 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The intranasal spray COVID-19 vaccine was made available for the first time in China, it is necessary to understand receivers’ satisfaction and experience toward the vaccine to help optimize vaccination service. A self-administered multicenter cross-sectional questionnaire survey was conducted in Beijing, China, in December 2022. The vaccination experience was evaluated through three dimensions: immediate tolerance, smooth progress, and time-saving. Vaccine acceptability was measured by receivers’ preference for the intranasal spray over intramuscular injection after vaccination and their recommendation willingness. Stepwise multinomial and binary logistic regression models were applied to investigate factors associated with vaccine acceptability. Among 10,452 participants included in the analysis, 92.6% felt no discomfort during the inoculation, 99.8% thought the vaccination process went well, and 89.4% deemed it a time-saving option. For vaccine acceptability, 5566 (53.3%) participants were willing to recommend the vaccine to others, 534 (5.1%) refused, and 4352 (41.6%) had not decided yet; 6142 (58.8%) participants preferred the intranasal spray, 873 (8.4%) preferred the intramuscular injection, and 3437 (32.9%) had no preferences. The most concerned aspects of the intranasal spray vaccine were vaccine effectiveness and safety. Receivers who perceived higher vaccine effectiveness or safety were more likely to recommend it to others (OR, 95%CI: 4.41, 3.24–6.00; 6.11, 4.52–8.27) or prefer it over intramuscular injection after vaccination (OR, 95%CI: 5.94, 4.62–7.65; 8.50, 6.70–10.78). Receivers showed good acceptability and experience toward the intranasal spray COVID-19 vaccine. Vaccine effectiveness and safety were the most concerned aspects, and corresponding publicity and education efforts may help improve vaccine acceptability.
Introduction
The coronavirus disease (COVID-19) pandemic has posed a great burden on human health worldwide. The rapid evolution of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the high transmissibility of new variants continuously give rise to new waves of infection.Citation1 Omicron, the fifth “Variant of Concern (VOC),” was found to have a higher reinfection risk than the Delta variant.Citation2 It indicated that the protective effect of existing COVID-19 vaccines or previous SARS-CoV-2 infections may have diminished because of the enhanced immune escape mechanism of the Omicron variant.Citation3 To improve and prolong protective immunity against the disease, several countries have launched second booster COVID-19 vaccination campaigns.Citation4–6 Sequential booster immunization, a vaccination strategy that uses a booster dose of a heterogeneous type of vaccine, has been recommended for its enhanced protection against COVID-19 infection.Citation4,Citation7
A vaccine platform is the underlying technology that can be used to develop vaccines. There are five types of COVID-19 vaccine platforms: the whole virus, viral vector, protein subunit, nucleic acid, and virus-like particles vaccines.Citation8 In China, multiple roadmaps are adopted to develop COVID-19 vaccines, and 15 vaccines (5 inactivated vaccines, 6 recombinant subunit vaccines, 3 viral vector vaccines, and 1 mRNA vaccine) have been conditionally approved for marketing or emergency use.Citation9,Citation10 Studies have indicated that mucosal vaccines can mimic the respiratory tract infection of SARS-CoV-2 and activate mucosal and local immunity,Citation11 which may protect against Omicron breakthrough infection.Citation12,Citation13 Up to date, five COVID-19 mucosal vaccines in India, Russia, and Iran, and two in China have been approved for use,Citation14,Citation15 including the orally inhaled vaccine developed by CanSino Biologics,Citation16 Influenza Virus Vector COVID-19 Vaccine for Intranasal Spray co-developed by Hong Kong University, Xiamen University, and Wantai Biopharmaceutical CompanyCitation17 in China.
Influenza Virus Vector COVID-19 Vaccine for Intranasal Spray was constructed using the attenuated influenza virus vector by inserting a gene encoding the receptor-binding domain (RBD) of the spike protein of SARS-CoV-2, which can also provide cross-protection against H1N1 and H5N1 influenza viruses.Citation17 Analysis of interim primary data from its phase 3 clinical trial shows that the vaccine offers good protection against Omicron variant strains BA.2, BA.4, and BA.5 (82%) and is well tolerated in adults.Citation18 The phase III clinical trial also shows that this vaccine is well-tolerated and can be an antibody-independent broad-spectrum effective tool to combat SARS-CoV-2 variants.Citation19,Citation20 This vaccine is applicable for people 18 years of age and older, including those who have not been immunized against any COVID-19 vaccine, and those who have received one, two, or three previous doses of other COVID-19 vaccines according to the vaccine instruction.
