccines are carried out in accordance with the provisions of the vaccine instruction and the principle of informed consent and voluntary self-payment. National immunization program has not yet been implemented, HPV vaccine supplies are inadequate, and vaccination rates are less than 1%.Citation12 In summary, preexisting low HPV vaccination rates combined with the impact of the Covid pandemic may have left many women of appropriate age unprotected by the HPV vaccine, thereby increasing susceptibility to HPV-related cancers.Citation13,Citation14 During the 2019 coronavirus (Covid-19) pandemic, HPV screening services have been greatly affected by the various restrictive measures put in place by the government to reduce the spread of the virus.Citation15–18 Therefore, it is expected that more domestic HPV vaccines should be commercially available and HPV vaccine price should be reduced to meet the demand and gradually achieve primary prevention.
The epidemiology of HPV infection and distribution patterns of HPV genotypes are important parameters for assessing cervical cancer risk in women.Citation19 There are differences in the genotypes of HPV infection in different regions and ethnic groups. The most common genotype that causes cervical cancer in Western countries, e.g., the United Kingdom, the United States, and Turkey, is HPV16, followed by HPV18, 45, 33, and 31. There are also differences in HPV infection among women in different regions with different economic development levels in China.Citation20 According to a systematic review by Yin et al.,Citation21 the rate of hrHPV infection caused by genotype types 16, 52, 58, 53, 18 was as high as 19.0% among women in the mainland China. Therefore, further longitudinal studies, including the prevalence and incidence screening, as well as age-stratified classification strategies in primary HPV screening, are necessary. Depending on the regional HPV epidemiology, vaccines that are more suitable for inhibiting hrHPV genotypes in the corresponding regions should be developed, making cervical cancer vaccines more effective.
This study aims to assess the prevalence and genotype distribution of HPV among women in eastern China during the COVID-19 pandemic period, and to systematically review the epidemiological model in order to provide a basis for the subsequent development of cervical cancer prevention and screening strategies and vaccine application in this region.
Materials and methods
Study population and samples
Female patients who underwent HPV screening in Third Provincial Hospital of Shandong Province from January to December 2021 and from January to December 2019 were selected as subjects. There were 13,902 cases in 2019 and 21,862 cases in 2021, with patients aged 16–92 years. Information on the patients’ HPV test results was collected retrospectively. This study was reviewed and approved by the Ethics Committee of our Hospital, the ethics approval number was KYLL-2023015. The review committee waived the need of patients’ consent due to anonymous analyses of the data. The management and publication of patient information in this study was strictly in accordance with the Declaration of Helsinki, including the confidentiality and anonymity.
Cervical sample collection
Cervical samples were collected with a dedicated cervical exfoliation cell collector. Cervix was exposed by using speculum or vaginal opener, and excessive secretions from the cervical opening were wiped off with a cotton swab. A cervical brush was placed at the opening of the cervix and rotated in one direction for 4~5 circles to obtain a sufficient sample of epithelial cells. The head of the cervical brush was placed into the elution tube, and the cervical brush handle was broken along the crease of the brush handle. The cap of the elution tube was tightened, the tube was labeled, and the elution tube was maintained in the upright position. All samples were sent to the laboratory within 12 hours, stored at 2–8°C, and tested for HPV within a week, according to the manufacturer’s instructions.
DNA extraction and HPV genotyping
HPV genotyping was performed for all collected samples by PCR-reverse dot hybridization method (Yaneng Bioscience, Shenzhen, China). DNA extraction, amplification, hybridization, membrane washing, and data interpretation were performed according to the kit instructions.Citation22 The PCR program included an initial denaturation step at 95°C for 10 min, followed by 40 cycles of 94°C for 30 s, 42°C for 90 s, 72°C for 30 s, and a final extension at 72°C for 5 min. Finally, the genotype information was obtained according to the position of the blue spot on the membrane strip. Human Papillomavirus Genotyping Kit For 23 Types (PCR-RDB) can qualitatively detect and genotype HPV. It can detect 23 HPV genotypes, including 17 high-risk types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) and 6 low-risk types (HPV6, 11, 42, 43, 81, and 83). DNA was extracted using an automatic nucleic acid extraction instrument (Yaneng Bioscience, Shenzhen, China). Amplification was performed using Roche LightCycler 480II fluorescence quantitative PCR instrument. An automatic nucleic acid molecular hybridization instrument (Yaneng Bioscience, Shenzhen, China) was used for reverse dot hybridization. Negative and positive controls were included throughout the experiment. At the same time, internal and external quality evaluations were also carried out to ensure that the results met the requirements of the laboratory.
Statistical analysis
The results were imported to an Excel spreadsheet, filtered, and stratified by age (≤25 years, 26–35 years, 36–45 years, 46–55 years, >55 years). SPSS 26 was used for data analysis, and chi-square test (χ2 test) was used to analyze the difference in HPV infection rates among different age groups. P values were two-sided at 95% CI. P value < .05 was considered statistically significant. Graphs were obtained using GraphPad Prism 9.0 software.
