ABSTRACT
The COVID-19 epidemic in December 2019 had a significant negative impact on people’s health and economies all across the world. The most effective preventive measure against COVID-19 is vaccination. Therefore, the development and production of COVID-19 vaccines is booming worldwide. This study aimed to analyze the current state of that research and its development tendency by bibliometrics. We conducted a thorough search of the Web of Science Core Collection. VOSviewer1.6.18 was used to perform the bibliometric analysis of these papers. A total of 6,325 papers were finally included. The USA maintained a top position worldwide. Shimabukuro Tom T and Harvard University were the most prolific author and institution. The Vaccines was the most published journal. The research hotspots of COVID-19 vaccines can be classified into vaccine hesitancy, vaccine safety and effectiveness, vaccine immunogenicity, and adverse reactions to vaccines. Studies on various vaccination types have also concentrated on efficacy against continuously developing virus strains, immunogenicity, side effects, and safety.
Introduction
The SARS-COV-2 virus infection that produced COVID-19, which initially manifested in Wuhan, China in December 2019 and was declared as a pandemic by the World Health Organization (WHO) in March 2020, spread quickly and globally.Citation1,Citation2 The epidemic, which started in 2019, has had a catastrophic impact on t people’s health all throughout the world.Citation3 SARS-CoV-2 was a single-stranded positive‐sense RNA virus which was extremely contagious and dangerous.Citation4,Citation5 The risk of transmission and exposure to SARS-CoV-2 depended on many factors, containing the route of disease transmission, patient characteristics, and environmental factors.Citation6 The most common clinical symptoms of COVID-19 include fever, cough, muscle soreness, and fatigue, as well as gastrointestinal symptoms such as anorexia, nausea, vomiting, and diarrhea.Citation7 It can also cause multiple organ damage, such as respiratory, circulatory, digestive, or nervous system failure.Citation8 To date, it has caused more than 650 million infections and more than 6.6 million deaths globally.Citation9 While social distancing, wearing masks, and washing hands can also mitigate and prevent the further spread of SARS-CoV-2,Citation10 we currently considered vaccination the most effective preventive intervention against COVID-19 and the best way to prevent serious illness.Citation11,Citation12 COVID-19 vaccination claimed to stop the epidemic with equitable access and optimum vaccination in all nations of the globe.Citation13
Vaccines were biological agents that gave recipients active immunity against a specific disease,Citation14 and it can be achieved by stimulating an immune response to an antigen (a molecule found on a pathogen).Citation13 To draw up a vaccine, it must provide complete information on the characteristics of antigens, adjuvants, vaccine production, and delivery systems.Citation14 On January 11, 2020,the genetic sequence -for SARS-CoV-2, the coronavirus that causes COVID-19, was issued.Citation15 On March 16,Moderna’s mRNA-based SARS-CoV-2 drug candidate entered Phase 1 clinical trials.Citation16 Because COVID-19 was novel to humans and little is known about the nature of the protective immune response, it is necessary to develop vaccination platforms and strategies parallelly to determine which vaccine approaches would be most effective.Citation17 To assist in the creation of a vaccine against COVID-19, the Alliance for Epidemic Preparedness Innovation (CEPI) collaborated with intern e acquired in the previous emergence of SARS-CoV-1 and MERS-CoV helped the SARS-CoV-2 vaccine advance quickly.Citation18 The technology platform for COVID-19 vaccine development was diverse, including messenger RNA, protein subunits, non-replicating viral vectors, inactivated viruses, live attenuated viruses, deoxyribonucleic acid, virus-like particles, and replicating viral vectors.Citation15,Citation19 Depending on the latest global statistics, they have developed a total of 242 vaccine candidates so far using a variety of platforms. It officially authorized 50 of them, 66 of which were in phase I clinical trials, 72 of which were in phase II, 92 of which were in phase III, and 12 of which were no longer advancing. In addition, 201 countries have authorized 11 vaccines that the World Health Organization added to its Emergency Use List (EUL). It was available to get the data at https://covid19.trackvaccines.org/vaccines/. Depending on reports on vaccination received by the World Health Organization, as of 21 December 2022, 13008,560,983 doses of vaccine had been administered globally.Citation9 However, the emergence of different SARS-CoV-2 variants has raised concerns about the reduced effectiveness of neutralizing antibodies and/or cell-mediated immunity induced by existing vaccines.Citation20 Studies have shown that boosted vaccination, especially heterologous booster vaccine, provided superior protection against SARS-CoV-2 infection.Citation21 It was also important to note that many people are hesitant to get vaccinated, potentially because of side effects.Citation22 Therefore, overcoming vaccination reluctance is still a difficult task.
