ABSTRACT
Infectious diseases are a leading cause of morbidity and mortality worldwide with vaccines playing a critical role in preventing deaths. To better understand the impact of low vaccination rates and previous epidemics on infectious disease rates, and how these may help to understand the potential impacts of the current coronavirus disease 2019 (COVID-19) pandemic, a targeted literature review was conducted. Globally, studies suggest past suboptimal vaccine coverage has contributed to infectious disease outbreaks in vulnerable populations. Disruptions caused by the COVID-19 pandemic have contributed to a decline in vaccination uptake and a reduced incidence in several infectious diseases; however, these rates have increased following the lifting of COVID-19 restrictions with modeling studies suggesting a risk of increased morbidity and mortality from several vaccine-preventable diseases. This suggests a window of opportunity to review vaccination and infectious disease control measures before we see further disease resurgence in populations and age-groups currently unaffected.
Introduction
Infectious diseases continue to be one of the leading causes of morbidity and mortality worldwide, accounting for 18.4% of deaths globally in 2019, with a higher proportion of deaths in low- and lower- to middle-income countries.Citation1,Citation2 Across all age groups, infectious diseases, including infectious diarrhea and lower respiratory infections, were among the top 10 causes of disease burden and deaths globally in 2019.Citation1,Citation3
Vaccines are recognized as having a critical role in preventing deaths and hospitalizations due to infectious diseases; estimates suggest that vaccines could have prevented nearly one-quarter (21.7%) of the 5.3 million deaths among children under the age of 5 years in 2019.Citation4 The role of vaccines in the global eradication of smallpox demonstrates the impact of successful global vaccination efforts, and other successes include the dramatic reduction and near elimination of polio in some regions of the world.Citation5,Citation6 Decreased rates of other childhood infectious diseases in regions with high vaccination rates have also been observed including diphtheria, pertussis, tetanus, measles, mumps, rotavirus, hepatitis-B, meningococcal, pneumococcal illness and rubella.Citation7–13 However, vaccine-preventable deaths continue to pose a significant economic burden to society, particularly in resource constrained communities. A 2018 analysis reported that four major vaccine-preventable diseases – rotavirus, pneumococcal disease, measles, and rubella – were estimated to collectively cost Africa (US) $13 billion annually, due to productivity losses resulting from premature death (US $10 billion) and prolonged sickness (US $2 billion), hospitalizations (US $260 million), and outpatient visits (US $73 million).Citation14
Disruptions in access to healthcare services, including problems with access to national immunization programs (NIP) and low vaccination uptake, can significantly impact the epidemiology of infectious diseases. Vaccine coverage may be impacted by changes to healthcare/vaccination policy, funding, safety concerns, patient noncompliance, and supply and administration issues.Citation15–18 Historically disruptions in access to NIPs and low vaccination uptake have had major impacts on the epidemiology of infectious diseases. One such recent example is the arrival of the coronavirus disease 2019 (COVID-19) pandemic where non-pharmacological interventions (NPI) were implemented globally to reduce the spread of the virus.Citation19–21 These included personal protection and hygiene measures (face masks, gloves and other personal protective equipment, hand hygiene, sanitizing contaminated surfaces) and social distancing (e.g., lockdowns, stay-at-home orders, bans/restrictions on travel and group gatherings/events).Citation19,Citation20,Citation22 However, in addition to controlling the spread of COVID-19, such measures also impacted the epidemiology of non-COVID-19 infectious diseases and the uptake of NIPs. Subsequently, the roll-out of COVID-19 vaccination programs has led to the relaxation of NPIs but the eventual long-term impact of the pandemic on vaccine-preventable diseases globally remains unclear.Citation21 It is therefore important to understand the potential impact of the COVID-19 pandemic and associated NPIs on the epidemiology of non-COVID infectious diseases. This targeted literature review (TLR) seeks to 1) identify past pandemics and corresponding NPIs and describe their impact on the epidemiology of infectious diseases; 2) identify historical examples of disruptions to NIPs and low vaccine uptake and characterize their impact on infectious diseases; 3) identify the impact of COVID-19 disruptions on vaccine uptake; 4) understand the impact of COVID-19 restrictions on vaccine preventable infectious disease epidemiology; and finally 5) apply these learnings to the COVID-19 pandemic and associated NPIs to build an understanding of their impact on the uptake of (non-COVID-19) vaccines and the current and future epidemiology of (non-COVID-19) infectious diseases.
Methods
A protocol-driven TLR was conducted to identify key evidence from real-world (observational) and mathematical modeling studies. Extensive literature searches of MEDLINE (OvidSP) and Embase (OvidSP) were conducted from inception to October 2021. Search strategies used a combination of indexing terms (Medical Subject Headings terms in MEDLINE and Emtree terms in Embase) as well as free-text keywords were used to identify studies reporting on factors causing disruption to NIPs and the epidemiology of vaccine-preventable infectious diseases in the general population. Separate search facets were developed using terms for infectious diseases, NPIs, outcomes, and study designs of interest, which were combined using Boolean operators and limited to studies in humans (see Supplemental File 1 for additional details). Gray literature searches were carried out to identify conference abstracts published from 2019 onward (indexed in Embase) and epidemiological/surveillance data reported by key public health websites (World Health Organization [WHO], Gavi, the Vaccine Alliance, United Kingdom Health Security Agency [UKHSA] formerly Public Health England [PHE]), Centers for Disease Control and Prevention [CDC], European Centre for Disease Prevention and Control) were also carried out. Pre-print databases (medRxiv, bioRxiv, Lancet preprints) were also searched for articles posted from 2019 to October 2021 to capture more recent COVID-specific data. Targeted hand searches for updated surveillance data and more recent publications were carried out in June 2022.
Articles at title/abstract and full text were systematically screened using DistillerSR® software and selected for inclusion by one reviewer with a random sample of 20% validated by a second, senior reviewer according to pre-defined population, interventions and comparisons, outcomes, and study design (PICOS) criteria (see Supplemental File 2 for additional details). Studies investigating the impact of NPIs, such as social distancing measures, general face mask use, and policy changes relevant to public health, were considered eligible for inclusion. Observational studies, epidemiological modeling studies (based on real-world data), disease surveillance, and public health reports were included regardless of geographical location if they reported on the dynamics of infectious disease epidemiology (with specific interest in vaccine-preventable diseases) resulting from any disruption to a vaccination program. Examples of disruptions could include any type of NPI, policy changes, vaccine hesitancy, or previous disease outbreaks. From those studies meeting the PICOS inclusion criteria, 50 key articles were prioritized for data extraction. To ensure a representative global sample of key studies across the five research questions, articles were prioritized based on the geographical location (i.e., COVID-related evidence from UK, North America and Europe, and global evidence on past pandemics), infectious disease investigated (i.e., infectious diseases relevant to the United Kingdom (UK)), and time-period of data collection. (i.e., last 10 years) to ensure a representative global sample of key studies across the five research questions. In addition, priority was given to those articles of most importance to public health, within the UK including articles on pneumococcal disease, influenza, human papilloma virus (HPV), pertussis, measles and shingles.Citation23 Data were extracted into a specially designed Microsoft Excel® spreadsheet by one reviewer and validated by a second reviewer. Key findings of the TLR were summarized qualitatively.
Results
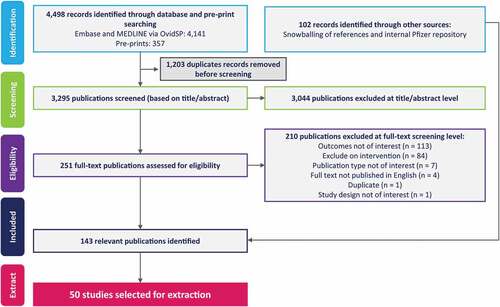
A total of 3,295 records from database searches were screened, and 251 records selected for full-text review, of which 41 records were included. In addition, 102 records were identified from gray literature searches, including searches of websites and citation chasing (). From the 143 included records 50 studies were prioritized for data extraction, including studies conducted in the UK (n = 13 studies), US (n = 12), and Europe (n = 10), followed by Africa (n = 6), Asia (n = 4), and Australia (n = 1) with four studies reporting on multiple global regions ().
Table 1. Impact of NPIs to tackle pre-COVID disease outbreaks on vaccine-preventable disease epidemiology.
Table 2. Historical disruptions in disease epidemiology due to NPIs (pre-COVID).
Table 3. Data on the impact of COVID-19 disruptions on vaccination uptake.
Table 4. Impact of lifting of COVID-19 restrictions on disease epidemiology.
Table 5. Potential future impacts of vaccination disruptions due to COVID-19 restrictions on disease burden.
