ABSTRACT
Post-transplant lymphoproliferative disorder (PTLD) is a potentially fatal complication following kidney transplantation, and there is a critical and unmet need for PTLD treatments associated with more pronounced and durable responses. To date, reports on the use of CD19-targeted chimeric antigen receptor (CAR) T (CAR-T) cells in patients after solid organ transplant (SOT) have been anecdotal, clinical presentations and outcomes have been heterogenous, and a longitudinal analysis of CAR-T cell expansion and persistence in PTLD patients has not been reported. Our report describes a patient with a history of renal transplant who received CD19-directed CAR-T cell therapy for the treatment of refractory PTLD, diffuse large B cell lymphoma (DLBCL)-type. We show that even with the background of prolonged immunosuppression for SOT, it is possible to generate autologous CAR-T products capable of expansion and persistence in vivo, without evidence of excess T-cell exhaustion. Our data indicate that CAR-T cells generated from a SOT recipient with PTLD can yield deep remissions without increased toxicity or renal allograft dysfunction. Future clinical studies should build on these findings to investigate CAR-T therapy, including longitudinal monitoring of CAR-T phenotype and function, for PTLD in SOT recipients.
Introduction
Post-transplant lymphoproliferative disorder (PTLD) is a potentially fatal complication following kidney transplantation. The risk of lymphoma in kidney-transplant recipients (KTRs) is 12-fold higher than in a similar, non-transplanted population.Citation1 PTLDs include a spectrum of B cell disorders, ranging from benign proliferation of lymphoid tissue to aggressive B cell lymphomas.Citation2 Risk factors for PTLD include intensity of posttransplant T cell suppression and chronic viral infection, particularly Epstein-Barr virus (EBV), especially in EBV-seronegative SOT recipients. PTLD treatments include reduction of immunosuppression, CD20-directed antibody therapy, and cytotoxic chemotherapy.Citation3 Despite recent advances in the prevention and treatment of PTLD, outcomes remain poor. Overall survival (OS) with PTLD is only around 60% in SOT recipients.Citation4 Therefore, there is a critical, unmet need for more effective PTLD therapies.
Adoptive cellular immunotherapy using EBV‐specific cytotoxic T cells (CTL) has been used successfully to treat PTLD in allogeneic hematopoietic stem cell transplant (HSCT) recipients. Two-thirds of SOT patients with refractory PTLD respond to EBV-CTL,Citation3,Citation5 however, these treatments are not readily available at most centers. As a potential alternative, CD19-directed chimeric antigen receptor (CAR) T cells (CAR-T) have significant clinical activity in relapsed/refractory B cell lymphomasCitation6 and may be effective in refractory B cell PTLD as well. It is unknown whether CAR-T products can be successfully manufactured from autologous T cells in immunosuppressed SOT recipients. Nor do we know whether these CAR-T cells would be capable of the requisite proliferation, target-cell killing, and persistence in vivo, given that after SOT, T cells are under chronic allo-antigenic stimulation, promoting cellular exhaustion.Citation7 Here, we report the case of a KTR who received autologous CD19-targeted CAR-T for refractory PTLD-DLBCL. Despite a long history of immunosuppression, the CAR-T cells demonstrated substantial in vivo expansion, without any signs of cellular exhaustion, and yielded a marked clinical response.
Materials and methods
Patients and samples
Samples: Blood was collected under an Institutional Review Board-approved research sample-acquisition protocol. Following informed consent, blood samples were collected; plasma was generated by centrifugation at 400 G and frozen immediately at −80°C. Peripheral blood mononuclear cells (PBMCs) were isolated using standard Ficoll-Hypaque density gradient centrifugation and frozen in liquid nitrogen.
Immunohistochemistry (IHC)
Histologic sections from formalin-fixed, paraffin-embedded tissue samples were processed with immunohistochemical and in situ hybridization stains using standard techniques.
Flow cytometry
Prior to the flow cytometric analysis of PBMC, staining was performed using a panel of monoclonal antibodies following manufacturer’s instructions (Supplementary Table 1). Live cells were identified by 7-AAD dye exclusion (Miltenyi #130-111-568). Samples were acquired using a Miltenyi MACSQuant Analyzer 10 Flow Cytometer.
CodePlex secretome analysis
Cytokine concentrations in patient sera were quantified using the CodePlex Secretome Human Adaptive Immune Panel kit (IsoPlexis # CODEPLEX-2L01). To carry out the CodePlex analysis, sera were loaded onto chip microchambers. The chip was then loaded into the Isolight reader (Isoplexis, Branford, CT); automated analysis of raw data was performed using IsoSpeak software (Isoplexis).