Numerous publications on COVID-19 vaccinations have been published as vaccine development and vaccination coverage have increased. Bibliometrics was a method that combined applied mathematics and statistics with the analysis of publications in a particular field. It can not only analyze and grasp the current situation and hot spots, but also evaluate the distribution of countries, authors, institutions, and journals within the field and establish the foundation for the future development and direction of research.Citation23,Citation24 This study aimed to reflect on the current issues and future development direction, explain the current hot spots and research status by extensive bibliometrics analysis, and give reference value for following research.
Methods
Data source and search strategy
The Web of Science Core Collection database was used as the data source for this study, and on November 10, 2022, a thorough search of the database was conducted. Appendix 1 provided specific search strategies. In addition, we finished the literature search in one day to prevent the divergence brought on by too rapid data updates.
In addition, we used the website covid19.who.int/ (accessed on 21 December 2022) to find out the number of confirmed cases, fatalities, and doses of the COVID-19 vaccine administered globally to date.Covid19.trackvaccines.org/ (accessed on 15 December 2022) was used to collect vaccine development situation, including the type of vaccine, the primary developers, and national approval. Moreover, lozierinstitute.org/,airtable.com/, and zh.herongyang.com websites that were viewed on December 15, 2022 were utilized to determine the vaccine’s place of origin.
Study selection
We included documents related to COVID-19 vaccines between January 2020 and 10 November 2022. 6 325 of the 9 943 Chinese and English publications that were found matched the requirements for inclusion. We excluded 3,604 publications (including conference abstracts, editorial materials, letters, book reviews, news, conference papers, notes, and corrections) and we further excluded 14 publications by browsing the titles and abstracts online.
Data extraction and visualization
For further processing, we imported all relevant data retrieved from the Web of Science Core Collection database into Microsoft Excel 2019 and VOSviewer1.6.18. Microsoft Excel 2019 is a software for constructing an evidence map that analyzes and charts basic information about countries, institutions, authors, journals, and different types of vaccines.
In this study, we used VOSviewer1.6.18 to visualize the collaboration of countries, institutions, authors, and co-occurrence of keywords. In the visual map, different nodes represented different elements (countries, authors, institutions, and keywords), the size of nodes reflected the number of publications or the frequency of keyword co-occurrence, The lines between nodes represented cooperative or co-occurrence relationships, and different colors represent different clustering.Citation25,Citation26 The wider the lines, the closer the partnership. CiteSpace6.1.3 was used to draw a dual-map overlay of journals. In addition, the journal impact factor (IF) is derived from the 2021 Journal Citation Report (JCR).
Results
Essential information
Analysis of publication outputs
The number of publications distributed showed the direction of COVID-19 vaccine research.Citation27 The specific year distribution of publications was 181 in 2020, 2,547 in 2021, and 3,572 in 2022. The results showed that the number of published COVID-19 vaccine-related articles increased rapidly from 2020 to 2022, which was closely related to the development of the COVID-19 pandemic. The research also revealed that the BNT162b2 vaccine has the most publications, with 1410, followed by Vaxzevria (430), mRNA-1273 (316), and CoronaVac (219).
