?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This open-labeled non-inferiority trial evaluated immunogenicity and reactogenicity of heterologous and homologous COVID-19 vaccination schedules in pregnant Thai women. 18–45-year-old pregnant women with no history of COVID-19 infection or vaccination and a gestational age of ≥12 weeks were randomized 1:1:1 into three two-dose primary series scheduled 4 weeks apart: BNT162b2-BNT162b2 (Group 1), ChAdOx1-BNT162b2 (Group 2), and CoronaVac-BNT162b2 (Group 3). Serum antibody responses, maternal and cord blood antibody levels at delivery, and adverse events (AEs) following vaccination until delivery were assessed. The 124 enrolled participants had a median age of 31 (interquartile range [IQR] 26.0–35.5) years and gestational age of 23.5 (IQR 18.0–30.0) weeks. No significant difference in anti-receptor binding domain (RBD) IgG were observed across arms at 2 weeks after the second dose. Neutralizing antibody geometric mean titers against the ancestral Wuhan strain were highest in Group 3 (258.22, 95% CI [187.53, 355.56]), followed by Groups 1 (187.47, 95% CI [135.15, 260.03]) and 2 (166.63, 95% CI [124.60, 222.84]). Cord blood anti-RBD IgG was correlated with, and equal to or higher than, maternal levels at delivery (r = 0.719, P < .001) and inversely correlated with elapsed time after the second vaccination (r = −0.366, P < .001). No significant difference in cord blood antibody levels between groups were observed. Local and systemic AEs were mild-to-moderate and more frequent in Group 2. Heterologous schedules of CoronaVac-BNT162b2 or ChAdOx1-BNT162b2 induced immunogenicity on-par with BNT162b2-BNT162b2 and may be considered as alternative schedules for primary series in pregnant women in mRNA-limited vaccine settings.
Introduction
COVID-19 has infected more than 685 million individuals and caused more than 6.84 million deaths globally as of April 2023.Citation1 Pregnant women are one of the high-risk groups for severe COVID-19.Citation2–4 Increased rates of intensive care unit (ICU) admission, maternal death, stillbirths, and preterm births were recorded in unvaccinated pregnant women compared to non-pregnant women or non-infected pregnant women.Citation3–8 This susceptibility is further exacerbated by comorbidities (i.e., hypertension, diabetes, obesity) that increase the risk of complications during pregnancy (i.e., preeclampsia, preterm births, cesarean delivery).Citation3 Despite this evident risk, this sub-population is typically excluded from COVID-19 vaccination studies.
With a growing body of evidence supporting vaccination in pregnant women,Citation4,Citation8–10 the World Health Organisation (WHO), American College of Obstetricians and Gynaecologists (ACOG), Society for Maternal-Fetal Medicine (SMFM), and Royal College of Obstetricians and Gynaecologists (RCOG) now recommend pregnant women get vaccinated – regardless of gestational age – to protect themselves and their infants against COVID-19.Citation11–13 Out of the nine vaccines approved by the WHO, three are widely used and have demonstrated efficacy and safety in healthy individuals,Citation14–18 including: chimpanzee, adenovirus-vectored ChAdOx1-nCoV-19 (Oxford/AstraZeneca™); BNT162b2 (Pfizer/BioNTech™), an mRNA vaccine with full-length SARS-CoV-2 spike protein; and inactivated, whole-virus CoronaVac (Sinovac/Biotech™). A recent systematic review and meta-analysis indicates that this efficacy and safety extends to pregnant women, with no reported adverse pregnancy outcomes.Citation4,Citation9,Citation19
Current COVID-19 vaccine studies in pregnant women emphasize the effects of mRNA vaccines (i.e., BNT162b2 and mRNA-1273 [Moderna™]).Citation4,Citation8–10,Citation20 There is limited data regarding the use of other vaccine types, despite the wider use of viral vector and inactivated vaccines in developing countries.Citation10 Heterologous schedules of ChAdOx1-BNT162b2 or CoronaVac-BNT162b2 generate similar or higher immunogenicity as homologous mRNA vaccination in healthy adults,Citation21,Citation22 and are less reactogenic than homologous BNT162b2 or ChAdOx1.Citation21–25 The sequence of heterologous regimens is crucial. We and others have previously shown that BNT162b2, when administered as a second dose following CoronaVac- or ChAdOx1-primed heterologous schedules, maximized immune response in heterologous primary series.Citation21,Citation22 Heterologous regimens also help address mRNA vaccine shortages in resource limited settings and provide flexible vaccine schedules.Citation22 WHO recommends heterologous scheduling of emergency use listed vaccines in such settings with those available vaccine platforms.Citation22 The implementation of such strategies, and the influence these regimens have on pregnant women’s altered immune systems, requires further evaluation in pregnant cohorts.Citation26
This study sought to evaluate the immunogenicity and reactogenicity of heterologous and homologous COVID-19 vaccination regimens of CoronaVac, ChAdOx1, and BNT162b2 in pregnant Thai women. We hypothesized that heterologous vaccination schedules would induce immunogenicity on-par with standard homologous schedules.