At the beginning of the Omicron wave in China, the second COVID-19 booster campaign was rolled out on December 14, 2022. Population groups with a high risk of infection, people aged 60 and above, people with serious underlying health conditions, and those with low immunity are advised to receive a second booster six months after the first one. Sequential booster immunization was prioritized. For example, those who have received three doses of inactivated vaccines were recommended for a different type of vaccine for the second booster,Citation21 namely a recombinant protein vaccine, an adenovirus vector vaccine, or an influenza virus vector vaccine. Influenza Virus Vector COVID-19 Vaccine for Intranasal Spray was one alternative for the second booster.Citation22
With the rapid evolution of the SARS-CoV-2 and the waning of the protective effect of previous vaccination, mucosal immunity may become a new trend for preventing infection. The intranasal spray COVID-19 vaccine was made available for the first time in Beijing, China,Citation23 and it is necessary to understand receivers’ satisfaction and experience toward the vaccine to help optimize vaccination service and promote vaccination. Therefore, we conducted a survey in Beijing as the vaccination campaign started, to investigate the acceptability of the intranasal spray COVID-19 vaccine and its associated factors among residents who have received this vaccine.
Materials and methods
Study design and participants
To investigate the receivers’ satisfaction and experience of the intranasal spray COVID-19 vaccine, a cross-sectional survey was jointly designed and conducted by the Beijing Center for Disease Prevention and Control and the School of Population Medicine and Public Health of the Chinese Academy of Medical Science & Peking Union Medical College in Beijing, China. The questionnaire was designed using the online survey platform “wjx” (https://www.wjx.cn). All the vaccination points in four districts (Chaoyang District, Haidian District, Changping District, and Huairou District) were included in this survey, covering urban, suburban, and outer suburban areas in Beijing. The survey started when the implementation plan for the second booster against COVID-19 was released on December 13, 2022. Data collection finished on December 31, 2022. Residents who have finished three doses of the whole-virion inactivated vaccine and came to receive the intranasal spray were invited to participate in the survey. Notably, participants without a recent SARS-CoV-2 infection history were enrolled according to the vaccination guideline that the next vaccine dose is recommended after 6 months of infection during the study period.Citation24,Citation25 In addition, vaccination staff routinely checked participants’ infection history before giving a vaccination. Participants with a SARS-CoV-2 infection history were those who got infected six months ago. The participants scanned the questionnaire’s quick response (QR) code to participate in this survey during their 30-minute post-vaccination observation period. Informed consent was obtained from each participant online before they completed the questionnaire. The sample size was calculated using the following formula with α = 0.05 and a permissible error of 0.01: . Assuming the acceptability of the intranasal spray COVID-19 will be 50%, a sample size of 9604 was determined. To account for attrition, the sample size was further increased by 10%. Therefore, the final sample size was 10,565.
Those who were aged ≥18 years, agreed to participate in the survey, had finished three doses of the whole-virion inactivated vaccine, and received an intranasal spray booster at one of the survey sites were included in this study. Participants included were all willing to receive the intranasal spray and provided written consent. If someone was unwilling to receive this vaccine, they can choose other types of vaccines later. Those who did not provide written consent, aged younger than 18 years, and whose IP address on the answer sheet was not located in Beijing city were excluded.
Data collection
The questionnaire collected information about sociodemographic characteristics, vaccination experience (immediate tolerance, smooth process, and time-saving), attitudes toward COVID-19 and the intranasal vaccine (perceived vaccine effectiveness and safety, whether prefer the intranasal spray over intramuscular injection after vaccination, and recommendation willingness, etc.), consideration of vaccine administration routes (favorable vaccine characteristics and concerns about the intranasal spray vaccine), social support (whether got family support to get the vaccine), and desired vaccine knowledge from publicity. To make our analysis clearer, the above information was grouped into “Sociodemographic information,” “Thinking and feeling,” “Social process,” “Practical issues,” and “Acceptability” by adapting the WHO’s behavioral and social drivers (BeSD) of vaccination framework.Citation26 In our study, whether receivers prefer the intranasal spray over the intramuscular injection after vaccination and their recommendation willingness were used as proxy indicators of vaccine acceptability or satisfaction.