Distribution by journals
1000 journals published 6325 publications, of which 353 journals published one article and 242 journals published two, making up 35.3% and 24.2% of the journal, respectively. Only 35 journals published 20 articles or more, accounting for 0.35%. This demonstrated that although several journals were engaged in COVID-19 vaccine research, only a small number of publications had a particular focus on it. The top 25 most influential and active journals published a total of 2,261 articles, accounting for 35.75% of the total publication output, which meant they were authoritative and recognized as mainstream journals in the field of research (). Vaccines (684) was the most prolific journal. The New England Journal of Medicine (19,148) has the most citations (19,148) and the greatest impact factor (IF = 176.082) of any journal. In addition, the top 25 were run by four countries, of which 10 are run by the USA, seven by the UK, seven by Switzerland, and one by South Korea. The dual-map overlay () showed four main reference paths. The published articles mainly covered molecular, biology, immunology, medicine, medical clinical, dermatology, and other fields, while the cited articles mainly focused on molecular, biology, genetics, medicine, health, nursing, sports and other fields.
Figure 1. The dual-map overlay of articles citing on COVID-19 vaccine research (The left side were the citing journal, the right side were the cited journal, and the curve showed the citing relationship).
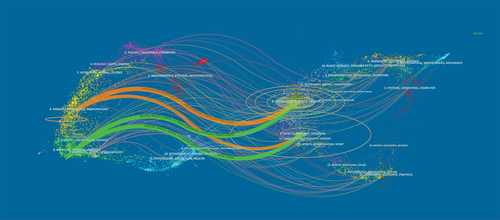
Table 1. The most prolific Journals in COVID-19 vaccine research.
Distribution by countries
162 different countries wrote and published all publications. and showed the top 25 countries in terms of publication volume and citation times. The country that produced the most publications was the USA, followed by China, the UK, Italy, and India. The most cited country was also the USA, followed by the UK, Germany, China, and South Africa. Since the USA, China, and the UK were both the most prolific and the most cited countries, in other words, they were the most influential countries in the field, and the USA was in the lead.
Figure 2. Leading country in COVID-19 vaccine research. (a) Annual output trends of the top 25 producing countries; (b)collaborative network and cluster distribution of countries on COVID-19 vaccines. (Number of publications ≥ 20).
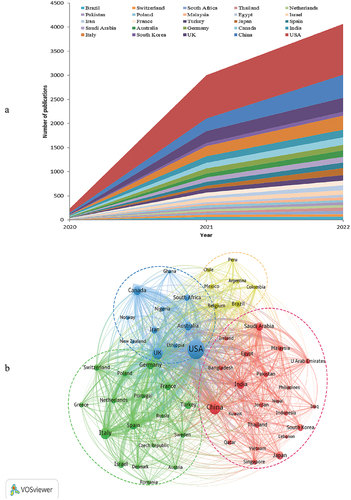
Table 2. Top 25 countries for COVID-19 vaccine research.
Strong international academic cooperation has been demonstrated to have significant positive effects on the scientific community.Citation28 International collaboration in the context of analytical research was highlighted by building a visual map of countries involved in COVID-19 vaccines.Citation29 showed the cooperation network of countries, which consisted of 4 clusters and 57 nodes. The cluster 1(shown in red) mainly included 22 countries. The cluster 2(shown in green) mainly included 17 countries. The cluster 3(shown in blue) mainly included 11 countries . The cluster 4 (shown in yellow) mainly included 7 countries. There was a close cooperative relationship among countries, especially the UK and the USA, followed by China and the USA, Canada and the USA.
Distribution by authors
A total of 36,622 authors participated in the publication of articles in this field, among which 31,442 published only one article, accounting for 85.86%. showed the top 25 authors. Among them, Shimabukuro Tom T. from the USA ranked first with 17 publications. In terms of citations, Lipsitch Marc from the USA ranked first with 2,204 citations.
Table 3. The most prolific authors in COVID-19 vaccine research.
Additionally, showed an author collaboration network visualization built using Vosviewer. 367 authors published at least five articles, but only 65 had coauthored with other authors. was composed of six clusters and 65 nodes, among which cluster 1 (red) was the largest cluster, composed mainly of 18 authors. Cluster 2 (green) included 14 authors, and these two clusters have almost no cooperation with other clusters. Cluster 3 (blue) included 13 authors, and the cooperation was sparse both within and between clusters. Cluster 4 (yellow) included eight authors, cluster 5 (purple) included seven authors, and cluster 6 (bright blue) included five authors, among which the cooperation between yellow and bright blue was relatively close.
