ABSTRACT
Healive® was the only Chinese WHO-prequalified inactivated vaccine for the hepatitis A virus, which has been widely used in national immunization programs in China. Long-term follow-up studies are needed to estimate the persistence of vaccine-induced antibody levels and the necessity for booster vaccines. During the trial, geometric mean concentrations (GMCs) and seroconversion rates (SRs) of anti-HAV antibodies were compared based on two different inactivated hepatitis A vaccines, Healive® and Havrix®. Four hundred children were randomly assigned to receive two doses of Healive® or Havrix® at 0 and 6 months. The current study assessed antibody persistence for both vaccines 15 years post-immunization. A mixed linear model was used to predict long-term antibody persistence. The GMCs were significantly higher for Healive® compared to Havrix® at 1, 6, 7, 66, 138 months (P < .001) and 186 months (P = .004 < .05) post-vaccination. Healive® and Havrix® reached a GMC of 164.8 mIU/ml and 105.7 mIU/ml post-15 years of vaccination, respectively. The seroconversion rates of both vaccines showed no statistically significant differences (97.9% for Healive® and 94.7% for Havrix®, P = .20). The prediction showed that Healive® would provide protection for a minimum of 30 years following immunization, with a lower limit of the 95% confidence intervals for GMCs greater than 20mIU/mL. Compared to Havrix®, the vaccine Healive® showed a stronger protective effect and better persistence among children at 15 years post-full immunization. Prediction indicated at least 30 years of antibody persistence for Healive® and at least 25 years for Havrix®.
Introduction
Hepatitis A is an inflammatory liver disease caused by Hepatitis A virus (HAV), and its common symptoms include jaundice, nausea, vomiting, and fever. HAV infection is primarily acquired via the fecal-oral route, either through ingestion of contaminated food or water or direct contact with an infectious person.Citation1 Hepatitis A is a vaccine preventable disease, and the effectiveness of vaccination against hepatitis A infection has been established worldwide.Citation2,Citation3 In 1991, the United States successfully developed the first inactivated hepatitis A vaccine Havrix®, which was then licensed in a number of countries.Citation4 The widely conducted vaccination program of hepatitis A vaccine in several countries, including the United States, Israel, and Argentina, has led to a dramatic decline in reported incidences of hepatitis A.Citation5 In 2002, China’s first self-developed hepatitis A inactivated vaccine, Healive® was launched in China.Citation6 In 2008, China incorporated the hepatitis A vaccine into the expanded immunization program, provided for free to all children older than 18 months.Citation7
To assess the immunogenicity and the long-term persistence of the Healive® vaccine, a randomized controlled clinical trial was conducted in 2006 in healthy children aged between 1–8 years with Havrix® (GlaxoSmithKline Biologicals) as a comparator. Four hundred children were enrolled and randomized into Healive® and Havrix® groups with a 3:1 ratio. Two doses of the vaccine were given at 0 and 6 months. Anti-HAV antibody was measured at 1, 6, 7, 66, 112, 138, and 186 months. Our previous research has evaluated the 5 years and 11 years geometric mean concentrations (GMCs) and seroconversion rates (SRs) after the full course of vaccination. Observed and predicted results revealed that both vaccines owned robust persistence along the follow-up period and may retain immunogenicity 30 years after the full-course vaccination.Citation8
Although predictions from previous studies showed that the protective effects of both vaccines could last for at least 30 years, evidence for the long-term protective effects of Havrix® and Healive® in a real-world setting is still scarce. One follow-up study in the US has evaluated the persistence of Havrix® vaccine-induced immunogenicity and found an 80 mIU/mL anti-HAV antibody GMC at 14 years of the trial (n = 5), and 43mIU/mL GMC at over 15 years of the trial (n = 1).Citation9 However, the extent and duration of long-term antibody persistence of Healive® in the Chinese population is still unknown. As Healive® is widely used in China, it is necessary to assess the long-term antibody persistence induced by Healive® vaccine in the real world and provide further recommendations on the boosting strategy of Healive®. As a result, this follow-up study will first assess the antibody level induced by Healive® 15 years post-vaccination and then compare the antibody persistence with predictions from previous mathematical models, aiming to offer some insights on the necessity of boosting vaccination.