Results
Overall and age-stratified HPV prevalence
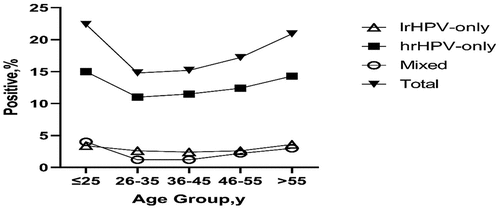
From January to December 2021, a total of 21,862 women subjects (aged from 16 to 92 years old) were tested for HPV infection in the molecular laboratory of the Third Provincial Hospital of Shandong Province. The results showed that 3,591 cases were HPV positive with an overall HPV infection rate of 16.4%. Positive rate of lrHPV-only was 2.7%, positive rate of hrHPV-only was 12.0%, and positive rate of lrHPV and hrHPV mixed infection (defined as mixed HPV) was 1.7%. The infection rate of hrHPV (including samples that are positive for hrHPV-only and positive for both hrHPV and lrHPV) was 13.7% (3005/21862), which was significantly higher than that of lrHPV (including samples that are positive for lrHPV-only and positive for both lrHPV and hrHPV) (4.4%, 965/21862) (P < .01) (). All the subjects were divided into five age groups (≤25, 26–35, 36–45, 46–55, >55), and the prevalence of HPV infection in each subgroup was calculated. As shown in , the majority of the subjects participating in the screening was between 26 and 55 years old, accounting for 86% (18723/21862). There was a bimodal distribution of HPV (lrHPV-only, hrHPV-only or mixed infection) infection rate in different age groups, with the first peak of infection rate in the age group of ≤25 years and the second peak in the age group >55 years. Similar bimodal distribution was also found in overall HPV infection rate, with infection rate of 22.4% in the age group ≤25 years old and the infection rate of 20.9% in the age group >55 years old, which were significantly higher than the overall infection rate of 16.4% in all subjects (P < .01).
Table 1. Age-stratified HPV prevalence in 21, 862 tested women.
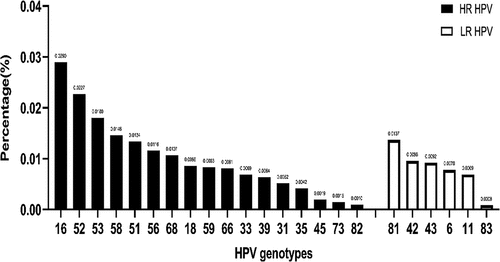
In addition, infections with hrHPV-only were very common, with an infection rate of more than 10% in all age groups, while mixed infections with rHPV and hrHPV were relatively rare, with an infection rate of less than 5% in all age groups (). The overall prevalence of each HPV genotype was shown in . The most common hrHPV genotype detected was HPV16 (2.9%), followed by HPV52 (2.3%), HPV53 (1.8%), HPV58 (1.5%), HPV51 (1.3%), HPV56 (1.2%), and HPV 68 (1.1%). As shown in , HPV18 was only the eighth most common hrHPV genotype. Furthermore, in subgroup analyses by age (≤25, 26–35, 36–45, 46–55, >55), HPV16, 52, and 53 were consistently the three most common hrHPV genotypes in all age groups, while HPV73 and 82 were the two least common hrHPV genotypes in all age groups. Among lrHPV genotypes, HPV6 (2.5%), HPV11 (1.8%), and HPV81 (1.8%) were the three most common types in the ≤25 age group (), while in other age groups, HPV81, HPV42, and HPV43 were the most common lrHPV genotypes. Among lrHPV genotypes, HPV83 had the lowest infection rate in all age groups.
Table 2. The prevalence of all HPV genotypes in 21,862 tested women.
summarized the age-stratified distribution of single and multiple (two or more) genotypes of HPV infections. Infections with single HPV genotype accounted for 75% of the 3591 HPV-positive cases. For infections with multiple HPV genotypes, there were 669 (18.6%), 169 (4.7%), 38 (1.1%), and 23 (0.6%) cases infected with double, triple, quadruple, and five or more HPV genotypes, respectively. As shown in , infection rates of multiple genotypes in the ≤25 and >55 age groups were significantly higher than those in other age groups (P < .01).
Figure 3. Proportion of infections with single, double, triple, quadruple and five or more genotypes.
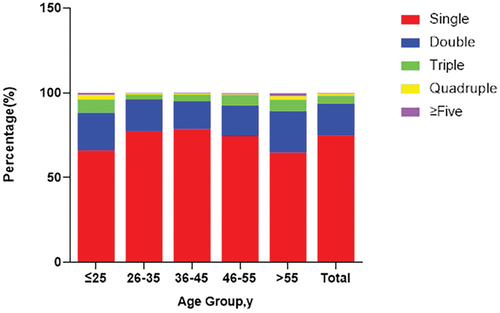
Table 3. Age-stratified distribution of single and multiple genotypes of HPV infection.
summarized the distribution of lrHPV-only, hrHPV-only, and mixed lrHPV and hrHPV in infections with single or multi-genotypes. In infections with single genotype, hrHPV-only accounted for 80%. In infections with double genotypes, hrHPV-only accounted for 58%. The proportion of mixed lrHPV and hrHPV increased significantly with the increase in the number of genotypes.