Thirty percent of studies were focused on measles (n = 15), 24% on pneumococcal pneumonia (n = 12), 10% on respiratory syncytial virus (RSV; n = 5), and 8% on polio (n = 4). Thirty-eight percent of the studies (n = 19) reported disease epidemiology in children, while the remainder reported data for the general population or other age-specific groups (e.g., adults, elderly). Out of the 50 included studies 56% were surveillance data studies (n = 28), 22% (n = 11) were modeling studies, 12% (n = 6) were retrospective cohort studies, 6% were (n = 3) literature reviews, and 4% (n = 2) were case–control investigations. One-third of the included articles were public health reports from UK PHE and the US CDC. Articles reported on data gathered before the COVID-19 pandemic and spanned a 20-year period from 1996 to 2019.
Impact of NPIs to tackle pre-COVID disease outbreaks on disease epidemiology
The impact of historical disease outbreaks (pre-COVID) on vaccine-preventable disease epidemiology was reported in three studies all of which focused on the impact of NPIs during the 2014 to 2015 Ebola outbreak across AfricaCitation24–26 ().
During the Ebola outbreak, the affected countries implemented various NPIs including curfews, border closures, and restrictions on free movement. In addition, the establishment of Ebola treatment centers and the re-deployment of healthcare workers to these centers led to the closure of healthcare facilities and the postponement of vaccination activities resulting in non-Ebola infectious disease outbreaks and a resurgence across various vaccine-preventable diseases.Citation24–26 In Liberia, the mean coverage of the first dose of measles-containing vaccine (MCV1) during the outbreak in 2015 was 16% lower than in the 2 years preceding the outbreak. Correspondingly, the incidence of measles increased from zero cases in 2013 to 2014, to 108.5 cases per million in 2015.Citation25 The incidence of measles in Sierra Leone increased from 6.9 per million in 2014, to 18.0 per million in 2015, during the Ebola outbreak, and remained high in 2016 and 2017.Citation25 A nationwide measles vaccination effort was initiated in June 2015 to combat the rising case numbers resulting in the vaccination of 1,205,865 children from 9 to 59 months of age (97.2% coverage).Citation25 Following continued outbreaks of measles involving children over 5 years of age, an expanded measles immunization program was implemented in May 2016, which reached 2,795,686 children aged 6 months to 14 years with a coverage at national level of 97.7% (95% confidence interval [CI]: 97.2% to 98%). A post-campaign survey revealed that 20.2% of the children received the vaccination for the first time.Citation25 In Guinea, estimates of coverage for the third dose of diphtheria-tetanus-pertussis (DTP3) vaccine, single-dose yellow fever vaccine, and MCV1 showed declines as a result of the 2014 to 2015 Ebola outbreak. DPT3 coverage was on average 48.5% in 2012 and 2013, 39.5% during the outbreak in 2014 and 2015, and 45% after the Ebola outbreak in 2016 and 2017. The single-dose yellow fever coverage was on average 45% in 2012 and 2013, 35.5% during the outbreak in 2014 and 2015, and 43% after the outbreak in 2016 and 2017. MCV1 was on average 45% in 2012 and 2013, 38% during the outbreak in 2014 to 2015, and 48% after the Ebola outbreak in 2016 and 2017. The incidence of measles increased from 2.7 per million in 2015 to 11.5 per million in 2016.Citation25
Historical disruptions in disease epidemiology due to NPIs (Pre-COVID)
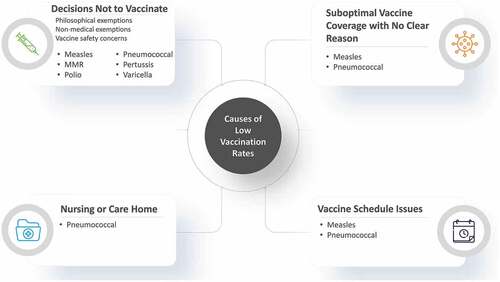
Twenty-one studies reported on the impact of low rates of vaccination on past disease epidemiology (pre-COVID) including eight on measles,Citation27–34 seven on pneumococcal disease,Citation38–44 three on polio,Citation35–37 and three on other infectious diseases ().Citation45–47 Low vaccination rates were caused primarily by changes in vaccine scheduleCitation30,Citation43 and individual decisions not to vaccinate, which included philosophical exemptions, non-medical exemptions, and vaccine safety concerns.Citation27,Citation29,Citation33,Citation35,Citation36,Citation40,Citation45–47 A number of studies also reported low vaccination rates in nursing or care homes,Citation38,Citation39,Citation42,Citation44 and some studies reported sub-optimal vaccine uptake with no clear reason despite availability of NIPs ().Citation28,Citation31,Citation32,Citation41
Figure 2. Causes of low vaccination rates.
Successful NIPs have seen measles and polio effectively eliminated from several regions across the globe, however periodically these diseases have re-emerged in recent years when vaccination rates have fallen below optimal levels. Local and regional outbreaks have resulted in increased disease-related morbidity/mortality in the Netherlands, Ireland, and several other countries across western Europe and Africa.Citation28,Citation30,Citation32,Citation34,Citation36 In the Netherlands, a large measles outbreak resulted in 2,766 reported cases, of which, 94% (n = 2,539) were reported in unvaccinated individuals, the majority due to religious reasons (84%; n = 2,135).Citation34 In response to this outbreak, early measles-mumps-rubella (MMR) vaccination was advised in infants too young to have already received their first dose (MMR1), as they represent a highly vulnerable population due to loss of maternal antibodies; a total of 5,800 infants received an early MMR1 vaccination. Another clear example of an outbreak linked to suboptimal uptake of measles vaccination occurred in Dublin from December 1999 to July 2000.Citation32 During this time, 1,407 cases were reported in Ireland, and within a single hospital 111 severely ill children were admitted, with 13 needing treatment in intensive care, seven requiring mechanical ventilation, and three children dying as a result of measles. Of the 111 children, 49 (44%) were >15 months of age and therefore eligible for their first MMR immunization, however only 18 (37%) had received this vaccination.
In 2011, measles outbreaks occurred in 36 out of 53 European countries. France reported the largest outbreak in the region, with 14,025 cases predominantly among individuals who were not vaccinated or those whose vaccination history was unknown.Citation28 In each of these examples, NIPs were in place, however sub-optimal uptake was observed, which resulted in a resurgence of vaccine-preventable illness.
Between 2010 and 2011, measles vaccination rates in the Democratic Republic of Congo (DRC) were poor with only three geographical areas achieving ≥89% coverage; subsequent epidemics resulted in 77,241 measles cases and 1,085 deaths.Citation30 The DRC is prone to measles outbreaks, with supplementary immunization activities (SIAs) having previously been implemented with the aim to increase measles vaccine coverage through catch-up programs targeting young children. Access to vaccinations as well as optimal uptake are critical to reduce the risk of outbreaks. Despite being planned in 2010, the SIAs were not implemented.Citation30
Polio outbreaks have also been observed due to the low uptake of vaccination programs.Citation36,Citation37 Polio cases were observed in Ukraine following a significant decline in the oral polio vaccine coverage from 91% in 2008 to 15% in 2015, over the subsequent year as vaccination rates and surveillance increased no further cases were reported.Citation36 Several factors contributed to the decline in vaccination against polio in the Ukraine, these included misconceptions around vaccine safety, anti-vaccine sentiments, as well as insufficient funding. Elsewhere in Afghanistan, following an ongoing ban on polio vaccine by anti-government elements, wild type 1 poliovirus (WPV1) cases increased from 13 cases observed in three provinces in 2019 to 26 cases from 12 provinces in 2020.Citation37
In the US, several outbreaks of measles were reported between 2000 and 2015, and vaccine refusal due to non-medical exemptions, such as religious belief, was a contributing factor to these outbreaks.Citation47 A detailed review of vaccination data for 970 measles cases revealed that 574 cases occurred in unvaccinated individuals who were eligible for vaccination, with 405 (70.6%) of these individuals having non-medical exemptions.Citation47 During this same period, several pertussis outbreaks were observed in the US, including eight outbreaks in populations where 59% to 93% of pertussis cases occurred in children who were intentionally unvaccinated.Citation47 Populations, including schools and communities/states with higher vaccination exemption rates, had correspondingly higher rates of pertussis, including among those who were fully vaccinated.Citation47
Low vaccination rates have also been associated with several outbreaks of invasive pneumococcal disease (IPD) in nursing homes across the US.Citation38,Citation39,Citation42,Citation44 Among 361 long-term care facilities assessed in 2001, 8% failed to meet state regulations requiring pneumococcal polysaccharide vaccinations (PPV) to be offered to all residents.Citation38 In addition, a survey of 54 nursing homes found that only 22% of residents had been vaccinated and the vaccination status was unknown for 66% of residents.Citation44 The underuse of PPV in nursing homes can be potentially attributed to a lack of prioritization by doctors, skepticism regarding vaccine effectiveness, and challenges when trying to obtain residents’ vaccination history.Citation38,Citation42,Citation44
Overall, previous evidence suggests young childrenCitation30,Citation32 and older adults, including those in nursing homes and long-term care facilities,Citation38,Citation42,Citation44 have been most affected by disease outbreaks due to low vaccination rates implying the need to maximize efforts on vaccination coverage and uptake in these vulnerable groups.
