ABSTRACT
Patients with pediatric rheumatic diseases (PRDs) have higher morbidity and mortality associated with infectious diseases. Vaccination is an effective way to prevent infection. This study aimed to understand the vaccination status, vaccination-related attitudes, and adverse reactions in patients with PRDs in one of the largest Pediatric Rheumatic and Immune centers in China. A cross-sectional study using an online questionnaire was conducted among the caregivers of patients with PRDs admitted to the Children’s Hospital of Chongqing Medical University. 189 valid questionnaires were collected. The most two common PRDs in this study were juvenile idiopathic arthritis (29.6%) and systemic lupus erythematosus (19.6%). Univariate analysis and multivariate logistic regression were used to identify potential factors associated with vaccination completion among these patients. Univariate analysis suggested that the age of onset, course of the disease, treatment duration, disease duration <1 month, disease duration ≥24 months, treatment duration <1 month, use of biological agents, at least one hospitalization, whether with one-time intravenous human immunoglobulin, caregiver concerns about vaccination before or after illness, and vaccine hesitancy may affect the scheduled vaccination completion by age in patients (p < .05). Multivariate logistic regression analysis showed that the age of onset (OR, 1.013; 95% CI, 1.005–1.022; p = .002) and caregiver concerns about vaccination before illness (OR, 0.600; 95% CI, 0.428–0.840; p = .003) independently influenced patients’ completion of scheduled vaccinations. This study suggests that rheumatic disease and treatment may influence age-appropriate vaccination. Appropriate education for patients and carers may improve vaccination cognition and attitudes.
Introduction
Rheumatic diseases (RDs) are chronic conditions with complex unknown causes affecting musculoskeletal, vascular and other tissues.Citation1 Patients with pediatric rheumatic diseases (PRDs) have a higher risk of infection due to their long-term use of immunosuppressive or immunomodulatory drugs and require vaccination to prevent serious infection. The indications and contraindications in the population with special healthcare have been revised.Citation2 Physicians have also noted that some kinds of vaccination could even lead to infection in certain conditions. The immunogenicity and safety of vaccination would be altered in these immunocompromised children. Therefore, vaccination decisions remain challenging in practice.
Vaccines are critical to controlling infectious disease outbreaks and reducing the risk of disease by working with the human body’s natural defenses. Immunization strategies have been gradually refined and popularized in different countries.Citation3,Citation4 It has been reported that the number of incompletely vaccinated children has increased by 5 million since 2019. Thus the World Health Organization (WHO) places a high priority on the prevention and treatment of infectious diseases.Citation5 The Immunization Agenda 2030 sets out an ambitious vaccination strategy around the world, requiring everyone to complete their immunization schedule to maintain good health.Citation3
China also has a national immunization program.Citation6 Children are regularly vaccinated for free according to the national immunization program before 7 years old. The unscheduled vaccines are self-paying that caregivers can selectively provide for their kids. Although great efforts have been made to improve vaccination completion by vaccinators’ and medical staff’s regular and scientific immunization recommendations, inconsistencies with published suggestions and factors potentially affecting the vaccination decision have led to differences in clinical practice.Citation7 Some case reports described the adverse events following immunization (AEFI) observed in patients with PRDs, primarily including IgA vasculitis (IgAV) and Kawasaki disease (KD).Citation8–10 Some researchers depicted the vaccination status in Chinese patients with inflammatory bowel disease, spinal muscular atrophies, and other adult chronic rheumatic diseases.Citation11–13 Several studies demonstrated the specific immunization of patients with PRDs.Citation14,Citation15 Yet, few studies have been conducted to explore the real knowledge, awareness, and attitudes on vaccination among the caregivers of these special health populations.
Hence, this study aims to provide a practical basis for future scientific commissioning and implementation by investigating the status and potential factors influencing vaccination in patients with PRDs in China.
Method
Study design and participants
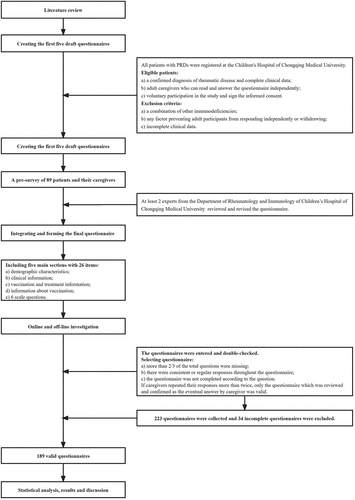
This study was led by a single center through web-based observational questionnaire survey of caregivers from 8 different provinces of China (i.e. Chong Qing, Gui Zhou, Yun Nan, Shan Xi, Si Chuan, Hu Nan, Guang Xi, and Qing Hai). All patients with PRDs were registered at the Children’s Hospital of Chongqing Medical University. Eligible patients must meet three conditions: a) a confirmed diagnosis of rheumatic disease and complete clinical data; b) adult caregivers who can read and answer the questionnaire independently; and c) voluntarily participate in the study and sign the informed consent. Exclusion criteria were: a) a combination of other immunodeficiencies; b) any factors preventing adult participants from responding independently or withdrawing; and c) incomplete clinical data. Participants were recruited from January 2021 to December 2021. Primary ethical approval was obtained from the Human Research Ethics Committee of the Children’s Hospital of Chongqing Medical University. The study has been registered on chictr.org.cn (ChiCTR2100045008).