Figure 3. Collaborative network and cluster distribution of authors on COVID-19 vaccines. (Number of publications ≥ 5).
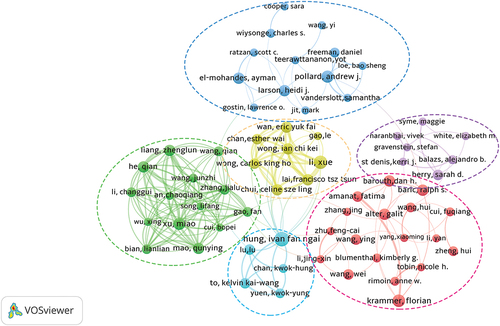
Taken together, the findings suggested that the number of contributing authors in this field was very large and that there was not much communication among authoritative authors.
Distribution by institutions
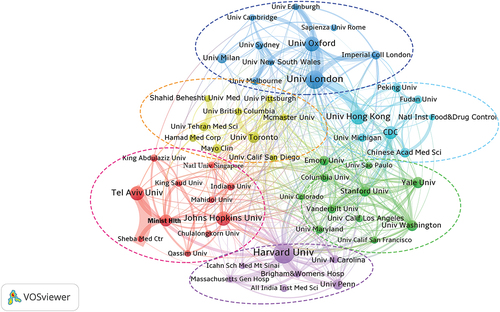
9,098 institutions participated in the research in this field, the most of them in just one study. The top 25 institutions, 21 of which were universities, were displayed in . Four institutions have published more than 100 articles. The most prolific institution was Harvard University in the USA with 175 articles, followed by the University of London in the UK (146), the University of Oxford in the UK (105), and the University of Hong Kong in China (104).
Table 4. Top 10 institutions for COVID-19 vaccine research.
Moreover, showed the collaborations of the institutions through network visualization using VOSviewer. It mainly contained of 6 clusters and 55 nodes. The institutions in the red cluster, such Tel Aviv University, were mostly located in Asia, but Stanford University and Harvard University were found in the green and purple clusters, respectively, and were mostly found in the United States. The three clusters’ institutions were strongly connected to one another. The majority of the institutions in the bright blue cluster were Chinese, as opposed to the majority of British institutions in the blue cluster. Along with the partnership between the clusters, the collaboration between the Universities of Hong Kong and London is also highlighted. Institutions in the yellow cluster were also more likely to carry out autonomous regional research than global research.
Topic analysis
Aggregate analysis
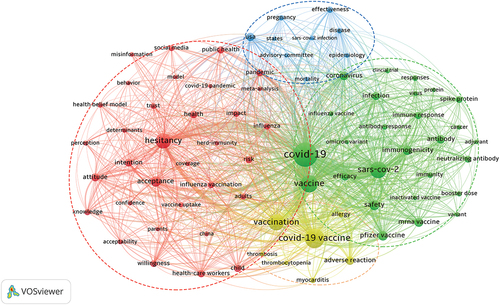
10582 COVID-19 vaccine research hotspots keywords were extracted from 6325 publications. In , 76 keywords that appeared more than 55 times are included and classified into four clusters in the map: The red cluster included 33 keywords such as hesitancy, acceptance, intention, attitude, willingness, health workers, children and adults, which mainly tended to study the attitudes of different populations toward vaccination against COVID-19. The green cluster included 27 keywords such as COVID-19, vaccine, SARS-COV-2, safety, efficacy, immunogenicity, Pfizer vaccine, mRNA vaccine, spike protein and antibody, and it mainly focused on the safety, efficacy and immunogenicity of different types of vaccines. The blue cluster included nine keywords such as USA, mortality, disease, pregnant women, and SARS-COV-2 infection, which mainly focused on the mortality rate of pregnant women infected with SARS-COV-2 in the United States during the COVID-19 pandemic. The yellow cluster included seven keywords, including COVID-19 vaccine, vaccination, adverse reactions, thrombosis, myocarditis, allergy and thrombocytopenia, which mainly involved the adverse reactions of COVID-19 vaccine. To sum up, the research hotspots of COVID-19 vaccines can be classified into vaccine hesitancy, vaccine safety and effectiveness, vaccine immunogenicity, and adverse reactions of vaccines.