Impact of COVID-19 disruptions on vaccination uptake
Ten studies reported on the impact of COVID-19 disruptions on vaccination uptake.Citation48–57 (). Three studies reported on multiple vaccine types,Citation55–57 two reported on measles vaccine,Citation48,Citation49 and the remaining studies reported on HPV,Citation51 meningococcal (MenACWY),Citation50 tetanus and diphtheria (Td)/inactivated poliovirus vaccine (IPV),Citation52 pertussisCitation53 and shingles vaccineCitation54 individually.
During the COVID-19 pandemic, mitigation measures such as lockdowns and school closures contributed to a sharp decline in the uptake of common childhood vaccinations (e.g., MMR, diphtheria-tetanus-pertussis [DTP], HPV), with the greatest impact felt in those countries with the strictest measures. In England, the operational delivery of all school-aged immunization programs was paused due to the COVID-19 pandemic, resulting in marked reductions in vaccination uptake. For example, a 29.7% reduction in meningococcal conjugate vaccine (MenACWY) was reported in year 9 students from 2019 to 2020 compared to levels in 2018 to 2019.Citation50 Similar reductions were reported for the priming dose of HPV for year 8 females (28.8%)Citation51 and Td/IPV (24%) in year 9 students.Citation52 Worldwide data indicated that the 2020 vaccine coverage for DTP3 dropped to 83%, leaving 22.7 million children unprotected.Citation57 MCV1 coverage decreased to 84%, whereas the second dose of the measles-containing vaccine (MCV2) coverage was relatively stable at 71% in 2019 and 70% in 2020.Citation56
Vaccinations in the older adults were also affected by the introduction of COVID-19 restrictions in England. The shingles vaccination program is open to adults aged between 70 and 79 years, and coverage in all ages was lower in the 2020 to 2021 financial year compared to 2019 to 2020.Citation54 For adults turning 70, those newly eligible to the shingles program, coverage dropped from 26.7% in June 2020 to 20.2% in June 2021.Citation54 Similarly, coverage decreased by 5.4% in 70-year-olds and 7.1% for 78-year-olds in 2021 compared with 2018 to 2019.Citation55
Impact of lifting of COVID-19 restrictions on disease epidemiology
Ten studies reported on changes to infectious disease epidemiology after COVID-19 restrictions were lifted.Citation58–67 The findings are summarized in .
Several diseases (especially respiratory diseases) saw a rapid reduction in cases after the introduction of COVID-19 restrictions due to reduced disease transmission rates in light of NPIs reducing social contact. However, research has suggested that although NPI measures have had a beneficial effect in reducing disease incidence, they may also have led to an increase in disease susceptibility, potentially due to waning immunity against some non-COVID-19 infectious diseases.Citation60,Citation62,Citation63 This immunity gap appears to have ultimately left populations at increased risk of subsequent vaccine-preventable disease outbreaks, with surges in cases, and changes to the seasonality of diseases, such as RSV, influenza, norovirus, and pneumococcal disease.Citation60,Citation62,Citation63
Low rates of norovirus infections were reported in England during periods where COVID-19 lockdowns were enforced, but this was likely accompanied by an increase in population susceptibility resulting in a rapid increase in infections (9% increase in symptomatic infections) as COVID-19 restrictions began to be lifted. The subsequent estimated annual incidence rate almost doubled when compared to that predicted before the arrival of COVID-19.Citation64 Although not a vaccine preventable infectious disease, the change in incidence of norovirus, highlights the impact of the COVID-19 pandemic and lifting of COVID-19 restrictions on infectious disease dynamics.
In Germany,Citation59 where NPIs included quarantine after exposure, restrictions on large gatherings, mask wearing, workplace/retail closures and travel restrictions, correlations were reported between reductions in IPD cases and increased stringency to NPIs. IPD incidence dropped sharply in the second quarter of 2020 but rebounded to pre-COVID-19 pandemic levels by the beginning of the third quarter of 2021.Citation59 In children ≤4 years of age, IPD levels began to return to pre-COVID-19 pandemic values in April 2021 and exceeded pre-COVID-19 pandemic levels by June 2021, showing a 9% increase over average monthly values for 2015 to 2019.Citation59 Similarly, for age groups 5 to 14 years, 15 to 24 years, and >80 years, increases in IPD cases began to be observed in spring 2021, crossing pre-COVID-19 pandemic levels in July 2021.Citation59 In Switzerland, a drastic decline in IPD isolates was observed from February 2020 (n = 139) to April 2020 (n = 22) and remained low until February 2021 (n = 19).Citation58 COVID-19 measures were relaxed by the Swiss government on March 1, 2021, and by June 2021, the number of IPD isolates had returned to pre-pandemic levels.Citation58
Reduced numbers of cases and shifts in seasonality for RSV have been reported globally.Citation60,Citation65,Citation67 Australia was the first country to report an increase in RSV cases accompanied by a shift in the seasonality and epidemiology of disease.Citation60 Similar effects have since been reported across North America and Europe.Citation62,Citation63,Citation65 A decrease in RSV cases beyond mean seasonal levels was observed in the US following the introduction of COVID-related NPIs. Prediction models have suggested that longer periods of NPI enforcement would subsequently reduce transmission rates, leading to an increase in susceptibility and ultimately resulting in larger RSV outbreaks. For example, modeling suggested that longer durations of NPIs (i.e., one year), would lead to larger RSV outbreak, and more importantly could also result in complex interactions affecting the normal seasonal pattern of disease.Citation65 The UK has also reported a rapid reemergence and increase in RSV bronchiolitis cases likely to be out of sync with the usual seasonal pattern of infections.Citation62 Conversely, in countries across Europe where a policy of keeping open primary schools and day care facilities was implemented (including France and Iceland), typical pre-COVID-19 pandemic RSV seasonality was observed in 2020 to 2021.Citation63 In the Netherlands, the RSV epidemic started 19 weeks after schools were reopened, also suggesting that school closures had an impact on RSV activity.Citation63
Potential future impacts of vaccination disruptions due to COVID-19 restrictions on disease burden
The potential impacts of COVID-19 restrictions on the future epidemiology of vaccine-preventable diseases including measles, meningitis, polio, and pneumococcal disease were examined in six studies ().Citation68–73
Data from the modeling studies suggested that disruptions to vaccination programs experienced during the COVID-19 pandemic could lead to an increase in number of cases and deaths from other infectious diseases. For example, the number of excess deaths due to measles is expected to range from 0.24 to 1.16 per 100,000 persons during 2020 to 2030 using data from Bangladesh, Chad, Ethiopia, Kenya, Nigeria, and South Sudan.Citation73 During 2020 to 2023, modeling studies have also predicted that the number of polio cases globally is projected to increase from 4,657 to 5,557 cases despite any timely recovery in vaccination programs. Further polio eradication activities are not expected to substantially impact the overall predicted trajectory.Citation71
COVID-19 lockdowns have had a profound effect on infectious disease incidence in the UK, and modeling studies suggest significant impacts will be felt moving forward. Based on an existing model of pneumococcal transmission in England and Wales, simulating the impact of a 40% reduction in vaccination coverage and 40% reduction in contact rates during the COVID-19 lockdowns introduced in Spring 2020 and Autumn/Winter 2020 to 2021,Citation69 a reduction in pneumococcal carriage prevalence and IPD incidence has been predicted to occur over a period of up to 5 years.Citation69 The reduction in transmission due to social distancing is predicted to offset any increase in IPD cases due to any reduction in vaccine coverage. Vaccination coverage has been shown to be a more important driver of vaccine mortality than the timing of vaccination, where high vaccination coverage can over-ride the effect of vaccination delay through herd immunity. Model scenarios for seven countries indicated that irrespective of delays, deaths averted by pneumococcal conjugate vaccine (PCV) were comparable when accounting for herd protection.Citation68
Discussion
This TLR summarizes key data and learnings from past and current disruptions to human activity and vaccination programs that can impact the epidemiology of infectious diseases to build a better understanding of the potential trend of infectious disease dynamics as we move out of the COVID-19 pandemic era.