Table 4. Distribution of lrHPV-only, hrHPV-only, and mixed lrHPV and hrHPV infections in infections with different genotypes.
HPV prevalence before and after COVID-19
From January to December 2019, a total of 1,3902 women subjects were tested for HPV infection in the molecular laboratory of the Third Provincial Hospital of Shandong Province. The results shown in demonstrated that HPV prevalence in 2021 was significantly lower than that in 2019 (P < .01).
Table 5. The prevalence of HPV in the year of 2019 and 2021.
Discussion
Persistent infection with high-risk human papillomaviruses (hrHPV) contributes to the development of invasive cervical cancer. The epidemiology of HPV infection and the distribution pattern of HPV genotypes are urgently needed parameters to assess the risk of cervical cancer in women.Citation23 Treatment delays during the COVID-19 pandemic can be due to multiple factors, including a shortage of hospital resources and workforce, or due to patients choosing to limit their potential exposure to SARS-CoV-2. In practice, the delays were probably different because of varying quarantine policies in different regions.Citation15 Previous data showed that the hrHPV infection rate in women aged 25–45 was 15.5% in China. Here, we analyzed a total of 21,862 women in Shandong Province from January to December 2021, and found that the overall HPV infection rate was 16.4%. Due to different cultural backgrounds and living habits, there are differences in the genotypes of HPV infection in different regions and different ethnic groups. Therefore, the prevalence of HPV infection varies from country to country, and may even vary between regions in the same country.Citation24
In addition, we analyzed a total of 13,902 female cases in Shandong Province from January to December 2019. The overall HPV infection rate was 22.0%, which is significantly higher than that in 2021, indicating that lockdown measures in China have significantly reduced HPV transmission. During the COVID-19 pandemic,strict quarantine and antimigration measures, keeping social distance, and vaccine injections have proved to be efficient approaches to stop the propagation of this pandemic. These strict lockdown measures and the restriction of bodily contact have led to negative influences on the psychological health and routine cancer screening and treatment. Nevertheless, these measures are also probably beneficial for the control of other seasonal respiratory virus infections and sexually transmitted infections (STIs). Studies have shown that throughout the SARS-CoV-2 pandemic, there was a dramatic decline in seasonal respiratory virus infections,Citation25 while the incidence of several STIs, such as syphilis, chlamydia, and gonorrhea, has also decreased.Citation26,Citation27 The trend of STIs infection rates during the pandemic is still debatable. This discrepancy was likely due to different data sources of patients with other STIs and the varying degrees of strict isolation policies in different regions during COVID-19 pandemic. Considering that HPV infections may increase rapidly after the pandemic.Citation28 We recommend the immediate implementation of effective HPV, cervical cancer screening, and HPV vaccine programs to reduce the spread of HPV infections. The global confrontation with COVID-19 has not only diverted current healthcare resources to deal with the infection but has also resulted in increased resources in the areas of testing and screening, as well as educating most of the global public of the benefits of vaccination. When the COVID-19 pandemic eventually recedes, the opportunity must not be missed to ensure that these newly created resources are maintained and redeployed for HPV-related disease prevention and control.Citation29
In this study, we showed that HPV infection was dominated by high-risk genotypes. The infection rate of hrHPV was more than 4 times that of lrHPV, and pure hrHPV infection rate (12.0%) was higher than pure lrHPV infection rate (2.7%) and mixed HPV infection rate (1.7%). The hrHPV infection rate varies in different regions of the world: 22.1% in Africa, 11.3% in North America, 8.1% in Europe, and 8% in Asia.Citation30 The hrHPV infection rate also varies in different regions of China. In this study, the hrHPV infection rate was 13.7%, which was lower than that previously reported in Henan Luoyang (19.02%)Citation31 and Zhejiang Taizhou (17.2%),Citation32 but was higher than that previously reported in Xinjiang (9.72%),Citation33 Beijing (9.9%),Citation34 and Jiangxi (12.26%).Citation35 Among the positive cases of HPV infection, single genotype infection was significantly higher than multiple genotype infection. The ≤25 and >55 age groups had significantly higher rates of multiple genotype infections than other age groups, which are consistent with previous studies and support the hypothesis that more sexual activities in young women may be associated with the spread of multiple HPV genotype. An epidemiological study of HPV infection among women in southern Mexico showed that co-infection of hrHPV genotypes of HPV-16, HPV-18, HPV-39, HPV-52, HPV-53, HPV-66, and HPV-69 with other HPV genotypes resulted in significantly higher risk of developing LSIL, HSIL, and cervical cancer.Citation36 Lee et al.Citation37 also observed that subjects with multiple genotype HPV infections had a 31.8-fold higher risk of developing cervical cancer. Infection with multiple HPV genotypes can increase the risk of developing precancerous lesions, which may accelerate cancer development.
