ABSTRACT
High-risk Human Papillomaviruses (HPVs) and Epstein – Barr virus (EBV) are present and involved in several types of human carcinomas, including cervical and, head and neck cancers. Nevertheless, their presence and association in the pathogenesis of colorectal cancer is still nascent. The current study explored the association between the high-risk HPVs and EBV and tumor phenotype in colorectal cancers (CRCs) in the Qatari population. We found that high-risk HPVs and EBV are present in 69/100 and 21/100 cases, respectively. Additionally, 17% of the cases showed a copresence of high-risk HPVs and EBV, with a significant correlation only between the HPV45 subtype and EBV (p = .004). While the copresence did not significantly associate with clinicopathological characteristics, we identified that coinfection with more than two subtypes of HPV is a strong predictor of advanced stage CRC, and the confounding effect of the copresence of EBV in such cases strengthens this association. Our results indicate that high-risk HPVs and EBV can co-present in human CRCs in the Qatari population where they could plausibly play a specific role in human colorectal carcinogenesis. However, future studies are essential to confirm their copresence and synergistic role in developing CRCs.
Introduction
It is generally perceived that nearly 20% of all cancers are caused by infectious agents,Citation1 of which more than 12% are caused by viruses, or more specifically ‘‘oncoviruses.’’Citation2,Citation3 The first human oncovirus, Epstein-Barr virus (EBV), was detected in Burkitt lymphoma cells by electron microscopy in 1964.Citation4 Since then, our understanding of viral oncology has grown considerably.
Interestingly, viral cancers are reported to arise nearly 15 to 40 years after initial infection;Citation1 However, in most oncoviral cancers, viral replication is usually absent.Citation1,Citation5 This is mainly due to the active replication resulting in host-cell lysis, thus preventing tumorogenesis.Citation5 Therefore, the viral nucleic acids either exist as naked plasmids inside the host cell or integrate into the host genome.Citation5 Oncogenic viruses may be DNA or RNA viruses; DNA oncoviruses include human papillomaviruses (HPVs), human herpesvirus-8 (HHV-8), hepatitis B virus (HBV), Merkel cell polyomavirus (MCPyV) and EBV. In contrast, RNA oncoviruses include human T-cell lymphotropic virus-1 (HTLV-1) and hepatitis C virus (HCV).Citation6 DNA oncoviruses’ genomes can integrate directly into the host genome. In contrast, RNA oncoviruses’ genomes must undergo reverse DNA transcription before integrating into the host genome.Citation6
HPVs are small, double-stranded, non-enveloped DNA viruses.Citation7,Citation8 HPV infections commonly start at the cutaneous or mucosal epithelium at the basal layer, often through lesions.Citation9 In most cases, infection is asymptomatic and potentially disappears within a two years interval.Citation10 Most HPV-associated cancers are caused by high-risk HPV (HR-HPV) types which include HPVs: 16, 18, 31, 33, 35, 39, 45, 51, 52, 55, 56, 58, 59, 68, 73, 82, and 83.Citation11 The HR-HPVs oncoproteins E6 and E7 are crucial for viral replication and the inhibition of cellular differentiation.Citation12,Citation13 In addition, they bind and inactivate tumor suppressor proteins (p53 and pRb), consequently leading to the degradation of these proteins via the ubiquitination pathway resulting in the deregulation of the transcription factor E2F, thereby causing uncontrolled cellular proliferation.Citation14
EBV belongs to a family of the herpes virus and is ubiquitous, as EBV infects almost 90% of the population.Citation14–16 EBV can exhibit dual tropism; where it can infect either epithelial cells and B-cellsCitation17 by switching its envelope proteins for specific entry mechanisms.Citation18 While infecting B-cells, EBV engages its gp350 protein to bind to the complement receptor type 2 protein, which is found on the membrane surface of B cells. However, the virus can switch to the gp40 envelope protein for epithelial cell infection, using it to bind to surface integrins.Citation19 EBV expresses specific viral oncoproteins that include the EBV-induced nuclear antigen 1/2 (EBNA1/2) and latent membrane proteins (LMP1, LMP2A, and LMP2B) that promote oncogenesis by facilitating genomic instabilities, inducing uncontrolled cell proliferation and blocking apoptosis via NF-κB, PI3K/Akt, MAPK and mTOR pathways.Citation20,Citation21
Oncoviruses, including HR-HPVs and EBV, are involved in cancer etiology and play a vital role in cancer metastasis, especially in the epithelial-to-mesenchymal transition (EMT) process.Citation22 Numerous studies have revealed the copresence of high-risk HPVs and EBV in various human malignancies, including cervical,Citation23 head and neck,Citation24,Citation25 breast,Citation26,Citation27 and gastric,Citation28 indicating their possible active role in cancer initiation and progression.
Recently, numerous reports worldwide have linked HPV and EBV as potential causative factors in colorectal cancer (CRC),Citation29–33 which is the third most common cancer and the second leading cause of cancer-related death globally.Citation34 However, only a few studies report the copresence of both EBV and HPV in colorectal cancer worldwide, including the Middle East region.Citation32,Citation35–37 Hence, we herein explore the copresence of HR-HPVs and EBV in relation to tumor phenotype in CRCs in the Qatari population.
Materials and methods
Sample collection and DNA extraction
The study included 100 formalin-fixed paraffin-embedded (FFPE) tissue samples diagnosed during 2018–2021 at the Department of Pathology, Hamad Medical Corporation (HMC). For the study, all cases were de-identified, and patients’ information was anonymized. The study was conducted based on the IBC approval of Qatar University’s IBC committee #IBC-2019/005 dated 29 Feb 2019.
Prior to PCR, all cases were re-reviewed by a board-certified pathologist to confirm the diagnosis and select appropriate FFPE tissues for the assays. The tumor grade was defined according to the College of American Pathologists Consensus Statement,Citation38 while the tumor stage was defined as per the American Joint Committee on Cancer (AJCC) TNM system (8th edition)Citation39
DNA was extracted from FFPE tissue samples (punch samples of 2 mm thickness) using the Thermo Scientific GeneJET FFPE DNA Purification Kit per the manufacturer’s instructions (ThermoFisher Scientific, USA). Briefly, enzymatic digestion of the FFPE sections was done using digestion buffer (200 µl) followed by lysing and release of genomic DNA using Proteinase K solution (20 µl). This was followed by heat incubation (90°C) for 40 min to de-crosslink the DNA. Then, the solution was centrifuged to obtain the supernatant containing DNA, to which binding buffer (200 µl) was added. Following binding, 96% ethanol, and lysate were added to the purification column and washed (wash Buffers 1 and 2) of adsorbed DNA to eradicate contaminants. Finally, DNA was eluted using the elution buffer (60 µl).