Prior to the COVID-19 pandemic (1996 to 2019), disruptions leading to low vaccination rates resulted in numerous disease outbreaks.Citation25,Citation26,Citation28–32,Citation34–36,Citation40,Citation43,Citation44,Citation47 Low vaccine uptake and/or coverage can have many causes (e.g., lack of vaccination policy, political conflicts, parental vaccine refusal, vaccine procurement problems, antivaccination sentiments, vaccine safety concerns, and changes in vaccine schedule), but regardless of the cause, evidence gathered in this TLR shows that low vaccination rates have contributed to increased infectious disease burden particularly in vulnerable population groups such as young children and older adults and in some cases leading to local and regional outbreaks of diseases previously brought under control.Citation24–47
The COVID-19 pandemic is still not completely resolved; however, mitigation measures including lockdowns and school closures have contributed to a sharp decline in vaccination uptake,Citation48–53,Citation55–57,Citation72 but also a decrease in disease burden due to reduced social contacts.Citation58–66
Although causality is difficult to prove especially with regard to individual NPIs, further studies published since completion of the targeted literature search (in October 2021) have provided further evidence that NPIs implemented during the COVID-19 pandemic have coincided with reductions in vaccination rates and disease numbers alongside in some cases changes in the seasonality of disease.Citation74–78
Overall, evidence suggests that the COVID-19 pandemic has significantly impacted the epidemiology of vaccine preventable infectious diseases at least in the short term and in many cases likely in the longer term.Citation79–102 As the COVID-19 pandemic has eased and NPIs have been lifted, a small recovery in vaccination uptake has been observed,Citation53,Citation57 though vaccination coverage still remains generally lower than pre-COVID-19 levels in key populations such as young children. As time progresses the signs of recovery of vaccination rates are variable in the extent and timing of this recovery by geographical region.Citation79,Citation81,Citation82,Citation85,Citation86,Citation93,Citation95,Citation97,Citation98,Citation103 In addition, increases in social contact have contributed to the spread of disease with at least initially a likely larger than normal pool of susceptible individuals. Using the example of pneumococcal disease, data from countries such as Germany and Switzerland initially reported reductions in cases during the pandemic, but cases have steadily increased since the relaxation of NPIs.Citation58,Citation59 Modeling the impact of COVID-19 NPIs on IPD cases in England and Wales predicted reductions in cumulative IPD cases for up to 5 years.Citation69 However, recent surveillance data from UKHSA show that the number of IPD cases in 2020/2021 in England has already increased to a similar level as previously reported in 2019/2020 in children less than 2 years () and to a higher level in children less than 15 years compared to pre-pandemic years 2017–2019.Citation104,Citation106 Within the UK, evidence suggests a similar trend is occurring in meningococcal and other diseases.Citation107 However, the relative contributions of low vaccination rates, increased population susceptibility, greater social contact, and the lifting of different NPIs is unclear and it is difficult to directly attribute the resurgence of vaccine preventable infectious diseases, like pneumococcal and meningococcal illness, to the lifting of NPIs, and/or the reduced vaccination rates during the pandemic.
Figure 3. Cumulative weekly number of reports of IPD in England due to any of the 13 serotypes covered by 13-valent Pneumococcal conjugate vaccine (PCV13).
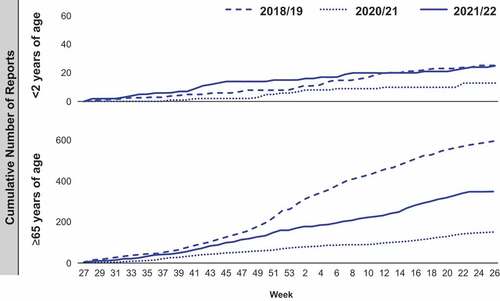
COVID-19 control measures continue to evolve as the pandemic and vaccination control measures change,Citation21 making it difficult to predict future trends in infectious disease epidemiology. However, some data from England (e.g., pneumococcal disease in age <15 years) already suggest that a resurgence in disease levels to pre-pandemic levels is occurring,Citation104,Citation106 and much earlier than predicted by modeling studies.Citation69
Though the studies identified in this TLR were unable to elucidate definitive causality between low vaccination levels, disease rates, and COVID-19 control measures, some speculated on potential causes,Citation48,Citation50,Citation52,Citation72 with the suggestion of a trend toward larger declines in disease in areas with more stringent COVID-19 response measures and in lower-income countries.Citation57 Consistent with this, multi-regional studies have shown that decreases in vaccination rates correlate with socioeconomic status,Citation81,Citation88,Citation100,Citation108 suggesting that changes in access to healthcare during the COVID-19 pandemic was a major contributor to decreases in vaccine uptake. Research from 170 countries has shown evidence of substantial disruptions to routine vaccination related to interrupted vaccination demand and supply, including reduced availability of healthcare staff.Citation82 The decline in vaccine uptake during the pandemic may also have contributed to a change in attitude toward vaccinations in general and concerns about the safety of vaccines,Citation109,Citation110 since rates remain low in some diseases despite the relaxation of NPIs.
Further hindering the interpretation of data is the presence of several confounding factors that could contribute to the reported changes in the number of infections after COVID-19 mitigation measures were implemented, including reductions in the reporting capacity of surveillance systems. Uncertainty also exists in general understanding of the measures that are the most influential in causing the observed changes in infectious disease incidence, and whether study designs are adequate to control for confounding factors. The aim of this review was also to capture both historical example of NPIs and the impact of recent COVID-19 related NPIs on disease epidemiology and national immunization programs. Although the electronic databases searches from which the evidence base was generated were conducted in October 2021, pre-print sources were included and additional manual ad-hoc searches for more recent evidence including surveillance data were conducted in June 2022. Given the last of the national lockdown restrictions were lifted in July 2021 the key effects of the COVID-19 restrictions should have been captured but given the target nature of our review our aim was not to capture all data during this period. Despite these issues, the burden of evidence suggests that the recent COVID-19 pandemic and the implementation of NPIs has led to significant impacts on non-COVID vaccine-preventable diseases in a similar manner to past examples such as Ebola.
The recent pandemic may have also positively affected vaccination efforts by encouraging an increase in vaccine awareness,Citation109,Citation111–117 which would explain increased vaccine uptake in selected groups (e.g., the elderly)Citation41,Citation118 and in certain geographical regions.Citation80,Citation86,Citation87,Citation103,Citation116,Citation119,Citation120 These positive effects of the pandemic on the public perception of vaccines are ongoing areas of research, but offer a public health opportunity to further improve rates of vaccine uptake and coverage and prevent future outbreaks of vaccine-preventable diseases. As the pandemic progresses, it is important to continue to monitor vaccination uptake and disease rates closely to prevent disease outbreaks. Even though causality is uncertain, a return to normal social mixing suggests the need for vigilance to maintain high vaccination levels and prevent future disease outbreaks. The drop in case numbers also offers a unique opportunity to reset the endemic equilibrium for vaccine-preventable diseases to levels lower than in the pre-COVID era. Consequently, there is a window of opportunity to review vaccination and disease rates before we see further disease resurgence in populations and age-groups currently unaffected. Actions to minimize interruptions in the delivery of immunization services and to plan and implement catch-up vaccinations are needed to mitigate the effects of the COVID-19 pandemic as recommended by a recent WHO report.Citation121 These actions include improving access to vaccines, increasing the efficiency of vaccination schedules, and harnessing opportunities for the simultaneous administration of multiple vaccines.Citation121
Abbreviations
| CDC | = | Centers for Disease Control and Prevention |
| CI | = | Confidence interval |
| DRC | = | Democratic Republic of Congo |
| DTP | = | Diphtheria-tetanus-pertussis |
| DTP3 | = | Third dose of diphtheria-tetanus-pertussis |
| HPV | = | Human papilloma virus |
| ICMJE | = | International Committee of Medical Journal Editors |
| IPD | = | Invasive pneumococcal disease |
| IPV | = | Inactivated poliovirus vaccine |
| MCV1 | = | First dose of measles-containing vaccine |
| MCV2 | = | Second dose measles-containing vaccine |
| MenACWY | = | Meningococcal conjugate vaccine |
| MMR | = | Measles-mumps-rubella |
| MMR1 | = | First dose of Measles-mumps-rubella |
| NIP | = | National immunization programs |
| NPI | = | Non-pharmacological interventions |
| PCV | = | pneumococcal conjugate vaccine |
| PHE | = | Public Health England |
| PICOS | = | Population, interventions and comparisons, outcomes, and study design |
| PPV | = | Pneumococcal polysaccharide vaccinations |
| RSV | = | Respiratory syncytial virus |
| SIA | = | Supplementary immunization activities |
| Td | = | Tetanus and diphtheria |
| TLR | = | Targeted literature review |
| WHO | = | World Health Organization |
| WPV1 | = | Wild type 1 poliovirus |
| UK | = | United Kingdom |
| UKHSA | = | United Kingdom Health Security Agency |
| US | = | United States |
Author contributions
All authors participated in data analysis and interpretation and contributed to the development of the manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval of the version to be published.