We divided the subjects into five groups by age (≤25 years old, 26–35 years old, 36–45 years old, 46–55 years old, >55 years old). There was a bimodal distribution of HPV infections (lrHPV-only, hrHPV-only, or mixed lrHPV and hrHPV) in different age groups, with the first infection rate peak in the ≤25 age group and the second peak in the >55 age group. A similar bimodal distribution pattern was also found for the overall HPV infection with the overall HPV infection rate in the ≤25 and >55 age groups, both of which were significantly higher than the infection rate (16.4%) in all age groups. These results are consistent with previous studies conducted in China.Citation38 In Europe and the United States, the HPV infection rate is very high under the age of 25, but in women over the age of 45, the infection rate is significantly reduced, and the infection pattern is unimodal.Citation39 Sexual activities and immature immune protection in younger women, as well as physiological and immune disturbances associated with hormonal changes during menopause in older women, could be responsible for these two peaks of HPV infection.Citation40
In addition, HPV screening is conducted mainly in the subjects between 26 and 55 years old, accounting for 86% of total subjects screened, which could be due to the fact that this age group has higher education and economic level, and health awareness.Citation41 With the improvement of medical care and living standards, the average life expectancy in China has increased from 77.0 years in 2018 to 77.3 years in 2019, indicating that China has entered an aging stage accompanied by the increased burden of cervical cancer in the elderly population.Citation42 Therefore, the prevention and control of cervical cancer in China should be emphasized, where resources permit, screening should be considered for women aged 50–65 who have never received cervical cancer screening, and more exams including cytology and even colposcopy should be performed in women aged >60 years to prevent cervical cancer.
In this study, we showed that the most prevalent hrHPV genotype was HPV16 (2.9%), followed by HPV52 (2.3%), HPV53 (1.8%), HPV58 (1.5%), and HPV51 (1.3%). In subgroup analyses by age, HPV16, 52 and 53 are consistently the three most common hrHPV genotypes in all age groups. In some other regions of China, e.g., in Luoyang of Henan Province, the most prevalent five genotypes are HPV16 (7.49%), HPV52 (3.04%), HPV58 (2.36%), HPV18 (1.65%), and HPV51 (1.61%).Citation31 In Taizhou of Zhejiang Province, the most prevalent genotype is HPV52 (19.7%), followed by HPV16 (11.9%), HPV58 (11.5%), HPV39 (7.2%), HPV18 (6.6%), and HPV56 (5.6%). For low-risk/undetermined-risk HPV genotypes, HPV53 was the most common type, with an overall prevalence of 7.8%, followed by HPV81 (7.5%), HPV61 (7.0%), HPV43 (5.9%), HPV06 (4.0%), and HPV44 (3.8%).Citation32 A survey of the prevalence of HPV genotypes in 145,918 subjects in Northwest China showed that the five most common HPV genotypes were HPV 16, 58, 52, 53, and 61 among all participants.Citation43 A study conducted by Beijing Chaoyang Hospital showed that among the 16 hrHPV genotypes, the 6 most prevalent HPV genotypes were HPV16、HPV52、HPV58、HPV18、HPV51 and HPV53, with infection rates of 23.3%, 14.8%, 13.3%, 9.8%, 9.2%, and 8.8%, respectively.Citation44 In addition, a study conducted in Hong Kong found that HPV58 (13.7%), HPV52 (11.7%), HPV53 (11.2%), HPV16 (10.0%), HPV18 (5.2%), and HPV51 (5.2%) were the 5 most prevalent hrHPV genotypes in women with abnormal cervical cytology.Citation45
In addition to cancer, anogenital warts are a global public health problem affecting young men and women and are mainly caused by two lrHPV genotypes, HPV06 and 11.Citation46 In this study, we showed that among lrHPV genotypes, HPV6, HPV11, and HPV81 were the three most common genotypes in the ≤25 years age group, while in other age groups HPV81, HPV42, and HPV43 were the three most common lrHPV genotypes. In Luoyang of Henan Province, the most important lrHPV genotypes were HPV81 (1.46%), HPV61 (1.36%), HPV54 (1.24%), HPV6 (0.58%), and HPV40 (0.53%).Citation31 These results highlight that in the development of HPV composite vaccines, HPV81-containing vaccines may be more effective in preventing HPV infection in China.
