ABSTRACT
Cancer represents a challenging medical problem worldwide. Several exploratory studies have been conducted to overcome these limitations. RNA vaccines play an important role in cancer prevention and treatment. Recent studies have shown that RNA vaccines play an important role in cancer prevention. However, there are currently no relevant bibliometric studies. This study aimed to apply bibliometrics to summarize the knowledge structure and research hotspots regarding the role of RNA vaccines in cancer. Publications related to RNA vaccines in cancer were searched on the web of science core collection (WoSCC) database. VOSviewers, CiteSpace and R package “bibliometrix” were used to conduct this bibliometric analysis. A total of 1399 articles were included, comprising 803 original articles and 596 reviews. The number of studies on RNA vaccines against cancer has been increasing annually. China and the United States were the principal countries of origin of publications. Johannes Gutenberg University Mainz, NCI, and Duke University were the main organizations. Frontiers in Immunology is the leading journal in the field. Hot keywords included tumor antigens, lipid nanoparticles, emerging roles, and dendritic cells. This is the bibliometric study to summarize the research trends and development of RNA vaccines for cancer. This information will provide a reference for researchers studying RNA vaccines against cancer.
Introduction
Cancer has one of the highest incidence and mortality rates among all diseases. By 2023, 1,958,310 new cancer cases and 609,820 cancer-related deaths are forecasted in the United States.Citation1 Therefore, it is necessary to prevent and treat these cancers. The traditional approach to cancer treatment is to discover and treat the tumor. Currently, treating cancer brings enormous pain and economic burden to people’s lives. Advancing cancer treatment before it occurs will improve survival and treatment of patients.
RNA vaccines can reduce the occurrence of cancer by regulating immunity, and may even have a therapeutic effect on cancer.Citation2–9 Owing to the rapid development of RNA vaccines in the field of cancer, it is necessary to systemize their knowledge.
Bibliometrics is a science that uses mathematical and statistical methods to analyze information in documents.Citation10–14 It not only helps to understand research achievements in specific fields, but also helps scholars grasp current research hotspots and frontiers as quickly as possible, and further guides them in selecting future research directions. In a bibliometric analysis, we can obtain detailed information about the literature, such as authors, keywords, countries, publishing institutions, and references. The commonly used software for bibliometrics includes CiteSpace, VOSviewer, and R package “bibliometric.”Citation15–28 The role of this software was to visualize the results of the literature. These tools have been applied in many research fields, such as cancer, surgery, and genetics. However, to the best of our knowledge, there are fewer bibliometric studies on RNA vaccines for cancer. Luofei ZhangCitation29 used bibliometric analysis to summarize vaccine and cancer prevention. However, this study is not about RNA vaccine. RNA vaccines have great potential to protect against cancer due to superiorities in safety, efficacy and industrial production. To fill this gap, we conducted a bibliometric analysis of the articles on cancer RNA vaccines.
Methods
Search strategy
Publications related to cancer RNA vaccines were retrieved from the Web of Science Core Collection (WoSCC) database (http://www.webofscience.com/wos/woscc/basic-search) on March 28, 2023. The search formula is TS= (Cancer) AND TS= (RNA vaccines) AND LA= (English), and the type of documents is set to “articles” and “review.”
VOSviewers, CiteSpace and the R package “bibliometrix” were used to conduct this bibliometric analysis.
Data analysis
VOSviewer (version 1.6.18) is bibliometric analysis software that can analyze key information from numerous publications.Citation30,Citation31 This software was used to build collaboration, co-citation, and co-occurrence networks. In our study, we used this software to complete a partial bibliometric analysis.
CiteSpace (version 6.2. R2) was developed by Professor Chen. We used this software to generate a dual-map overlay of journals and constructed a timeline of keywords.Citation21,Citation21,Citation32–38
The R package “bibliometrix” (version 4.1.2) was used to map a global distribution network of publications about RNA vaccines for cancer.Citation19,Citation39,Citation40
Microsoft Office Excel 2019 was used to conduct numerous analyses of publications.