Materials and methods
Study design and participants
This prospective, randomized, open-labeled non-inferiority trial was conducted between August 2021 and 2022 at the Faculty of Medicine, Siriraj Hospital – a tertiary care and referral center in Bangkok, Thailand. 18–45-year-old women with singleton pregnancies at a gestational age ≥12 weeks were included in the study. Participants with a history of: smoking, drinking, drug-abuse; preeclampsia, placental abnormalities, premature labor, or fetal growth restriction; abnormal vaginal bleeding more than twice during their current pregnancy; prior SARS-CoV-2 infection, COVID-19 vaccination before screening; prior blood, blood component, plasma, immunoglobulin, antiviral, or antibody transfusion ≤90 days of the study; immunocompromised status, immunosuppressant use; underlying diseases or concomitant disorders (i.e., diabetes mellitus type 1 or 2, hypertension); or with severe vaccine or drug allergies were excluded from the study. Post-enrollment, participants with high-risk pregnancies that were deemed inappropriate to continue the study were discontinued by principal investigators. This study was approved by its institutional ethics committee (COA no. Si 481/2021) and conducted according to the Belmont Report, Declaration of Helsinki, and International Council on Harmonization’s Good Clinical Practice.
It was registered under Thai Clinical Trials on the 17th of July, 2021 (TCTR20210717002, https://clinicaltrials.gov/).
Study procedure
Following written informed consent, participants were randomized 1:1:1 using Sealed Envelope™ and allocated into three vaccine arms, each designated to receive a two-dose (first-second dose) primary vaccination series four weeks apart: BNT162b2-BNT162b2 (Group 1), ChAdOx1-BNT162b2 (Group 2), and CoronaVac-BNT162b2 (Group 3). Vital signs were measured, and physical examinations performed, for participants prior to each dose. All were monitored for 30 minutes after each vaccination for immediate adverse reactions (AEs). Immunogenicity was determined through blood samples drawn from the participants at baseline (before vaccination), four weeks after the first vaccination, and two weeks after the second vaccination. Additional blood samples from mothers and their umbilical cords were collected at the time of delivery. Participants were instructed to self-monitor and digitally record local and systemic AEs for seven days after each vaccination. Solicited local (injection-site specific) AEs included pain or tenderness, swelling or induration, and erythema. Solicited systemic AEs included fever (oral temperature > 38°C), headache, fatigue or malaise, myalgia, arthralgia, and nausea or vomiting. The severity of solicited AEs were graded 1–4 according to the United States Food and Drug Administration’s (USFDA) Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials.Citation27 Any AEs that exacerbated preexisting conditions and resulted in a significant clinical deterioration in patients’ conditions were monitored and reported.
Chemiluminescent microparticle immunoassays
Chemiluminescent microparticle immunoassays (CMIA) were used to assess blood samples for SARS-CoV-2 spike protein anti-receptor binding domain (anti-RBD) IgG. This was performed using SARS-CoV-2 IgG II Quant (Abbott, Illinois, USA) detection assays that linearly measured antibody levels between 21.0 and 40,000.0 arbitrary units per mL (AU/mL). These units are subsequently converted to binding antibody units per mL (BAU/mL) per the WHO’s international standards through the equation . Antibody levels ≥ 50 AU/mL, or 7.1 BAU/mL, were defined as seropositive.
Microneutralization assays
Neutralizing antibodies (NAb) titers against SARS-CoV-2 were measured at baseline and two weeks after the second dose for all participants. Microneutralization assays were performed using ancestral Wuhan, Omicron BA.1, BA.2, and BA.5 strains. SARS-CoV-2 Wuhan/WA1/2020, Omicron BA.1, BA.2, and Vero-E6 cells were provided by Professor Florian Krammer and Dr. Juan Manuel Carreno Quiroz from the Icahn School of Medicine at Mount Sinai, NY, USA. The Omicron BA.5 variant was isolated from clinical samples collected at Siriraj Hospital, Mahidol University using Vero-E6 cell lines. Omicron spike genes were confirmed through sequencing. Viruses were passaged in Vero-E6 cells and stored at −80°C. Serum was heat inactivated at 56°C for 30 minutes. Heat inactivated sera were serially diluted 1:10–10,240 before adding 100 TCID50 of SARS-CoV-2 in MEM media with 0.5% BSA and incubation at room temperature for 1 hour. Residual viral infectivity in the serum-virus mixture was assessed in quadruplicate wells of Vero-E6 cells incubated in serum-free media at 37°C, 5% CO2. Viral cytopathic effect was read on the fifth day. NAb titers were calculated using the Reed-Muench method.Citation28–30 Non-linear regression curve fitting was performed to calculate 50% inhibitory dilution (ID50). All samples were analyzed in a blinded manner. The limit of detection of ID50 was at 10.