Questionnaire design
The questionnaire was designed by using a Uniform Resource Location, www.wjx.cn. It contained 26 items. We expanded 5–10 times and required 130–260 cases. The sample size was increased by 10–20% to account for unqualified questionnaires.Citation16
The questionnaire was self-administered and constructed after a literature review. The survey was designed to investigate the vaccination completion of patients with PRDs, including Chinese national scheduled and unscheduled vaccines. The initial draft of five questionnaires was designed separately for five distinct pediatric rheumatic diseases. A pre-survey of 89 patients’ caregivers revealed partial missing information due to the difficulty in keeping and recalling vaccination records. Therefore, we modified and integrated the questionnaires. The final questionnaire was reviewed and revised by at least 2 experts from the Department of Rheumatology and Immunology of Children’s Hospital of Chongqing Medical University, which included five main sections as followed.
First, the demographic characteristics collect the gender, age, relationship to the caregiver, place of residence of patients, and caregivers’ education level. Second, the clinical information contains the onset of age, duration of disease and treatment, diagnosis, and treatment strategy of patients. The third part investigates the vaccination during treatment. We wondered whether patients got inactivated or live attenuated vaccine during treatment, whether patients were vaccinated against varicella, whether a history of infecting varicella and when infected with varicella. Fourth, the information about vaccination refers to the scheduled vaccination completion according to age, reasons for not completing vaccinations, any adverse events following immunization, and the ways to learn how to vaccinate. Here, we use the ‘age-appropriate vaccination’ represented ‘vaccination schedule according to age,’ and the ‘completed group’ represented ‘completing the age-appropriate vaccination.’ Instead, the ‘incomplete group’ represented ‘not completing the age-appropriate vaccination.’ The fifth section includes 6 scale questions about caregivers’ attitudes to vaccination. That is the necessity of vaccination, concerns about vaccination before illness, vaccine hesitancy before illness, concerns about vaccination after illness, vaccine hesitancy after illness, and trust in physician recommendations. The questionnaire will take about 5–10 minutes to complete.
The online survey was conducted via a mini program from WeChat, Wenjuanxing. As well, face-to-face or telephone follow-ups were carried out by researchers after training. The questionnaires were entered and double-checked to ensure the authenticity and reliability of the data. The questionnaires would be considered invalid if more than 2/3 of the total questions were missing, consistent or regular responses throughout the questionnaire and not answered according to the questions. If caregivers repeated their responses more than twice, only the questionnaire reviewed and confirmed as the eventual edition by caregivers was valid ().
Statistical analysis
Excel was to record the results for a database of questionnaires. SPSS statistical software package (version 26.0; IBM) was utilized for statistical analysis. Quantitative data were presented as median and 25th–75th percentile interquartile range (IQR). Count data were described as the number of cases and percentage (%). The Chi-square test (χ2) or Fisher’s exact test was used to compare two groups of categorical variables. The Mann-Whitney U test was used to compare two groups of continuous variables. Variables with a p-value < .05 were considered as candidates. Logistic regression analysis was used for multivariate analysis to determine risk factors for not completing the vaccination schedule by age. Results were presented as adjusted odds ratios (ORs) with 95% confidence intervals (CI). A two-sided p-value < .05 was considered statistically significant in this study.
Results
Sociodemographic characteristics
A total of 223 questionnaires were collected. 34 incomplete questionnaires were excluded, leaving 189 questionnaires. The effective response rate was 89.6%. 96.3% (182/189) of the respondents were the parents of the patients. All patients had a median age of 109.0 (63.0 to 156.0) months. 50.8% (96/189) of the patients were male. 50.3% (95/189) lived in Chongqing. Most patients (65.1%, 123/189) were >7 years old. 106 caregivers provided the education levels, 11.3% (12/106) had completed primary school or lower, 41.5% (44/106) junior high school, 19.8% (21/106) senior high school, 10.4% (11/106) college, and 17.0% (18/106) bachelor’s degree or higher. There was no statistically significant difference in the relationship to caregivers, place of residence, gender and age ().
Table 1. General characteristics of the children.
67.2% (127/189) of the patients who completed their age-appropriate vaccinations at the time of answering the questionnaire. 189 patients were divided into three groups according to age (<2 years) and (≥7 years). In these three groups, higher rates were observed in the completed group than in the incomplete group, but without statistically significant differences. A further grouping was made by the cutoff point of age 2 or 7, respectively, and the differences between the two groups were not statistically significant ().
Clinical characteristics
The clinical characteristics are summarized in . Patients with juvenile idiopathic arthritis (JIA) accounted for the majority of cases (29.6%; 56/189), followed by systemic lupus erythematosus (SLE) (19.6%; 37/189), juvenile dermatomyositis (JDM) (17.5%; 33/189), Kawasaki disease (KD) (16.4%; 31/189) and IgA vasculitis (IgAV) (16.9%; 32/189). All patients had a median disease duration of 9.0 (1.0 to 26.0) months and a median treatment duration of 5.0 (0.0 to 18.5) months. Duration of treatment ‘0.0’ indicated the course of treatment was <1 month. Multiple response analysis showed that 35.7% (128/359) had received glucocorticosteroids, 30.1% (108/359) had received conventional disease-modifying antirheumatic drugs (cDMARDs), 17.3% (62/359) had received biological agents, and 17.0% (61/359) had received intravenous human immunoglobulin (IVIG).