Results
Quantitative analysis of publication
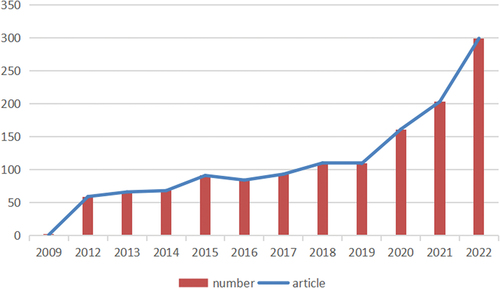
Our search strategy yielded 1399 articles comprising 803 original articles and 596 reviews. As shown in , the number of articles on cancer RNA vaccines has increased annually. There were no published articles on RNA vaccines for cancer between 1980 and 2008. From 2009 to 2019, the number of articles showed a slight upward trend. Explosive growth occurred from 2020 to 2022.
Country and institutional analysis
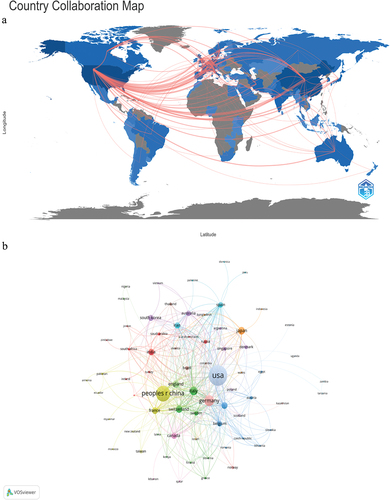
The publications were from 77 countries and 2160 institutions. The graph shows the relationship between the national publications. China and the United States were the largest contributors. Among them, the United States published 508 articles and China published 299 articles. shows the cooperation between various countries. The lines represent cooperation between countries; the thicker the lines, the closer the cooperation. shows that, although China has many publications, the only close relationship was between China and the United States. There is a lack of cooperation between China and other countries. Although the volume of publications from China is slightly lower than that of the United States, the volume of publications is still considerable.Different color present different countries.
Figure 2. Geographical distribution (a) and visualization of countries (b) on research of RNA vaccines for cancer.
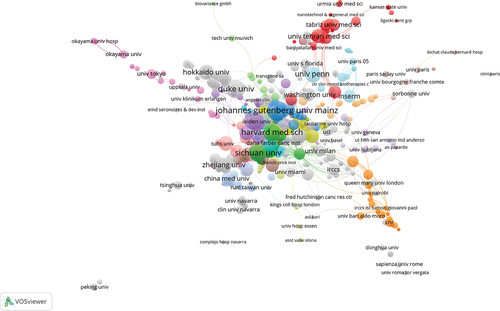
There were 2160 institutions involved. Johannes Gutenberg University Mainz (n = 28), NCI (n = 25), and Duke University (n = 24) were the primary publishers. From , it can be seen that there is a good cooperative relationship between domestic institutions. They also have cooperative relationships with foreign institutions. Different colors represents different clusters.
Authors and co-cited authors
8528 authors participated in the research on RNA vaccines for cancer. shows that most Chinese authors are closely related. This indicates that Chinese scholars have made significant research progress.
Figure 4. Visualization of authors (a) and co-cited authors (b) on research of RNA vaccines for cancer.
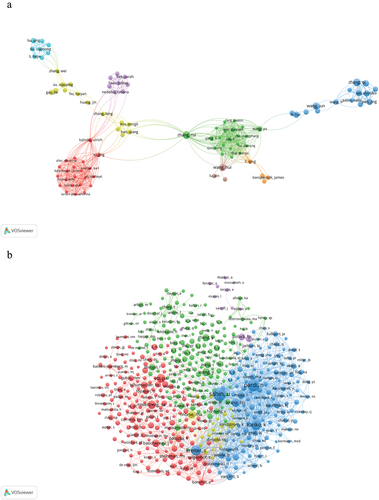
A total of 58,870 co-cited authors were identified. As shown in , there are active collaborations among different co-cited authors, such as U Sahin, N Pardi, K Kariko, et al. Different colors represents different clusters. The same color means the close relationship.
