ABSTRACT
In this phase 4 study we assessed boosting with fractional doses of heterologous COVID-19 vaccines in Brazilian adults primed with two doses of CoronaVac (Sinovac/Butantan, São Paulo, Brazil) at least 4 months previously. Participants received either full-dose of ChAdOx1-S (Group 1, n = 232), a half dose of ChAdOx1-S (Group 2, n = 236), or a half dose of BNT162b2 (Group 3, n = 234). The primary objective was to show 80% seroresponse rates (SRR) 28 d after vaccination measured as IgG antibodies against a prototype SARS-CoV-2 spike-protein. Safety was assessed as solicited and unsolicited adverse events. At baseline all participants were seropositive, with high IgG titers overall. SRR at Day 28 were 34.3%, 27.1% and 71.2%, respectively, not meeting the primary objective of 80%, despite robust immune responses in all three groups with geometric mean-fold rise (GMFR) in IgG titers of 3.39, 2.99 and 7.42, respectively. IgG immune responses with similar GMFR were also observed against SARS-CoV-2 variants, Alpha, Beta, Delta, Gamma and D614G. In subsets (n = 35) of participants GMFR of neutralizing immune responses against live prototype SARS-CoV-2 virus and Omicron BA.2 were similar to the IgG responses as were pseudo-neutralizing responses against SARS-CoV-2 prototype and Omicron BA.4/5 variants. All vaccinations were well tolerated with no vaccine-related serious adverse events and mainly transient mild-to-moderate local and systemic reactogenicity. Heterologous boosting with full or half doses of ChAdOx1-S or a half dose of BNT162b2 was safe and immunogenic in CoronaVac-primed adults, but seroresponse rates were limited by high baseline immunity.
Introduction
Although the numbers of cases of COVID-19 appeared to be declining steadily since 2021, new waves of infection due to Omicron variants in 2022 illustrated the potential threat of new outbreaks due to the emergence of new variants of SARS-CoV-2.Citation1 To move COVID-19 from a pandemic to an endemic disease, the global population has to have substantial protective immunity following extensive immunization campaigns and from widespread infection, giving hybrid immunity against the newest strains to emerge. However, while immunity to severe lower respiratory tract COVID-19 remains high, immunity to infection in the upper respiratory tract with successive emerging variants of the virus is limited as a result of a combination of waning antibodies,Citation2 and lower efficacy of postinfection immunity and the original vaccines against the new variants.Citation3 The latter is due to the successive accumulation of mutations in the spike protein (S-protein) of the new variants which is the main antigenic target of most vaccines.Citation4 This results in the original vaccines being less effective at preventing infection due to immune evasion by the new variants,Citation5 putting at risk frail individuals or those with significant comorbidities, just as with other respiratory viral infections in these cohorts. Ideally, new vaccines would be developed that match current and future variants, but as it is not possible to predict the next variant it is necessary to maintain a high level of immunity among the vulnerable. Current vaccines should be used to boost vaccine-induced immunity, while at the same time attempting to broaden the antibody response to minimize the impact of vaccine evasion by emerging variants.
Heterologous booster vaccination has been shown to be effective in increasing vaccine-derived immunity as well as increasing the breadth of the responses against new variants.Citation6–10 However, booster vaccination campaigns may be hampered by restricted availability and cost of COVID-19 vaccines leading to inequitable distribution of the global supply resulting in low coverage rates in many regions, including Southeast AsiaCitation11 and Africa.Citation12 Similar issues of restricted supply of other vaccines, particularly those widely used in low- and middle-income countries, have resulted in the use of fractional doses of those vaccines as booster doses; examples include inactivated poliovirus vaccine (IPV),Citation13 yellow fever vaccineCitation14 and malaria vaccine.Citation15 The present study was performed to assess the potential of two different COVID-19 vaccines, ChAdOx1-S and BNT162b2, as heterologous boosters in the Brazilian adult population following priming by two primary doses of the whole-virus inactivated COVID-19 vaccine, CoronaVac. This included the use of half doses to explore the potential of a fractional dose strategy to increase coverage with COVID-19 vaccines as heterologous booster doses in previously primed individuals.
Methods
This phase 4, single-blind, randomized, controlled trial was performed in two centers (Centro de Pesquisas Clínicas de Natal, Natal, and the Instituto Evandro Chagas, Belém) in Brazil from March 18, 2022, to July 11, 2022. The protocol was approved by the Oxford Tropical Research Ethics Committee, Oxford University, UK, and by the Brazilian National Ethical Committee. It was registered with the ISRCTN registry with reference number 47,074,508. All participants provided written informed consent. The trial was performed as a part of FRACT-COV, a platform trial approach supported by the Coalition for Epidemic Preparedness Innovations (CEPI) intended to fill in gaps in clinical research and to generate evidence to support pragmatic recommendations for COVID-19 vaccine use. The objectives were to ensure that fractional doses of heterologous COVID-19 vaccines were immunogenic and well tolerated in CoronaVac-primed adults.