Results
Patient history
Our patient was a 55-y-old woman with a history of end-stage renal disease who underwent an unrelated, living-donor kidney transplant in early 2021. Two months after SOT, immunosuppressive prophylaxis included mycophenolate mofetil 250 mg twice daily, tacrolimus 2 mg twice daily, and prednisone 5 mg daily. Three months after the transplant, she developed abdominal pain and night sweats. Esophagogastroduodenoscopy showed a gastric nodule. On biopsy, EBER staining was positive and IHC showed monomorphic PTLD, DLBCL type (). 18F-Fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET-CT) identified areas of PTLD in the stomach, bowel, and lungs. Mycophenolate was stopped, and tacrolimus was increased to 3 mg twice daily. The patient received three cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone). The next three cycles of chemotherapy omitted vincristine due to neuropathy. End-of-treatment PET-CT showed a mixed response and a biopsy confirmed persistent PTLD-DLBCL in the right upper lobe (January 2022). Immunosuppression was switched from tacrolimus to sirolimus, and treatment with PBR chemotherapy (polatuzumab, bendamustine, and rituximab) was initiated (March 2022), with subsequent disease progression. Prednisone was increased to 60 mg/d (April 2022) to control mass-associated pain. Consultation for adoptive T cell therapy options resulted in recommendation for CD19-directed CAR-T cell therapy with axicabtagene ciloleucel (axi-cel). The patient proceeded with bridging radiation therapy to the right-lung mass (May 2022), to facilitate rapid steroid taper prior to apheresis for CAR-T manufacture.
Clinical course
Following palliative radiation therapy, sirolimus was held for 19 d before MNC harvest by leukapheresis. CAR-T manufacturing yielded an axi-cel product fulfilling release criteria. Sirolimus was restarted after leukapheresis, then held again to permit complete clearance prior to CAR-T infusion (). Her kidney function was normal at the time, and lymphodepleting chemotherapy could be administered at a standard dose of fludarabine at 30 mg/m2 and cyclophosphamide at 500 mg/m2, followed by CAR-T infusion (June 2022). Low-dose prednisone (10 mg/d) was resumed on D +7. Additional immunosuppression such as sirolimus, tacrolimus, or mycophenolate mofetil was not restarted at any point after CAR-T.
Figure 1. CAR-T cell expansion and persistence in a patient with in a kidney transplant recipient with PTLD (a) Time course of reconstitution of white blood cell, neutrophil, and lymphocyte counts after lymphodepleting chemotherapy and CAR-T cell infusion. Timepoints where flow cytometry for the detection of CAR-T cells was performed are indicated by pink triangles. (b) Analysis of CAR-T cell numbers in the peripheral blood of our patient on d +27, +42, and +60 post CAR-T cell treatment. CAR-T cells were identified by staining of the expression of the CAR on the cell surface and co-staining with anti-CD3 and other T cell markers (Supplementary Table 1). (c) CAR-T cell memory subtypes were determined on d +42 and +60 post CAR-T treatment by co-staining for CD45RA and CD62L. Central memory (CM) and effector memory (EM) CAR-T cells are shown in the right lower and left lower quadrants, respectively. (d) Expression of exhaustion markers PD1, LAG3, and TIM3 on CAR-T cells was determined by flow cytometry on d +42 and +60 post CAR-T treatment. (e) Serum concentrations of 22 different T cell-related cytokines/chemokines were determined in our patient on d +27, +42 and +60 post CAR-T treatment using CodePlex secretome technology.
