ABSTRACT
The aim of this work is to describe the dynamics of influenza antibodies after vaccination in adults. We conducted a case-cohort serological study in the automobile manufacturing plants of the Renault España S.A. group in Valladolid and Palencia (Spain), including 550 workers (66.9%) previously vaccinated against influenza (group V), and 272 (33.1%) never vaccinated (group NV). A pre-vaccination serum sample was collected, another after 30–40 days and another after 6 months. The dynamics of antibodies were analyzed. A lower seroprotection of NV before vaccination was observed, but an antibody response between 2 and 4 times higher than in V was assessed. After 6 months, antibodies declined in both groups until equalize. Antibodies titers decrease with age, and no differences were found among underlying pathologies. Adults never vaccinated against influenza had lower seroprotection than those previously vaccinated, but influenza vaccination produces a more intense serological response in them, acquiring significantly higher antibody titers than those previously vaccinated. The antibodies, although in lower titers, persist and equalize among both groups at least 6 months after vaccination, which allows the individual to be protected during the entire circulation of the influenza virus in the same season.
KEYWORDS:
Introduction
Influenza is a viral infectious disease that causes around 3–5 million hospitalizations per year and 650,000 deaths worldwide.Citation1 However, these values are underestimated because influenza plays an essential role in the development and evolution of other pathologies, such as cardiovascular diseases, on which it has a direct and indirect impact.Citation2
The best way to prevent influenza is vaccination. There are different approaches to influenza vaccination. One approach is universal vaccination, such as the United States & Canada.Citation3,Citation4 Another approach is vaccination focused on the most vulnerable people,Citation5,Citation6 such as elderly, pregnant women and patients with underlying diseases.Citation7 A third approach is vaccination focused on workers in professions with a higher probability of contact with the most vulnerable people or zoonotic sources of influenza. An example is Spain, where health care workers (hospitals, pharmacies, nursing homes, social and health care centers, etc.), state security forces and corps, firemen, civil protection, health emergencies, and also farmers who are exposed to domestic fowl or pigs in poultry and livestock farms.Citation7 However, workers of other types can have recourse to influenza vaccination through their company’s occupational risk prevention services.
Although this practice is not very common, large companies carry out vaccination campaigns that bring the vaccine to the worker, and not the worker to the vaccine. This is important because some studies postulate that vaccination in the workplace has a clear impact in reducing the intensity of influenza epidemics.Citation8 At the same time, the low vaccine acceptability in adults makes it difficult to carry out serological and vaccine efficacy studies in this age group, since access to this type of samples is not easy and requires optimized logistics.
The aim of our study is to know the dynamics of the antibody response to influenza after vaccination of two groups of workers, one of whom had been vaccinated in the previous season, and another who had never been vaccinated against influenza.
Materials and methods
Study design
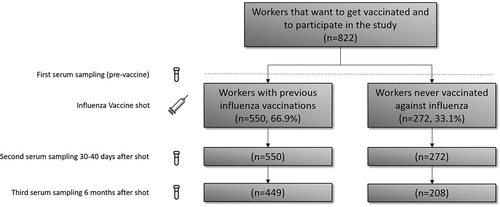
We designed a case-cohort study including workers between 18 and 65 years of age, from two automobile factories in Spain (Renault España S.A., hereinafter “The company”) in the cities of Valladolid and Palencia, who were vaccinated against influenza in the 2021–2022 season. To carry out this study, we included all individuals who agreed to participate in the study, the only exclusion criterion being the impossibility of being vaccinated against influenza due to the existence of contraindications. Recruitment was carried out by means of an appeal through e-mails and publicity about the vaccination campaign to all the workers of both factories (n = 8,043), carried out by the company. A total of 822 workers (10.2%) finally attended the vaccination, of whom 550 (66.9%) had been vaccinated against influenza at least the previous season (group V), and 272 (33.1%) had never been vaccinated against influenza previously (group NV). The vaccine (FluarixTetra, GSK®) was administered by the company’s medical team in both factories. The vaccine administered contained the influenza A and B strains recommended by WHO for vaccination in the 2021–2022 season for the northern hemisphere grown in egg (A(H1N1)pdm09, A/Victoria/2570/2019; A(H3N2), A/Cambodia/e0826360/2020; B/Victoria, B/Washington/02/2019; B/Yamagata, B/Phuket/3073/2013).Citation9
The company’s medical team collected a serum sample from each worker on the day of vaccination, an additional sample 30–40 days after vaccination and another sample 6 months after vaccination. The samples were sent to the National Influenza Center in Valladolid for serological analysis against the four human influenza viruses. In addition, during the first sample collection, the medical team collected information on sociodemographic variables (age and sex), history of influenza vaccination (Yes/No) and the existence of underlying diseases, which were differentiated into five different groups; Asthma, Cardiovascular Diseases (CVD), Diabetes (type I or II), Arterial Hypertension (AHT), Autoimmune/Inflammatory Diseases, and also nonexistence of underlying diseases. For the second sampling at approximately 1-month post-vaccination, samples were obtained from all individuals who participated in the study. For the third sampling 6-months after vaccination, only 449 individuals from group V (81.6%) and 208 from group NV (76.5%) participated ().