Compliance with ethical standards
Informed consent was not required for this study.
Supplemental Material
Download PDF (240.5 KB)Acknowledgments
Kate Halsby for contributing to the conceptualization of the TLR and the development of the TLR protocol and Anita Engh for contributing to manuscript writing.
Disclosure statement
EH, TM, JCK, DM, and CC are employed by Pfizer (and may own Pfizer stock or stock options). CF, AP, and PW are employed by Evidera, which provides consulting and other research services to pharmaceutical, medical device, and related organizations. In their salaried positions, they work with a variety of companies and organizations, and are precluded from receiving payment or honoraria directly from these organizations for services rendered. Evidera received funding from Pfizer to participate in the study and the development of this manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2219577.
Additional information
Funding
References
- WHO. World health statistics 2022: monitoring health for the SDGs, sustainable development goals. Geneva: World Health Organisation; 2022.
- WHO. The top 10 causes of death fact sheet. Geneva: World Health Organization; 2020.
- Murphy S, Kochanek K, Xu J, Arias E. Mortality in the United States, 2020. Hyattsville (MD): National Center for Health Statistics; 2021.
- Perin J, Mulick A, Yeung D, Villavicencio F, Lopez G, Strong KL, Prieto-Merino D, Cousens S, Black RE, Liu L. Global, regional, and national causes of under-5 mortality in 2000–19: an updated systematic analysis with implications for the sustainable development goals. Lancet Child Adolesc Health. 2022;6(2):106–17. doi:10.1016/S2352-4642(21)00311-4.
- Belongia EA, Naleway AL. Smallpox vaccine: the good, the bad, and the ugly. Clin Med Res. 2003;1(2):87–92. doi:10.3121/cmr.1.2.87.
- Larson HJ, Ghinai I. Lessons from polio eradication. Nature. 2011;473(7348):446–7. doi:10.1038/473446a.
- Castillo-Solorzano C, Marsigli C, Danovaro-Holliday MC, Ruiz-Matus C, Tambini G, Andrus JK. Measles and rubella elimination initiatives in the Americas: lessons learned and best practices. J Infect Dis. 2011;204(Suppl 1):S279–283. doi:10.1093/infdis/jir216.
- Peltola H, Heinonen OP, Valle M, Paunio M, Virtanen M, Karanko V, Cantell K. The elimination of indigenous measles, mumps, and rubella from Finland by a 12-year, two-dose vaccination program. N Engl J Med. 1994;331(21):1397–402. doi:10.1056/NEJM199411243312101.
- Nielsen A, Larsen SO. Whooping cough epidemiology in Denmark prior to and after the introduction of whooping cough vaccination. Protective effect of the vaccine and herd immunity. Ugeskr Laeger. 1990;152:597–604.
- Clarke KEN, MacNeil A, Hadler S, Scott C, Tiwari TSP, Cherian T. Global epidemiology of diphtheria, 2000-2017(1). Emerg Infect Dis. 2019;25(10):1834–42. doi:10.3201/eid2510.190271.
- Ridpath AD, Scobie HM, Shibeshi ME, Yakubu A, Zulu F, Raza AA, Masresha B, Tohme R. Progress towards achieving and maintaining maternal and neonatal tetanus elimination in the African region. Pan Afr Med J. 2017;27(Suppl 3):24. doi:10.11604/pamj.supp.2017.27.3.11783.
- van Panhuis WG, Grefenstette J, Jung SY, Chok NS, Cross A, Eng H, Lee BY, Zadorozhny V, Brown S, Cummings D, et al. Contagious diseases in the United States from 1888 to the present. N Engl J Med. 2013;369(22):2152–8. doi:10.1056/NEJMms1215400.
- Centers for Disease Control and Prevention (CDC). Impact of vaccines universally recommended for children–United States, 1990-1998. Morb Mort Wkly Rep. 1999;48(12):243–8.
- WHO. Business case for WHO immunization activities on the African continent, 2018-2030. Brazzaville: World Health Organization; 2018.
- Bangura JB, Xiao S, Qiu D, Ouyang F, Chen L. Barriers to childhood immunization in sub-Saharan Africa: a systematic review. BMC Public Health. 2020;20(1):1108. doi:10.1186/s12889-020-09169-4.
- Albers AN, Thaker J, Newcomer SR. Barriers to and facilitators of early childhood immunization in rural areas of the United States: a systematic review of the literature. Prev Med Rep. 2022;27:101804. doi:10.1016/j.pmedr.2022.101804.
- Aslam F, Ali I, Babar Z, Yang Y. Building evidence for improving vaccine adoption and uptake of childhood vaccinations in low- and middle-income countries: a systematic review. Drugs Ther Perspect. 2022;38(3):133–45. doi:10.1007/s40267-021-00890-7.
- Guzman-Holst A, DeAntonio R, Prado-Cohrs D, Juliao P. Barriers to vaccination in Latin America: a systematic literature review. Vaccine. 2020;38(3):470–81. doi:10.1016/j.vaccine.2019.10.088.
- Mendez-Brito A, El Bcheraoui C, Pozo-Martin F. Systematic review of empirical studies comparing the effectiveness of non-pharmaceutical interventions against COVID-19. J Infect. 2021;83(3):281–93. doi:10.1016/j.jinf.2021.06.018.
- Juneau CE, Pueyo T, Bell M, Gee G, Collazzo P, Potvin L. Lessons from past pandemics: a systematic review of evidence-based, cost-effective interventions to suppress COVID-19. Syst Rev. 2022;11(1):90. doi:10.1186/s13643-022-01958-9.
- Ritchie H, Mathieu E, Rodes-Guirao L, Appel C, Giattino C, Ortiz-Ospina E, Hasell J, Macdonald B, Beltekian D, Roser M. Coronavirus pandemic (COVID-19). Our World in Data; 2022[accessed 2022]. http://ourworldindata.org/coronavirus.
- Ayouni I, Maatoug J, Dhouib W, Zammit N, Fredj SB, Ghammam R, Ghannem H. Effective public health measures to mitigate the spread of COVID-19: a systematic review. BMC Public Health. 2021;21(1):1015. doi:10.1186/s12889-021-11111-1.
- The Green Book. 2022. https://www.gov.uk/government/publications/the-green-book-appraisal-and-evaluation-in-central-governent/the-green-book-2020.
- Gray KL, Walker NF, Martineau F, Bhadelia N, Harmon-Gray W-M, Skrip LA, DeMarco J, Konwloh P, Dunbar N. Interruption of tuberculosis detection and care during the Ebola virus disease epidemic (2014–2015) in Liberia: time-series analyses for 2013–2017. Int J Infect Dis. 2021;112:13–20. doi:10.1016/j.ijid.2021.08.041.
- Masresha BG, Luce R, Jr., Weldegebriel G, Katsande R, Gasasira A, Mihigo R. The impact of a prolonged Ebola outbreak on measles elimination activities in Guinea, Liberia and Sierra Leone, 2014-2015. Pan Afr Med J. 2020;35(Suppl 1):8. doi:10.11604/pamj.supp.2020.35.1.19059.
- Takahashi S, Metcalf CJE, Ferrari MJ, Moss WJ, Truelove SA, Tatem AJ, Grenfell BT, Lessler J. Reduced vaccination and the risk of measles and other childhood infections post-Ebola. Science. 2015;347(6227):1240–2. doi:10.1126/science.aaa3438.
- Asaria P, MacMahon E. Measles in the United Kingdom: can we eradicate it by 2010? BMJ. 2006;333(7574):890–5. doi:10.1136/bmj.38989.445845.7C.
- Centers for Disease Control and Prevention (CDC). Increased transmission and outbreaks of measles, European region, 2011. Wkly Epidemiol Rec. 2011;86(49):559–64.
- Dimala CA, Kadia BM, Nji MAM, Bechem NN. Factors associated with measles resurgence in the United States in the post-elimination era. Sci Rep. 2021;11(1):51. doi:10.1038/s41598-020-80214-3.
- Grout L, Minetti A, Hurtado N, François G, Fermon F, Chatelain A, Harczi G, de Dieu Ilunga Ngoie J, N’Goran A, Luquero FJ, et al. Measles in democratic Republic of Congo: an outbreak description from Katanga, 2010–2011. BMC Infect Dis. 2013;13(1):232. doi:10.1186/1471-2334-13-232.
- Majumder MS, Cohn EL, Mekaru SR, Huston JE, Brownstein JS. Substandard vaccination compliance and the 2015 measles outbreak. JAMA Pediatr. 2015;169(5):494–5. doi:10.1001/jamapediatrics.2015.0384.
- McBrien J, Murphy J, Gill D, Cronin M, O’Donovan C, Cafferkey MT. Measles outbreak in Dublin, 2000. Pediatr Infect Dis J. 2003;22(7):580–4. doi:10.1097/01.inf.0000073059.57867.36.