Eligible participants were adults 18 y of age or older who had previously received two doses of CoronaVac (Sinovac/Butantan) COVID-19 vaccine at least 4 months (120 d) before enrollment in this study. Main inclusion criteria were willingness and ability to comply with all study requirements and willingness of female participants to practice continuous contraception with an approved method for the duration of the study. The major exclusion criteria were any indication of acute illness on the day of enrollment, e.g., axillary temperature >37.5°C, any history of laboratory-confirmed COVID-19 infection within the previous 4 weeks, any history of an SAE or allergic reaction to the previous COVID-19 vaccinations, or any condition likely to affect the immune response either due to a chronic clinical disorder or recent treatment with immunosuppressive therapies. A full list of protocol-defined exclusion criteria is included in Supplementary material. A data safety monitoring board (DSMB) was convened from independent vaccine experts to monitor any serious adverse events (SAEs) and adverse events of special interest (AESIs) reported during the trial and advise accordingly on any necessary modifications of the trial for safety reasons.
Study vaccines
The AstraZeneca/Fiocruz COVID-19 vaccine (ChAdOx1-S/nCoV-19) is a recombinant replication-defective chimpanzee adenovirus expressing a codon-optimized coding sequence for spike protein (S-protein) from the SARS-CoV-2 genome sequence accession MN908947 with a leading tissue plasminogen activator (TPA) signal sequence. ChAdOx1-S was supplied in 10 dose vials stored at +2°C to +8°C. The standard dose of ChAdOx1-S is 5 × 1010 viral particles in 0.5 mL for intramuscular administration; the fractional dose used in this study was 2.5 × 1010 viral particles in 0.25 mL.
The Pfizer/BioNTech (BNT162b2) vaccine is a lipid nanoparticle-formulated, nucleoside-modified mRNA vaccine that encodes trimerized full-length SARS-CoV-2 spike glycoprotein modified by two proline mutations to lock it in the prefusion conformation and so more closely mimic the intact virus. The vaccine RNA is formulated in lipid nanoparticles (LNPs) for more efficient delivery into cells after intramuscular injection. BNT162b2 is supplied in vials with 6 doses per vial stored at −70°C (±10°C), with 0.9% sodium chloride diluent for injection. The standard dose is 30 μg in 0.30 mL, but the fractional dose used in this study was 15 μg contained in 0.15 mL injection volume.
Procedures
After screening enrolled volunteers were randomized (1:1:1) using a randomization algorithm in REDCapCitation16 to three groups with block sizes of 3, 6 or 9 to receive either a full dose of ChAdOx1-S or half doses of either ChAdOx1-S or BNT162b2. Randomization was stratified by site and participant-reported prior COVID-19 infection status. Following a baseline blood draw, study personnel administered the respective vaccine by intramuscular injection in the deltoid of the non-dominant arm and monitored the participant for 30 minutes. Subsets of approximately half of the participants from each group were requested to provide reactogenicity data. These participants were supplied with a thermometer, ruler and 7-d paper or electronic diary card soliciting local reactions (pain, redness and swelling) and systemic adverse events (chills, headache, fatigue, arthralgia, myalgia, nausea, loss of appetite, fever ≥ 38.0°C), with severity (mild, moderate, severe, potentially life-threatening). All participants were asked to report unsolicited adverse events occurring up to 28 d after vaccination, which were defined as being related or nonrelated based on causality assessments. Any serious adverse event (SAE) or adverse events of special interest (AESI) defined as a potential immune-mediated disease, were to be reported immediately to the investigator; SAEs the investigator considered to be related to vaccination were reported to the DSMB within 24 hours of the study investigator or sponsor being made aware of their occurrence.
Immunogenicity
Sera were prepared immediately from blood drawn on Days 0 and 28 and stored at −80°C. Aliquots were transported to the Pharmaceutical Product Development Bioanalytical Laboratory (PPD, Richmond, VA, USA) to assess immune responses as IgG antibodies against the S-protein of prototype SARS-CoV-2 and the Alpha (B.1.1.7), Beta (B.1.351), Delta (B.1.617.2), Gamma (P.1) and D614G variants by multiplexed electrochemiluminescence (ECL) immunoassay expressed as arbitrary units (AU/mL).Citation8 Serum aliquots of a subset of 35 participants from each study group were also sent for measurement of neutralizing antibody concentrations against prototype SARS-CoV-2 and Omicron BA.2 variant by the UK Health Security Agency (UKHSA) in a live neutralization assay, and to Monogram Biosciences (South San Francisco, CA, USA) to run the PhenoSense SARS-CoV-2 neutralizing antibody assay for prototype SARS-CoV-2 and Omicron BA.4/5 variant.