This work was approved by the Ethics Committee of the Eastern Health Area of Valladolid (Cod: PI 21–2442). All participants signed a written informed consent prior to vaccination and sampling. Also, this research was conducted according to the Declaration of Helsinki.
Culture of influenza viruses in eggs
For the serological assays, the reference viruses of the 2021–2022 influenza vaccine were cultured in embryonated chicken eggs to obtain viral stock to be used in the analyses. Briefly, a 1/10 pre-dilution of each virus was made in PBS and 100 µl was inoculated into the allantoic cavity of 9–11 days old embryos with a 22 G syringe.Citation10 The inoculated eggs were incubated for 48 h at 37°C, and after that they were euthanized at 4°C for 24 h. The top of the eggshell was then removed and the allantoic fluid was collected with a pipette. This liquid was stored at 4°C for 24 hours prior to being titrated using a hemagglutination assay with chicken blood, and then labeled and stored at −80°C until use.
Analysis of HA antibodies
The analysis of antibodies was performed through the haemagglutination inhibition assays, which is the reference assay to analyze antibodies response induced by vaccines recommended by WHO.Citation10 Upon arrival at the laboratory, serum samples were centrifuged at 2,5000 rpm for 10,’ and the supernatant was stored at −20°C. Antibody analysis against HA was performed using the hemagglutination inhibition assay. For each serum sample, 100 μl was added to 300 μl of receptor destroying enzyme (Denka Seiken, Tokyo, Japan) to remove nonspecific hemagglutination inhibitors. Following manufacturer’s instructions, this mixture was incubated at 37°C in a water bath for 12–18 hours, followed by enzyme inactivation for 1 h at 56°C.To perform HI, 50 µl serial 2-fold dilutions of each serum were made in 96 microwell V plates, and then 50 µl of a standard containing 4 units of hemagglutinin was added to each well and incubated for 30 min at room temperature. Finally, 50 µl of 0.5% chicken erythrocytes were added and incubated again at room temperature for another 30 min. The titer of Abs was defined as the highest dilution that caused complete inhibition of hemagglutination. The initial dilution was 1:10 and the lower limit of a detectable antibody titer is 10. When the antisera titer is below a detectable threshold, due to a shortage or lack of antibodies, it is conventionally expressed as 5, half of the lowest detection threshold.Citation11 Pre-vaccine, post-vaccine and 6-months antibodies titers were included in the database for study.
Statistical analysis
The results were analyzed using the classical serological parameters of the European Medicines Agency (EMA) for the evaluation of vaccine efficacy.Citation12 These include seroprotection rate (SPR), seroconversion rate (SCR) and geometric mean titers (GMTs and their increment [GMTi]) between post- and pre-vaccination serum samples. Seroprotection was considered when a titer of HI 1/40 was achievedCitation11 and seroconversion was defined as a titer increase of at least fourfold between pre- and post-vaccination sera. In addition, seroconversion was considered to have occurred in cases of negative pre-vaccination sera that achieved 1/40 titers after vaccination.Citation11 Values were described using the mean (95%CI). Volunteer characteristics, SPR and SCR were compared using χ2. GMTs were compared using Student’s t-test. GMT values were compared between underlying diseases and with healthy adults using Bonferroni’s test and Dunnett’s correction. Logistic regression was used to calculate the age-related increase/decrease in HIA titers. Statistical analysis was performed with SPSSV27 (IBM, Armonk, NY, USA) and GraphPad Prism V9 (GraphPad, San Diego, CA, USA). Statistical significance was set at p-value <.05.
Results
Patients characteristics
A total of 822 workers from both factories were included, of whom 550 belonged to group V (66.9%) and 272 to group NV (33.1%). The mean age of the V group was 42.2 years (CI95%, 41.2–43.0) and of the NV group was 44.3 (CI95%, 43.8–44.9), with significant differences between both groups (p < .0001) (). The percentage of males was similar among both groups (75.1% in V and 74.3% in NV). The percentage of workers that showed underlying diseases was significantly higher in the V group (18.5%) than in NV group (6.6%) (p < .0001). The only underlying disease that showed similar percentages was cardiovascular disease, with no significant differences in the proportion of these in both groups (p > .05).
Table 1. Demographic and clinic characteristics of patients included in the study.
Evolution of the response after influenza vaccination
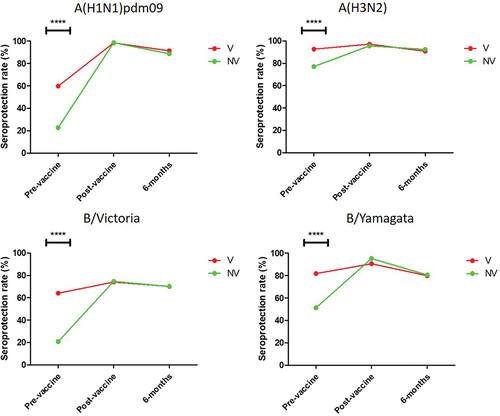
First, we evaluated the evolution of different serological parameters in the three serum samples obtained in the study in both groups of workers. The analysis of the seroprotection rate (SPR) showed that the pre-vaccination SPR was significantly higher against the four influenza viruses in group V than in NV (p < .0001) () (Supplementary material 1). However, no significant differences were found in any of the viruses in either the post-vaccine or 6-months sampling. The highest SPR in both V and NV was found against subtype A(H3N2), being 92.7% and 77.2% respectively, while the lowest was observed against subtype A(H1N1)pdm09 in group V (59.6%), and against B/Victoria in NV (20.6%).