Based on different types of vaccines
Appendix 2 listed detailed information on 47 different types of vaccines (including vaccine types, main developers, countries of origin, clinical trials, number of countries approved, and number of publications in WOS). The results in Appendix 2 showed that the largest number of countries approved for use are the BNT162b2 vaccine jointly developed by Pfizer and German Biotechnology, and Vaxzevria(AZD1222, ChAdOx1nCoV-19) vaccine jointly developed by Oxford University and AstraZeneca. They were approved for use in 149 countries. Ad26.COV2.S developed by Johnson & Johnson, covilo(BBIBP-CorV) developed by Sinopharm (Beijing), and mRNA-1273 developed by Moderna are approved for use in 113,93,88 countries, respectively. In the sites we looked at, 10 of those 47 vaccines were not approved, SCB 2019, covi-vac, mRNA-1273.211, BNT162C2, BNT162B1, BNT162A1, gx-19 vaccine, INO-4800, ndv hxp s(Butanvac) and CoviVac, respectively.
In terms of clinical trials, 45 vaccines were in Phase 1 trials, 46 were in Phase 2 trials, and 41 were in phase 3 trials. The top 5 vaccines in the number of trials were BNT162b2, Vaxzevria(AZD1222, ChAdOx1 nCoV-19), mRNA-1273, covilo(BBIBP-CorV) and coronavac. In terms of the number of articles published in Web of Science, the top 5 were BNT162b2, Vaxzevria(AZD1222, ChAdOx1 nCoV-19), mRNA-1273, coronavac, and Ad26.COV2.S, respectively. In addition, there were 10 vaccines for which no studies were available in the database. In other words, due to the limited number of publications, we constructed a visual network map for only the top 9 vaccines published.
A total of 1410 publications related to the BNT162b2 vaccine were retrieved from the Web of Science Core Collection database, and we constructed a co-occurrence cluster graph for 56 keywords with co-occurrence frequency ≥ 15 (Appendix Figure A1a), which mainly contained five clusters. The red cluster mainly tended to study the safety and side effects of vaccines. Green cluster was mainly about the impact of vaccines on dialysis patients. Blue cluster mainly studied the effectiveness of the vaccine against SARS-CoV-2 variant strains. Purple cluster mainly focused on the immunological research of vaccines. Yellow cluster mainly studied the serological immune response of the booster dose of vaccine.
There are 430 publications on the ChAdOx1 nCoV-19 vaccine in the Web of Science Core Collection database. Appendix Figure A1b was a co-occurrence network diagram composed of 31 keywords with co-occurrence frequency ≥ 2, which mainly included 4 clusters. The red cluster mainly involved vaccine safety and immunological research. Green, yellow, and blue mainly studied the side effects of the ChAdOx1 nCoV-19 vaccine.
316 papers referencing mRNA-1273 as a whole were retrieved. Using VOSviewer, a cluster map for 32 keywords with a keyword frequency of 2 was created in Appendix Figure A1c, which largely contained four clusters. The mRNA-1273 vaccine’s immunogenicity, safety, and side effects were the focus of the study. 219 Coronavac vaccination papers in all were located. Five clusters were included, as seen in Appendix Figure A2a. While the green cluster and yellow cluster primarily examined the antibody response to the Coronavac vaccine, the red cluster primarily focused on the immunogenicity of the Coronavac vaccine against SARS-CoV-2 variant strains. The purple and blue clusters focused on research on the safety and effectiveness of the Coronavac vaccine.
In relation to the Ad26.COV2.S vaccine, 88 articles have been made. The cluster graph in Appendix Figure A2b had 4 clusters and 26 keywords with a co-occurrence frequency of 2. In addition to illustrating the immunological and efficiency studies of the Ad26.COV2.S vaccine, this figure also showed a number of negative events, including cerebral venous thrombosis and thrombocytopenia.