Statistical analysis
The sociodemographic characteristics, vaccination experience, and vaccine acceptability of participants were analyzed. Continuous and categorical variables were presented as medians (interquartile ranges) and counts (percentages), respectively. The characteristics of participants grouped by vaccine acceptability were described, and chi-squared tests were applied to examine the differences among the groups. Bonferroni correction was applied for multiple comparisons, and the significance level was adjusted. Circle-track figuresCitation27 were drawn to describe attitudes and concerns about this vaccine in participants with different vaccine preferences. Venn diagram and the alluvial plot were drawn to show the overlap between the preference and the recommendation willingness regarding the route of vaccination. The health conditions of the participants with different immediate tolerance during vaccination were also described by Circle-track figures (Figure S1). All individuals have no missing data in this survey.
To investigate factors associated with vaccine acceptability, stepwise multinomial and binary logistic regression models were implemented. For preferences of administration routes, preferences of intranasal spray were compared to preferences of intramuscular injection, no preferences, and the combination of the two latter groups separately. For recommendation willingness, being willing to recommend the vaccine to others was compared to refusal, undecided, and the combination of the latter two groups separately. The independent variables included sociodemographic information, attitudes toward COVID-19 and the intranasal vaccine, vaccination experience, consideration of vaccine administration routes, and social support (). Variables were automatically selected by a stepwise algorithm in logistic regression models. After variable selection, logistic regression models with the selected variables were rebuilt, and the odds ratios (95% confidence intervals) were calculated.
Table 1. Sociodemographic characteristics of participants.
Table 2. Vaccination experience and acceptability of the intranasal spray COVID-19 vaccine.
Table 3. Factors associated with the preference of vaccine administration routes by stepwise multinomial and binary logistic regression analysis.
SAS (version 9.4; SAS Institute Inc., Cary, NC, USA) and RStudio (version 2022.2.1.461; RStudio Team, 2022) were used to perform data analysis. The stacked-column chart, circle-track figures, Venn diagram, and alluvial plot were drawn with R packages of “ggplot2,” “jjPlot,” “eulerr,” and “ggalluvial” respectively. All statistical tests were two-sided, and a p-value of .05 indicated statistical significance.
Results
Sociodemographic characteristics of the participants
In total, 10648 questionnaires were collected. After excluding those who did not provide the written consent form (n = 80), whose IP address was not located in Beijing city (n = 103), and who were younger than 18 years old (n = 13), 10452 participants were included in the final analysis. The median (interquartile range) age of the participants was 41.0 (34.0–49.0) years, with females accounting for 45.9%. Approximately half of the participants (48.7%) had an education level of high school and below, and 19.2% were healthcare workers. The vast majority of participants (85.0%) reported that they had no underlying diseases; 270 (2.6%) and 48 (0.5%) reported having sinusitis and asthma, respectively. More than 99% of the participants had no history of SARS-CoV-2 infection (). The sociodemographic characteristics of the participants with different administration route preferences are shown in Table S1.
Vaccination experience and acceptability of the intranasal spray COVID-19 vaccine
In our study, vaccination experience was measured by immediate tolerance, a smooth process, and time-saving. A total of 9673 (92.55%) participants felt no discomfort during vaccination, while 746 (7.14%) and 33 (0.32%) reported slight discomfort and strong discomfort respectively (). Compared with those who felt no discomfort during inoculation, the proportions of participants with asthma (1.0% VS 0.4%), sinusitis (5.9% vs 2.3%), coronary heart disease or cerebrovascular disease (2.2% VS 1.2%), and hypertension (14.8 VS 7.5%) were larger in those who felt discomfort (Table S3, Figure S1). Nearly all participants (99.75%) said that the vaccination process went well, and the vast majority of participants (89.41%) thought intranasal spray was more time-saving than intramuscular injection (). The vaccination experience among participants with different vaccine acceptability (whether prefer the intranasal spray over intramuscular injection after vaccination) was also shown. (Table S2).