In 2017, JKD, the largest independent pathology laboratory in Shandong Province, retrospectively analyzed all cervical HPV genotyping results reported from 2011 to 2017. The results showed that among 94,489 cases, the overall HPV positive rate was 28.4%. HPV16 (5.8%), HPV52 (5.1%), HPV58 (3.5%), HPV51 (2.6%), and HPV56 (2.3%) were the five most common hrHPV genotypes, and HPV81 (2.8%), HPV53 (2.8%), and HPV6 (2.3%) were the three most common lr-/urHPV genotypes. The results indicated that infection rates of HPV53, 51, 56, and 81 are high in the population in Shandong Province.Citation47 A recent survey in Guangxi Province showed that the HPV infection rate dropped from 21.99% in 2018 to 12.26% in 2021, and the six most common hrHPV genotypes were HPV52 (4.06%), 16 (2.70%), 58 (2.24%), 51 (1.87%), 39 (1.52%), and 53 (1.52%).Citation48
Since 2017, bivalent, quadrivalent, and nine-valent vaccines have been licensed in mainland China, and domestic vaccines against HPV16 and 18 were also approved in 2019. This also means that China has entered the post-vaccine era for the prevention of cervical cancer and other related cancers.Citation49 A national, multicenter, population-based study before the application of HPV vaccine showed that the prevalence of HR-HPV and LR-HPV was 12.1% and 5.2%, respectively, and the infection peaks were 20–24 years old and >50 years old. The five most common HPV genotypes were HPV52, HPV58, HPV53, HPV16, and HPV51.Citation50 Introduction of HPV vaccines into the population may alter predominant HPV genotypes through immunogenicity, cross-protection, or possible non-vaccine genotype substitution. Both previous studies and this study found that the infection rate of HPV51 and 53 was relatively high.Citation31–Citation32–43–Citation45, Citation47–Citation48–50 The nine-valent vaccine includes HPV6, 11, 16, 18, 31, 33, 45, 52, and 58.Citation51 Studies have shown that the nine-valent HPV vaccine can achieve 90.4%–100.0% preventive effect on the persistent infection of HPV31, 33, 45, 52, and 58 in East Asian female subgroups aged 9–26 years.Citation52 There is no evidence to prove that it provides a protective effect on the common genotypes HPV51 and 53 in China. HPV6 and 11 infection rate was high in the ≤25 years age group and was relatively low in other age groups, mainly HPV81. Therefore, this study provided reference for vaccine development.
Although in the literatures cited above, these HPV types (HPV51 and HPV53) appear as the fourth, the fifth or the sixth most prevalent, or even they are not mentioned, the infection rate in the population or the incidence rate of these genotypes in tissues with epithelial lesions ranked on the top list in China, which is obviously different from the situation in West countries where the genotypes such as HPV18/31/33/45 are more popular. We should pay more attention to this to avoid the consequences that our vaccines fail to protect these genotypes, resulting in higher infection rates with these genotypes.
Conclusion
China has a vast territory, complex and diverse natural environment, and various cultural phenomena. As of the end of 2021, the resident population of Shandong Province is 102 million,it is necessary for us to investigate the HPV prevalence and genotype distribution in eastern China. We focused on investigating the infection of patients who underwent HPV testing in our hospital during the COVID-19 pandemic and compared the infection rate with that in 2019, in order to understand the latest HPV infection rate in this area and provide guidance for the follow-up prevention and treatment of cervical cancer. The relationship between HPV infection types and cervical lesions, as well as the pathogenic mechanism of HPV will be the focus of our future studies.
Author contribution statement
Conception and design, acquisition of data, or analysis and interpretation of data: All authors. Drafting the article or revising it critically for important intellectual content: All authors. Final approval of the version to be published: All authors. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: XZ. All authors read and approved the final manuscript.
Disclosure statement
The authors declare that they have no conflicts of interest.
Additional information
Funding
References
- Stelzle D, Tanaka LF, Lee KK, Ibrahim Khalil A, Baussano I, Shah ASV, McAllister DA, Gottlieb SL, Klug SJ, Winkler AS, et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health. 2021;9(2):1–9. doi:10.1016/S2214-109X(20)30459-9. PMID: 33212031.
- Wei W, Zeng H, Zheng R, Zhang S, An L, Chen R, Wang S, Sun K, Matsuda T, Bray F, et al. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. 2020;21(7):e342–9. doi:10.1016/S1470-2045(20)30073-5.
- Marima R, Hull R, Lolas G, Syrigos KN, Kgoebane-Maseko M, Kaufmann AM, Dlamini Z. The catastrophic HPV/HIV dual viral oncogenomics in concert with dysregulated alternative splicing in cervical cancer. Int J Mol Sci. 2021;22(18):10115. doi:10.3390/ijms221810115. PMID:PMC8472041.
- Bhatla N, Singhal S. Primary HPV screening for cervical cancer. Best Pract Res Clin Obstet Gynaecol. 2020;65:98–108. doi:10.1016/j.bpobgyn.2020.02.008.
- Tumban E. A current update on human papillomavirus-associated head and neck cancers. Viruses. 2019;11(10):922. doi:10.3390/v11100922. PMID:PMC6833051.
- Shapiro GK. HPV vaccination: an underused strategy for the prevention of cancer. Curr Oncol. 2022;29(5):3780–92. doi:10.3390/curroncol29050303. PMID:PMC9140027.
- World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. 2nd ed. 2021. https://www.who.int/publications/i/item/9789240030824.
- Santesso N, Mustafa RA, Schünemann HJ, Arbyn M, Blumenthal PD, Cain J, Chirenje M, Denny L, De Vuyst H, Eckert LON, et al. World health organization guidelines for treatment of cervical intraepithelial neoplasia 2-3 and screen-and-treat strategies to prevent cervical cancer. Int J Gynecol Obstet. 2016;132(3):252–8. doi:10.1016/j.ijgo.2015.07.038.