Table 2. Clinical characteristics about diagnosis and treatment of the children.
It was found that patients who received a combination of biological agents had a lower completion rate, which differed without significance. Notably, the completion rate was higher in patients receiving only one-time IVIG treatment, and the difference was statistically significant (p = .012).
We also tested in several duration groups. Vaccination completion rates were higher in patients with a disease duration of fewer than 12 months including four groups: a) disease duration <1 month (p = .006), b) 1 month ≤ disease duration <3 months, c) 3 months ≤ disease duration <6 months, and d) 6 months ≤ disease duration <12 months.
In addition, the completion rate of age-appropriate vaccination was higher in three treatment duration groups: a) duration <1 month (p = . 011), b) 3 months ≤ duration <6 months, and c) ≥ 24 months, while fewer rates in four treatment duration groups, a) 1 month ≤ duration <3 months, b) 6 months ≤ duration <12 months, c) 12 months ≤ duration <18 months, and d) 18 months ≤ duration <24 months (). Notably, there were more patients not completing the age-appropriate vaccination in the group with treatment duration ≥24 months.
The completed group had an older age at disease onset (p = .018), shorter disease duration (p < .001), and shorter treatment duration (p = .003), with a statistically significant difference compared to the incomplete group ().
Reasons for not completing age-appropriate vaccinations
62 patients did not receive age-appropriate immunizations as scheduled. The four main reasons were: 26.0% (20/77) physician recommendations, 26.0% (20/77) vaccinator recommendations, 24.7% (19/77) caregiver concerns and vaccine hesitancy, and 23.4% (18/77) unclear reasons.
There were 65 responses among the patients with more than one hospitalization experience. The reasons focused on vaccinators’ and physicians’ recommendations. The remaining 12 responses from those with only one hospitalization experience presented the major reasons for vaccinator recommendations and other unclear reasons (Table S1A).
The main reasons for patients who became ill before 7 years old were physician recommendations, vaccinator recommendations, caregiver concerns, and vaccine hesitancy. For those with disease onset at or after 7 years old, the primary reasons were other unclear reasons and vaccinator recommendations (Table S1A).
The reasons for patients without completing the age-appropriate vaccination in various treatment groups showed that vaccinators, physicians and caregivers may differently affect vaccination decisions (Table S1B).
Description of adverse events following vaccination
16.9% (32/189) of the patients experienced adverse events following immunization. Multiple response analysis showed that the most common adverse events were allergy (5.9%, 2/34), nausea or vomiting or diarrhea (2.9%, 1/34), local redness with painful pus or ulcerated induration (11.8%, 4/34), and fever, rash or headache or dizziness or tiredness or general malaise (47.1%, 16/34), while 32.4% (11/34) were unclear adverse reactions ().
Table 3. Adverse events following immunization of children.
The most common AEFI among patients who completed the scheduled vaccinations according to age was fever, rash or headache or dizziness or tiredness or general malaise, with 10 person-times. The most common AEFI among patients not completing age-appropriate vaccinations was unclear events with 7 person-times. Caregiver concerns and vaccine hesitancy and other unclear reasons were the main reasons for not completing the vaccinations.
Multiple response analysis showed that of the 30 patients treated with medication, 33.3% (21/63) with glucocorticosteroids, 28.6% (18/63) with cDMARDs, 20.6% (12/63) with biologics, and the remaining 17.5% (11/63) with IVIG. The most common AEFI in 30 patients was fever, rash or headache or dizziness or fatigue, or general malaise. The patients with these adverse reactions mainly received glucocorticosteroids, cDMARDs, and IVIG ().
Information of varicella immunization and varicella infection
185 patients should be vaccinated against varicella according to their age. 88.1% (163/185) of patients received the varicella vaccine. 13.5% (25/185) of patients with previous varicella infection. 12.0% (3/25) of the patients had a history of infecting varicella during illness, and 1/3 (33.3%) of them didn’t get varicella vaccination. 88.0% (22/25) of patients infected with varicella before diagnosis, and 13.6% (3/22) of them didn’t receive varicella vaccination.
The proportion of varicella infection was higher in patients not received varicella vaccination (16.0%, 4/25) than in those received (11.3%, 18/160), but the difference was not statistically significant (p = .726). Patients with varicella infection during the illness were mainly treated with glucocorticosteroids (33.3%, 3/9) and cDMARDs (33.3%, 3/9). Similarly, patients with varicella infection before illness were mainly treated with glucocorticosteroids (46.9%, 23/49) and cDMARDs (30.6%, 15/49) (Table S2).
Although the varicella vaccine is a voluntary vaccine that families can give to their children, varicella vaccination coverage is over 80% in both completed and incomplete groups. However, none of the patients included in the study had received an annual seasonal influenza vaccination.
Description of status and attitudes related to vaccination
11 (5.8%) patients received vaccines during their illness. All received inactivated vaccines. As it was difficult for families to check and keep vaccination records, we selectively described some vaccinations with complete data. 8 of 11 patients completed age-appropriate vaccination, and 50% (4/8) of them had disease onset before 7 years old. The other 3 patients didn’t complete age-appropriate vaccination, and 100% (3/3) were onset before age 7.