Journals
Over the past decade, 498 journals have published papers related to research on cancer RNA vaccines. The top three were the Frontiers in Immunology, Cancer, and Journal for Immunotherapy of Cancer. Frontiers in Immunology had the largest number of published articles in 56 journals, 38 candidates, and 30 journals for understanding cancer. shows the relationship between the journals. The node represents the journal and the size of the node represents the number of articles posted. Relationships between the nodes are represented by curves. The more curves a node emits, the greater its influence. To understand the relationships between journals, we created a coupling diagram for journals, as shown in . In the diagram, the left side represents the cited journal, and the right side represents the citing journal. The lines between the left and right sides represent the cited relationships. Different colors represents different journals.
Keywords
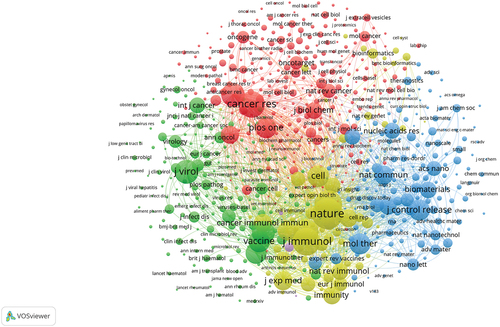
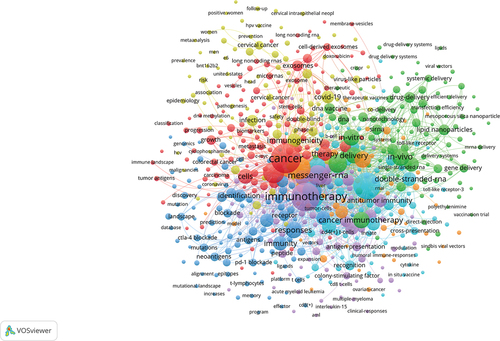
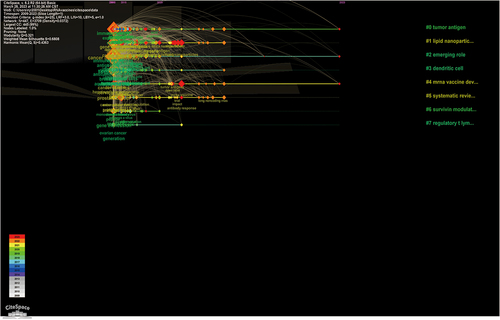
The keyword is the label of an article. The analysis of keywords can help readers quickly understand the topic of an article and grasp the research hotspots and frontiers in the field. To gain a more intuitive understanding of the research hotspots in this field, we used VOSviewer to analyze the keywords. The larger the dots, the greater the weight, as shown in . shows a timeline of the keywords. The timeline view lists documents that use keyword clustering. Each node represents a single document. Current research focuses on tumor antigens, lipid nanoparticles, emerging roles, and dendritic cells.Different colors represents different keywords.
Discussion
In recent years, since the occurrence of COVID-19, significant attention has been paid to the development of RNA vaccines, and research on RNA vaccines for cancer has attracted increasing attention. Therefore, it is necessary to conduct a phased summary and systematic analysis of research results in this field to provide guidance for scholars.Citation41–47
Bibliometrics aids researchers in efficiently and accurately mastering hot research in a field, and is widely loved by scholars. This study used Microsoft Excel 2019, CiteSpace, and VOSviewer to conduct bibliometric and visual analyses of research on the application of RNA vaccines in WoSCC. The number of publications in this field has been increasing annually, with recent rapid growth indicating that this field is receiving increasing attention, and related research is gradually maturing.