Statistics
The primary immunogenicity objective was to test the hypothesis that a booster dose of either a full dose of ChAdOx1-S or half doses of either ChAdOx1-S or BNT162b2 would provide a seroresponse in at least 80% of participants. The seroresponse rate (SRR) was calculated as the total proportion of each group that either demonstrated a fourfold or greater increase in IgG antibody concentration from baseline at Day 28 in participants with a detectable baseline antibody titer or had detectable antibody concentrations at Day 28 in those participants without detectable antibodies at baseline. If there were at least 200 evaluable participants per arm, the lower bound of the 95% Clopper-Pearson confidence interval would be above 80% for an SRR of 86% or higher. To allow for loss to follow up, we used a conservative increase of 15% of the sample size to 700 in total.
Geometric mean concentrations (GMC) of IgG or geometric mean titers (GMT) of neutralizing antibodies and the corresponding 95% confidence interval (95% CI) were calculated by back transformation of the arithmetic mean and its 95% CIs of the log transformed concentrations/titers. Similarly, the geometric mean-fold rises (GMFR) from Day 0 to Day 28 and the corresponding 95% CI were calculated by back transformation of the arithmetic mean and its 95% CIs of the change from baseline in log-transformed concentrations/titers. The incidence and associated 95% Clopper-Pearson CI were calculated for solicited adverse events occurring during days 0 to 7 following vaccination, all unsolicited adverse events occurring in the first 28 d and SAEs and AESIs throughout the duration of the trial.
The immunogenicity analysis population included all randomized participants who received a trial vaccination and provided immunogenicity data. The safety analysis population included all randomized participants who received a trial vaccination, and those who had at least one entry in a post-vaccination diary were included in the reactogenicity analysis population. All analyses were performed using R, version 4.2.0.
Results
Demographics
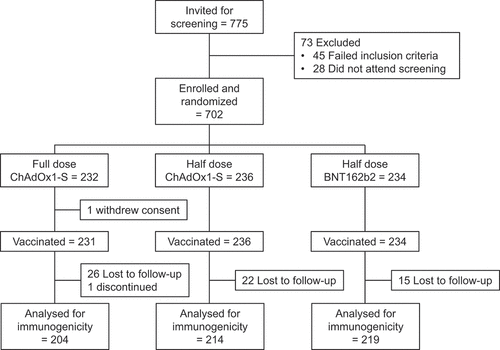
A total of 702 participants were enrolled from March 18, 2022, until July 11, 2022, and randomly assigned to the three groups, ChAdOx1-S full dose (n = 232), ChAdOx1-S half-dose (n = 236) and BNT162b2 half dose (n = 234). The demographics of the three groups were similar, with slightly more males (n = 382; 54%) than females (n = 320; 46%), a median age of 29.2 y (range 18.5 to 80.3) and an approximate equal distribution of ethnicities described as white, black, mixed, or other (). All participants had previously received two doses of CoronaVac approximately 28 d apart with the last dose approximately 6 months (median of 216 d) previously and all were seropositive at baseline, i.e., had detectable IgG antibodies against S-protein. All but one of the enrolled participants (full-dose ChAdOx1-S) received their assigned booster vaccination (), and 637 provided both Day 0 and 28 sera, although 40 (6%) of these were outside of the allowed time window of 42 d for their Day 28 sample.
Table 1. Baseline demographics of the enrolled and randomized population.
Safety and reactogenicity
All booster vaccinations were generally well tolerated, with six SAEs reported (two SAEs in each group) and only one AESI up to the data cutoff point (January 18, 2023). None of the SAEs was considered causally related to the trial vaccines (). The AESI was a case of COVID-19 with anosmia and ageusia as symptoms which resolved completely in the half-dose BNT162b2 group. Solicited local reactions were reported after 51.5% of full doses of ChAdOx1-S and at lower rates after half doses of ChAdOx1-S (38.1%) and BNT162b2 (42.1%) vaccines (). Local reactogenicity was similar in the three groups and, except for one case of moderate swelling after a half dose of ChAdOx1-S, consisted entirely of mild-to-moderate self-resolving injection site pain (). Solicited systemic AEs were reported at similar rates in all three groups (), the majority being described as mild or moderate. The most frequent systemic AEs were headache, myalgia and fatigue (). Participants reported unsolicited AEs more frequently after a half-dose of BNT162b2 vaccine (29.5%) than after full (22.9%) or half (26.7%) doses of ChAdOx1-S, but the proportions with AEs considered to be related to vaccination were low (3.4–4.3%) in all three groups. None of the infrequent severe unsolicited AEs was considered to be related to vaccination.
Figure 2. Incidences rates of participants reporting solicited local and systemic adverse events by highest severity, in days 0–7 after vaccination in the three study groups. One case of moderate swelling in the half-dose ChAdOx1 group is not shown.
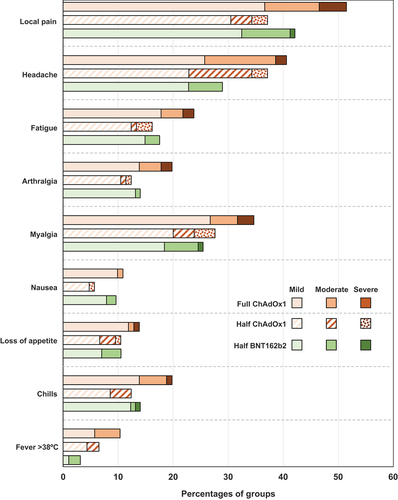
Table 2. Solicited (reactogenicity subset) and unsolicited adverse events, SAEs and AESIs (full safety set).