The vaccine acceptability was indicated by whether receivers preferred the intranasal spray over intramuscular injection after vaccination and their recommendation willingness. For preferences of vaccine administration routes, 6142 (58.8%) participants preferred the intranasal spray, 873 (8.4%) preferred the intramuscular injection, and 3437 (32.9%) had no preference between the two administration routes. For recommendation willingness, 5566 (53.3%) receivers showed a willingness to recommend the intranasal spray vaccine to others, 534 (5.1%) refused, and 4352 (41.6%) had not decided yet (). To visualize the overlap between the preference and willingness regarding the route of vaccination, the Venn diagram and the alluvial plot were also drawn (Figures S2 and S3).
Concerns about the intranasal COVID-19 vaccine
When participants chose an administration route, the top three factors were “Avoid undressing” (68.4%), “Needle-free” (64.8%), and “No discomfort after vaccination” (49.7%). There is little difference for participants with different vaccine acceptability. For example, those who preferred the intranasal spray prioritized “Needle-free” (74.8%), “Avoid undressing” (74.1%), and “More timesaving” (43.9%) while those who preferred intramuscular injection considered “No discomfort after vaccination” (61.6%), “Avoid undressing” (52.7%), and “Avoid taking off masks” (51.9%) in particular (, Table S4).
Figure 1. Considerations of vaccine administration routes (a) Priority factors for vaccination (b) Concerns about the intranasal spray vaccine.
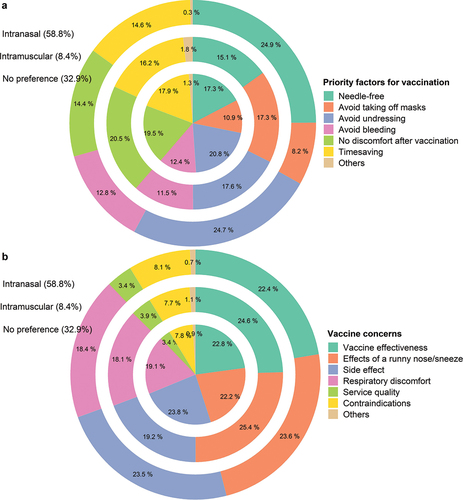
For this intranasal spray vaccine, participants were most concerned about the effect of a runny nose or sneeze on vaccine effectiveness (69.9%), vaccine side effects (69.6%), and Vaccine effectiveness (68.1%), followed by possible respiratory discomfort after inoculation (55.8%), contraindication (underlying respiratory diseases) (23.9%), service quality (10.3%), and others (2.4%). The concerns of different groups of people with different preferences are also shown (, Table S4). In addition, the topics of most interest include vaccine safety (72.7%), vaccine effectiveness (67.6%), how the intranasal spray vaccine works (55.1%), and whether the vaccination is free of cost (51.3%) in the future (Table S5).
Factors associated with vaccine acceptability
Compared with preferences for intramuscular injection or no preferences, the preference for intranasal spray was positively associated with high education level (odds ratio [OR]:1.29, 95% confidence interval [CI]:1.14–1.46) no discomfort during vaccination (OR:1.25, 95% CI:1.01–1.55), high perceived vaccine effectiveness (OR:5.08, 95% CI:4.44–5.81) and safety (OR:10.58, 95% CI:9.26–12.09). Besides, those who favored needle-free (OR:2.11, 95%CI:1.83–2.43), avoiding undressing (OR:1.65, 95% CI:1.44–1.91), no bleeding (OR:1.26, 95% CI:1.09–1.46), and more timesaving (OR:1.19, 95% CI:1.01–1.39), and those who got support from family members or friends (OR:1.81, 95% CI:1.61–2.03) were more likely to have a preference of intranasal spray. However, healthcare workers (OR:0.80, 95% CI:0.69–0.93), those with asthma (OR:0.39, 95% CI:0.18–0.85), and those preferring not taking off their masks when receiving vaccines (OR:0.69, 95% CI:0.59–0.81) showed less vaccine acceptability (). The willingness to recommend the intranasal spray vaccine to others was also investigated (). It is noticeable that healthcare workers also showed less acceptability in this dimension (OR: 0.71, 95% CI:0.62–0.80).