Figure 2. Evolution of the seroprotection rate against the four human influenza viruses type A and B in the group of workers who had been vaccinated at least the previous season (V), and in those who had never been vaccinated against influenza (NV). ****, p < .0001.
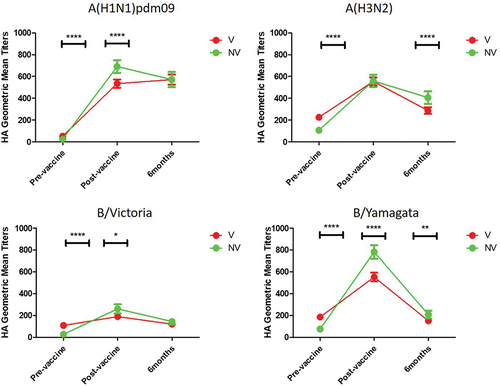
When we analyzed GMTs, we observed that this value was significantly higher before vaccination against all the viruses analyzed in group V with respect to NV (p < .0001) (). However, after vaccination, GMTs were significantly higher in the NV group in all cases (p < .05; p < .0001) with the exception of against subtype A(H3N2) (p > .05). After vaccination, the highest GMTs were found in the NV group against B/Yamagata virus [782.0 (CI95%, 719.2–844.8)] and the lowest in V group against B/Victoria lineage [(189.8 (CIV95%, 166.3–213.4)] (Supplementary material 2). In all cases, the antibodies titers after 6 months was reduced by 73.5% (in VN against B/Yamagata) to 16.8% (in VN against subtype A(H1N1)pdm09). The only exception was GMTs against subtype A(H1N1)pdm09 in group V, relative to the 1-month post-vaccination samples, the 6-month samples were 6.8% higher [(570.9 (C95%, 525.0–616.8)], but this difference was not statistically significant (p > .5).”
Figure 3. Evolution of the GMTs against the four human influenza viruses type A and B in the group of workers who had been vaccinated at least the previous season (V), and in those who had never been vaccinated against influenza (NV). *, p < .05; **, p > .01; ****, p < .0001.
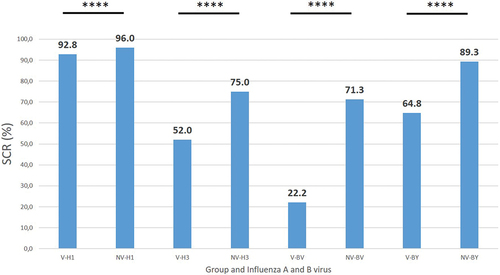
The seroconversion rate (SCR) between pre and post-vaccination sampling was significantly higher in the NV group against both influenza A subtypes and influenza B lineages (p < .0001) (). The highest SCR was observed against the A(H1N1)pdm09 subtype in both groups (92.8% group V and 96.0% group NV respectively), and the lowest against the B/Victoria lineage (22.2% group V and 71.3% group NV respectively).
Figure 4. Seroconversion rate (SCR) against the four human influenza viruses type A and B in the group of workers who had been vaccinated at least the previous season (V), and in those who had never been vaccinated against influenza (NV). H1, A(H1N1)pdm09; H3, A(H3N2); BV, B/Victoria; BY, B/Yamagata; ****, p < .0001.
Differences in the vaccine response and antibodies dynamics by age
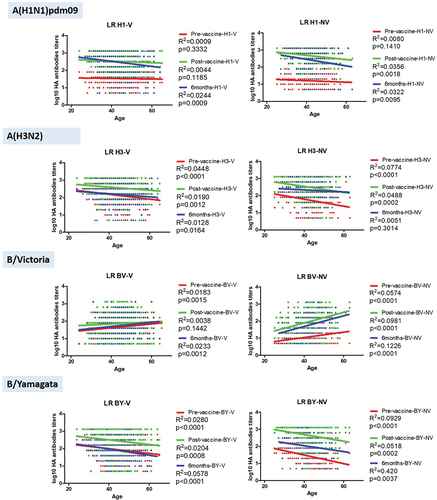
Secondly, we performed an age-dependent analysis of the dynamics of antibodies in both groups (N and NV) by means of logistic regression. We observed age-dependent significant differences against all viruses and in all samplings, except against subtype A(H1N1)pdm09 at pre-vaccine and post-vaccine samplings in V, and at pre-vaccine in NV (p > .05) (). In addition, no significant differences were observed against the B/Victoria lineage in V (p > .05). In those cases in which significant differences were observed, both in the influenza A(H1N1)pdm09 and A(H3N2) subtypes and in the B/Yamagata lineage, a decrease in antibody titer was observed with increasing age, of varying intensity among the different viruses analyzed. However, when the B/Victoria lineage was analyzed, an increase in antibodies titers was observed with increasing age. The highest R2 value was observed in the 6-month sampling against the B/Victoria (R2 = 0.1226) and B/Yamagata (R2 = 0.420) lineages in the NV. For subtype A(H3N2), the highest R2 was observed in the pre-vaccine sampling in both V (R2 = 0.0448) and NV (R2 = 0.0774). In the case of subtype A(H1N1)pdm09, in V the highest R2 was observed in the 6-month sampling (D6; R2 = 0.0244), while in NV it was in the post-vaccine sampling (D1; R2 = 0.0356).