Primary objective
The endpoint for the primary immunogenicity objective was the seroresponse rate against prototype SARS-CoV-2 virus at Day 28. Rates were 34.3% (95% CI: 27.8, 41.3), 27.1% (21.3, 33.6) and 71.2% (64.7, 77.1) for full-dose ChAdOx1-S, half-dose ChAdOx1-S and half-dose BNT162b2, respectively (). None of the groups met the 80% SRR anticipated in the study hypothesis. However, all three groups displayed marked increases in GMCs of IgG antibodies at Day 28, achieving GMCs greater than 100,000 AU/mL (), with geometric mean-fold rises (GMFR) of 3.39, 2.99 and 7.42, respectively. As this response may have been affected by the timing between booster vaccination and the post-booster blood draw, we performed a sensitivity analysis excluding 40 participants who provided their second serum sample more than 42 d after vaccination. This had little effect on the observed seroresponse rates and GMFRs of 3.41, 3.05 and 7.73 after full-dose ChAdOx1-S, half-dose ChAdOx1-S and half-dose BNT162b2, respectively (Supplementary Table S1).
Figure 3. Geometric mean concentrations (95% CI) of anti-prototype spike IgG antibodies in the three study groups before and after vaccination. Values above columns show geometric mean-fold rises (GMFR, with 95% CI) from Day 0 to Day 28. Panel a shows all samples per group, Panel B shows segregation according to self-reported history of prior COVID-19 infection at baseline.
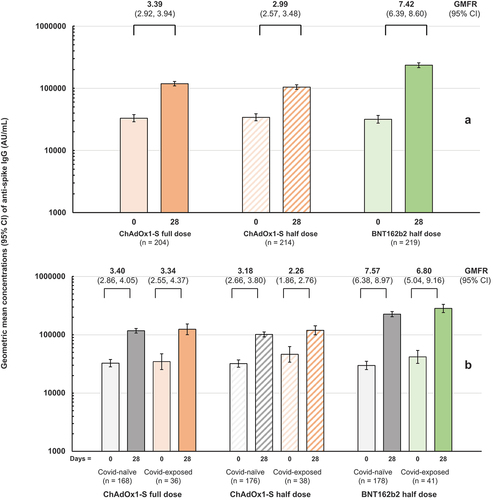
Table 3. Geometric mean concentrations (GMCs) at day 0 and 28 and geometric mean-fold rises (GMFRs) and seroresponse rates (SRRs)a for anti-spike IgG antibodies against the prototype and five variant SARS-CoV-2 viruses.
The seroresponse was measured as an increase above the pre-booster antibody concentration, which was much higher than anticipated from previous studies presumably due to both the previous vaccination with two doses of CoronaVac and enhanced hybrid immunity following several waves of natural exposure to circulating SARS-CoV-2 since the earlier measurements. To further explore this relationship, the impact of a self-reported history of COVID-19 infection on the responses was assessed. There were no meaningful differences observed in post-booster GMCs or GMFRs between those with or without a self-reported history of COVID-19 infection (, Supplementary Table S2).
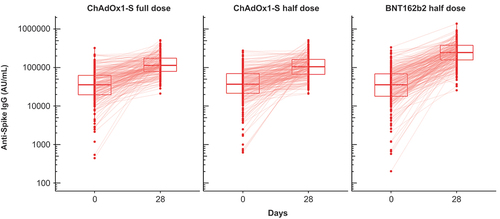
All participants had baseline antibodies against spike protein due to vaccine priming or hybrid immunity. When assessing individual responses, it appeared that in participants with high antibody concentrations before booster vaccination with full or half doses of ChAdOx1-S vaccine there was no or little increase in concentration post-booster – evident as horizontal lines between Days 0 and 28 in – while those with low pre-booster concentrations displayed marked increases. The effect was less evident in those who received a half dose of BNT162b2, although some individuals in that group did not show an increase.
Figure 4. Anti-spike IgG concentrations in the three study groups at Day 0 and 28, with lines between the two samples for each individual participant. Box plots represent median and 25th and 75th percentiles.