Table 4. Factors associated with the recommendation willingness of the intranasal spray vaccine.
Discussion
In China, the recent wave of the COVID-19 epidemic peaked in late December of 2022 and then maintained an almost uniform decline across all provinces. Considering the uncertainty of the epidemic trend and re-infection risk, sequential immunization has become urgent, especially for fragile populations.Citation28 Mucosal boost immunization may become a new trend for its ability to prevent infection by stopping the virus at the site of entry. This study can help us understand receivers’ vaccination experience, satisfaction, and concerns about the intranasal spray COVID-19 vaccine. Additionally, this study is conducted when the intranasal spray vaccination service was first offered in China, and it may also be valuable to other settings that will provide the intranasal spray vaccine service. In our study, about 90% of participants experienced a no-discomfort, time-saving, and smooth vaccination process. More than half of the participants showed a willingness to recommend the vaccine to others or preferred the intranasal spray vaccine over intramuscular injection after this vaccination. The participants’ experience with intranasal spray vaccination was generally good, with 92.6% feeling no discomfort, 99.8% thinking the vaccination process went well, and 89.4% thinking it was timesaving. Among those who experienced discomfort during vaccination, a history of respiratory diseases (such as asthma and sinusitis), cardiovascular diseases or hypertension was more common. It indicated that people with these health conditions were more likely to feel discomfort when vaccinated with the intranasal spray vaccine, but whether they are suitable for this administration route needs to be further investigated. Although people with asthma should use the intranasal vaccine with caution, studies have indicated that this intranasal vaccine was well tolerated and safe even in those with chronic conditions, such as asthma.Citation29
In this study, the vaccine acceptability was well. However, 5%-9% of participants refused to recommend the vaccine or preferred another administration route though they came to receive the intranasal spray. It might be caused by an unpleasant vaccination experience. Recent studies involving preferences of administration routes are mainly conducted in children or parents.Citation30,Citation31 For example, among children aged 8–12 years, 69% preferred the intranasal vaccine;Citation30 for parents of children with chronic illness, the proportion was 81%.Citation31 According to previous studies, the reasons behind the preference for intranasal vaccines were mainly the needle-free or less painful nature of this kind of vaccine,Citation31,Citation32 while easy administration, habit, and nasal problems were the common reasons behind the preference for intramuscular injection.Citation31 In our study, participants who preferred the intranasal spray vaccine valued the needle-free and convenient nature of this vaccine, while those who preferred the intramuscular injection were concerned about the infection risk if taking off masks.
The safety and effectiveness of this new vaccine are the most concerning aspects. About half of the participants showed confidence about the intranasal spray vaccine in terms of effectiveness and safety, which could be explained by that the advantages of the intranasal spray vaccine were widely publicized. For example, it is needle-free and less painful; it can stimulate immune responses in the intranasal and throat mucus membranes, which is highly overlapped with the infection site of the Omicron variant and is promising to block both infection and transmission of COVID-19.Citation29 We found that higher perceived vaccine effectiveness or safety was strongly associated with vaccine acceptability. The odds of preferring the intranasal vaccine over an intramuscular vaccine are nearly six times higher among those who believe the intranasal vaccine to be more effective. Meanwhile, lots of participants were also concerned about the vaccine’s side effects and vaccine effectiveness or worried that the vaccine effectiveness would be influenced by respiratory conditions such as runny nose, sinusitis, and asthma. It is reasonable that participants thought intranasal spray was superior to intramuscular injection in terms of effectiveness and safety but still had some concerns, given that it was newly approved and this type of vaccine was relatively rare. The positive results of the Phase 3 clinical trial for this vaccine may help improve the vaccine confidence of the public, which have been posted as a preprint on Research Square after our study period.Citation20
Factors associated with vaccine acceptability can be categorized into four domains of the behavioral and social drivers of the vaccination framework. For “thinking and feeling,” vaccine confidence was positively associated with vaccine acceptability. As mentioned above, people with high perceived vaccine effectiveness and safety were more likely to show satisfaction with this vaccine. For the “Social process,” social support is another crucial factor for vaccine acceptability.Citation26 As shown in our study, those who received support from family or friends were approximately two times more likely to prefer the intranasal spray vaccine than those who did not. For “practical issues,” better vaccination experience, preference for needle-free or not taking off masks were drivers for vaccine acceptability. For “sociodemographic information,” older age, male sex, and high education level were positively associated with vaccine acceptability, which is consistent with other studies.Citation33,Citation34 Besides, healthcare workers showed lower vaccine acceptability than non-healthcare workers for both recommendation willingness and preference for intranasal spray. The underlying reasons for their hesitancy need to be investigated further because of their essential role in vaccination promotion.Citation34,Citation35 In another survey conducted at the same time as this one (not yet published), we found that the primary barrier was fear of infection during the vaccination process. It implies that measures that can reduce SARS-CoV-2 infection risk during vaccination (e.g. outdoor vaccination setting) are warranted especially during a high incidence period.