Statistical analyses
Non-inferiority of heterologous schedules were demonstrated through lower bounds of 95% confidence intervals (CIs) of the ratio of anti-RBD SARS-CoV-2 IgG antibody geometric mean concentrations (GMC) two weeks after the second vaccination being at least 0.67, as recommended by the WHO.Citation31 Based on the parameters of the same immunogenicity study performed in healthy adults,Citation22 50 participants per group would provide 90% statistical power to determine non-inferiority of anti-RBD IgG GMCs between groups (assuming an equal ratio between two groups and considering a 30% dropout rate). AEs were recorded as frequencies (%) with two-sided 95% CIs. Anti-RBD IgG levels were reported as GMCs and geometric mean ratios (GMRs), both with 95% CIs, and compared with GMCs observed in healthy adults under the similar regimen from our previous study in the same setting (defined as reference cohort).Citation22 NAb levels were illustrated as geometric mean titers (GMT) with 95% CIs. Participants with anti-RBD IgG seropositive at baseline were excluded from the analysis. Statistical differences in immunological endpoints between and across study arms were analyzed using t-tests and one-way ANOVA, respectively. Statistical differences in AE endpoints were assessed using a Chi-square test or Fisher’s exact test. The Clopper-Pearson correlation coefficient was used to evaluate correlation between maternal and fetal anti-RBD IgG levels. All statistical analyses were performed using GraphPad™ Prism 9 (v9.2.0, 283; GraphPad™ Software, CA, USA) and STATA (v17; StataCorp™, LP, College Station, TX, USA) with a statistical significance of P ≤. 05. Anti-RBD IgG values of 0 were replaced with values of 0.01. ID50 values <10 were replaced by 10.
Results
One hundred and fifty participants were enrolled 1:1:1 into Groups 1, 2, and 3, respectively (). Twenty-six participants were excluded due to baseline anti-RBD IgG seropositivity, and 124 subjects included in subsequent analyses. The median (interquartile range [IQR]) age was 31 (26.0–35.5) years, gestational age was 23.5 (18.0–30.0) weeks, and BMI was 23.90 (21.2–27.6) kg/m2. No significant differences in baseline characteristics were observed between groups (). Antenatal screening revealed the following underlying conditions: thalassemia trait (n = 25), non-insulin-dependent gestational diabetes (n = 5), and hepatitis B inactive carrier (n = 2). Eighty-nine participants had deliveries at the study center, 10 were delivered before 37 weeks of gestation (all were ≥35 weeks), and six weighed <2,500 grams. None experienced miscarriages.
Figure 1. Consort flow diagram. One hundred and sixty-five participants were screened and 150 determined eligible to participate in the study. Participants were randomized 1:1:1 into three study arms (50 each) and assessed pre-vaccination (baseline), four weeks after the first vaccination, two weeks after the second vaccination, and upon delivery (after excluding baseline seropositive patients).
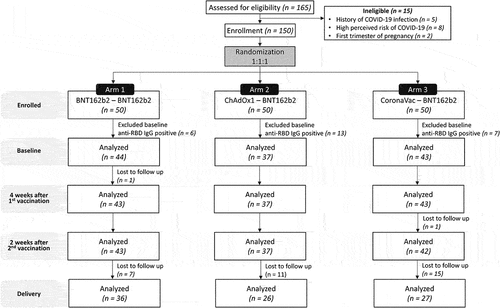
Table 1. Participant characteristics by COVID-19 vaccine regimen.
Maternal anti-SARS-CoV-2 RBD IgG and NAb responses
Four weeks after the first vaccination, anti-RBD IgG GMC was highest among Group 1 recipients (107.14 BAU/mL, 95% CI [79.41, 144.55]), followed by Group 2 (84.85 BAU/mL, 95% CI [60.13, 119.74]), and Group 3 (11.83 BAU/mL, 95% CI [9.05, 15.45]) recipients (P < .0001, , Supplementary Table S1). A similar trend was observed two weeks following the second vaccination, with IgG GMC recorded as 2072.84 BAU/mL (95% CI [1691.21, 2540.58]) for Group 1, 1754.61 BAU/mL for Group 2 (95% CI [1334.10, 2307.66]), and 1609.68 BAU/mL for Group 3 (95% CI [1334.83, 1941.13]) (P = .2379, , Supplementary Table S1). No significant difference in anti-RBD IgG levels was observed compared to healthy non-pregnant adults in the reference study.Citation22
Figure 2. Maternal humoral immune responses four weeks after the first dose and two weeks after the second dose. (a) Illustrates anti-SARS-CoV-2 receptor binding domain (RBD) IgG geometric mean concentrations (GMCs), with references of the same vaccine regimens in healthy, non-pregnant adults for comparison.Citation22 (b) Illustrates geometric mean titers (GMTs) of neutralizing antibodies (NAbs) from 50% inhibitory dilution (ID50) by microneutralization assays against the ancestral Wuhan strain and Omicron BA.1, BA.2, and BA.5 subvariants. Titers below the lower limit of detection (LLOD of 1:10) were replaced with a value of 10. Error bars represent geometric means with 95% confidence intervals (CIs). Abbreviations: BAU, Binding Antibody Units; ID50, 50% Inhibitory Dilution.
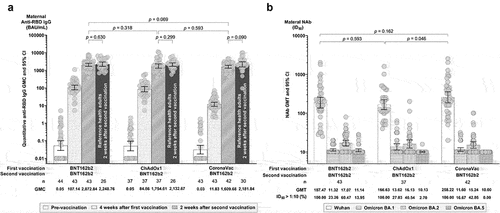
Two weeks after completing the second vaccination, all vaccination regimens generated high NAb GMTs against the ancestral Wuhan strain, with no statistical difference in titers observed across cohorts (P = .1284). Titers were highest in Group 3 (258.22, 95% CI [187.53, 355.56]), followed by Group 1 (187.47, 95% CI [135.15, 260.03]) and Group 2 (166.63, 95% CI [124.60, 222.84]) (, Supplementary Table S1). As expected, low NAb GMTs and seropositivity were observed against Omicron BA.1 (12.07, 95% CI [11.24, 12.97]; 22.13% seropositivity]), BA.2 (16.14, 95% CI [14.41, 18.07]; 48.36%), and BA.5 (10.43, 95% CI [10.09, 10.77]; 5.74%) across all three study arms (, Supplementary Table S1).