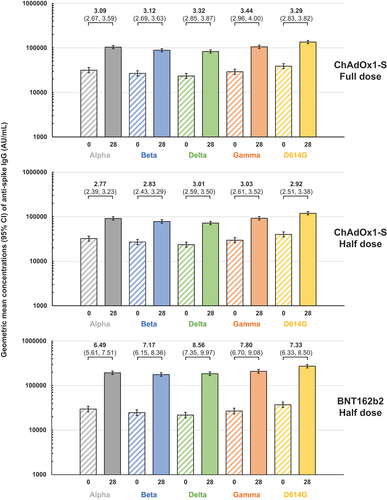
These assessments of immune response were made using the prototype SARS-CoV-2 virus which was the model virus on which the two different vaccines were designed. However, the current need is for booster vaccination to enhance immunity against the new SARS-CoV-2 variants, Alpha, Beta, Delta, Gamma, etc., which have emerged since the beginning of the pandemic and successively replaced the prototype virus with Omicron variants currently predominating in circulation. As each new variant appears to accumulate new mutations, principally in the S-protein, the immune response has been found to become less effective against these variants. In this study cohort of CoronaVac-primed adults, there was evidence of persisting cross-immunity at baseline against each of the five variant SARS-CoV-2 viruses tested (). Following a full dose of ChAdOx1-S as booster, there were increases in IgG antibodies against each of the variants, with GMFR greater than 3 in all cases. A half dose of ChAdOx1-S elicited similar responses, with GMFR ranging from 2.77 to 3.03 representing a marked increase in the immune response. The half dose of BNT162b2 elicited a more robust response against all five variants, with GMFR ranging from 6.49 to 8.56 and a higher range of GMCs at Day 28 than either booster dose of ChAdOx1-S.
Figure 5. Geometric mean concentrations (95% CI) of anti-spike IgG antibodies for the indicated SARS-CoV-2 variants in the three study groups before and after vaccination. Values above columns show geometric mean-fold rises (GMFR) with 95% CI) from Day 0 to Day 28.
On evaluation of individual responses against the five variants, as with those against the prototype SARS-CoV-2 virus, it was apparent that participants with high IgG concentrations at baseline had lower incremental responses to ChAdOx1-S boosters than those who had low baseline concentrations, but in the BNT162b2 group the trend to an increase in concentration after the booster was present in those with low or high concentrations at baseline (Supplementary figure).
Small subsets (35 per groups) of each group were also tested for antibodies against live prototype and Omicron SARS-CoV-2 viruses in neutralizing and pseudoneutralizing assays (), the results of which confirmed the observations of the IgG assays. Geometric mean-fold rises of neutralizing activity against prototype virus were 4.18 and 3.22 after full- and half-dose ChAdOx1-S boosters and 6.71 after the half-dose of BNT162b2. In the pseudo-neutralization assay, these factors were 4.13, 2.58 and 4.81, respectively. When assayed against the Omicron variant, baseline neutralizing titers were approximately ten-fold lower but rises after the booster doses were comparable in magnitude to those observed against prototype virus, so final titers were still lower. Baseline titers against Omicron in the pseudo-neutralization assay were about four-fold lower than against prototype and increased after the various boosters were similar in magnitude to those against prototype.
Table 4. Geometric mean neutralizing antibody titers (95% CI) at Days 0 and 28, with geometric mean fold rises (GMFR) in the three study groups measured by live neutralization and pseudo-neutralization assays with the prototype and Omicron variant SARS-CoV-2 viruses.
Discussion
The primary hypothesis of this study was that a full dose of ChAdOx1-S or fractional booster doses of ChAdOx1-S or BNT162b2 vaccines in CoronaVac-primed adults would elicit seroresponse rates (SRR) of 80%, a similar response to that observed in Brazilian adults who received a third dose of CoronaVac.Citation8 In this study, baseline antibody levels were high and none of the study groups achieved this level of response, with SRR of 27.1% and 34.3% for half- and full-doses of ChAdOx1-S and 71.2% for half-dose BNT162b2. However, all three groups had marked increases in IgG GMCs with GMFR of approximately 3 in the ChAdOx1-S groups and 7 in the BNT162b2 groups. Further, similar GMFRs were observed against the five SARS-CoV-2 variants tested suggesting that there was broad cross-reactive immunity induced against the different variants.
The lack of an anticipated 80% seroresponse rate was probably due to the high baseline concentrations of neutralizing activity which reflects the current real-world situation of COVID-19 circulation: high background levels of population immunity due to extensive immunization coverage and increasing levels of hybrid immunity following natural exposure to circulating SARS-CoV-2 viruses. The anticipated 80% seroresponse rate was based on previously observed responses to homologous and heterologous booster doses in CoronaVac-primed Brazilian adults measured in the same laboratory as in the present study.Citation8 In that study, done in August 2021 prior to the most recent two waves of COVID-19 in Brazil in January and June 2022,Citation17 the participants had low antibody concentrations at baseline with anti-spike IgG GMCs ranging from 3745 to 4433 AU/mL across the four study groups. In our study, despite a 6-month interval since their last vaccination, all participants were seropositive at baseline as illustrated in , with anti-spike IgG GMCs above 31,000 AU/mL (range 31,685 to 34,111 AU/mL) in each of the three study groups. This is approaching the GMC of 48,405 AU/mL achieved after a homologous CoronaVac booster in the earlier study.Citation8 This high pre-booster immunity makes it more difficult to achieve the four-fold increase required to meet the protocol definition of seroresponse. Those with low baseline titers responded well to the booster vaccination, but those with high titers responded less well. Although we attempted to eliminate hybrid immunity by removing those with documented COVID-19 infections in a sensitivity analysis, this had no effect on the results, suggesting that high rates of undocumented infection had already occurred in the population. In a population specifically selected to have received a full primary series of two doses of CoronaVac many infections may have been asymptomatic and would have naturally boosted the primed immune background, resulting in the high level of baseline immunity. It was notable that baseline GMCs in those with or without a history of COVID-19 infection were similar, indicating that selection based on reported infection failed to really isolate those with previous infection.