Underlying diseases as determinants of influenza vaccine antibody induction and duration
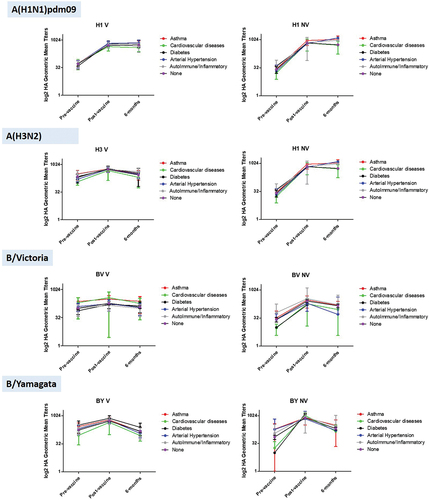
Additionally, we analyzed the response to vaccination (measured as SCR between the pre and post-vaccination samplings) and the evolution of antibody titers regarding the underlying diseases recorded for each subject in the study (Supplementary material 3). No significant differences were found in either SCR or GMTs in any sampling against any virus, neither between the different diseases among themselves, nor in comparison with the group of healthy individuals (p > .05) (). However, we found that the post-vaccination GMTs were significantly higher in healthy individuals than in the group with cardiovascular diseases against subtype A(H3N2) in the group V (p = .002). The Cardiovascular diseases and Diabetes groups could not be analyzed in the NV group because they had only 1 individual.
Discussion
Influenza vaccination in adults is not a priority in some European countries since adult healthy people are not, in general, included in any vaccine recommendation risk group.Citation13 Non-healthcare workers can benefit from vaccination through the prevention programs of their companies, but vaccination in work environment is very low, being below 11% in our case and in line with other studies published in Spain.Citation14
Our study showed that adults who have been previously vaccinated against influenza have an advantageous seroprotection against the four currently existing human influenza viruses. This advantage is observed in the pre-vaccination SPR, being, in general, 20–40% higher in those previously vaccinated compared to those who had never been vaccinated. This was also observed when analyzing the GMTs in both groups. Our data show that both V and NV had detectable antibodies before vaccination, but the V group showed titers 2–4 fold higher than NV, meaning that V are better prepared for the next influenza season than NV.
However, despite the V group started with such an advantage, vaccination produced a 2–5 fold stronger humoral response in VN group than in V in terms of increased antibodies titers. This left a similar SPR 30–40 days after vaccination between both groups (above 90% in most cases), but post-vaccination GMTs 1 to 1.3 times higher in NV and SCR 1 to 3 times more intense in NV, depending on the virus tested. This lower response found in the V group may be caused by the “negative interference” or “Hoskins paradox” effect.Citation15–19 This paradigm holds that repeated vaccination with similar influenza strains may lead to a lower response due to local antigen depletion by preexisting antibodies after vaccination.Citation20 Some authors have presented results showing that preexisting T-cell response is inversely correlated with pre-vaccination levels, thus the higher the baseline levels of preexisting T-cells, the lower the fold increase of those cells is observed after vaccination.Citation21 However, there are other scientific studies which suggest that preexistent immunity, through natural infections or vaccination, may increase the magnitude of the IgG response mediated by follicular CD4+ T cells.Citation22 Our results show a clear wider humoral response in people not previously vaccinated, supporting the negative interference effect. However, the existence of studies that show opposite results demonstrate that further research is needed to clarify the characteristics of this issue.
In our group, individuals who had been vaccinated at least the previous season probably have antibodies with high similarity to the strains used in the vaccine of the season in which the study was performed that could recognize the antigen and eliminate it. This can be corroborated by reviewing the phylogenetic groups to which the vaccine strains of the 2020–21 and 2021–22 seasons of subtype A(H1N1)pdm09 (6B.1A5a) and subtype A(H3N2) (3C.2a1b), and those of the B viruses, both Yamagata and Victoria, belong, since the same strains were administered in both seasons.Citation23 This supposed detrimental effect has made the efficacy of repetitive annual vaccination a subject of heated debate.Citation24–29 However, our data show that, although it seems that this effect also occurs in previously vaccinated adults, vaccination always increases antibody titer and seroprotection.The existence of this effect is not sufficient reason to avoid vaccination as it has more advantages than disadvantages, how other researchers had addressed such as reduction in median cost of absenteeism and the impact of adults vaccination in the reduction of influenza burden of disease.Citation8,Citation30
The benefit, however, for the previously unvaccinated group of people, is resounding, and shows the advantages of vaccinating healthy adults, and also those who have underlying diseases. The overall SPR achieved should be used to promote the benefits of influenza vaccination in the workplace. Companies are perfect places to promote adult vaccination due to the accessibility of this population to their medical and preventive services, as well as the logistical facilities available in these places to carry out massive vaccinations in the months before to the influenza epidemic. Some studies show that vaccination of workers can reduce the intensity of influenza epidemics, as well as reduce the associated costs and work absenteeism by up to 57%.Citation8,Citation30 Our results can serve as a public health tool to promote vaccination at the workplace.