Maternal serum and cord blood anti-SARS-CoV-2 RBD IgG levels at delivery
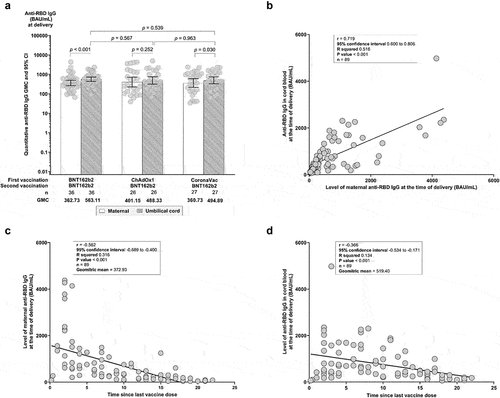
The median duration between the second vaccination and date of delivery across the three groups was 9 (3–15) weeks (n = 89). There were no significant differences in anti-RBD IgG GMCs in maternal serum at delivery (P = .9356) and cord blood (P = .7987) across groups (, Supplementary Table S1). However, anti-RBD IgG GMC of cord blood was significantly higher than maternal serum at delivery for Group 1 (563.11 vs 362.73 BAU/mL, P < .001) and Group 3 (494.89 vs 360.73 BAU/mL, P = .03), respectively. This was not seen for Group 2 (488.33 vs 401.15 BAU/mL, P = .252). Anti-RBD IgG levels measured in maternal serum and cord blood were strongly correlated (r = 0.719, P < .001, ). Anti-RBD IgG levels were inversely correlated with the time since the last vaccination for both maternal serum (r = −0.562, P < .001, ) and cord blood (r = −0.366, P < .001, ).
Figure 3. (a) Comparative geometric mean concentrations (GMCs) of maternal and cord blood anti-SARS-CoV-2 RBD IgG at delivery by vaccine regimen. (b) Pearson’s correlation coefficient (PCC) of umbilical cord anti-RBD IgG level against maternal anti-RBD IgG at delivery. (c) PCC of maternal anti-RBD IgG at time of delivery against time since the second vaccination. (d) PCC of umbilical cord blood anti-RBD IgG at the time of delivery against time since the second vaccination. (r) represents PCC.
Local and systemic AEs
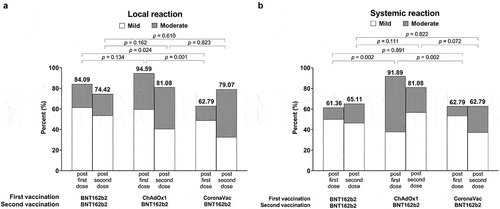
All AEs were reportedly mild or moderate (, Supplementary Figure S1) and self-resolved within 2–3 days. After the first vaccination, a significant difference in frequency and severity was observed for both local (P = .0013) and systemic reactions (P = .0036), particularly injection site reactions (P = .0013) and headaches (P < .0001) (, Supplementary Figure S1, Supplementary Table S2) between arms. This was particularly observed after ChAdOx1 administration for both moderate local (35.14%, 22.73%, and 13.95% for ChAdOx1, BNT162b2, and CoronaVac, respectively) and moderate systemic reactions (54.05%, 11.36%, and 9.30% for ChAdOx1, BNT162b2, and CoronaVac, respectively) (Supplementary Figure S1, Supplementary Table S2). After the second dose of BNT162b2 across groups, a significant difference in frequency and severity was observed only for specific systemic reactions in the heterologous ChAdOx1-BNT162b2 arm including: fatigue (P = .0123), headaches (P = .0189), and rashes (P = .0039) (Supplementary Figure S1, Supplementary Table S3). We found no association between AEs and gestational age.
Discussion
To our knowledge, this is the first study to assess immunogenicity and reactogenicity of various heterologous primary COVID-19 vaccination series in pregnant women. It is also one of few studies that explored ChAdOx1 administration in pregnant women. We found heterologous vaccination regimens of CoronaVac-BNT162b2 or ChAdOx1-BNT162b2 generated similar immunogenicity to homologous BNT162b2-BNT162b2, as previously noted in non-pregnant healthy adult cohorts.Citation22 Both heterologous and homologous vaccine arms induced robust NAbs against the ancestral Wuhan strain, but limited response against Omicron subvariants. While ChAdOx1-BNT162b2 regimens induced higher reactogenicity, heterologous regimens were safe and induced no concerned pregnancy outcomes. Cord blood and maternal serum antibody levels at delivery were not different across arms.