In a similar study to ours, Fadlyana et al. found that both the ChAdOx1-S and BNT162b2 vaccines used in the present study were able to induce robust booster responses when administered as full or half doses to CoronaVac-primed adults in Indonesia.Citation18 They observed lower responses in those who had been primed less than 6 months previously when compared with those primed 6–9 months previously, due to high baseline levels. Furthermore, they noted that “boosting appears to bring titers up to a certain level, irrespective of their baseline starting point.” This correlates with our observations of little or no response in those with high baseline antibody concentrations that were already at this “certain level” and could explain why the final GMCs in the different study groups were similar. As our population was primed only 6 months before receiving the booster doses, the baseline antibody concentrations were probably too high to make an 80% response rate biologically possible with these products.
Nonetheless, all groups achieved high levels of IgG antibodies (GMCs of 118,348, 104465 and 235,935 AU/mL in full-dose ChAdOx1-S, half-dose ChAdOx1-S and half-dose BNT162b2 groups, respectively) suggesting that the boosters would have conferred additional protection against COVID-19 infection. Although these values were lower than those achieved after heterologous boosting with full doses of ChAdOx1-S (335213 AU/mL) and BNT162b2 (674267 AU/mL) vaccines in the earlier study, they are higher than the 48,405 AU/mL achieved by a homologous CoronaVac booster in that study.Citation8 What this means in terms of protection against COVID-19 illness is unclear. The clinical efficacy of vaccination with current vaccines, and particularly the ChAdOx1-S and BNT162b2 vaccines used in the present study, was established at a time when tested populations were immunologically naive to SARS-CoV-2, not having been previously vaccinated or exposed to virus, especially not to the newly emerged variants which now predominate in global circulation.Citation19,Citation20 The Omicron variant has been shown to partly evade the neutralizing activity elicited by BNT162b2,Citation3 so in the absence of an accepted serologic correlation of protection for these vaccines, we cannot assume that the concentrations achieved will be protective, but it is noteworthy that the booster responses in all three groups were similar against all five variants tested. The nature of the responses themselves may also be different from those assessed in the original efficacy trials due to the natural exposure component contributing to the hybrid immunity observed.
Other studies have demonstrated variable responses to fractional doses of a variety of vaccines as primary immunizations; they may lead to inferior efficacy compared with the full dosesCitation21 or provide similar immune responses.Citation22 However, low doses of heterologous vaccines as booster doses generally lead to non-inferior responses compared with homologous full doses,Citation23–26 including half doses administered intradermally.Citation27–29
As alluded to above, the limitations of our study were the short interval of 6 months between the last priming dose and the booster, during which there were at least two surges of infections in Brazil probably resulting in high levels of circulating SARS-CoV-2 variants and so asymptomatic infections and natural boosting. Both factors would lead to high levels of antibodies at baseline which would limit the capacity to observe the anticipated 80% seroresponse rate against this background. However, the levels of antibodies achieved with three-fold increases in GMCs after boosting with half doses of ChAdOx1-S and BNT162b2 vaccines suggest that both would have increased immunity in the participants such that they would have more protection against new variants. Further, the hybrid immunity due to the vaccine boosters and natural exposure is likely to provide several months of protection against new variants.Citation30 However, we have only measured the immediate response to these fractional booster doses, and their effectiveness must also be monitored over the longer term with follow-up monitoring to allow assessment of waning of the induced antibodies and the cross-reactivity with any newly emerging variants in the future.
In conclusion, despite eliciting marked increases in IgG antibody concentrations against SARS-SoV-2 spike protein fractional (half) doses of ChAdOx1-S and BNT162b2 vaccines did not induce an 80% seroresponses in a population of adults primed with two doses of the inactivated whole virus COVID-19 vaccine, CoronaVac. This was likely due to high baseline levels of hybrid immunity resulting from the combination of the primary vaccination series and natural exposure to circulating SARS-SoV-2 virus. Nonetheless, the responses achieved suggest that protection would be extended by fractional booster doses of heterologous vaccines.
Data sharing
The datasets, including the redacted study protocol, redacted statistical analysis plan and individual participants data supporting the results reported in this article, will be made available to researchers who provide a methodologically sound research proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection and requirements for consent and anonymization.
Supplemental Material
Download MS Word (2.2 MB)Acknowledgments
We would like to thank all the study participants and study site staff, Oxford Vaccine Group staff, Intrials Clinical Research and CEPI for grant. We are grateful to Dr Keith Veitch (keithveitch communications, Amsterdam, the Netherlands) for assisting Professor Costa Clemens in drafting the manuscript.