The participants learned about the vaccination schedule in different ways, as follows: from vaccinators (64.8%), physicians (22.3%), themselves (9.5%), other caregivers (1.1%), schools (1.9%), and other ways (0.4%). Caregivers of patients who completed the program vaccine according to age were more likely to get vaccination information from the vaccinators or themselves rather than from physicians, other caregivers, schools, or other approaches. Fewer caregivers of the patients who had more than one hospitalization preferred to learn vaccination information from vaccinators, themselves, and other caregivers than from physicians, schools, or other ways (Table S3).
We assessed vaccination status and attitudes. Each Likert scale question has five levels: strongly agree, generally agree, neutral, generally disagree, and strongly disagree. These five scales corresponded to scoring 5, 4, 3, 2, and 1, respectively. Participants scored based on their judgment. The median scores for the necessity of vaccination, concerns about vaccination before illness, vaccine hesitancy before illness, concerns about vaccination after illness, vaccine hesitancy after illness, and trust in physician recommendations were 4 (4 to 5), 2 (2 to 2), 2 (1 to 2), 3 (2 to 4), 3 (2 to 4), and 4 (4 to 5), respectively.
Among the 32 patients who had experienced AEFI, the median scores for the necessity of vaccination, concern about vaccination before illness, vaccine hesitancy before illness, concern about vaccination after illness, vaccine hesitancy after illness, and trust in physician recommendations were 5 (4 to 5), 2 (2 to 4), 2 (1 to 3), 4 (3 to 5), 4 (3 to 5), and 4 (4 to 5). There was no statistical difference in whether the planned vaccination was completed by age.
Among the 62 patients who did not complete the vaccination schedule, the median scores for the necessity of vaccination, concern about vaccination before illness, vaccine hesitancy before illness, concern about vaccination after illness, vaccine hesitancy after illness, and trust in physician recommendations were 5 (4 to 5), 2 (2 to 4), 2 (1.8 to 2.3), 4 (2.8 to 5), 4 (2 to 5), 4 (4 to 5) ().
Table 4. Vaccination-related attitude information.
Among the 185 patients who should receive the varicella vaccination according to their age, the median scores for the necessity of vaccination, concern about vaccination before illness, vaccine hesitancy before illness, concern about vaccination after illness, vaccine hesitancy after illness, and trust in physician recommendations were 4 (4 to 5), 2 (2 to 2), 2 (1 to 2), 3 (2 to 4), 3 (2 to 4), 4 (4 to 5). 163 caregivers whose children had been vaccinated against varicella showed fewer concerns about vaccination before and after illness and vaccination hesitancy after illness than the rest 22 caregivers. There was no difference between 160 caregivers whose children had not been infected with varicella and the rest 25 caregivers ().
Factors that may affect the completion of vaccination according to age
Univariate analysis demonstrated significant differences in the completion of age-appropriate vaccination in 189 patients in terms of onset of age, duration of disease, duration of treatment, whether the first hospitalization, duration of disease <1 month, duration of disease ≥24 months, duration of treatment ≤1 month, whether biological agents were used, whether with one-time IVIG, concerns about vaccination before illness, concerns about vaccination after illness, and vaccine hesitancy after illness (p < .05).
Multivariate analysis showed that age at disease onset and how worried parents were before diagnosis were independent factors that influenced completing the age-appropriate vaccination (p < .05) ()
Discussion
Immunization programs for healthy children and patients with special health conditions are well-established in many countries and have effectively reduced the incidence of serious infectious diseases. In this article, we focused on describing and analyzing immunization completion of age-appropriate scheduled vaccines.
There is no statistical difference in general information. It might be related to the nationwide implementation of the Chinese immunization program.Citation6 All participants know about the vaccination procedures. Their kids are regularly informed and vaccinated by community vaccinators. Although we were unable to analyze the relationship between education level and vaccination completion rates, a lower completion rate was observed among illiterate caregivers. These results indicated that differences in vaccination rates influenced by regional, economic, cultural, and medical factors cannot be ignored.Citation17–20
In addition, the vaccination completion rate is higher for the patients more than 7 years old, which might be related to the requirement of finishing the scheduled vaccines before entering primary school in China. A lower vaccination completion rate was reported in the age group (2 years ≤ age <7 years) than in the other two age groups (<2 years) and (≥7 years). Although the difference is not statistically significant, it may indicate the need to increase the completion of early vaccination after birth to reduce the incidence of preventable diseases and ultimately improve child health.Citation21,Citation22
In 189 patients, glucocorticosteroids were the main drug treatment. Vaccination completion rates were lower in patients treated combined with biologics.Citation2,Citation23 Patients in the completed group had an older age of disease onset so that less impactful on finishing the immunization program. They also had a shorter duration of illness and treatment than those in the incomplete group. In particular, a higher completion rate was found in groups with disease duration (≤12 months) and treatment duration (≤1 month, 3 months ≤ duration <6 months).Citation2,Citation24 Regular scheduled vaccinations before illness might contribute to a higher completion rate, instead, a longer duration of disease and treatment might lead to a lower rate because of the decisions made by vaccinators or physicians to delay the immunization, based on disease activity and specific immunosuppressive agents.