- Xia C, Xu X, Zhao X, Hu S, Qiao Y, Zhang Y, Hutubessy R, Basu P, Broutet N, Jit M, et al. Effectiveness and cost-effectiveness of eliminating cervical cancer through a tailored optimal pathway: a modeling study. BMC Med. 2021;19(1):62. doi:10.1186/s12916-021-01930-9. PMID:PMC7927373.
- Zhang J, Zhao Y, Dai Y, Dang L, Ma L, Yang C, Li Y, Kong L, Wei L, Zhang S, et al. Effectiveness of high-risk human papillomavirus testing for cervical cancer screening in China. JAMA Oncol. 2021;7(2):263. doi:10.1001/jamaoncol.2020.6575.
- Ma Y, Wang C, Liu F, Lian G, Li S, He Q, Li T. Human papillomavirus vaccination coverage and knowledge, perceptions and influencing factors among university students in Guangzhou, China. Hum Vaccin Immunother. 2021;17(10):3603–12. doi:10.1080/21645515.2021.1927411. PMID:PMC8437546.
- Hu S, Xu X, Zhang Y, Liu Y, Yang C, Wang Y, Wang Y, Yu Y, Hong Y, Zhang X, et al. A nationwide post-marketing survey of knowledge, attitude and practice toward human papillomavirus vaccine in general population: implications for vaccine roll-out in mainland China. Vaccine. 2021;39(1):35–44. doi:10.1016/j.vaccine.2020.11.029.
- Silva T, Nogueira de Sa A, Beinner MA, Abreu MNS, Matozinhos FP, Sato APS, Vieira EWR. Impact of the COVID-19 pandemic on human papillomavirus vaccination in Brazil. Int J Public Health. 2022;67:1604224. doi:10.3389/ijph.2022.1604224. PMID:PMC9008128.
- Daniels V, Saxena K, Roberts C, Kothari S, Corman S, Yao L, Niccolai L. Impact of reduced human papillomavirus vaccination coverage rates due to COVID-19 in the United States: a model based analysis. Vaccine. 2021;39(20):2731–5. doi:10.1016/j.vaccine.2021.04.003. PMID:PMC8023201.
- Luo Q, O’Connell DL, Yu XQ, Kahn C, Caruana M, Pesola F, Sasieni P, Grogan PB, Aranda S, Cabasag CJ, et al. Cancer incidence and mortality in Australia from 2020 to 2044 and an exploratory analysis of the potential effect of treatment delays during the COVID-19 pandemic: a statistical modelling study. The Lancet Public Health. 2022;7(6):e537–48. doi:10.1016/s2468-2667(22)00090-1. PMID:PMC9159737.
- Chan A, Ashbury F, Fitch MI, Koczwara B, Chan RJ. Cancer survivorship care during COVID-19-perspectives and recommendations from the MASCC survivorship study group. Support Care Cancer. 2020;28(8):3485–8. doi:10.1007/s00520-020-05544-4. PMID:PMC7247777.
- Riera R, Bagattini ÂM, Pacheco RL, Pachito DV, Roitberg F, Ilbawi A. Delays and disruptions in cancer health care due to COVID-19 pandemic: systematic review. JCO Global Oncol. 2021;7:311–23. doi:10.1200/go.20.00639. PMID:PMC8081532 manuscript.
- Ryan G, Gilbert PA, Ashida S, Charlton ME, Scherer A, Askelson NM. Challenges to adolescent HPV vaccination and implementation of evidence-based interventions to promote vaccine uptake during the COVID-19 pandemic: “HPV is probably not at the top of our list”. Prev Chronic Dis. 2022;19:E15. doi:10.5888/pcd19.210378. PMID:PMC8992683.
- Richards TB, Lindley MC, Byron SC, Saraiya M. Human papilloma virus vaccination and cervical cancer screening coverage in managed care plans - United States, 2018. Prev Med. 2022;159:107019. doi:10.1016/j.ypmed.2022.107019. PMID:PMC9117512.
- Guo C, Du H, Qu X, Duan X, Li J, Li R, Jin H, Wang C, Zhao C, Bao J, et al. Prevalence of human papillomavirus among Chinese Han and Mongols minority women in inner Mongolia, China: reflected by self-collected samples in CHIMUST. Front Public Health. 2022;10:840879. doi:10.3389/fpubh.2022.840879. PMID:PMC9174663.
- Li K, Li Q, Song L, Wang D, Yin R. The distribution and prevalence of human papillomavirus in women in mainland China. Cancer. 2019;125(7):1030–7. doi:10.1002/cncr.32003.
- Zare-Bidaki M, Zardast M, Nadjafi-Semnani A, Nadjafi-Semnani M, Javanmard D, Ghafari S, Ghanbarzadeh N. Investigation of frequency and typing of human papillomavirus among genital warts using a reverse dot blot hybridization approach. BMC Infect Dis. 2022;22(1):278. doi:10.1186/s12879-022-07276-8. PMID:PMC8941769.