- Rodyna R. Measles situation in Ukraine during the period 2017-2019. Eur J Public Health. 2019;29(Supplement_4). doi:10.1093/eurpub/ckz186.496.
- Woudenberg T, van Binnendijk RS, Sanders EA, Wallinga J, de Melker HE, Ruijs WLM, Hahné SJM. Large measles epidemic in the Netherlands, May 2013 to March 2014: changing epidemiology. Euro Surveill. 2017;22(3). doi:10.2807/1560-7917.ES.2017.22.3.30443.
- Martinez M, Akbar IE, Wadood MZ, Shukla H, Jorba J, Ehrhardt D. Progress toward poliomyelitis eradication - Afghanistan, January 2019-July 2020. Morb Mortal Wkly Rep. 2020;69(40):1464–8. doi:10.15585/mmwr.mm6940a3.
- Khetsuriani N, Perehinets I, Nitzan D, Popovic D, Moran T, Allahverdiyeva V, Huseynov S, Gavrilin E, Slobodianyk L, Izhyk O, et al. Responding to a cVDPV1 outbreak in Ukraine: implications, challenges and opportunities. Vaccine. 2017;35(36):4769–76. doi:10.1016/j.vaccine.2017.04.036.
- Technical Advisory Group (TAG) on Polio Eradication in Afghanistan. Global polio eradication initiative technical advisory group (TAG) on polio eradication in Afghanistan: meeting report 2020. 2020.
- Centers for Disease C, Prevention. Outbreak of pneumococcal pneumonia among unvaccinated residents of a nursing home–New Jersey, April 2001. Morb Mortal Wkly Rep. 2001;50(33):707–10.
- Centers for Disease C, Prevention. Outbreaks of pneumococcal pneumonia among unvaccinated residents of chronic-care facilities–Massachusetts, October 1995, Oklahoma, February, 1996, and Maryland, May-June 1996. Morb Mortal Wkly Rep. 1997;46(3):60–2.
- Glanz JM, McClure DL, O’Leary ST, Narwaney KJ, Magid DJ, Daley MF, Hambidge SJ. Parental decline of pneumococcal vaccination and risk of pneumococcal related disease in children. Vaccine. 2011;29(5):994–9. doi:10.1016/j.vaccine.2010.11.085.
- PHE. Pneumococcal polysaccharide vaccine (PPV) coverage report (England), April 2019 to March 2020. 2021.
- Nuorti JP, Butler JC, Crutcher JM, Guevara R, Welch D, Holder P, Elliott JA. An outbreak of multidrug-resistant pneumococcal pneumonia and bacteremia among unvaccinated nursing home residents. N Engl J Med. 1998;338(26):1861–8. doi:10.1056/NEJM199806253382601.
- Picazo J, Ruiz-Contreras J, Casado-Flores J, Negreira S, Baquero F, Hernández-Sampelayo T, Otheo E, Méndez C. Effect of the different 13-valent pneumococcal conjugate vaccination uptakes on the invasive pneumococcal disease in children: analysis of a hospital-based and population-based surveillance study in Madrid, Spain, 2007-2015. PLoS One. 2017;12(2):e0172222. doi:10.1371/journal.pone.0172222.
- Quick RE, Hoge CW, Hamilton DJ, Whitney CJ, Borges M, Kobayashi JM. Underutilization of pneumococcal vaccine in nursing home in Washington state: report of a serotype-specific outbreak and a survey. Am J Med. 1993;94(2):149–52. doi:10.1016/0002-9343(93)90176-P.
- Atwell JE, Van Otterloo J, Zipprich J, Winter K, Harriman K, Salmon DA, Halsey NA, Omer SB. Nonmedical vaccine exemptions and pertussis in California, 2010. Pediatrics. 2013;132(4):624–30. doi:10.1542/peds.2013-0878.
- Glanz JM, McClure DL, Magid DJ, Daley MF, France EK, Hambidge SJ. Parental refusal of varicella vaccination and the associated risk of varicella infection in children. Arch Pediatr Adolesc Med. 2010;164(1):66–70. doi:10.1001/archpediatrics.2009.244.
- Phadke VK, Bednarczyk RA, Salmon DA, Omer SB. Association between vaccine refusal and vaccine-preventable diseases in the United States: a review of measles and pertussis. JAMA. 2016;315(11):1149–58. doi:10.1001/jama.2016.1353.
- PHE. Impact of COVID-19 on routine childhood immunisations early vaccine coverage data to July 2021 in England. 2021.
- PHE. Laboratory confirmed cases of measles, rubella and mumps, England April to June 2021. 2021.
- PHE. Meningococcal ACWY (MenACWY) vaccine coverage for the NHS adolescent vaccination programme in England, academic year 2019 to 2020. 2021.
- PHE. Human papillomavirus (HPV) vaccination coverage in adolescent females and males in England academic year 2019 to 2020. 2020.
- PHE. Td IPV school-leaver booster programme vaccine coverage, England, academic year 2019 to 2020. 2021.
- PHE. Pertussis vaccination programme for pregnant women update vaccine coverage in England, April to June 2021. 2021.
- PHE. Shingles vaccine coverage report (adults eligible from April to December 2020 and vaccinated to March 2021): England, Quarter 3 2020 to 2021. 2021.
- PHE. Herpes zoster shingles immunisation programme 2019 to 2020 evaluation reports. 2020.
- Centers for Disease Control and Prevention (CDC). Routine vaccination coverage — worldwide, 2020. 2021.
- WHO. Progresses and challenges with sustaining and advancing immunization coverage during the COVID-19 pandemic. 2021.
- Casanova C, Kuffer M, Leib SL, Hilty M. Re-emergence of invasive pneumococcal disease (IPD) and increase of serotype 23B after easing of COVID-19 measures, Switzerland, 2021. Emerg Microbes Infect. 2021;10(1):2202–4. doi:10.1080/22221751.2021.2000892.
- Perniciaro S, van der Linden M, Weinberger DM. Re-emergence of invasive pneumococcal disease in Germany during the spring and summer of 2021. Clin Infect Dis. 2022;75(7):1149–53. doi:10.1093/cid/ciac100.
- Foley DA, Phuong LK, Peplinski J, Lim SM, Lee WH, Farhat A, Minney-Smith CA, Martin AC, Mace AO, Sikazwe CT, et al. Examining the interseasonal resurgence of respiratory syncytial virus in Western Australia. Arch Dis Child. 2021;107(3):archdischild-2021–322507. doi:10.1136/archdischild-2021-322507.
- Halabi KC, Saiman L, Zachariah P. The epidemiology of respiratory syncytial virus in New York city during the coronavirus disease-2019 pandemic compared with previous years. J Pediatr. 2022;242:242–4 e241. doi:10.1016/j.jpeds.2021.10.057.
- Hussain F, Kotecha S, Edwards MO. RSV bronchiolitis season 2021 has arrived, so be prepared! Arch Dis Child. 2021;106(12):e51. doi:10.1136/archdischild-2021-322835.
- van Summeren J, Meijer A, Aspelund G, Casalegno JS, Erna G, Hoang U, Lina B, de Lusignan S, Teirlinck AC, Thors V, et al. Low levels of respiratory syncytial virus activity in Europe during the 2020/21 season: what can we expect in the coming summer and autumn/winter? Euro Surveill. 2021;26(29). doi:10.2807/1560-7917.ES.2021.26.29.2100639.
- O’Reilly KM, Sandman F, Allen D, Jarvis CI, Gimma A, Douglas A, Larkin L, Wong KL, Baguelin M, Baric RS, et al. Predicted norovirus resurgence in 2021-2022 due to the relaxation of nonpharmaceutical interventions associated with COVID-19 restrictions in England: a mathematical modelling study. BMC Med. 2021;19:299. doi:10.1186/s12916-021-02153-8.
- Baker RE, Park SW, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. The impact of COVID-19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proc Natl Acad Sci U S A. 2020;117(48):30547–53. doi:10.1073/pnas.2013182117.
- Redlberger-Fritz M, Kundi M, Aberle SW, Puchhammer-Stockl E. Significant impact of nationwide SARS-CoV-2 lockdown measures on the circulation of other respiratory virus infections in Austria. J Clin Virol. 2021;137:104795. doi:10.1016/j.jcv.2021.104795.
- Wan WY, Thoon KC, Loo LH, Chan KS, Oon LLE, Ramasamy A, Maiwald M. Trends in respiratory virus infections during the COVID-19 pandemic in Singapore, 2020. JAMA Netw Open. 2021;4(6):e2115973. doi:10.1001/jamanetworkopen.2021.15973.
- Carter ED, Tam Y, Walker N. Impact of vaccination delay on deaths averted by pneumococcal conjugate vaccine: modeled effects in 8 country scenarios. Vaccine. 2019;37(36):5242–9. doi:10.1016/j.vaccine.2019.07.063.