Serological analysis 6 months after vaccination revealed that both SPR and GMTs are reduced in both groups respecting to post-vaccination sampling, thus proving the waning of antibodies levels in both groups. This was previously analyzed in other works and is one of the main reasons for the reduction of influenza vaccine effectiveness during the same influenza season.Citation31,Citation32 However, this reduction does not occur with the same intensity. Although the SPR remains similar between both groups after waning, GMTs on 6-months sampling remain higher in NV against A(H3N2) and B/Yamagata, and identical against A(H1N1)pdm09 and B(Victoria). Thus, vaccination in previously unvaccinated individuals promotes the duration of the antibody response by up to 6 months at titers similar to or higher than in the previously vaccinated people.
Additionally, age-adjusted analyses showed that, although all subjects were adults, there are differences in the amount of antibodies detected as the age of the individual increases. The data showed a significant reduction in antibodies titer with increasing age against A(H1N1)pdm09, A(H3N2) and B/Yamagata subtypes. This effect could respond to the phenomenon known as immunosenescence,Citation33 which, although it is more linked to those over 65 years of age, is not a phenomenon that occurs from one day to the next, but has cumulative effects that increase as age increases.Citation34 However, the radically different behavior when analyzing the B/Victoria lineage is surprising, since we observed an increase in antibody titer as age increased, just the opposite of the rest of the subtypes. This curious difference could involve the effect known as “Original Antigenic Sin” or “OAS.”Citation35–37 This show that the first influenza infections determine how those that occur in the rest of life, responding better to viruses similar to the first encounter. In our particular case, we believe that the ancestral B type strain B/Lee/40, which circulated until 1983 and then split into the two lineages we currently know,Citation38 may be partly related to this effect. Although both lineages emerged at the same time, the B/Victoria lineage circulated more intense during the next two decades,Citation39,Citation40 whereas the B/Yamagata lineage prevailed during the 1990s.Citation41 Thus, older people within the adult group have probably been primed by the B/Victoria lineage, while younger people by B/Yamagata, giving rise to the observed age-dependent differences in antibody titer and presence. This phenomenon, together with the progressive immunosenescence discussed above, may also be behind the age-dependent differences in B/Yamagata being the most pronounced in the study, along with B/Victoria.
In general, the analysis adjusted by the existence of underlying diseases did not show significant differences between different pathologies when compared with each other and with the group of healthy individuals. Although it is true that the number of subjects is insufficient to make comparisons, our data show that the existence of certain pathologies in adults does not seem to have an impact either on previous protection, or on the vaccine response or the duration of antibodies. Our data only showed the existence of a lower mean antibody titer against subtype A(H3N2) in patients with cardiovascular pathologies compared to healthy patients, but these differences were subsequently annulled by vaccination. Cardiovascular pathology is of great interest in influenza since this virus is associated with the occurrence of certain cardiovascular events such as acute myocardial infarctionCitation42–44 or the occurrence of stroke.Citation44,Citation45 Therefore, the data, although limited, seem to indicate that, at least against A(H3N2), there is a seroprotection deficit against influenza in adult patients with cardiovascular pathology, so that adult patients with this type of pathology could be one of the greatest beneficiaries of influenza vaccination, as attested by some previous studies.Citation46,Citation47 Others underlying diseases, such as diabetes, seems to not have a clear impact in antibodies profiles in our groups, neither NV nor V. But, some authors had pointed out the importance of influenza vaccination in people living with diabetes. People with diabetes are more likely to suffer from severe disease and fatal influenza complications, because infection can cause chronic hyperglycemia allowing the virus to replicate easier and diminish the immune response.Citation48 Some researchers have described the benefit of influenza vaccination in type 2 diabetes to reduce all-cause mortality, among other outcomes.Citation49 Due to the low number of subjects with underlying diseases included in this work, further studies with a larger number of subjects with cardiovascular pathology are needed to corroborate that this deficit of protection prior to vaccination is generalized.
The main limitation of our study is that there was no opportunity to perform a 12-month follow-up to check for antibody drop before the next vaccination. This would be very useful to assess the protection of both groups just before the following epidemic. Additionally, the number of individuals with previous pathologies was very low in some cases, especially in the NV group, so more individuals are needed to obtain statistically significant conclusions. The main strength of our study is that two very large cohorts of young-adult population were included, which is not very common in this type of study.