HPV and EBV detection by PCR
High-risk HPVs and EBV in purified genomic DNA were detected by polymerase chain reaction (PCR) using specific primers for high-risk HPV types: 16, 18, 31, 33, 35, 45, 51, 52, and 59, as well as EBNA1, EBNA2, and LMP1 of EBV as previously described.Citation40,Citation41 For internal control, GAPDH was used. All the analyses were completed as described Our group.Citation40 For every single experiment, we used the respective positive and negative controls reported by our group previously.Citation41
PCR was carried out using the Invitrogen Platinum II Hot-Start Green PCR Master Mix (2×) (ThermoFisher Scientific, Waltham, MA, USA). Briefly, HPV and EBV virus genes were amplified for an initial denaturation (94°C for 2 min) followed by 40 cycles of 94°C for 30 s, annealing (temperatures ranging from 50°C to 62°C for 30 s) depending on each primer’s melting temperature as previously reportedCitation40,Citation41 and final extension (72°C for 10 min). The PCR product was resolved using 1.5% agarose gel electrophoresis and visualized using iBrightCL1000 Imaging System (ThermoFisher Scientific, Waltham, MA, USA). The samples expressing any two oncoproteins of EBV (EBNA1, EBNA2, and LMP1) were considered to determine EBV positivity in our CRC cohort.
Statistical analysis
Chi-square (χ2) test with Yates’ correction and Fisher’s exact test were performed to determine the significant association between the presence/copresence of high-risk HPVs and EBV with the clinicopathological data (tumor grade, tumor stage, and lymph node involvement). Statistical significance was achieved if p-values were ≤ .05 in two-tailed tests.
Further, we used logistic regression to estimate the association of EBV, HPV, and its subtypes and the coinfection of HPV & EBV with sociodemographic and clinical correlates. HPV and EBV infection was coded as 1 and 0, indicating the presence and absence of infection. Next, subtypes of HPV were combined to create a binary variable HPV-coinfection indicating more than two subtypes (2 or more) as present and less as absent. Also, the presence of HPV and EBV was combined to create a binary variable coinfection indicating the presence of both infections by two or more subtypes of HPV and EBV as present and any one infection absent. Stage of Cancer was classified as an advanced binary variable (stages 3 and 4) and others (stages 1 and 2). Statistical analysis and plotting of graphs were done using the Stata software (version 17).
Results
Clinicopathological characteristics of the cohort
summarizes the anatomical locations and clinicopathological characteristics of the CRC cohort.The study included samples from 66 male and 34 female patients. The mean age of patients was 57.1 years (standard deviation (SD), ±13.9 years), (age range = 23 to 96 years). All tumor samples were histologically confirmed as adenocarcinomas, of which 16% were metastatic cancers. Most of the samples (84%) were classified as grade 2 cancers. Moreover, 11%, 28%, 45%, and 16% of the cases were labeled as stages pT1, pT2, pT3, and pT4 tumors, respectively. Additionally, 58% of the cases were found to have metastasis in at least one or more local lymph nodes. Regarding the anatomical location of the cases, 23% of the specimens obtained were taken from the sigmoid colon, 17% from the rectosigmoid colon, 16% from the ascending colon, 14% from the descending colon, and 30% from other anatomic parts of the colon. summarizes the anatomical locations and clinicopathological characteristics of the CRC cohort.
Table 1. Clinicopathological characteristics of the CRC cohort.
The status of HPVs and EBV and their association with clinicopathological characteristics in CRC
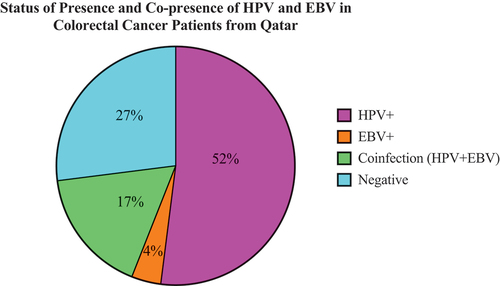
We found that high-risk HPVs are present in 69 of the 100 samples (); the most commonly present high-risk HPVs are 52, 18, 59, 31, 45, 51, 35, and 16, respectively. Furthermore, our investigation pointed out that 34/100 cases are positive for only one HPV subtype, while 35/100 were positive for more than one HPV subtype.Citation42 Finally, we noted that there is no correlation between HPV infection and clinicopathological characteristics of CRC; However, the coinfection of two or more HPV subtypes strongly correlated with advanced-stage CRC.Citation42 According to our data, HPV was found in 11 (16%), 10 (14%), 3 (4%), 18 (26%), 10 (14%), 6 (9%), 2 (3%), 4 (6%), 2 (3%), and 3 (5%) of the samples from the ascending colon, descending colon, transverse colon, sigmoid colon, rectosigmoid colon, cecum, rectum, hepatic flexure, splenic flexure and other colorectal regions respectively.
In addition, 21/100 of the samples were positive for EBV. EBNA1, EBNA2, and LMP1 of EBV are individually present in 54/100, 21/100, and 10/100 CRC cases, respectively. The presence of any two out of these genes in a single sample was classified as positive for the presence of EBV. In contrast, no significant association was observed between the presence of EBV and clinicopathological characteristics (i.e., tumor stage, grade, anatomic location of the tumor, the number of positive lymph nodes, and the presence of metastasis) of the CRC cohort. Our results identified the presence of EBV in 8 (13%), 7 (11%), 3 (5%), 18 (30%), 9 (15%), 4 (7%), 2 (3%), 5 (8%), 2 (3%), 3 (5%) of the samples from the ascending colon, descending colon, transverse colon, sigmoid colon, rectosigmoid colon, cecum, rectum, hepatic flexure, splenic flexure and other colorectal regions respectively.
Status of the copresence of HPVs with EBV and their association with clinicopathological characteristics
According to our data, 27 cases were negative for both virus types, while 52 cases showed the presence of only HPV, and 4 cases showed the presence of only EBV. More significantly, our data revealed the copresence of high-risk HPVs and EBV in 17% of CRC cases (); However, we found a significant correlation only between HPV45 and EBV (p = .004) (See ).