Materials and methods
Clinical trial methodology
This randomized, open-label clinical trial aimed to estimate the long-term differences in GMCs and SRs of anti-HAV antibodies induced by the inactivated hepatitis A vaccines Healive® and Havrix®. Four hundred healthy children with negative anti-HAV serum results were randomized in a 3:1 ratio to receive the Healive or Havrix® vaccine.Citation10 The Healive® vaccine group received two doses of Healive® (0.5 mL/dose) which contained 250 U (1IU = 13 U) of antigen and 0.25 mg of alum without preservatives.Citation11 The Havrix® vaccine group received Havrix® (0.5 mL/dose), which contained 720 ELISA units (El.U) of antigen and 0.25 mg alum with 2-phenoxyethanol as the preservative. Participants who completed the full course of immunization were followed up for 15 years. Blood samples were collected at 0, 1, 6, 7, 18, 30, 42, 54, 66, 112, 138 and 186 months, as well as at 0 and 6 months prior to vaccination for screening purposes. The data generated from 0 to 66 months were previously reported by Yu et al.Citation12 The data generated between 112 and 138 months were reported by Wang et al.Citation8 These obtained data will be used in this study for further descriptive and predictive analysis to demonstrate long-term patterns of anti-HAV antibody levels. Assay modifications were made during the previous 112 to 138 months follow-up study.Citation8 Up until 66 months post-vaccination, anti-HAV antibody levels were assessed by microparticle enzyme immunoassay (MEIA). While electro chemiluminescence immunoassay (ECLIA) was used between 112 and 186 months after vaccination. We used two commercially available kits for measuring anti-HAV antibodies, i.e., from Abbott Laboratories (HAVAB2.0, MEIA method) and Roche Diagnostics (Elecsys Anti-HAV, ECLIA method). For MEIA method of HAVAB2.0, the upper limit of quantification (ULOQ) is 100 mIU/mL and the lower limit of quantification (LLOQ) is 5 mIU/mL. For ECLIA method of Elecsys, the ULOQ of ELICA is 60 mIU/mL and LLOQ is 3 mIU/mL. The consistency of switching the methods and performance comparisons was previously discussed in Wang et al.Citation8 Seroprotection was defined as anti-HAV antibody levels of at least 20 mIU/mL.Citation13–15
Consent and study approval
The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Study protocols were approved by the Ethics Review Committee of the Changzhou Center for Disease Control and Prevention. Written informed consents were obtained from all participants prior to the performance of the study. The trial was registered at Clinical trials. gov (NCT00534885).
Statistical methods
Anti-HAV antibody GMCs and SRs were primarily estimated using the observed data without addressing missing data. Prior to the GMC calculation and 95% CI estimation, a logarithmic transformation (log10) was carried out. The Student’s t-test or Mann-Whitney U test was employed for GMC comparisons, and for SRs comparisons, the Chi-square test or Fisher’s exact test. Statistical significance was assessed by two-tailed analysis with P < .05. Sensitivity analysis using multiple imputation (MI) method was conducted for missing logged anti-HAV GMCs. Furthermore, based on published methodologies, a linear model with a change point throughout the antibody concentrations decline phase was used to estimate the long-term antibody persistence after the GMCs transformed into log10 form.Citation7,Citation10 Statistical analyses were performed using SPSS 26.0.