Knowledge base
In 1996, the first RNA vaccine against cancer was tested using DCs pulsed with RNA in vitro.Citation48 The RNA lipoplex vaccine is a hybrid carrier that combines an mRNA complex with a polycationic component within a lipid shell. Positively charged cationic lipids are naturally combined complexes with negatively charged mRNA and are endocytosed by antigen-presenting cells; therefore, they are often used for the construction of lipoplexes.Citation49
Frontiers and hotspots
Research on keywords can reveal trends for future research. Hot keywords included tumor antigens, lipid nanoparticles, emerging roles, and dendritic cells. These aspects can be found in the direction of the current hotspots. We found that the hot topic of RNA vaccines for cancer is tumor antigens.
Tumor antigens
RNA vaccines can increase the antigen reactiveness and induced innate immunity and adaptive immunity. RNA vaccines can also elicit human immune response without antibody-dependent enhancement(ADE) activity. RNA vaccines can be precise to activate immunityCitation50
Lipid nanoparticles(LNPs)
LNPs have been applied for the delivery of mRNA and present as the most clinical-translatable non-viral delivery vehicles. For cancer vaccine delivery, LNPs shall be planned to keep mRNA from extracellular RNase degradation and to deliver mRNA antigens to APCs. At the same time safety of vaccines is considered for cancer.
LNPs should meet the above condition.Citation51
RNA vaccines are bursting in research in cancer disease. RNA vaccine has some advantages.RNA vaccines can provide a intensified anti-tumor immunity. Compared with DNA vaccines, RNA vaccines are non-integrating, highly degradable. Compared to protein vaccines, RNA vaccines don’t have cellular and pathogenic viral components, without infectious possibilities.
Further in-depth perspectives based on the above bibliometric analysis
The importance and necessity of research in RNA vaccines for cancer can be further elucidated based on the bibliometric analysis. An ideal cancer vaccine should contain both a strong immunogenic cancer-specific or even patient-specific antigen and a chemical compound to activate cellular immunity. Therefore, one of the most important challenges in RNA vaccines is the ability to identify individual cancer neoantigens. Identifying tumor-specific mutations and predicting corresponding neoepitopes for individual HLA remains difficult. The polymorphism of the HLA system has been extensively studied in RNA vaccines for COVID-19 infection. However, the polymorphism of the HLA system has not been studied in RNA vaccines for cancer.Citation51–54 Therefore, the direction of RNA vaccines for cancer should be to promote the individualization and rapid industrialization of RNA vaccine. The relationship of RNA vaccines and HLA system should be studied. Another challenge is the instability of RNA cancer vaccines. To promote the widespread application of RNA vaccines, more strategies should be taken to improve the stability of RNA vaccine.Citation55,Citation56
Advantages and disadvantages
This study has several limitations. First, we focused on relevant research by providing bibliometrics for the first time. Second, we used three tools in tandem, which have been widely used in the field of bibliometrics to conduct surveys. Therefore, the data analysis process is likely to be objective. Finally, a bibliometric analysis provides a more comprehensive perspective. Compared to traditional reviews, we paid more attention to hotspots and frontiers in RNA vaccines.
This study has some limitations. First, the data in this study were only obtained from the WoSCC database, whereas other databases were ignored, which may have missed some relevant studies. Secondly, we searched for papers published in English and excluded non-English papers.
Conclusion
In summary, this study analyzed the current status and future development direction of cancer RNA vaccine research using a bibliometric method, which will help researchers gain a comprehensive understanding of this field, providing theoretical support and a reference basis for researchers to conduct further in-depth research.
Author contributions
XY, JK, ZX and YS wrote the manuscript. XY, JK, ZX, and YS contributed equally to this work and shared first authorship. ZL analyzed the data and drew the pictures. NL and JN reviewed and revised the manuscript. All the authors have read and approved the final version of the manuscript. All the authors contributed to the manuscript and approved the submitted version.