- Choi YH, Miller E. Impact of COVID-19 social distancing measures on future incidence of invasive pneumococcal disease in England and Wales: a mathematical modelling study. BMJ Open. 2021;11(9):e045380. doi:10.1136/bmjopen-2020-045380.
- Kitano T, Aoki H. The incremental burden of invasive pneumococcal disease associated with a decline in childhood vaccination using a dynamic transmission model in Japan: a secondary impact of COVID-19. Comput Biol Med. 2021;133:104429. doi:10.1016/j.compbiomed.2021.104429.
- Kalkowska DA, Voorman A, Pallansch MA, Wassilak SG, Cochi SL, Badizadegan K, Thompson KM. The impact of disruptions caused by the COVID-19 pandemic on global polio eradication. Vaccine. 2021;41(1).
- Causey K, Fullman N, Sorensen RJD, Galles NC, Zheng P, Aravkin ADanovaro-Holliday MC, Martinez-Piedra R, Sodha SV, Velandia-González MP, et al. Estimating global and regional disruptions to routine childhood vaccine coverage during the COVID-19 pandemic in 2020: a modelling study. Lancet. 2021;398(10299):522–34.
- Gaythorpe KA, Abbas K, Huber J, Karachaliou A, Thakkar N, Woodruff K, Li X, Echeverria-Londono S, Arsene Bita Fouda A, Cutts F, et al. Impact of COVID-19-related disruptions to measles, meningococcal A, and yellow fever vaccination in 10 countries. Elife. 2021;10:10. doi:10.7554/eLife.67023.
- Sun X, Xu Y, Zhu Y, Tang F. Impact of non-pharmaceutical interventions on the incidences of vaccine-preventable diseases during the COVID-19 pandemic in the eastern of China. Hum Vaccin Immunother. 2021;17(11):4083–9. doi:10.1080/21645515.2021.1956227.
- Fukuda Y, Tsugawa T, Nagaoka Y, Ishii A, Nawa T, Togashi A, Kunizaki J, Hirakawa S, Iida J, Tanaka T, et al. Surveillance in hospitalized children with infectious diseases in Japan: pre- and post-coronavirus disease 2019. J Infect Chemother. 2021;27(11):1639–47. doi:10.1016/j.jiac.2021.07.024.
- Yamamoto-Kataoka S, Kataoka Y, Tochitani K, Miyakoshi C, Yamamoto Y. Influence of anti-coronavirus disease 2019 policies on 10 pediatric infectious diseases. Pediatr Int. 2022;64(1):e14958. doi:10.1111/ped.14958.
- Pelletier JH, Rakkar J, Au AK, Fuhrman D, Clark RSB, Horvat CM. Trends in US pediatric hospital admissions in 2020 compared with the decade before the COVID-19 pandemic. JAMA Network Open. 2021;4(2):e2037227–e2037227. doi:10.1001/jamanetworkopen.2020.37227.
- Arvonen M, Raittinen P, Niemenoja O, Ilmonen P, Riihijärvi S, Särkkä S, Viitasaari L. Nationwide infection control strategy lowered seasonal respiratory infection rate: occupational health care perspective during the COVID-19 epidemic in Finland. Infect Dis (Lond). 2021;53(11):839–46. doi:10.1080/23744235.2021.1944661.
- Kiely M, Mansour T, Brousseau N, Rafferty E, Paudel YR, Sadarangani M, Svenson LW, Robinson JL, Gagneur A, Driedger SM, et al. COVID-19 pandemic impact on childhood vaccination coverage in Quebec, Canada. Hum Vaccin Immunother. 2022;18(1):2007707. doi:10.1080/21645515.2021.2007707.
- Wu L, Guo X, Liu J, Ma X, Huang Z, Sun X. Evaluation of influenza vaccination coverage in Shanghai city during the 2016/17 to 2020/21 influenza seasons. Hum Vaccin Immunother. 2022;18(5):2075211. doi:10.1080/21645515.2022.2075211.
- Colome-Hidalgo M, Campos JD, Gil de Miguel A. Tracking the impact of the COVID-19 pandemic on routine infant vaccinations in the Dominican Republic. Hum Vaccin Immunother. 2022;18(1):1972708. doi:10.1080/21645515.2021.1972708.
- Shet A, Carr K, Danovaro-Holliday MC, Sodha SV, Prosperi C, Wunderlich J, Wonodi C, Reynolds HW, Mirza I, Gacic-Dobo M, et al. Impact of the SARS-CoV-2 pandemic on routine immunisation services: evidence of disruption and recovery from 170 countries and territories. Lancet Glob Health. 2022;10(2):e186–e94. doi:10.1016/S2214-109X(21)00512-X.
- Kara A, Ilbay S, Topac O, Arabulan EA, Tezer H, Tavukçu N, Şimşek Ç. Alteration in vaccination rates and an evaluation of physicians’ perceptions of the possible impact of the SARS-CoV-2 pandemic on childhood vaccinations in Ankara, Turkey. Hum Vaccin Immunother. 2021;17(10):3457–62. doi:10.1080/21645515.2021.1923345.
- Miretu DG, Asfaw ZA, Addis SG. Impact of COVID-19 pandemic on vaccination coverage among children aged 15 to 23 months at Dessie town, Northeast Ethiopia, 2020. Hum Vaccin Immunother. 2021;17(8):2427–36. doi:10.1080/21645515.2021.1883387.
- Rahman SU, Haq FU, Imran M, Shah A, Bibi N, Khurshid R, Romman M, Gaffar F, Khan MI. Impact of the COVID-19 lockdown on routine vaccination in Pakistan: a hospital-based study. Hum Vaccin Immunother. 2021;17(12):4934–40. doi:10.1080/21645515.2021.1979380.
- Chiu NC, Lo KH, Chen CC, Huang S-Y, Weng S-L, Wang C-J, Kuo H-H, Chi H, Lien C-H, Tai Y-L, et al. The impact of COVID-19 on routine vaccinations in Taiwan and an unexpected surge of pneumococcal vaccination. Hum Vaccin Immunother. 2022;18(5):1–9. doi:10.1080/21645515.2022.2071079.
- Komori A, Mori H, Naito T. The COVID-19 pandemic increased the demand for pneumococcal vaccination in Japan. Hum Vaccin Immunother. 2021;17(11):4673–4. doi:10.1080/21645515.2021.1958612.
- Hoang U, de Lusignan S, Joy M, Sherlock J, Williams J, Bankhead C, Howsam G, Thomas M, Snape MD, Hobbs FDR, et al. National rates and disparities in childhood vaccination and vaccine-preventable disease during the COVID-19 pandemic: English sentinel network retrospective database study. Arch Dis Child. 2022;107(8):archdischild-2021–323630. doi:10.1136/archdischild-2021-323630.
- Mansour Z, Arab J, Said R, Rady A, Hamadeh R, Gerbaka B, Bizri AR. Impact of COVID-19 pandemic on the utilization of routine immunization services in Lebanon. PLos One. 2021;16(2):e0246951. doi:10.1371/journal.pone.0246951.
- Moreno-Montoya J, Ballesteros SM, Rojas Sotelo JC, Bocanegra Cervera CL, Barrera-Lopez P, De la Hoz-Valle JA. Impact of the COVID-19 pandemic on routine childhood immunisation in Colombia. Arch Dis Child. 2022;107(3):e4. doi:10.1136/archdischild-2021-321792.
- Abu-Rish EY, Bustanji Y, Abusal K. Nationwide routine childhood vaccination coverage during the COVID-19 pandemic in Jordan: current situation, reasons, and predictors of vaccination. Int J Clin Pract. 2022;2022:7918604. doi:10.1155/2022/7918604.
- Babatunde OA, Olatunji MB, Omotajo OR, Ikwunne OI, Babatunde AM, Nihinlola ET, Patrick GF, Dairo DM. Impact of COVID-19 on routine immunization in Oyo State, Nigeria: trend analysis of immunization data in the pre- and post-index case period; 2019-2020. Pan Afr Med J. 2022;41:54. doi:10.11604/pamj.2022.41.54.28575.
- Connolly E, Boley EJ, Fejfar DL, Varney P, Aron M, Fulcher I, Lambert W, Ndayizigiye M, Law M, Mugunga J-C, et al. Childhood immunization during the COVID-19 pandemic: experiences in Haiti, Lesotho, Liberia and Malawi. Bull World Health Organ. 2022;100(2):115–26C. doi:10.2471/BLT.21.286774.