Results
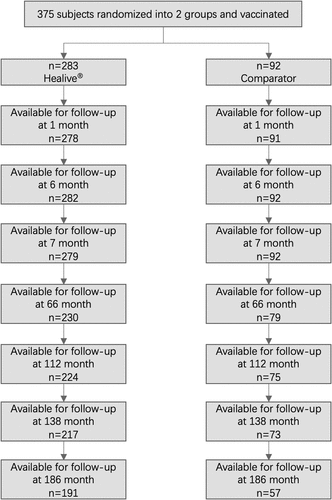
The original clinical trial enrolled 400 participants, with 300 in the Healive® vaccine group and 100 in the Havrix® control group. Of the 400 participants, 375 completed full vaccination and were enrolled in follow-up studies, with 283 participants in the Healive® vaccination group and 92 in the Havrix® control group. Of the 375 fully-vaccinated participants, 248 were followed up 15 years post-vaccination, with 191 in the Healive® vaccine group and 57 in the Havrix® control group ().
Of the 375 participants included in the follow-up analysis, the mean age at the initiation of the study for the Healive® and Havrix® group was 3.8 years and 3.7 years, respectively. 142 out of the 283 participants in the Healive® group and 48 out of the 92 participants in the control group were male. Of the 248 participants followed up at 186 months (15 years after full vaccination), the mean age of the participants was 18.8 years and 18.5 years for the Healive® group and Havrix® control group, respectively, and 94 out of the 191 and 34 out of the 57 of the participants were male, respectively. The demographic characteristics of both groups were normally distributed and well comparable at baseline ().
Table 1. Demographic characteristics of the study population.
and summarize the GMCs and SRs of the anti-HAV antibodies for the two groups at 1, 6, 7, 66, 112, 138, and 186 months. The results at 1, 6, 7, 66, 112 and 138 months have been analyzed and published in previous studies.Citation8,Citation12 At 186 months (15 years after full vaccination), GMCs in the Healive® vaccine group and Havrix® control group reached 164.8 (95% CI, 142.7–190.4) mIU/mL and 105.7 (95% CI, 80.6–138.7) mIU/mL, respectively. Compared to the Havrix® control group, the Healive® vaccine group had significantly higher GMCs at each time point from 1 to 186 months post-vaccination (P = .004 < .01). At 186 months, the SRs in the Healive® and control group were 97.9% and 94.7%, respectively. The Healive® group had higher or equal SRs at each time point from 1 to 186 months of vaccination compared to the control group.
Figure 2. Geometric mean concentration over time.
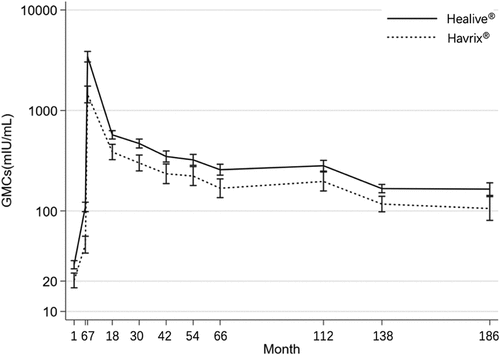
Table 2. Geometric mean concentrations and seroprotection rates over time.
The sensitivity analysis was performed by multiple imputations. Results showed at 138 months after full vaccination, GMCs in the Healive® and Havrix® control group were 173.8 mIU/mL and 120.2 mIU/mL, respectively. At 186 months after full vaccination, GMCs in the Healive® and Havrix® control group were 154.9 mIU/mL and 100.0 mIU/mL, respectively, consistent with the above results.
shows the predicted GMCs and SRs from the linear mixed model at 246 to 426 months based on the observed 15-year follow-up data. The model was formerly published by Yu et al. in the 5-year-follow-up study.Citation10 The observed and predicted GMCs at 186 months after vaccination were 164.8mIU/mL and 141 mIU/mL for the Healive® group, respectively. In the Havrix® comparator group, the observed and predicted GMCs were 105.7 mIU/mL and 94.8 mIU/mL. displays the observed values. These numbers demonstrate a remarkable concordance between the predicted and observed GMCs. The GMCs (95% CI) for the Healive® group were estimated to be 40.0 (31.7–50.5) mIU/mL at 366 months and 26.3 (19.9–34.7) mIU/mL at 426 months. For the Havrix® group, GMCs (95% CI) were predicted to be 26.7 (17.6–40.4) mIU/mL at 366 months and 17.5 (10.6–28.8) mIU/mL at 426 months. GMCs for the Healive® groups were predicted to have a lower limit of at least 20 mIU/mL at 366 months (30 years after full vaccination), while the Havrix® group had a lower limit of at least 20 mIU/mL at 306 months (25 years after full vaccination). Compared to the Havrix® group, the Healive® group also showed higher GMC at 426 months (35 years after full vaccination) with a lower limit of predicted GMC of 19.9 mIU/mL(). At 186 months after immunization for the two groups, the observed and anticipated SRs were consistent. shows the predicted SRs, and the SRs in the Healive® group were higher than the Havrix® group for up to 426 months after the full course of immunization.