This study had several limitations. First, it was a convenience sampling survey conducted in only one province in China. However, this multicenter study had a large sample size and covered urban, suburban, and outer suburban areas in Beijing city, which may have increased the representativeness of our study population. Besides, only a small number of participants aged over 60 years were included, and the population representativeness may be insufficient for this group of people. Though no association between age and vaccine acceptability was found, the acceptability of the intranasal spray vaccine could be different for aged people. Second, vaccine side effects were not investigated in this study. Considering that the survey was conducted during the post-vaccination observation period of participants, we only asked them whether they felt discomfort during vaccination. Third, the acceptability for the intranasal spray vaccine may change over time as more information becomes available to the public, but this was not investigated because of the limited study duration.
In conclusion, the intranasal spray COVID-19 vaccine has good acceptability and vaccination experience. However, it should be done with caution in people with respiratory conditions because of possible discomfort during vaccination. Vaccine effectiveness and safety are major concerns about the vaccine, which were found to be strongly associated with vaccine acceptability. Therefore, publicity and education efforts to increase vaccine confidence may help to improve vaccine acceptability. Additionally, the underlying reasons for healthcare workers’ less vaccine acceptability need to be further investigated because of their crucial role in public vaccination behavior.
Author’s contributions
JL, JW, and LF contributed equally as co-last authors. JL, LF, YM, YC, JW conceptualized and designed the study. YM and WL conducted the statistical analysis. YM wrote the first draft of the manuscript. RS, BJ, HW, LY, LS conducted data collection. JL, LF, JW, WY, WL supervised all the writing and editing of the manuscript. All authors read and approved the final manuscript.
Ethical approval statement
The study was approved by the Medical Ethics Committee of the Chinese Academy of Medical Sciences and Peking union Medical College, Beijing, China (CAMS&PUMC-IEC-2022-019). Written informed consent was obtained from all participants.
Supplemental Material
Download PDF (502.6 KB)Acknowledgments
We would like to thank Editage (www.editage.com) for writing support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon request.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2235963.
Additional information
Funding
References
- Araf Y, Akter F, Tang Y, Fatemi R, Parvez MSA, Zheng C, Hossain MG. Omicron variant of SARS-CoV-2: genomics, transmissibility, and responses to current COVID-19 vaccines. J Med Virol. 2022;94(5):1825–12. doi:10.1002/jmv.27588.
- Loo K-Y, Letchumanan V. COVID-19: understanding the new variants of concern. Prog Micobes Mol Biol. 2022;5(1). doi:10.36877/pmmb.a0000282.
- Zhou Z, Zhu Y, Chu M. Role of COVID-19 vaccines in SARS-CoV-2 variants. Front Immunol. 2022;13:898192. doi:10.3389/fimmu.2022.898192.
- Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, Mizrahi B, Alroy-Preis S, Ash N, Milo R, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–400. doi:10.1056/NEJMoa2114255.
- Walensky RP. CDC recommends Pfizer booster at 5 months, additional primary dose for certain immunocompromised children. 2022 Jan 4 [accessed 2022 Dec 21]. https://stacks.cdc.gov/view/cdc/113081.
- All adults to be offered COVID-19 boosters by end of January. Department of Health and Social Care; 2021 Nov 30 [accessed 2023 Jan 10]. https://www.gov.uk/government/news/all-adults-to-be-offered-covid-19-boosters-by-end-of-january.