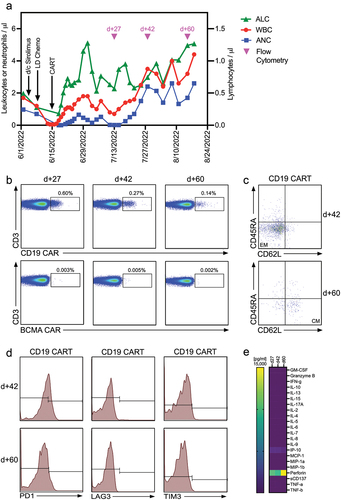
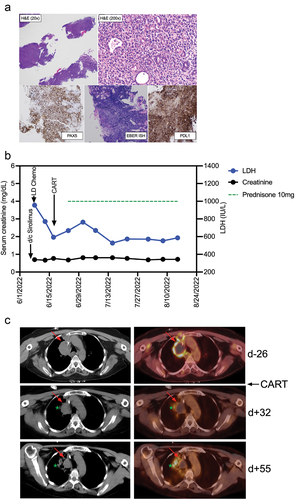
Figure 2. Diagnosis and clinical course of PTLD in a kidney transplant recipient treated with CD19 CAR-T cells histologic images from the gastric antral soft tissue nodule biopsy, examination at low power magnification demonstrates thin cores of tissue with a focal area of intact gastric mucosa (right side of figure), and architectural effacement of the remaining tissue by a hypercellular diffuse lymphoid infiltrate (H&E stain, 20× magnification). Examination at higher magnification reveals an infiltrate of large atypical lymphoid cells forming sheets and infiltrating residual mucosal epithelium, with moderate cytologic atypia and pleomorphism (H&E stain, 200× magnification). Immunohistochemical and in situ hybridization (ISH) stains demonstrate strong diffuse positivity of the CD20-expressing lymphocytes for PAX5 (40× magnification), EBER ISH (40× magnification), and PDL1 with negativity for CD10 and Ki-67-positivity in 90% of cells (data not shown). (b) Clinical course after discontinuation of sirolimus, lymphodepleting (LD) chemotherapy with fludarabine/cyclophosphamide, and CAR-T cell infusion. Post-CAR-T corticosteroid dosing is shown as a green dotted line, serum concentrations of lactate dehydrogenase (LDH) and creatinine are shown in blue and black, respectively. (c) PET-CT Images from before CAR-T cell treatment (d-26), and two timepoints (d + 32 and d + 55) after CAR-T cell treatment. The upper panel (pre-CAR-T; d-26) shows an index right upper mediastinal mass (left: CT, right: fused PET/CT) measuring 5.0 × 3.6 cm with central photopia and peripheral increased F-fluorodeoxyglucose (FDG) uptake on PET representing active lymphoma (indicated by red arrow). The middle panel (d + 32) shows a decreased size (3.4 × 3.0 cm) of the index lesion with mild residual peripheral FDG uptake representing partial metabolic response to the treatment. The green star indicates a new infectious pulmonary process in the right upper lobe. The lower panel (d + 55) shows a further decrease in size (3.0 × 2.6 cm) of the mediastinal index lesion with resolution of the peripheral FDG uptake, representing a complete metabolic response.
CAR-T expansion and persistence
Following CAR-T, chemotherapy-induced leukopenia improved quickly; peripheral blood lymphocyte counts peaked on post-CAR-T D +15 (). On post-CAR-T D +27, CAR-T cell expansion and persistence were assessed by flow cytometry, and a clear population of CD19-targeting CAR-T cells could be distinguished in the peripheral blood (). Contemporaneously, clinical assessment found that the LDH had decreased () and PET-CT demonstrated a partial response (). Peripheral blood assessment on D +42 continued to detect a CAR-T population () consisting of effector/memory (EM)-type T cells (). Exhaustion markers, such as PD1, LAG3, or TIM3, were not identified (). On CAR-T D +60, memory-type CAR-T cells could still be detected in the peripheral blood () and remained negative for exhaustion markers (). At that time, imaging showed a complete metabolic response to CAR-T cell treatment (). Throughout the treatment course, no treatment-related adverse events, e.g., cytokine release syndrome or neurotoxicity, were clinically apparent nor were any cytokine peaks detected in the peripheral blood (). Additionally, the patient’s renal function remained stable (). Unfortunately, 2 months after CAR-T cell therapy, our patient developed a SARS-CoV-2 infection. She initially responded to therapy but subsequently worsened, dying of complications of COVID-19 pneumonia 3 months after CAR-T infusion.
Discussion
Reports on the use of CD19-targeted CAR-T cells in patients after SOT have been anecdotal,Citation8–12 clinical presentations and outcomes have been heterogenous, and a longitudinal analysis of CAR-T expansion and persistence in PTLD patients has not been previously reported. The case presented herein demonstrates that it is possible to produce autologous CAR-T in SOT recipients that are capable of expansion and persistence in vivo, without signs of T cell exhaustion even in the setting of prolonged immunosuppressive prophylaxis. Our data suggest that post-SOT CAR-T cells are able to produce deep and potentially durable remissions of PTLD. Future clinical studies should prospectively evaluate CD19-directed CAR-T cell therapy for PTLD. Our patient’s course also demonstrates that, in the context of lymphodepleting preparative chemotherapy prior to CAR-T infusion, immunosuppressive prophylaxis may be safely withheld in order to maximize T cell expansion, and effector function may be safely accomplished, without allograft rejection or reduced function in KTRs.
Unfortunately, our case also illustrates the profound immune dysfunction resulting from effective CD19-directed CAR-T therapy, as well as the need for – and limitations of – optimal antimicrobial prophylaxis and social distancing, especially in the context of the COVID-19 pandemic.