There were 79 studies on the Covishield vaccine. Appendix Figure A2c contained four clusters. The red cluster focused on the study of people’s attitude toward Covishield vaccine, the green cluster mainly studied the antibody reaction of Covishield vaccine, the blue cluster was related to the efficacy of Covishield vaccine, and the yellow cluster was mainly related to the adverse reaction of Covishield vaccine.
A total of 66 Covilo-vaccine-related studies were found. Appendix Figure A3a was a keyword co-occurrence network constructed by using VOSviewer, which mainly included four clusters. The red cluster mainly involved the safety and immunokinetic of the Covilo vaccine for adolescents. The green cluster mainly studied the effect of a booster dose of Covilo vaccine on SARS-CoV-2 variants, while the blue cluster mainly included multiple sclerosis and adverse reactions, which are mainly related to adverse reactions of Covilo vaccine. Yellow cluster mainly focused on immunological determination of the vaccine by health care workers.
The Covaxin vaccine has been studied in 66 studies. We constructed a cluster graph (Appendix Figure A3b) for 31 keywords whose co-occurrence frequency was greater than or equal to 2, which contained 3 clusters altogether. Clusters shown in red focus on vaccine side effects, green clusters relate to the efficacy of the vaccine against the SARS-CoV-2 variant, and blue clusters study vaccine rejection.
There were 38 studies on the Sputnik V vaccine. Appendix Figure A3c showed the cluster graph constructed by us for 23 keywords with co-occurrence frequency ≥ 2, including three clusters. The red clusters focused on the vaccine’s efficacy against Delta variants, the green cluster on immunology, and the blue cluster on safety and side effects.
Discussion
Research status
As we all know, the COVID-19 pandemic has had a severe impact on people’s health and economies around the globe. Vaccination has been a major achievement and has become a global strategy for the effective prevention of infectious diseases and proactive control of emerging pathogens.Citation30 And the continued evolution and escape of the SARS-CoV-2 variant highlighted the importance of developing a vaccine. To effectively respond to the COVID-19 pandemic, extensive efforts have been made to develop and manufacture the COVID-19 vaccine. An analysis of publications on COVID-19 vaccines showed a significant increase in the overall trend of research, possibly due to the emergence of evolving variants and the reduced protection of existing vaccines against newly emerging variants, prompting researchers to investigate this area.
To explore the current research status of the COVID-19 vaccine, this paper conducted a bibliometric analysis and visualization of published studies on the COVID-19 vaccine. Vaccines were the journal that publishes the most research on COVID-19 vaccines. In addition, researches showed that the top 25 journals mainly dealt with medicine and immunology, and 2/5 of the journals were run by the USA. We found after analyzing the top 25 organizations that the majority are related to colleges and that more than half are situated in the USA. Harvard University in the USA and the University of London in the UK, as the most active institutions, were playing a more important role than other institutions in COVID-19 vaccine research. In general, some American and British institutions and authors had a prominent position in the research process and had particular advantages in this field. Research on COVID-19 vaccines was spread around the world, but there were still some differences and imbalances. The USA played a crucial role in the research of the COVID-19 vaccine, ranking first in the number of publications, which was consistent with the study of Chen Y et al.Citation31 This may be related to solid medical research conditions in the USA, which included adequate funding, cutting-edge technology, and professional researchers.Citation31 Because research has shown that funding was one of the potential mechanisms for encouraging and boosting productivity.Citation32 In addition, the network diagram showed that the primary author and the affiliated institution were interrelated but not closely, which may be one of the reasons for the lower research efficiency. Due to the ability to exchange information and the findings of scientific research, as well as to improve both the number and quality of scientific and technical advancements, collaboration between them should be strengthened in future study.Citation32,Citation33
Research hotspot