Table 2. Correlation of EBV and HPV subtypes in colorectal cancer patients from Qatar.
In addition, 7 cases were found to show the copresence of EBV with a single HPV type. Of these seven cases, the most noted copresence was HPV18+EBV (4 cases). Moreover, 4 cases showed the copresence of EBV with two HPV subtypes; here, the most frequent combination was HPV52 & HPV45 + EBV (2 cases). Only a single triple-HPV infection was noted in copresence with EBV (HPV18, HPV31 & HPV52 + EBV). However, EBV was found to be co-present in three and two cases in combination with four and five HPV subtypes, respectively. summarizes the observed frequencies of the copresence of EBV with different HPV subtypes.
Table 3. Coinfection of various HPV subtypes with EBV.
Initial analysis revealed a higher association between coinfection of HPV (2 or more subtypes) and EBV with advanced colorectal cancer (odds ratio of 1.56). However, after adjusting for potential confounding factors such as age, gender, and histological grade, the association was weaker and not statistically significant, with an odds ratio of 1.01. We found no statistically significant association between advanced colorectal cancer and coinfection with two or more subtypes of HPV along with EBV. (Odds ratio of 1.01, 95% CI 0.22–4.61) (). However, a coinfection of two or more high-risk subtypes of human papillomavirus (HPV) is a powerful predictor, and EBV infection confounds this association resulting in 4.39 higher odds of developing colorectal cancer (CRC) [OR = 4.39 (95% CI: 1.6–11.98)] compared to individuals without this coinfection. ().
Table 4. Association of advanced* colorectal cancer with HPV, two or more subtypes of HPV and EBV coinfection. (n = 100).
Table 5. Association of advanced colorectal cancer with a coinfection of HPV high-risk subtype as the main predictor and EBV. (n = 100).
Discussion
Detection of HPV among CRC FFPE samples from a Qatari cohort
This study is the first to report the individual presence and co-presence of HPV and EBV in colorectal cancer in Qatar. We show that 69% of the 100 colorectal cancer samples from the Qatari population tested positive for HR-HPVs. This is consistent with previous studies that have identified the presence of HPV in many colorectal cancer samples globally, with positivity rates ranging from 40% to 80% of CRC samples.Citation43–45
The highest prevalence of HPV has been reported in Turkey and Iran; studies have reported the presence of HPV in 81–83% of the CRC samples.Citation43,Citation45,Citation46 The HPV positivity observed in our study (69%) is concordant with data obtained from studies conducted in SyriaCitation35 and Lebanon,Citation36 where HPV-DNA positivity in CRC was observed in (54%) and (64%) of the samples, respectively. Additionally, a moderate HPV prevalence (12–44%) was reported in CRC samples from countries in the Middle East region.Citation32,Citation47–51 In contrast, a few studies have reported low HPV prevalence (<10%),Citation37,Citation52–56 while some did not detect the presence of HPV in CRC.Citation57–60 The variation in HPV positivity rates reported in different studies is partly due to focusing on the presence of specific types of HPV, mostly 16 and 18.Citation61–63 However, these studies fail to capture the full range of HPV types present in colorectal cancer samples, leading to an underestimation of overall HPV positivity.
Most significantly, we noted a correlation between the advanced stage of CRC with a coinfection of two or more HPV subtypes in our CRC cohort from Qatar (Fernandes et al. submitted). Thus, our results are comparable to other reports stating a similar association between HPV coinfections and disease severity and reduced treatment response in patients.Citation64–66 These findings suggest that HPV coinfection can have an important role in CRC progression.
Detection of EBV among CRC samples in a Qatar population
Several studies worldwide have linked EBV to the pathogenesis of CRC and reported EBV positivity to fall within the range of 20% to 50%.Citation32,Citation67–73 In our study, we report the presence of EBV in 21 out of 100 (21%) CRC samples, which falls within the lower end of the range of EBV positivity identified globally. Our study is comparable to data obtained from other Middle East countries like Iran,Citation67 Syria,Citation32 and Lebanon,Citation36 where EBV was found in 38%, 36%, and 29% of the CRC cases, respectively. While a few studies have reported a low prevalence of EBV positivity (1–8%) in CRCCitation74–76 other studies conducted in the Middle East and other regions of the world have failed to detect EBV in CRC.Citation68,Citation77–81 These contrasting results suggest that the association between EBV and CRC may vary depending on the population and region under study.
EBV has been confirmed as an etiologic factor in the development of several cancers, including nasopharyngeal carcinoma, classical Hodgkin lymphoma, Burkitt lymphoma, and a subset of gastric carcinomas. However, its role in other cancers is still debated. Many studies have shown an association of EBV with advanced stage and aggressive tumor phenotype in cancer.Citation82–87 However, an equal number of reports have denied such an association.Citation88–92 As our study did not identify a significant association between the presence of EBV and clinicopathological characteristics, We herein support the latter viewpoint and add to the growing evidence suggesting a lack of association between EBV and clinical indicators of severity and prognosis.
Copresence of EBV with different HPV subtypes
In this study, 17% of colorectal cancer (CRC) samples from Qatar contained both HPV and EBV. This co-presence has been reported in other studies worldwide, with rates ranging from 12% to 50% in various types of cancers.Citation25,Citation26,Citation30,Citation38,Citation76,Citation85–88 In comparison, our data presents a comparatively low co-presence of HPV & EBV (17%). However, similar rates have been reported in other studies from the Middle East and MENA region; For example, the copresence of HPV and EBV was detected in 17% and 28% of the CRC samples from SyriaCitation32 and Lebanon,Citation36 respectively.
In addition, in our study, EBV was found to be co-present in 7, 4, 1, 3, and 2 cases, along with single, double, triple, quadruple, and quintuple HPV subtypes, respectively. Interestingly, despite HPV52 being the most detected HPV subtype in our sample population (41/100 cases (41%), EBV was found to be co-present almost equally with HPV18 (10/17 cases) as compared to HPV52 (11/17 cases). Moreover, all seven cases positive for HPV45 were found to be co-infected with EBV; Wherein we noted a significant (p = .004) correlation between the two. Our findings suggest that EBV may have a stronger ability to co-infect with certain HPV subtypes, such as HPV18 or HPV 45, to activate more putative oncogenic mechanisms. However, additional analysis is needed to elucidate the mechanisms of HPV and EBV coinfection within the context of HPV-type variations.