In summary, although adult individuals who have never been vaccinated against influenza have a lower seroprotection than those who have been previously vaccinated, influenza vaccination produces a more intense serological response in them, acquiring significantly higher antibody titers than those previously vaccinated. The antibodies, although in lower titers, persist with similar titers in both groups until at least 6 months after vaccination, which allows the individual to be protected during the entire circulation of the influenza virus in the same season. Age is a limiting factor in the response to influenza vaccines and in the persistence of antibodies, even below 65 years of age, with a gradual fall in antibody titer with increasing age for most viruses, except for the B/Victoria lineage. In general, underlying diseases do not have a negative impact on influenza immunization in adults, although cardiovascular diseases may result in lower pre-vaccination protection against subtype A(H3N2) compared to healthy individuals, a fact that is immediately corrected by vaccination. Those results have clear implications in public health advising for companies to recommend influenza vaccination in those who usually got it, and those who not.
Author’s contributions
ISM, CL, ACP, LTF, JIE, JCS and JME designed the study, CL, YML, ACP, LTF and JIE recruited the subjects and obtained the samples; ISM, JSM, CPG, SRR, MDGG, VFE and CHG performed the experiments and analyzed the data; ISM, SRR, MDG, CPG, VFE and CHG written the manuscript; ISM, JCS and JME revised the manuscript; all the authors revised and approved the final version of the manuscript.
Supplemental Material
Download PDF (481.6 KB)Acknowledgments
We acknowledge all the medical and nurse staff involved in the recruitment and extraction of samples in Renault España S.A. Valladolid and Palencia sites.
Disclosure statement
The authors CL, YML, ACP, LJT and JIE work for Renault España S.A. The rest of the authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2236537.
Additional information
Funding
References
- Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–10. doi:10.1016/S0140-6736(17)33293-2.
- Warren-Gash C, Smeeth L, Hayward AC. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis. 2009;9(10):601–10. doi:10.1016/S1473-3099(09)70233-6.
- Canada Ministry of Health. 2022/2023 Universal Influenza Immunization Program (UIIP) [Internet]. 2022 [accessed 2023 Feb 27]. https://www.health.gov.on.ca/en/pro/programs/publichealth/flu/uiip/ .
- CDC. Summary: ‘prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2022-23’ [Internet]. 2022 [accessed 2023 Feb 27]. https://www.cdc.gov/flu/professionals/acip/summary/summary-recommendations.htm .
- WHO. WHO regional office for Europe recommendations on influenza vaccination for the 2021/2022 season during the ongoing COVID-19 pandemic [Internet]. 2021 [accessed 2023 Feb 27]. https://apps.who.int/iris/bitstream/handle/10665/347890/WHO-EURO-2021-3920-43679-61434-eng.pdf?sequence=1&isAllowed=y .
- ECDC. Seasonal influenza vaccination strategies [Internet]. 2023 [accessed 2023 Feb 27]. https://www.ecdc.europa.eu/en/seasonal-influenza/prevention-and-control/vaccines/vaccination-strategies .
- Ministerio de Sanidad. Recomendaciones de vacunación frente a la gripe Temporada 2020-2021 [Internet]. 2020 [accessed 2022 July 6]. https://www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/docs/Recomendaciones_vacunacion_gripe.pdf .
- Verelst F, Beutels P, Hens N, Willem L. Workplace influenza vaccination to reduce employee absenteeism: an economic analysis from the employers’ perspective. Vaccine. 2021;39(14):2005–15. doi:10.1016/j.vaccine.2021.02.020.
- WHO. Recommended composition of influenza virus vaccines for use in the 2021-2022 northern hemisphere influenza season [Internet]. 2021 [accessed 2023 Feb 16]. https://cdn.who.int/media/docs/default-source/influenza/who-influenza-recommendations/vcm-northern-hemisphere-recommendation-2021-2022/202102_recommendation.pdf?sfvrsn=2af603d8_12&download=true .
- WHO Global Influenza. Surveillance network. Manual for the laboratory diagnosis and virological surveillance of influenza [Internet]. 2011 [accessed 2022 Feb 14]. https://apps.who.int/iris/handle/10665/44518 .
- Trombetta CM, Perini D, Mather S, Temperton N, Montomoli E. Overview of serological techniques for influenza vaccine evaluation: past, present and future. Vaccines (Basel). 2014;2(4):707–34. doi:10.3390/vaccines2040707.
- EMA. Note for guidance on harmonisation of requirements for influenza vaccines (CPMP/BWP/214/96) [Internet]. 1997 [accessed 2023 Feb 20]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf .
- Aguolu OG, Willebrand K, Elharake JA, Qureshi HM, Kiti MC, Liu CY, Restrepo Mesa A, Nelson K, Jenness S, Melegaro A, et al. Factors influencing the decision to receive seasonal influenza vaccination among US corporate non-healthcare workers. Hum Vaccin Immunother. 2022;18(6):2122379. doi:10.1080/21645515.2022.2122379.