Homologous mRNA vaccine regimens were previously reported as safe and immunogenic in pregnant women.Citation32,Citation33 It is recommended by both the US and EU for pregnant women due to the high vaccine efficiency it confers in non-pregnant adult populations.Citation26 Previous studies reported that, for this same population, heterologous ChAdOx1-BNT162b2 vaccination induced similar or higher humoral and cellular immune responses than homologous ChAdOx1-ChAdOx1 or BNT162b2-BNT162b2 regimens.Citation34 Our findings extend this humoral response to pregnant women administered ChAdOx1-BNT162b2 or CoronaVac-BNT162b2 vaccine regimens. Some studies report lower anti-RBD IgG levels in pregnant populations.Citation9,Citation20,Citation22,Citation32 This could be from the relatively immunosuppressed state of pregnant women themselves.Citation9 However, we noted no difference in anti-RBD antibody responses between pregnant and non-pregnant healthy adult cohorts. Similar conclusions were drawn by Gray et al. (as cited inCitation20) and Bookstein et al.Citation9
Limited NAbs generated against Omicron subvariants support the need for boosters in pregnant women. A previous report indicated higher neonatal and maternal NAb concentrations post-booster dose compared to primary series administered within the first trimester.Citation20 Healthy Thai adults who received heterologous COVID-19 vaccination also demonstrated anamnestic antibody responses subsequent to BNT162b2 boosting.Citation22 Real-world studies confirmed the effectiveness of heterologous regimens with boosters in Thailand.Citation35,Citation36 Additionally, previous studies also suggest that administering CoronaVac after ChAdOx1 or BNT162b2 priming resulted in poorer immune responses.Citation22,Citation37 This highlights the importance of vaccine administration sequencing toward heterologous scheduling efficacy and need for stringent clinical trial evaluation.
Previously reported positive correlations between maternal serum, cord blood, breast milk anti-RBD IgG levels are consistent with the strong correlation reported in this study.Citation4,Citation20,Citation26,Citation38 We found higher cord blood anti-RBD IgG levels than maternal levels at delivery, with statistical significance in Groups 1 and 3. This indicates that there is vertical transplacental transfer of SARS-CoV-2 antibodies after COVID-19 vaccination, conferring primary protection to new-born babies against infection.Citation39,Citation40 The degree of infant protection depends on the placental transfer ratio, which increases with gestational age.Citation20,Citation26 Cord blood and maternal antibody concentrations are influenced by the interval between delivery and vaccination.Citation20 The inverse correlation between cord blood and elapsed time from vaccination in our study further supports booster administration during the third trimester of pregnancy to optimize fetal antibody transfer and ensure sufficient neonatal protection.Citation20 The duration of this protection requires further evaluation.Citation26
COVID-19 vaccinations are safe for healthy pregnant women and are not associated with adverse pregnancy outcomes.Citation41–43 Similar local and systemic AEs were observed in pregnant sub-populations compared to non-pregnant populations.Citation3,Citation44 Fetal complications were consistent with expected ranges described in previous literature,Citation3,Citation20,Citation41,Citation44,Citation45 and no additional AEs were observed specifically for pregnant women.Citation9 Badell et al.Citation20 noted AEs in pregnant cohorts to be typically mild-to-moderate, occurring on the day of vaccination and resolving a couple days thereafter. While no significant differences in AE frequency were reported across first, second, and third trimesters,Citation9 a greater frequency and severity has been recorded after the second dose (i.e., uterine contractions, injection-site pain, headaches, myalgia, and fatigue).Citation9,Citation20 Consistent with previous studies in non-pregnant adults in Thailand and other countries,Citation22,Citation46–48 higher systemic AEs such as fever and fatigue were more commonly reported following ChAdOx1 vaccination compared with BNT162b2. Nevertheless, no serious AEs (SAEs) or concerning adverse pregnancy outcomes were observed in our study. Our findings support flexible vaccination regimens and contribute to the growing body of evidence that heterologous vaccination schedules during pregnancy are safe and immunogenic. However, there is some evidence that suggests a potential risk between ChAdOx1 administration and vaccine-induced immune thrombotic thrombocytopenia (VITT) at a very low frequency.Citation49,Citation50 ChAdOx1 has since been discontinued for use in routine vaccination practices in Thailand. With this, we discourage ChAdOx1 administration in pregnant women.
This study has some limitations. First, the unblinded nature of our study risks observer bias, particulary for reactogenicity. However, immunogenicity would not be affected by an open-labeled design. Second, we excluded women with high-risk pregnancies and restricted our study to two heterologous regimens which had been found to be highly immunogenic in non-pregnant adults. These two heterologous regimens have also been used routinely in Thailand. Our results may not be generalizable to women with high-risk pregnancies or other heterologous COVID-19 regimens. Third, we did not evaluate cellular immune responses, which may be altered due to their pregnant status. Fourth, we may not have been able to study rare AEs due to the limited number of enrolled subjects. Fifth, several participants were excluded from analysis due to prior SARS-CoV-2 infection or lost to follow up during delivery. This reduced the statistical power from 90% to 85%. Our data must be interpreted with caution due to the smaller sample size. However, the number of evaluable subjects by the time of delivery is still sufficient to demonstrate differences in immunogenicity. Lastly, we did not include non-pregnant, healthy adults in this study as a control. However, our previous study in non-pregnant, healthy adults vaccinated under similar regimens and settings served as a reference for anti-RBD IgG response.Citation22
To conclude, our findings that heterologous primary vaccine series of CoronaVac-BNT162b2 and ChAdOx1-BNT162b2 offers immunogenicity on-par with homologous BNT162b2-BNT162b2 regimens grants flexibility to both vaccine management and utilization in countries with limited mRNA vaccine supplies. This strongly supports homologous or heterologous COVID-19 vaccination recommendations during pregnancy to confer protection to both mother and child. With the exception of ChAdOx1 vaccination, the lack of SAEs and adverse pregnancy outcomes also further alleviates concerns of heterologous regimens in pregnant women. Further multicenter trials with large cohorts are required to verify the results of this study as well as determine long-term infant immunity and safety.