We did not observe the correlation between the coinfection of HPV and EBV and clinicopathological characteristics. This is in contrast to other studies from around the world.Citation82,Citation83
Studies from the MENA regionCitation32,Citation93–95 have reported an association between the coinfection of HPV & EBV and advanced stage, grade, and tumor phenotypes in various types of cancer. However, other studies, including ours, reported a lack of correlation between the copresence of HPV and EBV and the clinicopathological characteristics of tumors. In particular, there was no association between HPV and EBV coinfection and the location of tumors. Interestingly, although the mode of entry of oncoviruses is suspected to be through the anus/rectum, according to our data, coinfection was found to be the highest in the sigmoid colon. Although non-significant, these results do imply the clinical importance of identifying the location of the coinfection within the colon/rectum. In summary, the low prevalence of EBV positivity observed in CRC cases in Qatar may explain the lack of association found in our study.
The association between EBV and HPV subtypes with advanced colorectal cancer is inconclusive due to study limitations like the small sample size and potential confounding factors. The wide confidence interval for the coinfection of EBV and multiple HPV subtypes and advanced colorectal cancer suggests a need for larger studies to determine the true association. Despite these limitations, the current results suggest a potentially significant role for examining coinfection as a predictor of colorectal cancer prognosis, warranting further investigation.
Our study found that coinfection of high-risk subtypes of human papillomavirus (HPV) is a strong predictor of advanced colorectal cancer (CRC). The copresence of Epstein-Barr virus (EBV) infection was identified as an important confounding factor in this association. This highlights the importance of considering these coinfections and potential risk factors for developing advanced stages of CRC. These findings are supported by previous research, which has shown that the coinfection between EBV and multiple high-risk HPV subtypes is linked to advanced tumor phenotypes in CRC.Citation32,Citation93–95 Coinfection of HPV and EBV is known to play varying roles in the pathogenesis of cancers, ranging from oncogenesis to EMT and metastasis.Citation31 Both oncoviruses are known to infect epithelial cells similarly, thus enabling them to transform normal cells into malignant ones. In addition, both viruses can infect and replicate in upper aero-digestive epithelial cells and in the epithelia of the colon and rectum, where they are known to trigger the lytic phases of the HPV and EBV life cycle.Citation31 It is generally postulated that EBV can cooperate with other oncoviruses to promote and boost oncogenic transformation and progression in various human carcinomas.Citation31,Citation96,Citation97 Such cooperative effects among various oncoviruses are found to be plausible drivers of various cancers.Citation31
Moreover, coinfection with HPV and EBV, in particular, stimulates EBV persistence via either enhanced viral replication, latency, or through triggering HPV oncoproteins expression.Citation98 In addition, both oncoviruses are known to trigger common signaling pathways, such as JAK/STAT/SRC, RAS/MEK/ERK, WNT/β-catenin, and PI3k/Akt/mTOR pathways.Citation31,Citation99,Citation100 These results emphasize the need to carefully evaluate the role of coinfections in larger studies and consider their putative role in the development of CRC, as this can significantly impact the clinical management of patients.
Conclusions
Our study, for the first time, reports the copresence of high-risk HPVs and EBV in colorectal cancer in Qatar. However, further research involving a larger sample size from various countries in the Gulf region is required to confirm these findings. It is also crucial to understand the cellular and molecular mechanisms that underline the role of coinfection of HPV and EBV in the development of CRC. This study opens the door for further research within the aspect of cooperative oncoviral infections. Moreover, it can also help in assessing the efficacy of high-risk HPVs and future EBV vaccines in preventing CRC progression worldwide and in the Middle East.
Author contributions
Conceptualization, A.-E.A.M.; writing – original draft preparation, Q.F., and I.G.; Sample Processing, Q.F., I.G., K.M., H.A.S., and M.P. Statistical Analysis, G.R.B., Review and editing, S.V., H.A.-T., G.R.B., and A.-E.A.M. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We would like to thank A. Kassab for her critical reading of the manuscript. Special Thanks to Dr. Said Dermime for his scientific advice and support. Open Access funding provided by the Qatar National Library.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Zur Hausen H. The search for infectious causes of human cancers: where and why. Virology. 2009;392(1):1–9. doi:10.1016/j.virol.2009.06.001.
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, Ghissassi FE, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, et al. A review of human carcinogens—Part B: biological agents. Lancet Oncol. 2009;10(4):321–2. doi:10.1016/s1470-2045(09)70096-8.
- de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607–15. doi:10.1016/s1470-2045(12)70137-7.
- Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from burkitt’s lymphoma. Lancet. 1964;283(7335):702–3. doi:10.1016/s0140-6736(64)91524-7.
- Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer. 2010;10(12):878–89. doi:10.1038/nrc2961.
- Akram N, Imran M, Noreen M, Ahmed F, Atif M, Fatima Z, Bilal Waqar A. Oncogenic role of tumor viruses in humans. Viral Immunol. 2017;30(1):20–7. doi:10.1089/vim.2016.0109.
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–50. doi:10.1038/nrc798.
- Zur Hausen H. Papillomaviruses in the causation of human cancers — a brief historical account. Virology. 2009;384(2):260–5. doi:10.1016/j.virol.2008.11.046.
- Zheng Y, Li X, Jiao Y, Wu C. High-risk human papillomavirus oncogenic E6/E7 mRNAs splicing regulation. Front Cell Infect Microbiol. 2022;12:929666. doi:10.3389/fcimb.2022.929666.
- Richardson H, Kelsall G, Tellier P, Voyer H, Abrahamowicz M, Ferenczy A, Coutlée F, Franco EL. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev. 2003;12:485–90.
- Bernard HU, Burk RD, Chen Z, van Doorslaer K, Zur Hausen H, de Villiers EM. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401(1):70–9. doi:10.1016/j.virol.2010.02.002.
- Doorbar J. The papillomavirus life cycle. J Clin Virol. 2005;32:7–15. doi:10.1016/j.jcv.2004.12.006.
- Johansson C, Schwartz S. Regulation of human papillomavirus gene expression by splicing and polyadenylation. Nat Rev Microbiol. 2013;11(4):239–51. doi:10.1038/nrmicro2984.
- Münger K, Basile JR, Duensing S, Eichten A, Gonzalez SL, Grace M, Zacny VL. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene. 2001;20(54):7888–98. doi:10.1038/sj.onc.1204860.