- Díez-Domingo J, Redondo Margüello E, Ortiz de Lejarazu Leonardo R, Gil de Miguel Á, Guillén Ortega JM, Rincón Mora J, Martinón-Torres F. A tool for early estimation of influenza vaccination coverage in Spanish general population and healthcare workers in the 2018–19 season: the Gripómetro. BMC Public Health. 2022;22(1):825. doi:10.1186/s12889-022-13193-x.
- Keitel WA, Cate TR, Couch RB. Efficacy of sequential annual vaccination with inactivated influenza virus vaccine. Am J Epidemiol. 1988;127(2):353–64. doi:10.1093/oxfordjournals.aje.a114809.
- Hoskins TW, Davies JR, Smith AJ, Miller CL, Allchin A. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza at Christ´s hospital. Lancet. 1979;313(8106):33–5. doi:10.1016/S0140-6736(79)90468-9.
- Hoskins TW, Davies JR, Smith AJ, Allchin A, Miller CL, Pollock TM. Influenza at Christ´s hospital: March, 1974. Lancet. 1976;307(7951):105–8. doi:10.1016/S0140-6736(76)93151-2.
- Beyer WEP, de Bruijn IA, Palache AM, Westendorp RGJ, Osterhaus ADME. The plea against annual influenza vaccination? ‘The Hoskins’ paradox’ revisited. Vaccine. 1998;16(20):1929–32. doi:10.1016/S0264-410X(98)00123-6.
- Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine. 1997;15(10):1114–22. doi:10.1016/S0264-410X(97)00003-0.
- Bedford T, Suchard MA, Lemey P, Dudas G, Gregory V, Hay AJ, McCauley JW, Russell CA, Smith DJ, Rambaut A, et al. Integrating influenza antigenic dynamics with molecular evolution. Elife. 2014;3:e01914. doi:10.7554/eLife.01914.
- Janssens Y, Joye J, Waerlop G, Clement F, Leroux-Roels G, Leroux-Roels I. The role of cell-mediated immunity against influenza and its implications for vaccine evaluation. Front Immunol. 2022;13:959379. doi:10.3389/fimmu.2022.959379.
- Wild K, Smits M, Killmer S, Strohmeier S, Neumann-Haefelin C, Bengsch B, Krammer F, Schwemmle M, Hofmann M, Thimme R, et al. Pre-existing immunity and vaccine history determine hemagglutinin-specific CD4 T cell and IgG response following seasonal influenza vaccination. Nat Commun. 2021;12(1):6720. doi:10.1038/s41467-021-27064-3.
- Worlwide Influenza Centre. The Francis Crick Institute. Report prepared for the WHO annual consultation on the composition of influenza vaccine for the southern hemisphere 2022 [Internet]. 2021 [accessed 2023 June 22]. https://www.crick.ac.uk/sites/default/files/2021-10/SH%20Sept%202021%20WIC%20web%20report%20v2.pdf .
- Sullivan SG, Kelly H. Stratified estimates of influenza vaccine effectiveness by prior vaccination: caution required. Clin Infect Dis. 2013;57(3):474–6. doi:10.1093/cid/cit255.
- Leung VKY, Fox A, Carolan LA, Aban M, Laurie KL, Druce J, Deng Y-M, Slavin MA, Marshall C, Sullivan SG, et al. Impact of prior vaccination on antibody response and influenza-like illness among Australian healthcare workers after influenza vaccination in 2016. Vaccine. 2021;39(24):3270–8. doi:10.1016/j.vaccine.2021.04.036.
- Thompson MG, Naleway A, Fry AM, Ball S, Spencer SM, Reynolds S, Bozeman S, Levine M, Katz JM, Gaglani M. Effects of repeated annual inactivated influenza vaccination among healthcare personnel on serum hemagglutinin inhibition antibody response to A/Perth/16/2009 (H3N2)-like virus during 2010–11. Vaccine. 2016;34(7):981–8. doi:10.1016/j.vaccine.2015.10.119.
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, Serres GD. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines. 2017;16(7):723–36. doi:10.1080/14760584.2017.1334554.
- Ramsay LC, Buchan SA, Stirling RG, Cowling BJ, Feng S, Kwong JC, Warshawsky BF. The impact of repeated vaccination on influenza vaccine effectiveness: a systematic review and meta-analysis. BMC Med. 2019;17(1):9. doi:10.1186/s12916-018-1239-8.
- Jones-Gray E, Robinson EJ, Kucharski AJ, Fox A, Sullivan SG. Does repeated influenza vaccination attenuate effectiveness? A systematic review and meta-analysis. Lancet Respir Med. 2023;11(1):27–44. doi:10.1016/S2213-2600(22)00266-1.
- Ferro A, Bordin P, Benacchio L, Fornasiero F, Bressan V, Tralli V, Moretti F, Majori S. Influenza vaccination and absenteeism among healthy working adults: a cost-benefit analysis. Ann Ig. 2020;32(3):234–44. doi:10.7416/ai.2020.2346.