According to the keyword cluster analysis, we can find that vaccine hesitancy, vaccine safety and effectiveness, vaccine immunogenicity, and adverse reactions of vaccines has become a research hotspot in this field. Identifying and understanding COVID-19 vaccine hesitancy may contribute to future public health messages.Citation34 The uncertainty of vaccine efficacy and fear of adverse events were the main problems leading to vaccine hesitancy.Citation35 However, knowing the safety of vaccines can reduce public hesitation about vaccination.Citation36 In addition, the topic of how the emergence of different variants may affect known vaccine efficacy has also surfaced in 2022. The global spread of COVID-19 variants has raised widespread public concern about the vaccine effectiveness of these variants.Citation37 Changes or mutations in the virus, while not making the vaccine completely ineffective, can reduce its protection against infection.Citation38 If existing vaccines proved less effective against emerging strains, the composition of the vaccine will need to be changed to prevent these strains.Citation39
According to the analysis of the clustering network diagram of different vaccines, the research directions of different vaccines were basically the same, mainly focusing on the safety, immunogenicity, adverse reactions and effectiveness against new variants of vaccines. In terms of vaccine side effects, injection site pain, fever, muscle aches, and fatigue were the most common adverse effects of almost all vaccines. Although there were some rare complications associated with COVID-19 vaccination, such as myocarditis with mRNA vaccine, thrombosis with thrombocytopenia with Janssen vaccine and AstraZeneca vaccine, and Gulan-Barre syndrome with Janssen vaccine, the benefits of vaccination far outweigh the side effects.Citation39 It was important to note that the continued evolution and evasion of SARS-CoV-2 variants could further compromise the efficacy of current COVID-19 vaccines and lead to a surge in breakthrough infections or re-infections.Citation40 While the presence of the variant reduces the effectiveness of the vaccine, a booster may provide a higher level of protection against severe and fatal diseases.Citation41
Further in-depth inspiration based on the above bibliometric analysis
There were several areas for future development in COVID-19 vaccine: First, due to the impact of the continuous evolution of SARS-CoV-2 variants on the efficacy of current vaccines, we must look for robust T memory cell responses for long-term prevention of COVID-19. Second, the side effects of vaccines are still an important research direction, and further studies are needed in the future to minimize adverse reactions and improve safety and efficacy. Third, parents’ attitudes to vaccinating their children remain a difficult issue, so there is a need to develop vaccines specifically for children in the future. It would be a privilege if our research could provide a valuable reference for decision-making by clinicians, epidemiologists and those developing vaccines.
Limitations
There were some limitations to this study. First of all, we only searched one database (Web of Science), and although the amount of data we analyzed was large enough to highlight the current research status, it cannot be ruled out that available articles only included in other databases (e.g., PubMed, Embase) was missed. In addition, we only included reviews and articles, which may also cause data bias. Finally, this study only included articles published before November 10, 2022, so it will miss some of the most recent research. Therefore, all explanations in this study were due to the consideration of these limitations.
Conclusion
In the immediate aftermath of the outbreak, research on COVID-19 vaccines focused on their safety, efficacy, and immunogenicity. After the rapid development of vaccines, vaccine hesitancy and adverse reactions became the focus of research in this period. Finally, as different variants emerge, researchers lean toward the effectiveness of the current vaccine against the virus variants. Due to the continuous evolution of the SARS-CoV-2 variant and the natural immunity of the variant, the influence of the new variant on the effectiveness of the vaccine has become a major obstacle in this field of research, so the vaccine research and development should be fully promoted in the later stage.
Author contributions
ZT and CX conducted literature search and screening, data integration and mapping, and wrote the first draft. ZT and JT were responsible for the concept and design, and oversaw the study. LC, MZ, JX, QZ, JZ, WL and CS were responsible for data collection and analysis. All authors reviewed and approved the final submitted version.
Supplemental Material
Download Zip (43.4 MB)Supplemental Material
Download PDF (239.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2242747.
Additional information
Funding
References
- Shimohata T. Neuro-COVID-19. Clin Exp Neuroimmunol. 2022;13(1):17–11. doi: 10.1111/cen3.12676.
- Velavan TP, Meyer CG. The COVID-19 epidemic. Trop Med Int Health. 2020;25(3):278–80. doi:10.1111/tmi.13383.
- Toubasi AA, Al-Sayegh TN, Obaid YY, Al-Harasis SM, AlRyalat SAS. Efficacy and safety of COVID-19 vaccines: a network meta-analysis. J Evid Based Med. 2022;15(3):245–62. doi:10.1111/jebm.12492.