Immunosuppressed patients are more susceptible to developing infectious diseases. The availability of vaccines is largely dependent on the generation of an effective immune response, the protection provided by vaccination, and the occurrence of adverse reactions. The guidelines state that patients with PRDs treated with glucocorticosteroids, disease-modifying antirheumatic drugs (DMARDs) and/or anti-tumor necrosis factor (TNF)-alpha could receive inactivated vaccines according to national immunization guidelines.Citation2 For those patients treated with high-dose glucocorticosteroids or rituximab, post-vaccination testing of antigen-specific antibody concentrations is recommended as an indicator to assess adequate immune response.Citation2 However, in our study, only 11 patients had received inactivated vaccines during the treatment, and post-vaccination monitoring of specific antibody concentrations was not performed. None of the patients had received consecutive influenza vaccinations annually, which is not consistent with guideline recommendations. The associated factors in influencing the current low seasonal influenza vaccination rate and the strategy to increase influenza vaccination among children with RDs in China need further investigation.Citation2,Citation25–27
Varicella zoster virus (VZV) is one of the eight herpesviruses. Primary infection occurs as varicella. In otherwise well children, it is self-limiting and rarely life threatening, excepting some special conditions, such as sJIA complicated with macrophage activation syndrome.Citation28,Citation29 Some forms of immunosuppression increase the risk of serious primary infection and dissemination of infection after reactivation in patients without previous exposure to the virus.Citation28 A meta-analysis showed that varicella was one of the most frequent serious infections on biologics in JIA.Citation30 An increase in viral infection rates over time was also seen.Citation31 Those who developed varicella infection before or during treatment were treated with glucocorticosteroids and cDMARDs. And our data showed that patients who received the varicella vaccine had a lower rate of infection. Considering the potential seriousness of varicella infection in immunocompromised children and the recent vaccination recommendations of the European League Against Rheumatism (EULAR) for adult patients with RDs, these results provide the rationale for routine immunization to reduce the varicella risk.Citation29,Citation32,Citation33 Nevertheless, varicella vaccine is live attenuated. It must be noted that vaccines do not always protect against infection. Hence, asking patients about their history of varicella infection and vaccination should be valued to decide whether to supplement varicella vaccination.Citation2 Additionally, in the era of new outbreak, the impact of the new coronavirus vaccine on VZV reactivation in children with RDs remains to be studied.Citation34
Medical personnel and vaccinators are more influential in vaccinating children with special health conditions. Much of the failure to vaccinate on time is due to a lack of professional involvement in the assessment. Caregiver concerns and vaccine hesitancy should also be taken into account. So it is important to increase training and establish communication channels among not only vaccinators and physicians but also caregivers.
The most common AEFIs that occurred during vaccination in 189 patients were fever, rash or headache or dizziness or fatigue or general malaise, and other unknown adverse reactions. Some evidence demonstrates that vaccines are generally well tolerated and effective in patients with RDs, yet antibody titers are frequently lower than in healthy controls.Citation35,Citation36 Relatively, the data on the safety of live attenuated vaccines are limited.Citation35 More information is needed on new vaccines and antirheumatic therapies. The precise identification of AEFIs may help to determine the authenticity of adverse vaccine reactions and the recurrence or worsening of the disease after vaccination.Citation37
There was a shift from a preference for vaccinator advice to a preference for physician advice in the way caregivers learned about vaccination after treatment. However, the percentage of those who received vaccination information from school education was low.Citation21 The median rating of the necessity of vaccination by caregivers was 4 or 5, indicating a relatively high level of awareness and importance of vaccination among them. Caregiver concerns about vaccination and vaccine hesitancy before or after illness were rated higher among those who had an AEFI or had not completed age-appropriate vaccinations.Citation3,Citation38,Citation39 Moreover, the trust in physician recommendations was high, with a median score of 4. Hence, healthcare providers should deliver vaccination instructions to patients and caregivers, and use multiple channels to enhance health education during hospitalization. The risk of primary infection versus vaccine infection must depend on individuals to determine the vaccination strategy. Meanwhile, vaccinators should collaborate with rheumatologists to develop individual vaccination strategies for patients with PRDs based on their specific treatment.Citation21,Citation40,Citation41
This study has several limitations. First, it was a single-center study from Chongqing with a small sample size, and it was a web-based study. These may cause bias in the patients responding to the survey, so further multicenter studies and larger cohorts are desired to confirm our findings. Second, our study population was mainly from the Children’s Hospital of Chongqing Medical University, which may have led to selection bias. Although there were no significant differences in age, gender, or residence in whether age-appropriate vaccinations were completed. Incomplete information due to education level in our study may have influenced the understanding of vaccination perceptions and attitudes. Despite regular communication of vaccination information by vaccinators, there may still be delays or non-vaccination for parental reasons, and further research on the exact knowledge of vaccination information by caregivers may be needed. Third, the survey found that caregivers had relatively complete information about varicella vaccination and influenza vaccination. However, we did not concentrate on the immunologic changes of varicella and influenza infection, which may affect vaccination guidance. Future clinical trials on children with RDs and the immune response to vaccination should be conducted.
Conclusion
This study investigated factors associated with age-appropriate immunization completion in patients with PRDs in a single center. The results indicated the spread of the national immunization program in China has made an important contribution to improving the current status of vaccination. Special health populations delay vaccinations due to their own diseases and treatments. Administering planned vaccinations before illness and timely catch-up vaccinations within the first 12 months of treatment course and promoting immunization for patients treated for more than 2 years may increase the age-appropriate vaccination rate. Inactivated vaccines are routinely recommended, but caution is still needed when administering or catching up on live attenuated vaccines (including the varicella vaccine). For patients in the early or active stages of rheumatic disease, especially those treated with biological agents, caregiver concerns and vaccine hesitancy remain potential factors influencing childhood vaccine completion.