- Rebolj M, Rimmer J, Denton K, Tidy J, Mathews C, Ellis K, Smith J, Evans C, Giles T, Frew V, et al. Primary cervical screening with high risk human papillomavirus testing: observational study. BMJ. 2019;364:l240. doi:10.1136/bmj.l240. PMID:PMC6364146.
- Gupta S, Purwar S, Gupta P, Halder A, Gupta A, Pushpalatha K, John JH. Burden and associated genotype patterns of high-risk human papilloma virus infection and cervical cytology abnormalities among women in Central India. Infect Dis Obstet Gynecol. 2022;2022:3932110. doi:10.1155/2022/3932110. PMID:PMC9132658.
- Batiha O, Al‐deeb T, Al‐zoubi E, Alsharu E. Impact of COVID‐19 and other viruses on reproductive health. Andrologia. 2020;52(9). doi:10.1111/and.13791.
- Ogunbodede OT, Zablotska-Manos I, Lewis DA. Potential and demonstrated impacts of the COVID-19 pandemic on sexually transmissible infections. Curr Opin Infect Dis. 2021;34(1):56–61. doi:10.1097/QCO.0000000000000699.
- Tao J, Napoleon SC, Maynard MA, Almonte A, Silva E, Toma E, Chu CT, Cormier K, Strong S, Chan PA. Impact of the COVID-19 pandemic on sexually transmitted infection clinic visits. Sex Transm Dis. 2021;48(1):e5–7. doi:10.1097/OLQ.0000000000001306. PMID:PMC7736141.
- Liu H, Yao Q, Li D, Zhao Z, Li Y. Impact of COVID-19 outbreak on the gynecological outpatients HPV infection rate in Wuhan, China: a retrospective observational study. Front Med (Lausanne). 2022;9:799736. doi:10.3389/fmed.2022.799736. PMID:PMC9035827.
- Vorsters A, Bosch FX, Poljak M, Waheed DE, Stanley M, Garland SM, Prevention HPV, control B. S the international papillomavirus. HPV prevention and control - the way forward. Prev Med. 2022;156:106960. doi:10.1016/j.ypmed.2022.106960. PMID:PMC8772134.
- Bruni L, Diaz M, Castellsague X, Ferrer E, Bosch FX, de Sanjose S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202(12):1789–99. doi:10.1086/657321.
- Wang X, Han S, Li X, Wang X, Wang S, Ma L. Prevalence and distribution of human papillomavirus (HPV) in Luoyang city of Henan province during 2015-2021 and the genetic variability of HPV16 and 52. Virol J. 2022;19(1):37. doi:10.1186/s12985-022-01759-5. PMID:PMC8896270.
- Xu HH, Lin A, Chen YH, Dong SS, Shi WW, Yu JZ, Yan WH. Prevalence characteristics of cervical human papillomavirus (HPV) genotypes in the Taizhou area, China: a cross-sectional study of 37 967 women from the general population. BMJ Open. 2017;7(6):e014135. doi:10.1136/bmjopen-2016-014135. PMID:PMC5577888.
- Wang J, Tang D, Wang K, Wang J, Zhang Z, Chen Y, Zhang X, Ma C. HPV genotype prevalence and distribution during 2009-2018 in Xinjiang, China: baseline surveys prior to mass HPV vaccination. BMC Womens Health. 2019;19(1):90. doi:10.1186/s12905-019-0785-3. PMID:PMC6615222.
- Li M, Du X, Lu M, Zhang W, Sun Z, Li L, Ye M, Fan W, Jiang S, Liu A, et al. Prevalence characteristics of single and multiple HPV infections in women with cervical cancer and precancerous lesions in Beijing, China. J Med Virol. 2019;91(3):473–81. doi:10.1002/jmv.25331.
- Li C, Wu M, Wang J, Zhang S, Zhu L, Pan J, Zhang W. A population-based study on the risks of cervical lesion and human papillomavirus infection among women in Beijing, People’s Republic of China. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2655–64. doi:10.1158/1055-9965.EPI-10-0212.
- Alarcon-Romero LDC, Organista-Nava J, Gomez-Gomez Y, Ortiz-Ortiz J, Hernandez-Sotelo D, Del Moral-Hernandez O, Mendoza-Catalan MA, Antano-Arias R, Leyva-Vazquez MA, Sales-Linares N, et al. Prevalence and distribution of human papillomavirus genotypes (1997-2019) and their association with cervical cancer and precursor lesions in women from Southern Mexico. Cancer Control. 2022;29:10732748221103331. doi:10.1177/10732748221103331. PMID:PMC9136461.
- Ah Lee S, Kang D, Soo Seo S, Kim Jeong J, Young Yoo K, Tark Jeon Y, Weon Kim J, Hyun Park N, Beom Kang S, Pyo Lee H, et al. Multiple HPV infection in cervical cancer screened by HPVDNAChip™. Cancer Lett. 2003;198(2):187–92. doi:10.1016/s0304-3835(03)00312-4.