- DeSilva MB, Haapala J, Vazquez-Benitez G, Daley MF, Nordin JD, Klein NP, Henninger ML, Williams JTB, Hambidge SJ, Jackson ML, et al. Association of the COVID-19 pandemic with routine childhood vaccination rates and proportion up to date with vaccinations across 8 US health systems in the vaccine safety datalink. JAMA Pediatr. 2022;176(1):68–77. doi:10.1001/jamapediatrics.2021.4251.
- Ji C, Piche-Renaud PP, Apajee J, Stephenson E, Forte M, Friedman JN, Science M, Zlotkin S, Morris SK, Tu K. Impact of the COVID-19 pandemic on routine immunization coverage in children under 2 years old in Ontario, Canada: a retrospective cohort study. Vaccine. 2022;40(12):1790–8. doi:10.1016/j.vaccine.2022.02.008.
- Lee DID, Vanderhout S, Aglipay M, Birken CS, Morris SK, Piché-Renaud P-P, Keown-Stoneman CDG, Maguire JL. Delay in childhood vaccinations during the COVID-19 pandemic. Can J Public Health. 2022;113(1):126–34. doi:10.17269/s41997-021-00601-9.
- MacDonald SE, Paudel YR, Kiely M, Rafferty E, Sadarangani M, Robinson JL, Driedger SM, Svenson LW. Impact of the COVID-19 pandemic on vaccine coverage for early childhood vaccines in Alberta, Canada: a population-based retrospective cohort study. BMJ Open. 2022;12(1):e055968. doi:10.1136/bmjopen-2021-055968.
- Moura C, Truche P, Sousa Salgado L, Meireles T, Santana V, Buda A, Bentes A, Botelho F, Mooney D. The impact of COVID-19 on routine pediatric vaccination delivery in Brazil. Vaccine. 2022;40(15):2292–8. doi:10.1016/j.vaccine.2022.02.076.
- Brooks HE, McLendon LA, Daniel CL. The impact of COVID-19 on pediatric vaccination rates in Alabama. Prev Med Rep. 2021;22:101320. doi:10.1016/j.pmedr.2021.101320.
- Jain R, Chopra A, Falezan C, Patel M, Dupas P. COVID-19 related immunization disruptions in Rajasthan, India: a retrospective observational study. Vaccine. 2021;39(31):4343–50. doi:10.1016/j.vaccine.2021.06.022.
- Shen AK, Bramer CA, Kimmins LM, Swanson R, Vranesich P, Orenstein W. Vaccine coverage across the life course in Michigan during the COVID-19 pandemic: JanuarySeptember 2020. Am J Public Health. 2021;111(11):2027–35. doi:10.2105/AJPH.2021.306474.
- Silveira MM, Conrad NL, Leivas Leite FP. Effect of COVID-19 on vaccination coverage in Brazil. J Med Microbiol. 2021;70(11). doi:10.1099/jmm.0.001466.
- Chen PY, Chuang PN, Chiang CH, Chang HH, Lu CW, Huang KC. Impact of coronavirus infectious disease (COVID-19) pandemic on willingness of immunization-A community-based questionnaire study. PLos One. 2022;17(1):e0262660. doi:10.1371/journal.pone.0262660.
- UK Health Security Agency. Pneumococcal disease: cases caused by strains covered by Prevenar 13 vaccine. 2022 [accessed 2022 Jun]. https://www.gov.uk/government/publications/pneumococcal-disease-cases-caused-by-strains-covered-by-prevenar-13-vaccine/pneumococcal-disease-cases-caused-by-strains-covered-by-prevenar-13-vaccine.
- Institute for Government. Timeline of UK government coronavirus lockdowns and restrictions. [accessed 2022 June]. https://www.instituteforgovernment.org.uk/charts/uk-government-coronavirus-lockdowns.
- Bertran M, Amin-Chowdhury Z, Sheppard CL, Eletu S, Zamarreño DV, Ramsay ME, Litt D, Fry NK, Ladhani SN. Increased incidence of invasive pneumococcal disease among children after COVID-19 pandemic, England. Emerg Infect Dis. 2022;28(8):1669–72. doi:10.3201/eid2808.220304.
- Hadley L, Karachaliou A, Christensen H, Ramsay M, Trotter C. Modelling the impact of COVID-19 on meningococcal carriage and disease in the UK. SSRN Electron J. 2021. doi:10.2139/ssrn.3889421.
- Walker B, Anderson A, Stoecker C, Shao Y, LaVeist TA, Callison K. COVID-19 and routine childhood and adolescent immunizations: evidence from Louisiana medicaid. Vaccine. 2022;40(6):837–40. doi:10.1016/j.vaccine.2021.12.022.
- Breuker C, Guedj AM, Allan M, Coinus L, Molinari N, Chapet N, Roubille F, Le Quintrec M, Duhalde V, Jouglen J, et al. The COVID-19 pandemic led to a small increase in changed mentality regarding infection risk without any change in willingness to be vaccinated in chronic diseases patients. J Clin Med. 2021;10(17):3967. doi:10.3390/jcm10173967.
- Cavaliere AF, Zaami S, Pallottini M, Perelli F, Vidiri A, Marinelli E, Straface G, Signore F, Scambia G, Marchi L. Flu and tdap maternal immunization hesitancy in times of COVID-19: an Italian survey on multiethnic sample. Vaccines (Basel). 2021;9(10):1107. doi:10.3390/vaccines9101107.
- Song Z, Liu X, Xiang P, Lin Y, Dai L, Guo Y, Liao J, Chen Y, Liang Y, Sun Y. The current status of vaccine uptake and the impact of COVID-19 on intention to vaccination in patients with COPD in Beijing. Int J Chron Obstruct Pulmon Dis. 2021;16:3337–46. doi:10.2147/COPD.S340730.
- Redondo Marguello E, Trilla A, Munguira ILB, Lopez-Herce AJ, Cotarelo Suarez M. Knowledge, attitudes, beliefs and barriers of healthcare professionals and adults >/= 65 years about vaccine-preventable diseases in Spain: the ADult vaccination drIvers and barriErs (ADVISE) study. Hum Vaccin Immunother. 2022;18(1):2025007. doi:10.1080/21645515.2021.2025007.
- Loubet P, Rouviere J, Merceron A, Launay O, Sotto A. On behalf of the Avnir G. patients’ perception and knowledge about influenza and pneumococcal vaccination during the COVID-19 pandemic: an online survey in patients at risk of infections. Vaccines (Basel). 2021;9(11):1372. doi:10.3390/vaccines9111372.
- Loubet P, Sotto A, Group A. Covid-19 pandemic: an opportunity to seize to increase awareness and vaccine coverage in at-risk populations. Hum Vaccin Immunother. 2021;17(10):3472–3. doi:10.1080/21645515.2021.1926760.
- Ozer M, Baskaya N, Bostanci I. Attitudes towards influenza and pneumococcal vaccines in parents of asthmatic children during the COVID-19 pandemic. Pediatr Pulmonol. 2022;57(4):871–7. doi:10.1002/ppul.25818.
- Zhang M, Chen H, Wu F, Li Q, Lin Q, Cao H, Zhou X, Gu Z, Chen Q. Heightened willingness toward pneumococcal vaccination in the elderly population in Shenzhen, China: a cross-sectional study during the COVID-19 pandemic. Vaccines (Basel). 2021;9(3):212. doi:10.3390/vaccines9030212.
- Domnich A, Cambiaggi M, Vasco A, Maraniello L, Ansaldi F, Baldo V, Bonanni P, Calabrò GE, Costantino C, de Waure C, et al. Attitudes and beliefs on influenza vaccination during the COVID-19 pandemic: results from a representative Italian survey. Vaccines (Basel). 2020;8(4):711. doi:10.3390/vaccines8040711.
- Gallant AJ, Nicholls LAB, Rasmussen S, Cogan N, Young D, Williams L. Changes in attitudes to vaccination as a result of the COVID-19 pandemic: a longitudinal study of older adults in the UK. PLos One. 2021;16(12):e0261844. doi:10.1371/journal.pone.0261844.
- Zhou M, Zhan J, Kong N, Campy KS, Chen Y. Factors associated with intention to uptake pneumococcal vaccines among Chinese elderly aged 60 years and older during the early stage of COVID-19 pandemic. Psychol Health Med. 2022;27(1):91–105. doi:10.1080/13548506.2021.1905862.
- McQuaid F, Mulholland R, Sangpang Rai Y, Agrawal U, Bedford H, Cameron JC, Gibbons C, Roy P, Sheikh A, Shi T, et al. Uptake of infant and preschool immunisations in Scotland and England during the COVID-19 pandemic: an observational study of routinely collected data. PLoS Med. 2022;19(2):e1003916. doi:10.1371/journal.pmed.1003916.
- WHO. Mitigating the impact of COVID-19 on control of vaccine-preventable diseases: a health risk management approach focused on catch-up vaccination. Copenhagen: WHO Regional Office for Europe; 2020.