In summary, healthcare providers and immunization departments play a key role in providing scientific guidance to these patients and caregivers should consider the suggestions from medical staff and vaccinators. It is more beneficial to provide scientific guidance on vaccination through various approaches to enhance vaccination education, dynamic assessment and updating of indications, active follow-up of vaccine effectiveness, together with enhanced systems for monitoring and reporting of adverse reactions.Citation40,Citation42–44
CRediT authorship contribution statement
Xiwen Luo, Jing Tao designed the questionnaire, analyzed the results and wrote the manuscript. Xiwen Luo, Jing Tao and Min Pang conducted the survey. Zhiyong Zhang and Xuemei Tang designed, supervised and edited the manuscript.
Supplemental Material
Download PDF (252.9 KB)Acknowledgments
We are very grateful to the patients and their families for their cooperation and for giving consent to participate in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2215108.
Additional information
Funding
References
- Warren RW, Perez MD, Wilking AP, Myones BL. Pediatric rheumatic diseases. Pediatr Clin North Am. 1994;41(4):1–12. doi:10.1016/S0031-3955(16)38808-3.
- Heijstek MW, Ott de Bruin LM, Bijl M, Borrow R, van der Klis F, Koné-Paut I, Fasth A, Minden K, Ravelli A, Abinun M, et al. EULAR recommendations for vaccination in paediatric patients with rheumatic diseases. Ann Rheum Dis. 2011;70(10):1704–12. doi:10.1136/ard.2011.150193.
- Piot P, Larson HJ, O’Brien KL, N’kengasong J, Ng E, Sow S, Kampmann B. Immunization: vital progress, unfinished agenda. Nature. 2019;575(7781):119–29. doi:10.1038/s41586-019-1656-7.
- Robinson CL, Bernstein H, Poehling K, Romero JR, Szilagyi P. Advisory committee on immunization practices recommended immunization schedule for children and adolescents aged 18 years or younger - United States, 2020. Morb Mortal Wkly Rep. 2020;69(5):130–2. doi:10.15585/mmwr.mm6905a3.
- Rachlin A, Danovaro-Holliday MC, Murphy P, Sodha SV, Wallace AS. Routine vaccination coverage - worldwide, 2021. Morb Mortal Wkly Rep. 2022;71(44):1396–400. doi:10.15585/mmwr.mm7144a2.
- National Health Commission Of The People’s Republic Of China. Childhood immunization schedule for national immunization program vaccines - China (version 2021). China CDC Wkly. 2021;3(52):1101–8. doi:10.46234/ccdcw2021.270.
- Janow G, Ilowite NT. Pediatric rheumatic disease: vaccination in pediatric rheumatic disease–risks and benefits. Nat Rev Rheumatol. 2012;8(4):188–90. doi:10.1038/nrrheum.2012.13.
- Shu M, Liu Q, Wang J, Ao R, Yang C, Fang G, Wan C, Guo W. Measles vaccine adverse events reported in the mass vaccination campaign of Sichuan province, China from 2007 to 2008. Vaccine. 2011;29(18):3507–10. doi:10.1016/j.vaccine.2009.10.106.
- Zhu ZG, Zheng Y, Lu S, Hu Q, Fang Y. Rabies post-exposure prophylaxis for a male with severe Henoch Schönlein purpura following rabies vaccination. Hum Vaccin Immunother. 2018;14(11):2666–8. doi:10.1080/21645515.2018.1486354.
- Alsager K, Khatri Vadlamudi N, Jadavji T, Bettinger JA, Constantinescu C, Vaudry W, Tan B, Sauvé L, Sadarangani M, Halperin SA, et al. Kawasaki disease following immunization reported to the Canadian immunization monitoring program ACTive (IMPACT) from 2013 to 2018. Hum Vaccin Immunother. 2022;18(5):2088215. doi:10.1080/21645515.2022.2088215.
- Esposito S, Antoniol G, Labate M, Passadore L, Alvisi P, Daccò V, Ghizzi C, Colombo C, Principi N. Vaccines in children with inflammatory bowel disease: brief review. Vaccines (Basel). 2021;9(5):487. doi:10.3390/vaccines9050487.
- Qu YJ, Tian YL, Song F, Wang J, Bai JL, Cao YY, Jin YW, Wang H, Cheng MM. Coverage rate and adverse reactions of national immunization program vaccines in children with spinal muscular atrophy: a cross-sectional retrospective cohort study. Zhonghua Er Ke Za Zhi. 2020;58(4):308–13. doi:10.3760/cma.j.cn112140-20200108-00016.
- Sen P, Naveen R, Houshmand N, Moghadam Kia S, Joshi M, Saha S, Jagtap K, Agarwal V, Nune A, Nikiphorou E, et al. Vaccine hesitancy decreases, long term concerns remain in myositis, rheumatic disease patients: a comparative analysis of the COVAD surveys. Rheumatology (Oxford). Published online 2023 Feb. doi:10.1093/rheumatology/kead057.