Author contributions
D.A. designed the study, performed experiments, analyzed the data, made figures, and wrote the manuscript. T.I., D.O., E.G., and N.D. processed patient samples and performed experiments. K.K., J.M.B., K.A.D., J.A.Y., K.H., S.D., S.V.N., K.D., N.M.H., and A.P.R. analyzed data and wrote the manuscript. W.C., M.E.K., M.K., and X.F. performed experiments, analyzed the data, and wrote the manuscript. T.L. analyzed the data, prepared figures, and wrote the manuscript.
Supplemental Material
Download PDF (17.3 KB)Disclosure statement
S.D. serves on advisory boards for Bristol-Myers Squibb, Incyte, and Atara Biotherapeutics. N.M.H. serves on advisory boards for InCyte and Kite-Gilead and is a member of the DSMB for American Gene Technologies. The remaining authors declare that they do not have any competing interests.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2216116
Additional information
Funding
References
- Sprangers B, Riella LV, Dierickx D. Posttransplant lymphoproliferative disorder following kidney transplantation: a review. Am J Kidney Dis. 2021;78(2):272–5. doi:10.1053/j.ajkd.2021.01.015.
- Abbas F, Kossi ME, Shaheen IS, Sharma A, Halawa A. Post-transplantation lymphoproliferative disorders: current concepts and future therapeutic approaches. World J Transplant. 2020;10(2):29–46. doi:10.5500/wjt.v10.i2.29.
- Allen UD, Preiksaitis JK, A.S.T.I.D.C.O. Practice. Post-transplant lymphoproliferative disorders, Epstein-Barr virus infection, and disease in solid organ transplantation: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33(9):e13652. doi:10.1111/ctr.13652.
- Lindsay J, Othman J, Heldman MR, Slavin MA. Epstein–Barr virus posttransplant lymphoproliferative disorder: update on management and outcomes. Curr Opin Infect Dis. 2021;34(6):635–45. doi:10.1097/QCO.0000000000000787.
- Liu JY, Zhang J-M, Zhan H-S, Sun L-Y, Wei L. EBV-specific cytotoxic T lymphocytes for refractory EBV-associated post-transplant lymphoproliferative disorder in solid organ transplant recipients: a systematic review. Transpl Int. 2021;34(12):2483–93. doi:10.1111/tri.14107.
- Holstein SA, Lunning MA. CAR T-Cell therapy in hematologic malignancies: a voyage in progress. Clin Pharmacol Ther. 2020;107(1):112–22. doi:10.1002/cpt.1674.
- Angeletti A, Cantarelli C, Riella LV, Fribourg M, Cravedi P. T-cell exhaustion in organ transplantation. Transplant. 2022;106(3):489–99. doi:10.1097/TP.0000000000003851.
- Feng G, Li Q, Zhu H, Jiang Y, Yuan J, Fu Y, Deng Q. Safety and efficacy of anti-CD19-chimeric antigen receptor T cell combined with programmed cell Death 1 inhibitor therapy in a patient with refractory post-transplant lymphoproliferative disease: case report and literature review. Front Oncol. 2021;11:726134. doi:10.3389/fonc.2021.726134.
- Hernani R, Sancho A, Amat P, Hernández-Boluda JC, Pérez A, Piñana JL, Carretero C, Goterris R, Gómez M, Saus A, et al. CAR-T therapy in solid transplant recipients with post-transplant lymphoproliferative disease: case report and literature review. Curr Res Transl Med. 2021;69(4):103304. doi:10.1016/j.retram.2021.103304.
- Krishnamoorthy S, Ghobadi A, Santos RD, Schilling JD, Malone AF, Murad H, Bartlett NL, Alhamad T. CAR-T therapy in solid organ transplant recipients with treatment refractory posttransplant lymphoproliferative disorder. Am J Transplant. 2021;21(2):809–14. doi:10.1111/ajt.16367.
- Luttwak E, Hagin D, Perry C, Wolach O, Itchaki G, Amit O, Bar-On Y, Freund T, Kay S, Eshel R, et al. Anti-CD19 CAR-T therapy for EBV-negative posttransplantation lymphoproliferative disease—a single center case series. Bone Marrow Transplant. 2021;56(5):1031–7. doi:10.1038/s41409-020-01145-1.
- Wang T, Feng M, Luo C, Wan X, Pan C, Tang J, Xue F, Yin M, Lu D, Xia Q, et al. Successful treatment of pediatric refractory Burkitt lymphoma PTLD after liver transplantation using anti-CD19 chimeric antigen receptor T-cell therapy. Cell Transplant. 2021;30:963689721996649. doi:10.1177/0963689721996649.