Table 3. Comparison of Predicted GMCs and SRs based on 15-year follow-up period.
Discussion
This study compared the followed-up GMCs and SRs of anti-HAV antibodies elicited by the inactivated vaccines Healive® and Havrix® after 15 years of full-course of vaccination. Long-term immunogenic persistence was predicted using a linear mixed model reported by the previous study.Citation10
The results showed that the GMCs in the Healive® vaccine group were significantly higher than those in the Havrix® control group along the time points from 1 to 186 months after the full course of vaccination. The GMCs remain relatively stable from 138 months to 186 months, compared to the 112 months to 138 months (), which might indicate both vaccines’ long-term persistence performed well. Based on that, the confidence interval for Healive® and Havrix® was not overlapped, which might suggest Healive® could provide better long-term antibody persistence than Havrix®. The Healive® group had higher or equal SRs compared to the control group at most time points during the 15-year follow-up study. There was no statistically significant difference in SRs between the two groups after full vaccination (i.e., 1 and 6 months). The SRs for both groups reached 100% at 7 months, the SRs for the Healive® group remained highly stable, with the lower limit of the SR sustaining above 90% throughout the follow-up period. While the SRs for the Havrix® groups were less stable with lower limits of SRs below 90% at 112 months and 186 months. This indicated that Healive® might maintain a better seroprotective anti-HAV antibody level than Havrix® 15 years after vaccination. In addition, the 15-year effects assessed in this study fill the gap in the long-term follow-up of Havrix® in children and provide the latest data support for the immunogenicity and antibody persistence of the Healive® vaccine.
The observed GMCs results in the Healive® and Havrix® groups at 186 months after full-course vaccination were consistent with the predicted GMCs based on the 11-year follow-up data, which indicated that the statistic model Yu et al. used was reliable in predicting the long-term protective effects of Healive®. According to the latest prediction in this study, immunogenicity induced by Healive® would last for at least 30 years and thus a booster dose is not currently recommended. Compared to 11-year prediction of antibody, the predicted GMCs based on data with 15-year follow-up showed longer antibody persistence. Furthermore, the SRs for Healive® could maintain as high as 95% for 30 years post-full-course vaccination, while the SRs for Havrix® might remain to have 90% for 20 years post-full-course vaccination. These results suggested that the effect of Healive® was not only superior to Havrix® in the observed 15-year follow-up assessments of immunogenicity and anti-HAV antibody persistence but might also maintain all these features and advantages in future years.
The study also has several limitations. First, as a long-term clinical trial, loss of follow-up of the participants was increased over time. The reasons for loss of follow-up include going to school or work somewhere else, and loss of contact. It is possible that the reduced sample size is not sufficient to detect a significant difference between two groups. While there was no statistical difference from the baseline characteristic between the loss-of-follow-up participants and the non-loss-of-follow-up participants, which might suggest that the impact of the attrition on the results is relatively limited.
In conclusion, the Chinese first licensed inactivated hepatitis A vaccine with a 0–6-month vaccination schedule, is more immunogenic than Havrix® 15 years after full-course immunization in children. The Healive® vaccine might offer stronger long-term persistence and protection in healthy Chinese children. According to the antibody persistence prediction analysis, the Healive® vaccine might remain effective for at least 30 years.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Martin A, Lemon SM. Hepatitis A virus: from discovery to vaccines. Hepatology. 2006;43(S1):S164–6. doi:10.1002/hep.21052.