Acknowledgments
We thank all the authors who participated in the study on RNA vaccines for cancer.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The original contributions of this study are included in the article and supplementary material at http://www.webofscience.com/wos/woscc/basic-search. Further inquiries can be directed to the corresponding authors.
Additional information
Funding
References
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–9. doi:10.3322/caac.21763.
- Zhang S, Xia K, Chang Y, Wei Y, Xiong Y, Tang F, Peng J, Ouyang Y. LRP2 and DOCK8 are potential antigens for mRNA vaccine development in immunologically ‘cold’ KIRC tumours. Vaccines. 2023;11(2):396. doi:10.3390/vaccines11020396.
- Zaleska J, Kwasnik P, Paziewska M, Purkot J, Szabelak A, Jurek M, Masny N, Dziatkiewicz I, Pronobis-Szczylik B, Piebiak A, et al. Response to anti-SARS-CoV-2 mRNA vaccines in multiple myeloma and chronic lymphocytic leukemia patients. Int J Cancer. 2023;152(4):705–12. doi:10.1002/ijc.34209.
- Yang W, Mixich L, Boonstra E, Cabral H. Polymer-based mRNA delivery strategies for advanced therapies. Adv Healthcare Mater. 2023;12(15):e2202688. doi:10.1002/adhm.202202688.
- Yang W, Cao J, Cheng H, Chen L, Yu M, Chen Y, Cui X. Nanoformulations targeting immune cells for cancer therapy: mRNA therapeutics. Bioact Mater. 2023;23:438–70. doi:10.1016/j.bioactmat.2022.11.014.
- Yang D, Wu Y, Turan I, Keil J, Li K, Chen MH, Liu R, Wang L, Sun XL, Chen GY. Targeting intracellular Neu1 for coronavirus infection treatment. iScience. 2023;26(2):106037. doi:10.1016/j.isci.2023.106037.
- Yan F, Cowell LG, Tomkies A, Day AT. Therapeutic vaccination for HPV-mediated cancers. Curr Otorhinolaryngol Rep. 2023;11(1):44–61. doi:10.1007/s40136-023-00443-8.
- Xu H, Zhao F, Wu D, Zhang Y, Bao X, Shi F, Cai Y, Dou J. Eliciting effective tumor immunity against ovarian cancer by cancer stem cell vaccination. Biomed Pharmacother = Biomed Pharmacotherapie. 2023;161:114547. doi:10.1016/j.biopha.2023.114547.
- Xu C, Cao CH, Tan T, Deng ST, Wu BH, Yang Q, Wu MW, Wu H. mRNA vaccine-a new cancer treatment strategy. Curr Cancer Drug Targets. 2023. doi:10.2174/1568009623666230222124424.
- Youn BY, Lee SY, Cho W, Bae KR, Ko SG, Cheon C. Global trends of nutrition in cancer research: a bibliometric and visualized analysis study over the past 10 years. Int J Environ Res Public Health. 2022;19(7):4165. doi:10.3390/ijerph19074165.
- Xing J, Liu J, Han M, Jiang Y, Jiang J, Huang H. Bibliometric analysis of traditional Chinese medicine for smoking cessation. Tob Induc Dis. 2022;20:97. doi:10.18332/tid/154961.
- Wu F, Gao J, Kang J, Wang X, Niu Q, Liu J, Zhang L. Knowledge mapping of exosomes in autoimmune diseases: a bibliometric analysis (2002-2021). Front Immunol. 2022;13:939433. doi:10.3389/fimmu.2022.939433.
- Wang Z, Bai C, Hu T, Luo C, Yu H, Ma X, Liu T, Gu X. Emerging trends and hotspot in gut-lung axis research from 2011 to 2021: a bibliometrics analysis. Biomed Eng Online. 2022;21(1):27. doi:10.1186/s12938-022-00987-8.