- World Health Organization. Interim recommendations for heterologous COVID-19 vaccine schedules. 2021 Dec 16 [accessed 2022 Dec 21]. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-recommendation-heterologous-schedules.
- COVID-19 vaccine tracker. 2022 Dec 2 [accessed 2022 Dec 21]. https://covid19.trackvaccines.org/agency/who/.
- National Health Commission of the People’s Republic of China. Notice on the issuance of the work plan for vaccination in response to the recent outbreak of novel coronavirus infection. 2023 Apr 10 [accessed 2023 June 20]. http://www.nhc.gov.cn/xcs/zhengcwj/202304/c8db1a9da0204fdb87c88d7f70b284c0.shtml.
- Xinhua News Agency. These progress have been made in the development of a novel coronavirus vaccine in parallel with multiple technological routes. 2022 Oct 12 [accessed 2022 Dec 21]. http://www.gov.cn/xinwen/2022-10/12/content_5717961.htm.
- Rubin R. COVID-19 vaccine nasal spray. Jama. 2021;326(12):1138–. doi:10.1001/jama.2021.14996.
- Havervall S, Marking U, Svensson J, Greilert-Norin N, Bacchus P, Nilsson P, Hober S, Gordon M, Blom K, Klingström J, et al. Anti-spike mucosal IgA protection against SARS-CoV-2 omicron infection. N Engl J Med. 2022;387(14):1333–6. doi:10.1056/NEJMc2209651.
- Liew F, Talwar S, Cross A, Willett BJ, Scott S, Logan N, Siggins MK, Swieboda D, Sidhu JK, Efstathiou C, et al. SARS-CoV-2-specific nasal IgA wanes 9 months after hospitalisation with COVID-19 and is not induced by subsequent vaccination. eBioMedicine. 2023;87:104402. doi:10.1016/j.ebiom.2022.104402.
- Emily W. How nasal-spray vaccines could change the pandemic. Nature. 2022;609(7926):240–2. doi:10.1038/d41586-022-02824-3.
- Knisely JM, Buyon LE, Mandt R, Farkas R, Balasingam S, Bok K, Buchholz UJ, D’Souza MP, Gordon JL, King DFL, et al. Mucosal vaccines for SARS-CoV-2: scientific gaps and opportunities—workshop report. NPJ Vaccines. 2023;8(1):53. doi:10.1038/s41541-023-00654-6.
- Emily W. China and India approve nasal COVID vaccines — are they a game changer? Nature. 2022;609(7927):450. doi:10.1038/d41586-022-02851-0.
- Chen J, Wang P, Yuan L, Zhang L, Zhang L, Zhao H, Chen C, Wang X, Han J, Chen Y, et al. A live attenuated virus-based intranasal COVID-19 vaccine provides rapid, prolonged, and broad protection against SARS-CoV-2. Sci Bull. 2022;67(13):1372–87. doi:10.1016/j.scib.2022.05.018.
- Wantai Biopharmaceutical Company. Prompt announcements to get key data from phase III clinical trial of influenza virus vector COVID-19 vaccine for intranasal spray (Identifier: 2022-092). 2022 Oct 11 [accessed 2022 Dec 21]. https://www.ystwt.com/zxgg.
- Beijing Wantai Biological Pharmacy Enterprise Co.,Ltd. A phase III clinical trial of influenza virus vector COVID-19 vaccine for intranasal spray (DelNS1-2019-nCoV-RBD-OPT1). Identifier ChiCTR2100051391. 2021 Dec 4 [accessed 2023 June 18]. https://www.chictr.org.cn/showproj.html?proj=133897.
- Xia N, Zhu F, Huang S, Liu X, Chen Q, Zhuang C, Han J, Jaen A, Do TH, Peter J, et al. The efficacy and safety of an intranasal spray COVID-19 vaccine in a randomized double-blind placebo-controlled phase III trial during omicron period. Res Sq. 2023 Feb 19 [accessed 2023 June 20]. doi:10.21203/rs.3.rs-2407050/v1.