Disclosure statement
A.J.P. is Chair of the UK Department of Health and Social Care’s (DHSC) Joint Committee on Vaccination & Immunisation (JCVI) but does not participate in the JCVI COVID-19 committee and was a member of the WHO SAGE until 2022. The views expressed in this article do not necessarily represent the views of DHSC, JCVI, or WHO. The University of Oxford has entered into a partnership with AstraZeneca on coronavirus vaccine development.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2233400
Additional information
Funding
References
- Kirby T. New variant of SARS-CoV-2 in UK causes surge of COVID-19. Lancet Respir Med. 2021;9(2):e20–12. doi:10.1016/S2213-2600(21)00005-9.
- Taylor L. Covid-19: Omicron drives weekly record high in global infections. BMJ. 2022;376:o66. doi:10.1136/bmj.o66.
- Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, San JE, Cromer D, Scheepers C, Amoako DG, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602(7898):654–6. doi:10.1038/s41586-021-04387-1.
- Liu L, Iketani S, Guo Y, Chan JFW, Wang M, Liu L, Luo Y, Chu H, Huang Y, Nair MS, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602(7898):676–81. doi:10.1038/s41586-021-04388-0.
- Planas D, Saunders N, Maes P, Guivel-Benhassine F, Planchais C, Buchrieser J, Bolland W-H, Porrot F, Staropoli I, Lemoine F, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602(7898):671–5. doi:10.1038/s41586-021-04389-z.
- Behrens GMN, Barros-Martins J, Cossmann A, Ramos GM, Stankov MV, Odak I, Dopfer-Jablonka A, Hetzel L, Köhler M, Patzer G, et al. BNT162b2-boosted immune responses six months after heterologous or homologous ChAdOx1nCoV-19/BNT162b2 vaccination against COVID-19. Nat Commun. 2022;13(1):4872. doi:10.1038/s41467-022-32527-2.
- Low EV, Tok PSK, Husin M, Suah JL, Tng BH, Thevananthan T, Appannan MR, Yahaya H, Mohd Zin S, Muhamad Zin F, et al. Assessment of heterologous and homologous boosting with inactivated COVID-19 vaccine at 3 months compared with homologous boosting of BNT162b2 at 6 months. JAMA Netw Open. 2022;5(8):e2226046. doi:10.1001/jamanetworkopen.2022.26046.
- Costa Clemens SA, Weckx L, Clemens R, Almeida Mendes AV, Ramos Souza A, Silveira MBV, da Guarda SNF, de Nobrega MM, de Moraes Pinto MI, Gonzalez IGS, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399(10324):521–9. doi:10.1016/S0140-6736(22)00094-0.
- Atmar RL, Lyke KE, Deming ME, Jackson LA, Branche AR, El Sahly HM, Rostad CA, Martin JM, Johnston C, Rupp RE, et al. Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386(11):1046–57. doi:10.1056/NEJMoa2116414.
- Chen C-J, Yang L-Y, Chang W-Y, Huang Y-C, Chiu C-H, Shih S-R, Huang C-G, Huang KYA. A randomized controlled trial of heterologous ChAdOx1 nCoV-19 and recombinant subunit vaccine MVC-COV1901 against COVID-19. Nat Commun. 2022;13(1):5466. doi:10.1038/s41467-022-33146-7.
- Chu DT, Vu Ngoc SM, Vu Thi H, Nguyen Thi Y-V, Ho T-T, Hoang V-T, Singh V, Al-Tawfiq JA. COVID-19 in Southeast Asia: current status and perspectives. Bioengineered. 2022;13(2):3797–809. doi:10.1080/21655979.2022.2031417.
- Lawal L, Aminu Bello M, Murwira T, Avoka C, Yusuf Ma’aruf S, Harrison Omonhinmin I, Maluleke P, Tsagkaris C, Onyeaka H. Low coverage of COVID-19 vaccines in Africa: current evidence and the way forward. Hum Vaccines Immunother. 2022;18(1):2034457. doi:10.1080/21645515.2022.2034457.
- Mashunye TR, Ndwandwe DE, Dube KR, Shey M, Shelton M, Wiysonge CS. Fractional dose compared with standard dose inactivated poliovirus vaccine in children: a systematic review and meta-analysis. Lancet Infect Dis. 2021;21(8):1161–74. doi:10.1016/S1473-3099(20)30693-9.
- Nnaji CA, Shey MS, Adetokunboh OO, Wiysonge CS. Immunogenicity and safety of fractional dose yellow fever vaccination: A systematic review and meta-analysis. Vaccine. 2020;38(6):1291–301. doi:10.1016/j.vaccine.2019.12.018.
- Moon JE, Greenleaf ME, Regules JA, Debois M, Duncan EH, Sedegah M, Chuang I, Lee CK, Sikaffy AK, Garver LS, et al. A phase IIA extension study evaluating the effect of booster vaccination with a fractional dose of RTS,S/AS01E in a controlled human malaria infection challenge. Vaccine. 2021;39(43):6398–406. doi:10.1016/j.vaccine.2021.09.024.