- Ferdinands JM, Gaglani M, Martin ET, Monto AS, Middleton D, Silveira F, Talbot, HK, Zimmerman, R, Patel, M. Waning vaccine effectiveness against influenza-associated hospitalizations among adults, 2015–2016 to 2018–2019, United States hospitalized adult influenza vaccine effectiveness network. Clin Infect Dis. 2021;73(4):726–9. doi:10.1093/cid/ciab045.
- Hu W, Sjoberg PA, Fries AC, DeMarcus LS, Robbins AS. Waning vaccine protection against influenza among department of defense adult beneficiaries in the United States, 2016-2017 through 2019-2020 influenza seasons. Vaccines (Basel). 2022;10(6):888. doi:10.3390/vaccines10060888.
- Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007;211(2):144–56. doi:10.1002/path.2104.
- Lawton G. You’re only as young as your immune system. New Sci. 2020;245(3275):44–8. doi:10.1016/S0262-4079(20)30646-1.
- Zhang A, Stacey HD, Mullarkey CE, Miller MS. Original antigenic sin: how first exposure shapes lifelong anti–influenza virus immune responses. J Immunol. 2019;202(2):335–40. doi:10.4049/jimmunol.1801149.
- Haaheim LR. Original antigenic sin. A confounding issue? Dev Biol (Basel). 2003;115:49–53.
- Monto AS, Malosh RE, Petrie JG, Martin ET. The doctrine of original antigenic sin: separating good from evil. J Infect Dis. 2017;215(12):1782–8. doi:10.1093/infdis/jix173.
- Ye F, Chen XJ, Guan WD, Pan SH, Yang ZF, Chen RC. Analysis of influenza B virus lineages and the HA1 domain of its hemagglutinin gene in Guangzhou, southern China, during 2016. Virol J. 2018;15(1):175. doi:10.1186/s12985-018-1085-5.
- Chen JM, Guo YJ, Wu KY, Guo JF, Wang M, Dong J, Zhang Y, Li Z, Shu Y-L. Exploration of the emergence of the Victoria lineage of influenza B virus. Arch Virol. 2007;152(2):415–22. doi:10.1007/s00705-006-0852-6.
- Hay AJ, Gregory V, Douglas AR, Lin YP. The evolution of human influenza viruses. Philos Trans R Soc Lond B. 2001;356(1416):1861–70. doi:10.1098/rstb.2001.0999.
- Xu C, Chan KH, Tsang TK, Fang VJ, Fung ROP, Ip DKM, Cauchemez S, Leung GM, Peiris JM, Cowling BJ. Comparative epidemiology of influenza B Yamagata- and Victoria-lineage viruses in households. Am J Epidemiol. 2015;182(8):705–13. doi:10.1093/aje/kwv110.
- Barnes M, Heywood AE, Mahimbo A, Rahman B, Newall AT, Macintyre CR. Acute myocardial infarction and influenza: a meta-analysis of case-control studies. Heart. 2015;101(21):1738–47. doi:10.1136/heartjnl-2015-307691.
- García-Lledó A, Rodríguez-Martín S, Tobías A, García-de-Santiago E, Ordobás-Gavín M, Ansede-Cascudo JC, Alonso‐Martín J, De Abajo FJ. Relationship between influenza, temperature, and type 1 myocardial infarction: an ecological time-series study. J Am Heart Assoc. 2021;10(8):e019608. doi:10.1161/JAHA.120.019608.
- Blackburn R, Zhao H, Pebody R, Hayward A, Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004-2015. Clin Infect Dis. 2018;67(1):8–17. doi:10.1093/cid/cix1144.
- Boehme AK, Luna J, Kulick ER, Kamel H, Elkind MSV. Influenza-like illness as a trigger for ischemic stroke. Ann Clin Transl Neurol. 2018;5(4):456–63. doi:10.1002/acn3.545.
- de Abajo FJ, Rodríguez-Martín S, Barreira D, Rodríguez-Miguel A, Fernández-Antón E, Gil M, García-Lledó A. Influenza vaccine and risk of acute myocardial infarction in a population-based case–control study. Heart. 2022 de junio de 10;108(13):1039–45. doi:10.1136/heartjnl-2021-319754.
- Fröbert O, Götberg M, Erlinge D, Akhtar Z, Christiansen EH, MacIntyre CR, Oldroyd KG, Motovska Z, Erglis A, Moer R, et al. Influenza vaccination after myocardial infarction: a randomized, double-blind, placebo-controlled, multicenter trial. Circulation. 2021;144(18):1476–84. doi:10.1161/CIRCULATIONAHA.121.057042.
- Macias AE, McElhaney JE, Chaves SS, Nealon J, Nunes MC, Samson SI, Seet BT, Weinke T, Yu H. The disease burden of influenza beyond respiratory illness. Vaccine. 2021;39:A6–14. doi:10.1016/j.vaccine.2020.09.048.
- Modin D, Claggett B, Køber L, Schou M, Jensen JUS, Solomon SD, Vardeny O, Knop FK, Nielsen SD, Fralick M, et al. Influenza vaccination is associated with reduced cardiovascular mortality in adults with diabetes: a nationwide cohort study. Diabetes Care. 2020;43(9):2226–33. doi:10.2337/dc20-0229.