Author contributions
Conceptualization, Methodology, and Supervision, Kulkanya Chokephaibulkit; Data Curation and Investigation, Chenchit Chayachinda, Kanokwaroon Watananirun, Chayawat Phatihattakorn, Sanitra Anuwutnavin, Suvimol Niyomnaitham, Wanatpreeya Phongsamart, Keswadee Lapphra, Orasri Wittawatmongkol, Supattra Rungmaitree, Kobporn Boonnak, Patimaporn Wongprompitak, Sansnee Senawong; Formal Analysis, Kulkanya Chokephaibulkit, Laddawan Jansarikit; Software and Visualization, Laddawan Jansarikit; Writing – Original Draft, Avishek Upadhya, Kulkanya Chokephaibulkit; Writing – Review & Editing, all authors.
Informed consent statement
All participants signed paper informed consent forms prior to participanting in the clinical study.
Institutional review board ststament
This study was approved by its institutional ethics committee (COA no. Si 481/2021) and conducted according to the Belmont Report, Declaration of Helsinki, and International Council on Harmonization’s Good Clinical Practice.
Supplemental Material
Download PDF (268.2 KB)Acknowledgments
We would like to thank Professor Florian Krammer and Dr. Juan Manuel Carreno, from the Icahn School of Medicine, Mount Sinai, New York, USA for their contributions to the microneutralization assays.
Disclosure statement
No potential conflicts of interest were reported by the authors.
Data availability statement
Deidentified individual patient information and other datasets, study protocols, and documents generated and/or analyzed during this study will be made available after publication and shared to researchers with a methodologically sound proposal to achieve their aims upon request, by e-mail, to the corresponding author.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2228670.
Additional information
Funding
References
- COVID-19 coronavirus pandemic. Worldometer; 2023 Apr 15. https://www.worldometers.info/coronavirus/.
- Phoswa WN, Khaliq OP. Is pregnancy a risk factor of COVID-19? Eur J Obstet Gynecol Reprod Biol. 2020;252:605–9. doi:10.1016/j.ejogrb.2020.06.058.
- Riley LE. mRNA Covid-19 vaccines in pregnant women. N Engl J Med. 2021;384(24):2342–3. doi:10.1056/NEJMe2107070.
- Nunes MC, Madhi SA. COVID-19 vaccines in pregnancy. Trends Mol Med. 2022;28(8):662–80. doi:10.1016/j.molmed.2022.04.012.
- Oakes MC, Kernberg AS, Carter EB, Foeller ME, Palanisamy A, Raghuraman N, Kelly JC. Pregnancy as a risk factor for severe coronavirus disease 2019 using standardized clinical criteria. Am J Obstet Gynecol MFM. 2021;3(3):100319. doi:10.1016/j.ajogmf.2021.100319.
- Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, Roggero P, Prefumo F, Do Vale MS, Cardona-Perez JA, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 Infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817–26. doi:10.1001/jamapediatrics.2021.1050.
- Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, Zhou D, Coomar D, Sheikh J, Lawson H, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi:10.1136/bmj.m3320.
- Januszek SM, Faryniak-Zuzak A, Barnaś E, Łoziński T, Góra T, Siwiec N, Szczerba P, Januszek R, Kluz T. The approach of pregnant women to vaccination based on a COVID-19 systematic review. Medicina. 2021;57(9):977. doi:10.3390/medicina57090977.
- Bookstein Peretz S, Regev N, Novick L, Nachshol M, Goffer E, Ben‐David A, Asraf K, Doolman R, Levin EG, Regev Yochay G, et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obst Gyn. 2021;58(3):450–6. doi:10.1002/uog.23729.
- Kalafat E, Heath P, Prasad S, Pat O, Khalil A. COVID-19 vaccination in pregnancy. Am J Obstet Gynecol. 2022;227(2):136–47. doi:10.1016/j.ajog.2022.05.020.
- WHO. Coronavirus disease (COVID-19): Vaccines. World Health Organization; 2022. https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-vaccines.
- The American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. The American College of Obstetricians and Gynecologists; 2021. https://www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals#:~:text=%E2%80%9CACOG%20is%20recommending%20vaccination%20of,complications%2C%20and%20because%20it%20is.
- The Royal College of Obstetricians & Gynaecologists. Coronavirus (COVID-19) infection in pregnancy. Royal College of Obstetricians & Gynaecologists; 2022. p. 1–61. https://www.rcog.org.uk/guidance/coronavirus-covid-19-pregnancy-and-women-s-health/coronavirus-covid-19-infection-in-pregnancy/.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi:10.1056/NEJMoa2034577.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi:10.1016/S0140-6736(20)32661-1.
- Tanriover MD, Doganay HL, Akova M, Guner HR, Azap A, Akhan S, Köse Ş, Erdinç FŞ, Akalın EH, Tabak ÖF, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–22. doi:10.1016/S0140-6736(21)01429-X.
- Zeng B, Gao L, Zhou Q, Yu K, Sun F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: a systematic review and meta-analysis. BMC Med. 2022;20(1):200. doi:10.1186/s12916-022-02397-y.
- Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385(7):585–94. doi:10.1056/NEJMoa2108891.
- Prasad S, Kalafat E, Blakeway H, Townsend R, O’Brien P, Morris E, Draycott T, Thangaratinam S, Le Doare K, Ladhani S, et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat Commun. 2022;13(1):2414. doi:10.1038/s41467-022-30052-w.
- Badell ML, Dude CM, Rasmussen SA, Jamieson DJ. Covid-19 vaccination in pregnancy. bmj. 2022;378:e069741. doi:10.1136/bmj-2021-069741.
- Liu X, Shaw RH, Stuart AS, Greenland M, Aley PK, Andrews NJ, Cameron JC, Charlton S, Clutterbuck EA, Collins AM, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398(10303):856–69. doi:10.1016/S0140-6736(21)01694-9.
- Niyomnaitham S, Quan Toh Z, Wongprompitak P, Jansarikit L, Srisutthisamphan K, Sapsutthipas S, Jantraphakorn Y, Mingngamsup N, Licciardi PV, Chokephaibulkit K, et al. Immunogenicity and reactogenicity against the SARS-CoV-2 variants following heterologous primary series involving CoronaVac, ChAdox1 nCov-19 and BNT162b2 plus BNT162b2 booster vaccination: an open-label randomized study in healthy Thai adults. Hum Vaccin Immunother. 2022;18(6):2091865. doi:10.1080/21645515.2022.2091865.
- Powell AA, Power L, Westrop S, McOwat K, Campbell H, Simmons R, Ramsay ME, Brown K, Ladhani SN, Amirthalingam G. Real-world data shows increased reactogenicity in adults after heterologous compared to homologous prime-boost COVID-19 vaccination, March−June 2021, England. Eurosurveillance. 2021;26(28):2100634. doi:10.2807/1560-7917.ES.2021.26.28.2100634.
- Jara A, Undurraga EA, Zubizarreta JR, González C, Pizarro A, Acevedo J, Leo K, Paredes F, Bralic T, Vergara V, et al. Effectiveness of homologous and heterologous booster doses for an inactivated SARS-CoV-2 vaccine: a large-scale prospective cohort study. Lancet Glob Health. 2022;10(6):e798–e806. doi:10.1016/S2214-109X(22)00112-7.
- Atmar RL, Lyke KE, Deming ME, Jackson LA, Branche AR, El Sahly HM, Rostad CA, Martin JM, Johnston C, Rupp RE, et al. Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386(11):1046–57. doi:10.1056/NEJMoa2116414.
- Dagan N, Barda N, Biron-Shental T, Makov-Assif M, Key C, Kohane IS, Hernán MA, Lipsitch M, Hernandez-Diaz S, Reis BY, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med. 2021;27(10):1693–5. doi:10.1038/s41591-021-01490-8.
- United States Food and Drug Administration. Guidance for industry toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials . 2007. p. 10. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/toxicity-grading-scale-healthy-adult-and-adolescent-volunteers-enrolled-preventive-vaccine-clinical.
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–11. doi:10.1038/s41591-021-01377-8.
- Subbarao K, McAuliffe J, Vogel L, Fahle G, Fischer S, Tatti K, Packard M, Shieh W-J, Zaki S, Murphy B, et al. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J Virol. 2004;78(7):3572–7. doi:10.1128/JVI.78.7.3572-3577.2004.
- Houser KV, Gretebeck L, Ying T, Wang Y, Vogel L, Lamirande EW, Bock KW, Moore IN, Dimitrov DS, Subbarao K, et al. Prophylaxis with a middle east respiratory syndrome coronavirus (MERS-CoV)–specific human monoclonal antibody protects rabbits from MERS-CoV infection. J Infect Dis. 2016;213(10):1557–61. doi:10.1093/infdis/jiw080.
- World Health Organization. Guidelines on clinical evaluation of vaccines: regulatory expectations. WHO Technical Report Series 1004, Annex 9, 2017: World Health Organization; 2020. https://www.who.int/publications/m/item/WHO-TRS-1004-web-annex-9.
- Blakeway H, Amin‐Chowdhury Z, Prasad S, Kalafat E, Ismail M, Abdallah F, Rezvani A, Amirthalingam G, Brown K, Le Doare K, et al. Evaluation of immunogenicity and reactogenicity of COVID-19 vaccines in pregnant women. Ultrasound Obst Gyn. 2022;60(5):673–80. doi:10.1002/uog.26050.
- Fu W, Sivajohan B, McClymont E, Albert A, Elwood C, Ogilvie G, Money D. Systematic review of the safety, immunogenicity, and effectiveness of COVID‐19 vaccines in pregnant and lactating individuals and their infants. Int J Gynecol Obstet. 2022;156(3):406–17. doi:10.1002/ijgo.14008.
- Pozzetto B, Legros V, Djebali S, Barateau V, Guibert N, Villard M, Peyrot L, Allatif O, Fassier J-B, Massardier-Pilonchéry A, et al. Immunogenicity and efficacy of heterologous ChAdOx1–BNT162b2 vaccination. Nature. 2021;600(7890):701–6. doi:10.1038/s41586-021-04120-y.