- Wang X, Lu J, Song Z, Zhou Y, Liu T, Zhang D. From past to future: bibliometric analysis of global research productivity on nomogram (2000-2021). Front Public Health. 2022;10:997713. doi:10.3389/fpubh.2022.997713.
- Vittori A, Cascella M, Leonardi M, Monaco F, Nocerino D, Cuomo A, Ottaiano A, Perri F, Mascilini I, Francia E, et al. Vosviewer-based bibliometric network analysis for evaluating research on Juvenile Primary Fibromyalgia Syndrome (JPFS). Children (Basel, Switzerland). 2022;9(5):637. doi:10.3390/children9050637.
- Vargas JS, Livinski AA, Karagu A, Cira MK, Maina M, Lu YL, Joseph AO. A bibliometric analysis of cancer research funders and collaborators in Kenya: 2007-2017. J Cancer Policy. 2022;33:100331. doi:10.1016/j.jcpo.2022.100331.
- Sun HL, Feng Y, Zhang Q, Li JX, Wang YY, Su Z, Cheung T, Jackson T, Sha S, Xiang YT. The microbiome–gut–brain axis and dementia: a bibliometric analysis. Int J Environ Res Public Health. 2022;19(24):16549. doi:10.3390/ijerph192416549.
- Sun HL, Bai W, Li XH, Huang H, Cui XL, Cheung T, Su ZH, Yuan Z, Ng CH, Xiang YT. Schizophrenia and inflammation research: a bibliometric analysis. Front Immunol. 2022;13:907851. doi:10.3389/fimmu.2022.907851.
- Srivastava R, Srivastava S. Bibliometric analysis of Indian journal of palliative care from 1995 to 2022 using the VOSviewer and bibliometrix software. Indian J Palliat Care. 2022;28(4):338–53. doi:10.25259/ijpc_30_2022.
- Song L, Zhang J, Ma D, Fan Y, Lai R, Tian W, Zhang Z, Ju J, Xu H. A bibliometric and knowledge-map analysis of macrophage polarization in atherosclerosis from 2001 to 2021. Front Immunol. 2022;13:910444. doi:10.3389/fimmu.2022.910444.
- Liu X, Zhao S, Tan L, Tan Y, Wang Y, Ye Z, Hou C, Xu Y, Liu S, Wang G. Frontier and hot topics in electrochemiluminescence sensing technology based on CiteSpace bibliometric analysis. Biosens Bioelectron. 2022;201:113932. doi:10.1016/j.bios.2021.113932.
- Li B, Hu K, Lysenko V, Khan KY, Wang Y, Jiang Y, Guo Y. A scientometric analysis of agricultural pollution by using bibliometric software VoSViewer and Histcite™. Environ Sci Pollut Res Int. 2022;29(25):37882–93. doi:10.1007/s11356-022-18491-w.
- Leal Filho W, Ternova L, Parasnis SA, Kovaleva M, Nagy GJ. Climate change and zoonoses: a review of concepts, definitions, and bibliometrics. Int J Environ Res Public Health. 2022;19(2):893. doi:10.3390/ijerph19020893.
- He Z, Dai L, Zuo Y, Chen Y, Wang H, Zeng H. Hotspots and frontiers in pulmonary arterial hypertension research: a bibliometric and visualization analysis from 2011 to 2020. Bioengineered. 2022;13(6):14667–80. doi:10.1080/21655979.2022.2100064.
- Hadid M, Elomri A, El Mekkawy T, Kerbache L, El Omri A, El Omri H, Taha RY, Hamad AA, Al Thani MHJ. Bibliometric analysis of cancer care operations management: current status, developments, and future directions. Health Care Manag Sci. 2022;25(1):166–85. doi:10.1007/s10729-021-09585-x.
- Geng Y, Zhu R, Maimaituerxun M. Bibliometric review of carbon neutrality with CiteSpace: evolution, trends, and framework. Environ Sci Pollut Res Int. 2022;29(51):76668–86. doi:10.1007/s11356-022-23283-3.