- Evans AS. The spectrum of infections with Epstein-Barr virus: a hypothesis. J Infect Dis. 1971;124(3):330–7. doi:10.1093/infdis/124.3.330.
- Williams H, Crawford DH. Epstein-Barr virus: the impact of scientific advances on clinical practice. Blood. 2006;107(3):862–9. doi:10.1182/blood-2005-07-2702.
- Borza CM, Hutt-Fletcher LM. Alternate replication in B cells and epithelial cells switches tropism of Epstein–Barr virus. Nat Med. 2002;8(6):594–9. doi:10.1038/nm0602-594.
- Tsang CM, Tsao SW. The role of Epstein-Barr virus infection in the pathogenesis of nasopharyngeal carcinoma. Virol Sin. 2015;30(2):107–21. doi:10.1007/s12250-015-3592-5.
- Shannon-Lowe C, Rickinson A. The global landscape of EBV-Associated tumors. Front Oncol. 2019;9. doi:10.3389/fonc.2019.00713.
- Farrell PJ. Epstein–Barr virus and cancer. Annu Rev Pathol. 2019;14(1):29–53. doi:10.1146/annurev-pathmechdis-012418-013023.
- Gaur N, Gandhi J, Robertson ES, Verma SC, Kaul R. Epstein-Barr virus latent antigens EBNA3C and EBNA1 modulate epithelial to mesenchymal transition of cancer cells associated with tumor metastasis. Tumour Biol. 2015;36(4):3051–60. doi:10.1007/s13277-014-2941-6.
- Chen X, Bode AM, Dong Z, Cao Y. The epithelial–mesenchymal transition (EMT) is regulated by oncoviruses in cancer. FASEB J. 2016;30(9):3001–10. doi:10.1096/fj.201600388r.
- Abudoukadeer A, Niyazi M, Aikula A, Kamilijian M, Sulaiman X, Mutailipu A, Abudula A. Association of EBV and HPV co-infection with the development of cervical cancer in ethnic Uyghur women. Eur J Gynaecol Oncol. 2015;36:546–50.
- Fernandes Q, Merhi M, Raza A, Inchakalody VP, Abdelouahab N, Zar Gul AR, Uddin S, Dermime S. Role of Epstein–Barr virus in the pathogenesis of head and neck cancers and its potential as an immunotherapeutic target. Front Oncol. 2018;8(JUL). doi:10.3389/fonc.2018.00257.
- Vanshika S, Preeti A, Sumaira Q, Vijay K, Shikha T, Shivanjali R, Shankar SU, Mati GM. Incidence of HPV and EBV in oral cancer and their clinico-pathological correlation– a pilot study of 108 cases. J Oral Biol Craniofacial Res. 2021;11(2):180–4. doi:10.1016/j.jobcr.2021.01.007.
- Lawson JS, Glenn WK. Multiple oncogenic viruses are present in human breast tissues before development of virus associated breast cancer. Infect Agent Cancer. 2017;12(1):55. doi:10.1186/s13027-017-0165-2.
- Nagi K, Gupta I, Jurdi N, Jabeen A, Yasmeen A, Batist G, Vranic S, Al-Moustafa A-E. High-risk human papillomaviruses and Epstein–Barr virus in breast cancer in Lebanese women and their association with tumor grade: a molecular and tissue microarray study. Cancer Cell Int. 2021;21(1):308. doi:10.1186/s12935-021-02009-4.
- Wang R, Liu K, Chen X-Z. Group S research. Associations between gastric cancer risk and virus infection other than Epstein-Barr virus: the protocol of a systematic review and meta-analysis based on epidemiological studies. Medicine (Baltimore). 2019;98(32):e16708–e16708. doi:10.1097/MD.0000000000016708.
- Burnett-Hartman AN, Newcomb PA, Potter JD. Infectious agents and colorectal cancer: a review of Helicobacter pylori, Streptococcus bovis, JC virus, and human papillomavirus. Cancer Epidemiol Biomarkers Prev. 2008;17(11):2970–9. doi:10.1158/1055-9965.EPI-08-0571.
- Chen H, Chen X-Z, Waterboer T, Castro FA, Brenner H. Viral infections and colorectal cancer: a systematic review of epidemiological studies. Int J Cancer. 2014;137(1):12–24. doi:10.1002/ijc.29180.
- Fernandes Q, Gupta I, Vranic S, Al Moustafa AE. Human papillomaviruses and Epstein–Barr virus interactions in colorectal cancer: a brief review. Pathogens. 2020;9(4):300. doi:10.3390/pathogens9040300.
- Malki MI, Gupta I, Fernandes Q, Aboulkassim T, Yasmeen A, Vranic S, Al Moustafa A-E, Al-Thawadi HA. Co-presence of Epstein–Barr virus and high-risk human papillomaviruses in Syrian colorectal cancer samples. Hum Vaccines Immunother. 2020;16(10):2403–7. doi:10.1080/21645515.2020.1726680.
- Song LB, Zhang X, Zhang CQ, Zhang Y, Pan Z-Z, Liao W-T, Li M-Z, Zeng M-S. Infection of Epstein-Barr virus in colorectal cancer in Chinese. Ai Zheng. 2006;25:1356–60.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. doi:10.3322/caac.21660.
- Al Moustafa A-E, Ghabreau L, Segal E, Yasmeen A, Kassab A, Akil N. High-risk human papillomavirus infections in colorectal cancer in the Syrian population and their association with Fascin, Id-1 and P-cadherin expressions: a tissue microarray study. Clin Cancer Investig J. 2012;1(1):26. doi:10.4103/2278-0513.95016.
- Nagi K, Gupta I, Jurdi N, Yasmeen A, Vranic S, Batist G, Moustafa AEA. Copresence of high-risk human papillomaviruses and Epstein–Barr Virus in colorectal cancer: a tissue microarray and molecular study from Lebanon. Int J Mol Sci. 2021;22(15):8118. doi:10.3390/ijms22158118.
- Mahmoudvand S, Safaei A, Erfani N, Sarvari J. Presence of human papillomavirus DNA in colorectal cancer tissues in Shiraz, Southwest Iran. Asian Pacific J Cancer Prev. 2015;16(17):7883–7. doi:10.7314/apjcp.2015.16.17.7883.
- Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, Hammond MEH, Henson DE, Hutter RVP, Nagle RB, et al. Prognostic factors in colorectal cancer: college of American pathologists consensus statement 1999. Arch Pathol Lab Med. 2000;124(7):979–94. doi:10.5858/2000-124-0979-PFICC.
- Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol. 2018;25(6):1454–5. doi:10.1245/s10434-018-6462-1.
- Gupta I, Al Farsi H, Jabeen A, Skenderi F, Al-Thawadi H, AlAhmad YM, Abdelhafez I, Al Moustafa A-E, Vranic S. High-risk human papillomaviruses and Epstein–Barr virus in colorectal cancer and their association with clinicopathological status. Pathog (Basel, Switzerland). 2020;9(6):452. doi:10.3390/pathogens9060452.
- Gupta I, Ulamec M, Peric-Balja M, Ramic S, Al Moustafa A-E, Vranic S, Al-Farsi HF. Presence of high-risk HPVs, EBV, and MMTV in human triple-negative breast cancer. Hum Vaccin Immunother. 2021;17(11):4457–66. doi:10.1080/21645515.2021.1975452.
- Fernandes Q, Gupta I, Murshed K, Abo Samra H, Al-Thawadi H, Vranic S, Petkar M, Babu GR, Al Moustafa A-E. Coinfection of HPVs is associated with advanced stage in colorectal cancer patients from Qatar. Pathogens. 2023;12(3):424. doi:10.3390/pathogens12030424.
- Buyru N, Tezol A, Dalay N. Coexistence of K-ras mutations and HPV infection in colon cancer. Bmc Cancer. 2006;6(1):115. doi:10.1186/1471-2407-6-115.
- Bernabe-Dones RD, Gonzalez-Pons M, Villar-Prados A, Lacourt-Ventura M, Rodríguez-Arroyo H, Fonseca-Williams S, Velazquez FE, Diaz-Algorri Y, Lopez-Diaz SM, Rodríguez N, et al. High prevalence of human papillomavirus in colorectal cancer in Hispanics: a case-control study. Gastroenterol Res Pract. 2016;2016:1–8. doi:10.1155/2016/7896716.
- Damin DC, Caetano MB, Rosito MA, Schwartsmann G, Damin AS, Frazzon AP, Ruppenthal RD, Alexandre COP. Evidence for an association of human papillomavirus infection and colorectal cancer. Eur J Surg Oncol. 2007;33(5):569–74. doi:10.1016/j.ejso.2007.01.014.
- Salepci T, Yazici H, Dane F, Topuz E, Dalay N, Onat H, Aykan F, Seker M, Aydiner A. Detection of human papillomavirus DNA by polymerase chain reaction and southern blot hybridization in colorectal cancer patients. J Buon. 2009;14(3):495–499.
- Tavakolian S, Goudarzi H, Eslami G, Dayyani F, Kazeminezhad B, Faghihloo E. Prevalence of human papilloma virus and Epstein–Barr virus in tumorous and adjacent tissues of colorectal cancer in Iran. Gene Reports. 2020;20:100774. doi:10.1016/j.genrep.2020.100774.
- El-Seidi EA, Sorour AE, Gamil M. Human papillomavirus in patients with colorectal cancer. Egypt J Med Microbiol. 2014;23(1):59–67. doi:10.12816/0024260.
- Afshar RM, Deldar Z, Mollaei HR, Arabzadeh SA, Iranpour M. Evaluation of HPV DNA positivity in colorectal cancer patients in Kerman, Southeast Iran. Asian Pacific J Cancer Prev. 2018;19(1). doi:10.22034/APJCP.2018.19.1.193.
- Karbasi A, Borhani N, Daliri K, Kazemi B, Manoochehri M. Downregulation of external death receptor genes FAS and DR5 in colorectal cancer samples positive for human papillomavirus infection. Pathol Res Pract. 2015;211(6):444–8. doi:10.1016/j.prp.2015.02.001.
- Kadhem Mallakh M, Mohammed Mahmood M, Hasan Mohammed Ali S. Immunomolecular investigation of human papillomavirus genotypes (16, 18) and P63 expression in patients with malignant and non-malignant colorectal tumors. Arch Razi Inst. 2022;77(1):383–90. doi:10.22092/ARI.2021.356608.1879.
- Karbalaie Niya MH, Keyvani H, Safarnezhad Tameshkel F, Salehi-Vaziri M, Teaghinezhad-S S, Bokharaei Salim F, Monavari SHR, Javanmard D. Human papillomavirus type 16 integration analysis by real-time PCR assay in associated cancers. Transl Oncol. 2018;11(3):593–8. doi:10.1016/j.tranon.2018.02.017.
- Gazzaz F, Mosli MH, Jawa H, Sibiany A. Detection of human papillomavirus infection by molecular tests and its relation to colonic polyps and colorectal cancer. Saudi Med J. 2016;37(3):256–61. doi:10.15537/smj.2016.3.13514.
- Meshkat M, Tayyebi Meibodi N, Sepahi S, Fadaee N, Salehpour M, Meshkat Z. The frequency of human papillomaviruses in colorectal cancer samples in Mashhad, northeastern Iran. TURKISH J Med Sci. 2014;44:501–3. doi:10.3906/sag-1303-81.
- Ranjbar R, Saberfar E, Shamsaie A, Ghasemian E. The aetiological role of human papillomavirus in colorectal carcinoma: an Iranian population- based case control study. Asian Pacific J Cancer Prev. 2014;15(4):1521–5. doi:10.7314/apjcp.2014.15.4.1521.
- Toru S, Bilezikçi B. Early changes in carcinogenesis of colorectal adenomas. West Indian Med J. 2012;61(1):10–16. doi:10.7727/wimj.2011.136.
- Gornick MC, Castellsague X, Sanchez G, Giordano TJ, Vinco M, Greenson JK, Capella G, Raskin L, Rennert G, Gruber SB, et al. Human papillomavirus is not associated with colorectal cancer in a large international study. Cancer Causes Control. 2010;21(5):737–43. doi:10.1007/s10552-010-9502-0.
- Khabaz MN. HPV and the development of colorectal cancer. Glob J Health Sci. 2016;9(4):251. doi:10.5539/gjhs.v9n4p251.
- Taherian H, Tafvizi F, Fard ZT, Abdirad A. Lack of association between human papillomavirus infection and colorectal cancer. Prz Gastroenterol. 2014;9(5):280–4. doi:10.5114/pg.2014.46163.