- Zhang L. Hepatitis A vaccination. Hum Vacc Immunother. 2020;16(7):1565–73. doi:10.1080/21645515.2020.1769389.
- Herzog C, Herck KV, Damme PV. Hepatitis A vaccination and its immunological and epidemiological long-term effects – a review of the evidence. Hum Vacc Immunother. 2021;17(5):1496–519. doi:10.1080/21645515.2020.1819742.
- Peetermans J. Production, quality control and characterization of an inactivated hepatitis A vaccine. Vaccine. 1992;10:S99–101. doi:10.1016/0264-410X(92)90557-Z.
- WHO. WHO position paper on hepatitis A vaccines – October 2022. Wkly Epidemiol Rec. 2022;97:493–512.
- Sinovac. Hepatitis A vaccine (human diploid cell), inactivated - Healive®-SINOVAC: supply vaccines to eliminate human diseases [Internet]. [accessed 2023 Feb 13]. http://www.sinovac.com/product/showproduct.php?id=43&lang=en.
- China NHC of the PR of. The Ministry of Health formulated an implementation plan for expanding the national immunization program, and 15 infectious diseases including hepatitis A were included in the national immunization program [Internet]. [accessed 2023 Feb 13]. http://www.nhc.gov.cn/wjw/ghjh/200804/33518.shtml.
- Wang Y, Qi Y, Xu W, Hu Y, Wang L, Yu Y, Jiang Z, Xia J, Zeng G, Wang Y. Immunogenicity persistence in children of hepatitis A vaccines Healive® and Havrix®: 11 years follow-up and long-term prediction. Hum Vacc Immunother. 2020;16(10):2559–64. doi:10.1080/21645515.2020.1715687.
- Raczniak GA, Thomas TK, Bulkow LR, Negus SE, Zanis CL, Bruce MG, Spradling PR, Teshale EH, McMahon BJ. Duration of protection against hepatitis A for the current two-dose vaccine compared to a three-dose vaccine schedule in children. Vaccine. 2013;31(17):2152–5. doi:10.1016/j.vaccine.2013.02.048.
- Jiang W-P, Chen J-T, Wang X, Wang Y-L, Liu Y, Chen W-Y, Xu W-G, Qiu Y-Z, Yin W-D. Immunogenicity and safety of three consecutive lots of a new preservative-free inactivated hepatitis A vaccine (Healive®): a double-blind, randomized and controlled trial. Vaccine. 2008;26(18):2297–301. doi:10.1016/j.vaccine.2007.11.008.
- Bhave S, Bavdekar A, Sapru A, Bawangade S, Pandit A. Immunogenicity of single dose live attenuated hepatitis A vaccine. Indian Pediatr. 2011;48(2):135–7. doi:10.1007/s13312-011-0039-4.
- Yu C, Song Y, Qi Y, Li C, Jiang Z, Li C, Zhang W, Wang L, Xia J. Comparison of immunogenicity and persistence between inactivated hepatitis A vaccine Healive® and Havrix® among children: a 5-year follow-up study. Hum Vacc Immunother. 2016;12(10):2595–602. doi:10.1080/21645515.2016.1197450.
- Cui F, Liang X, Wang F, Zheng H, Hutin YJ, Yang W. Development, production, and postmarketing surveillance of hepatitis A vaccines in China. J Epidemiol. 2014;24:169–77. doi:10.2188/jea.JE20130022.
- Rao S, Mao JS, Motlekar S, Fangcheng Z, Kadhe G. A review of immunogenicity and tolerability of live attenuated hepatitis A vaccine in children. Hum Vacc Immunother. 2016;12(12):3160–5. doi:10.1080/21645515.2016.1216286.
- André F, Damme PV, Safary A, Banatvala J. Inactivated hepatitis a vaccine: immunogenicity, efficacy, safety and review of official recommendations for use. Expert Rev Vaccines. 2002;1(1):9–23. doi:10.1586/14760584.1.1.9.