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi:10.1016/j.jbi.2019.103208.
- WHO Health Emergency Dashboard. Brazil situation. [accessed 2023 Feb 13]. https://covid19.who.int/region/amro/country/br.
- Fadlyana E, Setiabudi D, Kartasasmita CB, Putri ND, Rezeki Hadinegoro S, Mulholland K. Immunogenicity and safety in healthy adults of full dose versus half doses of COVID-19 vaccine (ChAdox1-S or BNT162b2) or full-dose CoronaVac administered as a booster dose after priming with CoronaVac: a randomised, observer-masked, controlled trial in Indonesia. Lancet Infect Dis. 2023;23(5):S1473-3099(22)00800–3. doi:10.1016/S1473-3099(22)00800-3.
- Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi:10.1016/S0140-6736(20)32661-1.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. doi:10.1056/NEJMoa2035389.
- WieRceka W, Ahujab A, Chaudhuria E, Kremer M, Simoes Gomes A, Snyder CM, Tabarrok A, Tan BJ. Testing fractional doses of COVID-19 vaccines. PNAS. 2022;119(8):e2116932119. doi:10.1073/pnas.2116932119.
- Valim V, Martins-Filho OA, Gouvea MPG, Camacho LAB, Villela DAM, de Lima SMB, Azevedo AS, Neto LFP, Domingues CMAS, de Medeiros Junior NF, et al. Effectiveness, safety, and immunogenicity of half dose ChAdOx1 nCoV-19 COVID-19 vaccine: Viana project. Front Immunol. 2022;13:966416. doi:10.3389/fimmu.2022.966416.
- Deng J, Ma Y, Liu Q, Du M, Liu M, Liu J. Comparison of the effectiveness and safety of heterologous booster doses with homologous booster doses for SARS-CoV-2 vaccines: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19(17):10752. doi:10.3390/ijerph191710752.
- Tan CS, Collier AY, Yu J, Liu J, Chandrashekar A, McMahan K, Jacob-Dolan C, He X, Roy V, Hauser BM, et al. Durability of heterologous and homologous COVID-19 vaccine boosts. JAMA Netw Open. 2022;5(8):e2226335. doi:10.1001/jamanetworkopen.2022.26335.
- Wang X, Zhao X, Song J, Wu J, Zhu Y, Li M, Cui Y, Chen Y, Yang L, Liu J, et al. Homologous or heterologous booster of inactivated vaccine reduces SARS-CoV-2 Omicron variant escape from neutralizing antibodies. Emerg Microb Infect. 2022;11(1):477–81. doi:10.1080/22221751.2022.2030200.
- Nanthapisal S, Puthanakit T, Jaru-Ampornpan P, Nantanee R, Sodsai P, Himananto O, Sophonphan J, Suchartlikitwong P, Hiransuthikul N, Angkasekwinai P, et al. A randomized clinical trial of a booster dose with low versus standard dose of AZD1222 in adult after 2 doses of inactivated vaccines. Vaccine. 2022;40(18):2551–60. doi:10.1016/j.vaccine.2022.03.036.
- Intapiboon P, Seepathomnarong P, Ongarj J, Surasombatpattana S, Uppanisakorn S, Mahasirimongkol S, Sawaengdee W, Phumiamorn S, Sapsutthipas S, Sangsupawanich P, et al. Immunogenicity and safety of an intradermal BNT162b2 mRNA vaccine booster after two doses of inactivated SARS-CoV-2 vaccine in healthy population. Vaccines (Basel). 2021;9(12):1375. doi:10.3390/vaccines9121375.
- Tawinprai K, Siripongboonsitti T, Porntharukchareon T, Wittayasak K, Thonwirak N, Soonklang K, Sornsamdang G, Auewarakul C, Mahanonda N. Immunogenicity and safety of an intradermal fractional third dose of ChAdOx1 nCoV-19/AZD1222 vaccine compared with those of a standard intramuscular third dose in volunteers who previously received two doses of CoronaVac: a randomized controlled trial. Vaccine. 2022;40(12):1761–7. doi:10.1016/j.vaccine.2022.02.019.
- Niyomnaitham S, Chatsiricharoenkul S, Toh ZQ, Senawong S, Pheerapanyawaranun C, Phumiamorn S, Licciardi PV, Chokephaibulkit K. Evaluation of the safety and immunogenicity of fractional intradermal COVID-19 vaccines as a booster: a pilot study. Vaccines. 2022;10(9):1497. doi:10.3390/vaccines10091497.
- Malato J, Ribieiro RM, Fernandes E, Leite PP, Casaca P, Antunes C, Fonseca VR, Gomes MC, Graca L. Stability of hybrid versus vaccine immunity against BA.5 infection over 8 months. Lancet Infect Dis. 2023;23(2):148–50. doi:10.1016/S1473-3099(22)00833-7.