- The Joint Prevention and Control Mechanism of the State Council in Response to the Novel Coronavirus Pneumonia. Notice on the implementation plan for vaccination of second booster dose of COVID-19 vaccines. 2022 Dec 14 [accessed 2023 June 18]. http://www.nhc.gov.cn/xcs/zhengcwj/202212/acd8ba68d934488983909e81642dc337.shtml.
- National Health Commission of the People’s Republic of China. A notice on the issuance of the implementation plan for the second dose of the novel coronavirus vaccine. 2022 Dec 14 [accessed 2022 Dec 21]. http://www.nhc.gov.cn/xcs/yqfkdt/202212/acd8ba68d934488983909e81642dc337.shtml.
- Wantai Biopharmaceutical Company. Prompt announcement on the inclusion of the nasal spray influenza virus vector COVID-19 vaccine for emergency use (Identifier: 2022-102). 2022 Dec 6 [accessed 2023 June 18]. https://www.ystwt.com/zxgg/
- National Health Commission of the People’s Republic of China. Technical guidance on COVID-19 vaccination (First edition). 2021 Mar 29 [accessed 2023 Jun 18]. http://www.nhc.gov.cn/xcs/yqfkdt/202103/c2febfd04fc5498f916b1be080905771.shtml.
- Department of Health and Aged Care, Australian Government. Vaccination after COVID-19 infection. 2023 Apr 5 [accessed 2023 June 18]. https://www.health.gov.au/our-work/covid-19-vaccines/getting-your-vaccination/vaccination-after-covid-19-infection.
- WHO. Understanding the behavioural and social drivers of vaccine uptake WHO position paper - May 2022. Wkly Epidemiol Rec. 2022;97(20):209–24.
- Z J. jjPlot: some geom/stat functions to produce funny graphs. 2022 [accessed 2022 Dec 21]. https://github.com/junjunlab/jjPlot.
- Burki TK. Omicron variant and booster COVID-19 vaccines. Lancet Respir Med. 2022;10(2):e17. doi:10.1016/S2213-2600(21)00559-2.
- Zhu F, Zhuang C, Chu K, Zhang L, Zhao H, Huang S, Su Y, Lin H, Yang C, Jiang H, et al. Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Respir Med. 2022;10(8):749–60. doi:10.1016/S2213-2600(22)00131-X.
- Flood EM, Ryan KJ, Rousculp MD, Beusterien KM, Block SL, Hall MC, Mahadevia PJ. A survey of children’s preferences for influenza vaccine attributes. Vaccine. 2011;29(26):4334–40. doi:10.1016/j.vaccine.2011.04.018.
- Marien AG, Hochart A, Lagrée M, Diallo D, Martinot A, Dubos F. Parental acceptance of an intranasal vaccine: example of influenza vaccine. Archives de Pédiatrie. 2019;26(2):71–4. doi:10.1016/j.arcped.2018.11.002.
- Ryan KA, Filipp SL, Gurka MJ, Zirulnik A, Thompson LA. Understanding influenza vaccine perspectives and hesitancy in university students to promote increased vaccine uptake. Heliyon. 2019;5(10):e02604. doi:10.1016/j.heliyon.2019.e02604.
- Wong E-Y, Qiu H, Chien WT, Wong J-L, Chalise HN, Hoang H-X, Nguyen HT, Chan P-S, Wong M-S, Cheung A-L, et al. COVID-19 vaccine willingness and related factors among health care workers in 3 Southeast Asian jurisdictions. JAMA Netw Open. 2022;5(8):e2228061. doi:10.1001/jamanetworkopen.2022.28061.
- Li L, Ma Y, Li W, Tang G, Jiang Y, Li H, Jiang S, Zhou Y, Yang Y, Zhang T, et al. Caregiver willingness to vaccinate children with pneumococcal vaccines and to pay in a low-resource setting in China: a cross-sectional study. Vaccines [Internet]. 2022;10(11):1897. doi:10.3390/vaccines10111897.
- Rong H, Lai X, Ma X, Hou Z, Li S, Jing R, Zhang H, Peng Z, Feng L, Fang H. Seasonal influenza vaccination and recommendation: the difference between general practitioners and public health workers in China. Vaccines [Internet]. 2020;8(2):265. doi:10.3390/vaccines8020265.