- Cao Z, Zhang Y, Luo JH, Liao WQ, Cheng X, Zhan JH. A bibliometric analysis of publications on burn sepsis using VOSviewer. Front Med. 2022;9:971393. doi:10.3389/fmed.2022.971393.
- Barbosa MLO, Galembeck E. Mapping research on biochemistry education: A bibliometric analysis. Biochem Mol Biol Educ. 2022;50(2):201–215. doi:10.1002/bmb.21607.
- Luofei Z, Kefu Y, Bin Z, Shenghui M, Jiping H, Zhigang Z. Trends in research related to vaccine and cancer prevention from 1992 to 2022: a 30-years bibliometric analysis. Hum Vaccin Immunother. 2023;2207441. doi:10.1080/21645515.2023.2207441.
- Fu R, Xu H, Lai Y, Sun X, Zhu Z, Zang H, Wu Y. A VOSviewer-based bibliometric analysis of prescription refills. Front Med. 2022;9:856420. doi:10.3389/fmed.2022.856420.
- van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–38. doi:10.1007/s11192-009-0146-3.
- Wu N, Li M. A CiteSpace-based analysis of the development trends affecting clinical research nurses in China: a systematic review. J Multidiscip Healthc. 2022;15:2363–74. doi:10.2147/jmdh.S363741.
- Shi Y, Luo J, Wang X, Zhang Y, Zhu H, Su D, Yu W, Tian J. Emerging trends on the correlation between neurotransmitters and tumor progression in the last 20 years: a bibliometric analysis via CiteSpace. Front Oncol. 2022;12:800499. doi:10.3389/fonc.2022.800499.
- Pang XM, Peng ZY, Zheng X, Shi JJ, Zhou BC. Analysis of research hotspots in COVID-19 genomics based on citespace software: bibliometric analysis. Front Cell Infect Microbiol. 2022;12:1060031. doi:10.3389/fcimb.2022.1060031.
- Lin J, Ling F, Huang P, Chen M, Song M, Lu K, Wang W. The development of GABAergic network in depression in recent 17 years: a visual analysis based on CiteSpace and VOSviewer. Front Psychiatry. 2022;13:874137. doi:10.3389/fpsyt.2022.874137.
- Li J, Mao Y, Ouyang J, Zheng S. A review of urban microclimate research based on CiteSpace and VOSviewer analysis. Int J Environ Res Public Health. 2022;19(8):4741. doi:10.3390/ijerph19084741.
- Zhou Q, Kong HB, He BM, Zhou SY. Bibliometric analysis of bronchopulmonary dysplasia in extremely premature infants in the web of science database using CiteSpace software. Front Pediatr. 2021;9:705033. doi:10.3389/fped.2021.705033.
- Dang Q, Luo Z, Ouyang C, Wang L. First systematic review on health communication using the CiteSpace software in China: exploring its research hotspots and frontiers. Int J Environ Res Public Health. 2021;18(24):13008. doi:10.3390/ijerph182413008.
- Oyewola DO, Dada EG. Exploring machine learning: a scientometrics approach using bibliometrix and VOSviewer. SN Appl Sci. 2022;4(5):143. doi:10.1007/s42452-022-05027-7.
- Arruda H, Silva ER, Lessa M, Proença D, Jr, Bartholo R. VOSviewer and bibliometrix. J Med Libr Assoc. 2022;110(3):392–5. doi:10.5195/jmla.2022.1434.
- Krause W. mRNA—from COVID-19 treatment to cancer immunotherapy. Biomedicines. 2023;11(2):308. doi:10.3390/biomedicines11020308.
- Helfgott DC, Racine-Brzostek S, Short KJ, Zhao Z, Christos P, Nino I, Niu T, Contreras J, Ritchie EK, Desai P, et al. Immunogenicity of COVID-19 mRNA vaccines in patients with acute myeloid leukemia and myelodysplastic syndrome. Leuk Lymphoma. 2023;64(3):662–70. doi:10.1080/10428194.2022.2131414.