- Bao H, Ma L, Zhao Y, Song B, Di J, Wang L, Gao Y, Ren W, Wang S, Wu J, et al. Age-specific effectiveness of primary human papillomavirus screening versus cytology in a cervical cancer screening program: a nationwide cross-sectional study. Cancer Commun (Lond). 2022;42(3):191–204. doi:10.1002/cac2.12256. PMID:PMC8923126.
- Donkoh ET, Asmah RH, Agyemang-Yeboah F, Dabo EO, Wiredu EK. Prevalence and distribution of vaccine-preventable genital human papillomavirus(HPV) genotypes in Ghanaian women presenting for screening. Cancer Control. 2022;29:10732748221094721. doi:10.1177/10732748221094721. PMID:PMC9096183.
- Althoff KN, Paul P, Burke AE, Viscidi R, Sangaramoorthy M, Gravitt PE. Correlates of cervicovaginal human papillomavirus detection in perimenopausal women. J Women Health. 2009;18(9):1341–6. doi:10.1089/jwh.2008.1223. PMID:PMC2825723.
- Zhao F, Qiao Y. Cervical cancer prevention in China: a key to cancer control. Lancet. 2019;393(10175):969–70. doi:10.1016/s0140-6736(18)32849-6.
- Yao P, Millwood I, Kartsonaki C, Mentzer AJ, Allen N, Jeske R, Butt J, Guo Y, Chen Y, Walters R, et al. Sero-prevalence of 19 infectious pathogens and associated factors among middle-aged and elderly Chinese adults: a cross-sectional study. BMJ Open. 2022;12(5):e058353. doi:10.1136/bmjopen-2021-058353. PMID:PMC9086621.
- Lin X, Chen L, Zheng Y, Yan F, Li J, Zhang J, Yang H. Age-specific prevalence and genotype distribution of human papillomavirus in women from Northwest China. Cancer Med. 2022;11(22):4366–73. doi:10.1002/cam4.4732.
- Gong M, Chen C, Zhao H, Guo L, Sun M, Song M. Analysis of distributions of HPV infection in females with cervical lesions in the Western district of Beijing Chaoyang hospital. J Healthc Eng. 2022;2022:5422748. doi:10.1155/2022/5422748. PMID:PMC8938050.
- Wong EL, Cheung AW, Chen Z, Wong AY, Yeung AC, Yau PS, Chan PK. Molecular epidemiology of human papillomavirus infection among Chinese women with cervical cytological abnormalities. Front Public Health. 2022;10:820517. doi:10.3389/fpubh.2022.820517. PMID:PMC9152134.
- Hao S, Wang C, Liu S, He J, Jiang Y. HPV genotypic spectrum in Jilin province, China, where non-vaccine-covered HPV53 and 51 are prevalent, exhibits a bimodal age-specific pattern. PLos One. 2020;15(3):e0230640. doi:10.1371/journal.pone.0230640. PMID:PMC7313545.
- Jiang L, Tian X, Peng D, Zhang L, Xie F, Bi C, Wang R, Wang J, Qi D. HPV prevalence and genotype distribution among women in Shandong province, China: analysis of 94,489 HPV genotyping results from Shandong’s largest independent pathology laboratory. PLos One. 2019;14(1):e0210311. doi:10.1371/journal.pone.0210311. PMID:PMC6336302 sharing data and materials.
- Wei L, Ma L, Qin L, Huang Z. The prevalence and genotype distribution of human papillomavirus among women in Guangxi, southern China. Infect Agent Cancer. 2022;17(1):19. doi:10.1186/s13027-022-00431-5. PMID:PMC9022619.
- Qiao YL, Wu T, Li RC, Hu YM, Wei LH, Li CG, Chen W, Huang SJ, Zhao FH, Li MQ, et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced bivalent human papillomavirus vaccine: an interim analysis of a randomized clinical trial. J Natl Cancer Inst. 2020;112(2):145–53. doi:10.1093/jnci/djz074. PMID:PMC7019098.
- Bao HL, Jin C, Wang S, Song Y, Xu ZY, Yan XJ, Li LM, Ning Y, Wang HJ. Prevalence of cervicovaginal human papillomavirus infection and genotypes in the pre-vaccine era in China: a nationwide population-based study. J Infect. 2021;82(4):75–83. doi:10.1016/j.jinf.2021.02.017.
- Hampson IN. Effects of the prophylactic HPV vaccines on HPV type prevalence and cervical pathology. Viruses. 2022;14(4):757. doi:10.3390/v14040757. PMID:PMC9029410.
- Garland SM, Pitisuttithum P, Ngan HYS, Cho CH, Lee CY, Chen CA, Yang YC, Chu TY, Twu NF, Samakoses R, et al. Efficacy, immunogenicity, and safety of a 9-valent human papillomavirus vaccine: subgroup analysis of participants from Asian Countries. J Infect Dis. 2018;218(1):95–108. doi:10.1093/infdis/jiy133. PMID:PMC5989602.