- Akgün Ö, Kayaalp GK, Demirkan FG, Çakmak F, Tanatar A, Guliyeva V, Sönmez HE, Ayaz NA. Exploring the attitudes, concerns, and knowledge regarding COVID-19 vaccine by the parents of children with rheumatic disease: cross-sectional online survey. Vaccine. 2022;40(12):1829–36. doi:10.1016/j.vaccine.2022.01.061.
- Jansen MH, Rondaan C, Legger G, Minden K, Uziel Y, Toplak N, Maritsi D, van den Berg M, Berbers G, Bruijning P, et al. Efficacy, immunogenicity and safety of vaccination in pediatric patients with autoimmune inflammatory rheumatic diseases (pedAIIRD): a systematic literature review for the 2021 update of the EULAR/PRES recommendations. Front Pediatr. 2022;10:910026. doi:10.3389/fped.2022.910026.
- Biccard BM, Gopalan PD, Miller M, Michell WL, Thomson D, Ademuyiwa A, Aniteye E, Calligaro G, Chaibou MS, Dhufera HT, et al. Patient care and clinical outcomes for patients with COVID-19 infection admitted to African high-care or intensive care units (ACCCOS): a multicentre, prospective, observational cohort study. Lancet. 2021;397(10288):1885–94. doi:10.1016/S0140-6736(21)00441-4.
- Ni ZL, Tan XD, Shao HY, Wang Y. Immunisation status and determinants of left-behind children aged 12-72 months in central China. Epidemiol Infect. 2017;145(9):1763–72. doi:10.1017/S0950268817000589.
- Stein-Zamir C, Israeli A. Age-appropriate versus up-to-date coverage of routine childhood vaccinations among young children in Israel. Hum Vaccin Immunother. 2017;13(9):2102–10. doi:10.1080/21645515.2017.1341028.
- Tang XY, Yan XX, Wei X, Qin QL, Lin YD, Geater A, Deng Q-Y, Zhong G, Li Q. Timeliness, completeness, and timeliness-and-completeness of serial routine vaccinations among rural children in Southwest China: a multi-stage stratified cluster sampling survey. Vaccine. 2021;39(24):3236–49. doi:10.1016/j.vaccine.2021.04.048.
- Xiong Y, Xue Y, Jiao G, Xie J, Cheng J. Comparative analysis of the status and influencing factors of immunization among children between registered and floating population. Front Public Health. 2022;10:872342. doi:10.3389/fpubh.2022.872342.
- Wightman P, McCue K, Sabo S, Annorbah R, Jiménez D, Pilling V, Butler M, Celaya MF, Rumann S. Community health worker intervention improves early childhood vaccination rates: results from a propensity-score matching evaluation. BMC Public Health. 2022;22(1):1854. doi:10.1186/s12889-022-14239-w.
- Pavlopoulou ID, Michail KA, Samoli E, Tsiftis G, Tsoumakas K. Immunization coverage and predictive factors for complete and age-appropriate vaccination among preschoolers in Athens, Greece: a cross–sectional study. BMC Public Health. 2013;13(1):908. doi:10.1186/1471-2458-13-908.
- Brenol CV, Azevedo VF, Bonvehi PE, Coral-Alvarado PX, Granados J, Muñoz-Louis R, Pineda C, Vizzotti C. Vaccination recommendations for adults with autoimmune inflammatory rheumatic diseases in Latin America. J Clin Rheumatol. 2018;24(3):138–47. doi:10.1097/RHU.0000000000000624.
- Papp KA, Haraoui B, Kumar D, Marshall JK, Bissonnette R, Bitton A, Bressler B, Gooderham M, Ho V, Jamal S, et al. Vaccination guidelines for patients with immune-mediated disorders taking immunosuppressive therapies: executive summary. J Rheumatol. 2019;46(7):751–4. doi:10.3899/jrheum.180784.
- Lai X, Li M, Hou Z, Guo J, Zhang H, Wang J, Fang H. Factors associated with caregivers’ hesitancy to vaccinate children against influenza: a cross-sectional survey in China. Vaccine. 2022;40(29):3975–83. doi:10.1016/j.vaccine.2022.05.023.
- Du M, Tao L, Liu J. Association between risk perception and influenza vaccine hesitancy for children among reproductive women in China during the COVID-19 pandemic: a national online survey. BMC Public Health. 2022;22(1):385. doi:10.1186/s12889-022-12782-0.
- Wu D, Jin C, Bessame K, Tang FFY, Ong JJ, Wang Z, Xie Y, Jit M, Larson HJ, Chantler T, et al. Effectiveness of a pay-it-forward intervention compared with user-paid vaccination to improve influenza vaccine uptake and community engagement among children and older adults in China: a quasi-experimental pragmatic trial. Lancet Infect Dis. 2022;22(10):1484–92. doi:10.1016/S1473-3099(22)00346-2.
- Cates M, Donati M, Gillet S, Ustianowski A, Galloway J. Managing varicella zoster virus contact and infection in patients on anti-rheumatic therapy. Rheumatology (Oxford). 2018;57(4):596–605. doi:10.1093/rheumatology/kex189.
- Kearsley-Fleet L, Klotsche J, van Straalen JW, Costello W, D’Angelo G, Giancane G, Horneff G, Klein A, Láday M, Lunt M, et al. Burden of comorbid conditions in children and young people with juvenile idiopathic arthritis: a collaborative analysis of 3 JIA registries. Rheumatology (Oxford). 2022;61(6):2524–34. doi:10.1093/rheumatology/keab641.