- Zeng C, Evans JP, Chakravarthy K, Qu P, Reisinger S, Song NJ, Rubinstein MP, Shields PG, Li Z, Liu SL. COVID-19 mRNA booster vaccines elicit strong protection against SARS-CoV-2 Omicron variant in patients with cancer. Cancer Cell. 2022;40(2):117–19. doi:10.1016/j.ccell.2021.12.014.
- Wu JT, La J, Branch-Elliman W, Huhmann LB, Han SS, Parmigiani G, Tuck DP, Brophy MT, Do NV, Lin AY, et al. Association of COVID-19 vaccination with SARS-CoV-2 infection in patients with cancer: a US nationwide veterans affairs study. JAMA Oncol. 2022;8(2):281–6. doi:10.1001/jamaoncol.2021.5771.
- Wong TY, Lee KS, Russ BP, Horspool AM, Kang J, Winters MT, Allison WM, Rader NA, Miller OA, Shiflett M, et al. Intranasal administration of BReC-CoV-2 COVID-19 vaccine protects K18-hACE2 mice against lethal SARS-CoV-2 challenge. NPJ Vaccines. 2022;7(1):36. doi:10.1038/s41541-022-00451-7.
- Šušol O, Hájková B, Zelená H, Hájek R. Third dose of COVID-19 vaccine restores immune response in patients with haematological malignancies after loss of protective antibody titres. Br J Haematol. 2022;197(3):302–5. doi:10.1111/bjh.18073.
- Su E, Fischer S, Demmer-Steingruber R, Nigg S, Güsewell S, Albrich WC, Rothermundt C, Silzle T, Kahlert CR. Humoral and cellular responses to mRNA-based COVID-19 booster vaccinations in patients with solid neoplasms under active treatment. ESMO Open. 2022;7(5):100587. doi:10.1016/j.esmoop.2022.100587.
- Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184(2):465–72. doi:10.1084/jem.184.2.465.
- Lorentzen CL, Haanen JB, Met Ö, Svane IM. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol. 2022;23(10):e450–e8. doi:10.1016/s1470-2045(22)00372-2.
- Wang Y, Zhang Z, Luo J, Han X, Wei Y, Wei X. Wei X. mRNA vaccine: a potential therapeutic strategy. Mol Cancer. 2021;20(1):33. doi:10.1186/s12943-021-01311-z.
- Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20(1):41. doi:10.1186/s12943-021-01335-5.
- Sahin U, Oehm P, Derhovanessian E, Jabulowsky RA, Vormehr M, Gold M, Maurus D, Schwarck-Kokarakis D, Kuhn AN, Omokoko T, et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585(7823):107–12. doi:10.1038/s41586-020-2537-9.
- Duan LJ, Wang Q, Zhang C, Yang DX, Zhang XY. Potentialities and challenges of mRNA vaccine in cancer immunotherapy. Front Immunol. 2022;13:923647. doi:10.3389/fimmu.2022.923647.
- Crocchiolo R, Gallina AM, Pani A, Campisi D, Cento V, Sacchi N, Miotti V, Gagliardi OM, D’Amico F, Vismara C, et al. Polymorphism of the HLA system and weak antibody response to BNT162b2 mRNA vaccine. Hla. 2022;99(3):183–91. doi:10.1111/tan.14546.
- Barbier AJ, Jiang AY, Zhang P, Wooster R, Anderson DG. The clinical progress of mRNA vaccines and immunotherapies. Nat Biotechnol. 2022;40(6):840–54. doi:10.1038/s41587-022-01294-2.
- Liu X, Zhang Y, Zhou S, Dain L, Mei L, Zhu G. Circular RNA: an emerging frontier in RNA therapeutic targets, RNA therapeutics, and mRNA vaccines. J Control Release. 2022;348:84–94. doi:10.1016/j.jconrel.2022.05.043.