- Yavuzer D, Karadayi N, Salepci T, Baloglu H, Dabak R, Bayramicli OU. Investigation of human papillomavirus DNA in colorectal carcinomas and adenomas. Med Oncol. 2010;28(1):127–32. doi:10.1007/s12032-010-9416-4.
- Temesgen MM, Alemu T, Shiferaw B, Legesse S, Zeru T, Haile M, Gelanew T. Prevalence of oncogenic human papillomavirus (HPV 16/18) infection, cervical lesions and its associated factors among women aged 21–49 years in Amhara region, Northern Ethiopia. PLos One. 2021;16(3):e0248949–e0248949. doi:10.1371/journal.pone.0248949.
- Torres-Ibarra L, Cuzick J, Lorincz AT, Spiegelman D, Lazcano-Ponce E, Franco EL, Moscicki AB, Mahmud SM, Wheeler CM, Rivera-Paredez B, et al. Comparison of HPV-16 and HPV-18 genotyping and cytological testing as triage testing within human papillomavirus–based screening in Mexico. JAMA Netw Open. 2019;2(11):e1915781–e1915781. doi:10.1001/jamanetworkopen.2019.15781.
- Gnanamony M, Peedicayil A, Subhashini J, Ram TS, Rajasekar A, Gravitt P, Abraham P. Detection and quantitation of HPV 16 and 18 in plasma of Indian women with cervical cancer. Gynecol Oncol. 2010;116(3):447–51. doi:10.1016/j.ygyno.2009.10.081.
- Badawi H, Ahmed H, Ismail A, Diab M, Moubarak M, Badawy A, Saber M. Role of human papillomavirus types 16, 18, and 52 in recurrent cystitis and urinary bladder cancer among Egyptian patients. MedGenMed Medscape Gen Med. 2008;10(10):232.
- Kaliff M, Sorbe B, Mordhorst LB, Helenius G, Karlsson MG, Lillsunde-Larsson G. Findings of multiple HPV genotypes in cervical carcinoma are associated with poor cancer-specific survival in a Swedish cohort of cervical cancer primarily treated with radiotherapy. Oncotarget. 2018;9(27):18786–96. doi:10.18632/oncotarget.24666.
- Munagala R, Doná MG, Rai SN, Jenson AB, Bala N, Ghim SJ, Gupta RC. Significance of multiple HPV infection in cervical cancer patients and its impact on treatment response. Int J Oncol. 2009;34(1):263–71. doi:10.3892/ijo_00000148.
- Tafvizi F, Fard ZT, Assareh R. Original paper Epstein-Barr virus DNA in colorectal carcinoma in Iranian patients. Polish J Pathol. 2015;2:154–60. doi:10.5114/pjp.2015.53012.
- Fiorina L, Ricotti M, Vanoli A, Luinetti O, Dallera E, Riboni R, Paolucci S, Brugnatelli S, Paulli M, Pedrazzoli P, et al. Systematic analysis of human oncogenic viruses in colon cancer revealed EBV latency in lymphoid infiltrates. Infect Agent Cancer. 2014;9(1):18. doi:10.1186/1750-9378-9-18.
- Karpinski P, Myszka A, Ramsey D, Kielan W, Sasiadek MM. Detection of viral DNA sequences in sporadic colorectal cancers in relation to CpG island methylation and methylator phenotype. Tumour Biol. 2011;32(4):653–9. doi:10.1007/s13277-011-0165-6.
- Militello V, Trevisan M, Squarzon L, Biasolo MA, Rugge M, Militello C, Palù G, Barzon L. Investigation on the presence of polyomavirus, herpesvirus, and papillomavirus sequences in colorectal neoplasms and their association with cancer. Int J Cancer. 2009;124(10):2501–3. doi:10.1002/ijc.24224.
- Park JM, Choi MG, Kim SW, Chung I-S, Yang CW, Kim YS, Jung CK, Lee KY, Kang J-H. Increased incidence of colorectal malignancies in renal transplant recipients: a case control study. Am J Transplant. 2010;10(9):2043–50. doi:10.1111/j.1600-6143.2010.03231.x.
- Salyakina D, Tsinoremas NF. Viral expression associated with gastrointestinal adenocarcinomas in TCGA high-throughput sequencing data. Hum Genomics. 2013;7(1):23. doi:10.1186/1479-7364-7-23.
- Bedri S, Sultan AA, Alkhalaf M, Al Moustafa A-E, Vranic S. Epstein-Barr virus (EBV) status in colorectal cancer: a mini review. Hum Vaccin Immunother. 2019;15(3):603–10. doi:10.1080/21645515.2018.1543525.
- Sarvari J, Mahmoudvand S, Pirbonyeh N, Safaei A, Hosseini SY. The very low frequency of Epstein-Barr JC and BK viruses DNA in colorectal cancer tissues in Shiraz, Southwest Iran. Polish J Microbiol. 2018;67(1):73–9. doi:10.5604/01.3001.0011.6146.
- Shah KV, Daniel RW, Simons JW, Vogelstein B. Investigation of colon cancers for human papillomavirus genomic sequences by polymerase chain reaction. J Surg Oncol. 1992;51(1):5–7. doi:10.1002/jso.2930510104.
- Grinstein S, Preciado MV, Gattuso P, Chabay PA, Warren WH, De Matteo E, Gould VE. Demonstration of Epstein-Barr virus in carcinomas of various sites. Cancer Res. 2002;62:4876–8.
- Mehrabani-Khasraghi S, Ameli M, Khalily F. Demonstration of herpes simplex virus, cytomegalovirus, and Epstein-Barr virus in colorectal cancer. Iran Biomed J. 2016;20(5):302–6. doi:10.22045/ibj.2016.08.
- Kijima Y, Hokita S, Takao S, Baba M, Natsugoe S, Yoshinaka H, Aridome K, Otsuji T, Itoh T, Tokunaga M, et al. Epstein-Barr virus involvement is mainly restricted to lymphoepithelial type of gastric carcinoma among various epithelial neoplasms. J Med Virol. 2001;64(4):513–8. doi:10.1002/jmv.1079.
- Boguszakova L, Hirsch I, Brichacek B, Faltýn J, Fric P, Dvoráková H, Vonka V. Absence of cytomegalovirus, Epstein-Barr virus, and papillomavirus DNA from adenoma and adenocarcinoma of the colon. Acta Virol. 1988;32:303–8.