- Aeschlimann FA, Chong SL, Lyons TW, Beinvogl BC, Góez-Mogollón LM, Tan S, Laxer RM. Risk of serious infections associated with biologic agents in juvenile idiopathic arthritis: a systematic review and meta-analyses. J Pediatr. 2019;204. doi:10.1016/j.jpeds.2018.08.065.
- Nossent JC, Keen HI, Preen DB, Inderjeeth CA. Trends in hospitalization for tuberculosis and other opportunistic infections in Australian patients with inflammatory joint diseases. Rheumatol Ther. Published online 2023 Feb;10(3):563–73. doi:10.1007/s40744-023-00534-4.
- Krasselt M, Baerwald C, Liebert UG, Seifert O. Humoral immunity to varicella zoster virus in patients with systemic lupus erythematosus and rheumatoid arthritis compared to healthy controls. Vaccines (Basel). 2021;9(4):325. doi:10.3390/vaccines9040325.
- Furer V, Rondaan C, Heijstek MW, Agmon-Levin N, van Assen S, Bijl M, Breedveld FC, D’Amelio R, Dougados M, Kapetanovic MC, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;79(1):39–52. doi:10.1136/annrheumdis-2019-215882.
- Chen J, Li F, Tian J, Xie X, Tang Q, Chen Y, Ge Y. Varicella zoster virus reactivation following COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a cross-sectional Chinese study of 318 cases. J Med Virol. 2023;95(1):e28307. doi:10.1002/jmv.28307.
- Sousa S, Duarte AC, Cordeiro I, Ferreira J, Gonçalves MJ, Meirinhos T, Meirinhos, TM, Romão, VC, Santos, MJ et al. Efficacy and safety of vaccination in pediatric patients with systemic inflammatory rheumatic diseases: a systematic review of the literature. Acta Reumatol Port. 2017;42(1). https://pubmed.ncbi.nlm.nih.gov/28133957.
- Bühler S, Jaeger VK, Adler S, Bannert B, Brümmerhoff C, Ciurea A, Distler O, Franz J, Gabay C, Hagenbuch N, et al. Safety and immunogenicity of tetanus/diphtheria vaccination in patients with rheumatic diseases—a prospective multi-centre cohort study. Rheumatology (Oxford). 2019;58(9):1585–96. doi:10.1093/rheumatology/kez045.
- Sattui SE, Liew JW, Kennedy K, Sirotich E, Putman M, Moni TT, Akpabio A, Alpízar-Rodríguez D, Berenbaum F, Bulina I, et al. Early experience of COVID-19 vaccination in adults with systemic rheumatic diseases: results from the COVID-19 global rheumatology alliance vaccine survey. RMD Open. 2021;7(3):e001814. doi:10.1136/rmdopen-2021-001814.
- Heijstek MW, van Gageldonk PGM, Berbers GAM, Wulffraat NM. Differences in persistence of measles, mumps, rubella, diphtheria and tetanus antibodies between children with rheumatic disease and healthy controls: a retrospective cross-sectional study. Ann Rheum Dis. 2012;71(6):948–54. doi:10.1136/annrheumdis-2011-200637.
- Nakafero G, Grainge MJ, Myles PR, Mallen CD, Zhang W, Doherty M, Nguyen-Van-Tam JS, Abhishek A. Effectiveness of inactivated influenza vaccine in autoimmune rheumatic diseases treated with disease-modifying anti-rheumatic drugs. Rheumatology (Oxford). 2020;59(12):3666–75. doi:10.1093/rheumatology/keaa078.
- Curtis JR, Cofield SS, Bridges SL, Bassler J, Deodhar A, Ford TL, Huffstutter J, Jankeel A, Kivitz A, Kamal S, et al. The safety and immunologic effectiveness of the live varicella-zoster vaccine in patients receiving tumor necrosis factor inhibitor therapy: a randomized controlled trial. Ann Intern Med. 2021;174(11):1510–18. doi:10.7326/M20-6928.
- Tang XY, Cheng M, Geater A, Deng QY, Zhong G, Lin YD, Chen N, Lan T, Jiang L-Y, Zhu M-T, et al. Multi-level determinants of failure to receive timely and complete measles vaccinations in Southwest China: a mixed methods study. Infect Dis Poverty. 2021;10(1):102. doi:10.1186/s40249-021-00885-6.
- Nayar RK, Nair AT, Shaffi M, Swarnam K, Kumar A, Abraham M, Lordson J, Grace C. Methods to overcome vaccine hesitancy. Lancet. 2019;393(10177):1203–4. doi:10.1016/S0140-6736(19)30218-1.
- Colmegna I, Valerio V, Gosselin-Boucher V, Lacoste G, Labbe S, Lavoie KL, Hazel E, Ward B, Hudson M, Peláez S. Barriers and facilitators to influenza and pneumococcal vaccine hesitancy in rheumatoid arthritis: a qualitative study. Rheumatology (Oxford). 2021;60(11):5257–70. doi:10.1093/rheumatology/keab471.
- Benis A, Khodos A, Ran S, Levner E, Ashkenazi S. Social media engagement and influenza vaccination during the COVID-19 pandemic: cross-sectional survey study. J Med Internet Res. 2021;23(3):e25977. doi:10.2196/25977.