- Suphanchaimat R, Nittayasoot N, Jiraphongsa C, Thammawijaya P, Bumrungwong P, Tulyathan A, Cheewaruangroj N, Pittayawonganon C, Tharmaphornpilas P. Real-world effectiveness of mix-and-match vaccine regimens against SARS-CoV-2 delta variant in Thailand: a nationwide test-negative matched case-control study. Vaccines. 2022;10(7):1080. doi:10.3390/vaccines10071080.
- Sritipsukho P, Khawcharoenporn T, Siribumrungwong B, Damronglerd P, Suwantarat N, Satdhabudha A, Chaiyakulsil C, Sinlapamongkolkul P, Tangsathapornpong A, Bunjoungmanee P, et al. Comparing real-life effectiveness of various COVID-19 vaccine regimens during the delta variant-dominant pandemic: a test-negative case-control study. Emerging Microbes Infect. 2022;11(1):585–92. doi:10.1080/22221751.2022.2037398.
- Cohen G, Jungsomsri P, Sangwongwanich J, Tawinprai K, Siripongboonsitti T, Porntharukchareon T, Wittayasak K, Thonwirak N, Soonklang K, Sornsamdang G, et al. Immunogenicity and reactogenicity after heterologous prime-boost vaccination with CoronaVac and ChAdox1 nCov-19 (AZD1222) vaccines. Hum Vaccin Immunother. 2022;18(5):2052525. doi:10.1080/21645515.2022.2052525.
- Gloeckner S, Hornung F, Heimann Y, Schleussner E, Deinhardt-Emmer S, Loeffler B, Zoellkau J. Newborns’ passive humoral SARS-CoV-2 immunity following heterologous vaccination of the mother during pregnancy. Am J Obstet Gynecol. 2022;226(2):261–2. doi:10.1016/j.ajog.2021.10.006.
- Halasa NB, Olson SM, Staat MA, Newhams MM, Price AM, Pannaraj PS, Boom JA, Sahni LC, Chiotos K, Cameron MA, et al. Maternal vaccination and risk of hospitalization for Covid-19 among infants. N Engl J Med. 2022;387(2):109–19. doi:10.1056/NEJMoa2204399.
- Halasa NB, Olson SM, Staat MA, Newhams MM, Price AM, Boom JA. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19–associated hospitalization in infants aged< 6 months—17 states, July 2021–January 2022. Morb Mort Wkly Rep 2022; 71:264.
- Sadarangani M, Soe P, Shulha HP, Valiquette L, Vanderkooi OG, Kellner JD, Muller MP, Top KA, Isenor JE, McGeer A, et al. Safety of COVID-19 vaccines in pregnancy: a Canadian national vaccine safety (CANVAS) network cohort study. Lancet Infect Dis. 2022;22(11):1553–64. doi:10.1016/S1473-3099(22)00426-1.
- DeSilva M, Haapala J, Vazquez-Benitez G, Vesco KK, Daley MF, Getahun D, Zerbo O, Naleway A, Nelson JC, Williams JTB, et al. Evaluation of acute adverse events after Covid-19 vaccination during pregnancy. N Engl J Med. 2022;387(2):187–9. doi:10.1056/NEJMc2205276.
- Fell DB, Dhinsa T, Alton GD, Török E, Dimanlig-Cruz S, Regan AK, Sprague AE, Buchan SA, Kwong JC, Wilson SE, et al. Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. Jama. 2022;327(15):1478–87. doi:10.1001/jama.2022.4255.
- Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, Marquez PL, Olson CK, Liu R, Chang KT, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273–82. doi:10.1056/NEJMoa2104983.
- Ellington S, Olson CK. Safety of mRNA COVID-19 vaccines during pregnancy. Lancet Infect Dis. 2022;22(11):1514–15. doi:10.1016/S1473-3099(22)00443-1.
- Alghamdi AN, Alotaibi MI, Alqahtani AS, Al Aboud D, Abdel-Moneim AS. Bnt162b2 and ChAdOx1 SARS-CoV-2 post-vaccination side-effects among Saudi vaccinees. Front Med. 2021;8:760047. doi:10.3389/fmed.2021.760047.
- Bae S, Lee YW, Lim SY, Lee J-H, Lim JS, Lee S, Park S, Kim S-K, Lim Y-J, Kim EO, et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. 2021;36(17). doi:10.3346/jkms.2021.36.e115.
- Klemis V, Schmidt T, Schub D, Mihm J, Marx S, Abu-Omar A, Ziegler L, Hielscher F, Guckelmus C, Urschel R, et al. Comparative immunogenicity and reactogenicity of heterologous ChAdOx1-nCoV-19-priming and BNT162b2 or Mrna-1273-boosting with homologous COVID-19 vaccine regimens. Nat Commun. 2022;13(1):4710. doi:10.1038/s41467-022-32321-0.
- Clarke L, Brighton T, Chunilal SD, Lee CS, Passam F, Curnow J, Chen VM, Tran HA. Vaccine-induced immune thrombotic thrombocytopenia post dose 2 ChAdOx1 nCov19 vaccination: less severe but remains a problem. Vaccine. 2023;41(20):3285–91. doi:10.1016/j.vaccine.2023.03.071.
- Pavord S, Scully M, Hunt BJ, Lester W, Bagot C, Craven B, Rampotas A, Ambler G, Makris M. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385(18):1680–9. doi:10.1056/NEJMoa2109908.