- Cho YJ, Chang MS, Park SH, Kim HS, Kim WH. In situ hybridization of Epstein-Barr virus in tumor cells and tumor-infiltrating lymphocytes of the gastrointestinal tract. Hum Pathol. 2001;32(3):297–301. doi:10.1053/hupa.2001.22766.
- Wong N, Herbst H, Herrmann K, Kirchner T, Krajewski AS, Moorghen M, Niedobitek F, Rooney N, Shepherd NA, Niedobitek G. Epstein-Barr virus infection in colorectal neoplasms associated with inflammatory bowel disease: detection of the virus in lymphomas but not in adenocarcinomas. J Pathol. 2003;201(2):312–8. doi:10.1002/path.1442.
- de Lima MAP, Neto PJN, Lima LPM, Gonçalves Júnior J, Teixeira Junior AG, Teodoro IPP, Facundo HT, da Silva CGL, Lima MVA. Association between Epstein-Barr virus (EBV) and cervical carcinoma: a meta-analysis. Gynecol Oncol. 2018;148(2):317–28. doi:10.1016/j.ygyno.2017.10.005.
- Tavakoli A, Monavari SH, Solaymani Mohammadi F, Kiani SJ, Armat S, Farahmand M. Association between Epstein-Barr virus infection and gastric cancer: a systematic review and meta-analysis. Bmc Cancer. 2020;20(1). doi:10.1186/s12885-020-07013-x.
- Yahia ZA, Adam AAM, Elgizouli M, Hussein A, Masri MA, Kamal M, Mohamed HS, Alzaki K, Elhassan AM, Hamad K, et al. Epstein Barr virus: a prime candidate of breast cancer aetiology in Sudanese patients. Infect Agent Cancer. 2014;9(1). doi:10.1186/1750-9378-9-9.
- Chen X-Z, Chen H, Castro FA, Hu J-K, Brenner H. Epstein-Barr virus infection and gastric cancer: a systematic review. Medicine (Baltimore). 2015;94(20):e792. doi:10.1097/MD.0000000000000792.
- Aguayo F, Khan N, Koriyama C, González C, Ampuero S, Padilla O, Solís L, Eizuru Y, Corvalán A, Akiba S. Human papillomavirus and Epstein-Barr virus infections in breast cancer from chile. Infect Agent Cancer. 2011;6(1). doi:10.1186/1750-9378-6-7.
- Mazouni C, Fina F, Romain S, Ouafik L, Bonnier P, Brandone J-M, Martin P-M. Epstein-Barr virus as a marker of biological aggressiveness in breast cancer. Br J Cancer. 2011;104(2):332–7. doi:10.1038/sj.bjc.6606048.
- Kadivar M, Monabati A, Joulaee A, Hosseini N. Epstein-Barr virus and breast cancer: lack of evidence for an association in Iranian women. Pathol Oncol Res. 2011;17(3):489–92. doi:10.1007/s12253-010-9325-z.
- Perrigoue JG, Den Boon JA, Friedl A, Newton MA, Ahlquist P, Sugden B. Lack of association between EBV and breast carcinoma. Cancer Epidemiol Biomarkers Prev. 2005;14(4):809–14. doi:10.1158/1055-9965.EPI-04-0763.
- Torabizadeh Z, Nadji A, Naghshvar F, Nosrati A, Parsa M. Association between Epstein - Barr virus (EBV) and breast cancer TT. Res-Mol-Med. 2014;2(4):24–9. doi:10.18869/acadpub.rmm.2.4.24.
- Morales-Sánchez A, Molina-Muñoz T, Martínez-López JLE, Hernández-Sancén P, Mantilla A, Leal YA, Torres J, Fuentes-Pananá EM. No association between Epstein-Barr virus and mouse mammary tumor virus with breast cancer in Mexican women. Sci Rep. 2013;3(1). doi:10.1038/srep02970.
- Zekri ARN, Bahnassy AA, Mohamed WS, El-Kassem FA, El-Khalidi SJ, Hafez MM, Hassan ZK. Epstein-Barr virus and breast cancer: epidemiological and molecular study on Egyptian and Iraqi women. J Egypt Natl Canc Inst. 2012;24(3):123–31. doi:10.1016/j.jnci.2012.06.001.
- Gupta I, Jabeen A, Al-Sarraf R, Farghaly H, Vranic S, Sultan AA, Al Moustafa A-E, Al-Thawadi H. The co-presence of high-risk human papillomaviruses and Epstein-Barr virus is linked with tumor grade and stage in Qatari women with breast cancer. Hum Vaccin Immunother. 2021;17(4):982–9. doi:10.1080/21645515.2020.1802977.
- Al Moustafa AE, Al-Antary N, Aboulkassim T, Akil N, Batist G, Yasmeen A. Co-prevalence of Epstein–Barr virus and high-risk human papillomaviruses in Syrian women with breast cancer. Hum Vaccines Immunother. 2016;12(7):1–4. doi:10.1080/21645515.2016.1139255.
- Gupta I, Ghabreau L, Al-Thawadi H, Yasmeen A, Vranic S, Al Moustafa A-E, Malki MI. Co-incidence of human papillomaviruses and Epstein–Barr virus is associated with high to intermediate tumor grade in human head and neck cancer in Syria. Front Oncol. 2020;10. doi:10.3389/fonc.2020.01016.
- Al Moustafa A-E, Chen D, Ghabreau L, Akil N. Association between human papillomavirus and Epstein-Barr virus infections in human oral carcinogenesis. Med Hypotheses. 2009;73(2):184–6. doi:10.1016/j.mehy.2009.02.025.
- Guidry JT, Birdwell CE, Scott RS. Epstein-Barr virus in the pathogenesis of oral cancers. Oral Dis. 2018;24(4):497–508. doi:10.1111/odi.12656.
- Guidry JT, Scott RS. The interaction between human papillomavirus and other viruses. Virus Res. 2017;231:139–47. doi:10.1016/j.virusres.2016.11.002.
- Ferreira AR, Ramalho AC, Marques M, Ribeiro D. The interplay between antiviral signalling and carcinogenesis in human papillomavirus infections. Cancers (Basel). 2020;12(3):646. doi:10.3390/cancers12030646.
- Al-Antary N, Farghaly H, Aboulkassim T, Yasmeen A, Akil N, Al Moustafa A-E. Epstein-Barr virus and its association with Fascin expression in colorectal cancers in the Syrian population: a tissue microarray study. Hum Vaccin Immunother. 2017;13(7):1573–8. doi:10.1080/21645515.2017.1302046.