- Campos DMO, Fulco UL, de Oliveira CBS, Oliveira JIN. SARS-CoV-2 virus infection: targets and antiviral pharmacological strategies. J Evid Based Med. 2020;13(4):255–60. doi:10.1111/jebm.12414.
- He QY, Shi YM, Tang Q, Xing H, Zhang H, Wang M, Chen XP. Herbal medicine in the treatment of COVID-19 based on the gut–lung axis. Acupunct Herb Med. 2022;2(3):172–83. doi:10.1097/hm9.0000000000000038.
- Li Y, Tan J, Tan S, Zhou Y, Sai B, Dai B, Lu X. Infection rate and factors affecting close contacts of COVID-19 cases: a systematic review. J Evid Based Med. 2022;15(4):385–97. doi:10.1111/jebm.12508.
- Luo X, Lv M, Zhang X, Estill J, Yang B, Lei R, Ren M, Liu Y, Wang L, Liu X, et al. Clinical manifestations of COVID-19: An overview of 102 systematic reviews with evidence mapping. J Evid Based Med. 2022;15(3):201–15. doi:10.1111/jebm.12483.
- Xia M, Pang B, Yi S, Shan X, Deng S, Qin Y, Jiang T, Lu H. Moxibustion for COVID-19: a systematic scoping review. Acupunct Herb Med. 2022;2(3):162–71. doi: 10.1097/HM9.0000000000000044.
- WHO. Coronavirus disease (COVID-19) dashboard [ website]. [accessed 2022 Dec 21]. https://covid19.who.int/.
- Fernandes A, Chaudhari S, Jamil N, Gopalakrishnan G. COVID-19 vaccine. Endocr Pract. 2021;27(2):170–2. doi:10.1016/j.eprac.2021.01.013.
- Holness NA, Powell-Young YM, Torres E, DuBois S, Giger JN. Covid-19, pregnancy, and vaccinations. J Natl Black Nurses Assoc. 2021;32:1–9.
- Hahn WO, Wiley Z. COVID-19 vaccines. Infect Dis Clin North Am. 2022;36(2):481–94. doi:10.1016/j.idc.2022.01.008.
- Ndwandwe D, Wiysonge CS. COVID-19 vaccines. Curr Opin Immunol. 2021;71:111–16. doi:10.1016/j.coi.2021.07.003.
- Noruzi A, Gholampour B, Gholampour S, Jafari S, Farshid R, Stanek A, Saboury AA. Current and future perspectives on the COVID-19 vaccine: a scientometric review. J Clin Med. 2022;11(3):750. doi:10.3390/jcm11030750.
- Thanh Le T, Andreadakis Z, Kumar A, Gómez Román R, Tollefsen S, Saville M, Mayhew S. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–6. doi:10.1038/d41573-020-00073-5.
- Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382(21):1969–73. doi:10.1056/nejmp2005630.
- Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20(10):615–32. doi:10.1038/s41577-020-00434-6.
- Azamor T, Horbach IS, Brito E, Cunha D, Melgaço JG, Silva AMVD, Tubarão LN, Azevedo AS, Santos RT, Alves NDS, et al. Protective immunity of COVID-19 vaccination with ChAdOx1 nCoV-19 following previous SARS-CoV-2 infection: a humoral and cellular investigation. Viruses. 2022;14(9):1916. doi:10.3390/v14091916.
- Wouters OJ, Shadlen KC, Salcher-Konrad M, Pollard AJ, Larson HJ, Teerawattananon Y, Jit M. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397(10278):1023–34. doi:10.1016/S0140-6736(21)00306-8.
- Hadj Hassine I. Covid-19 vaccines and variants of concern: a review. Rev Med Virol. 2022;32(4):e2313. doi:10.1002/rmv.2313.
- Zhang X, Wang Y, Hu C, Xu P, Ma L, Liu L, Sun J, Liu Y, Yang H, Pan F, et al. Effectiveness of a booster dose of COVID-19 vaccines during an outbreak of SARS-CoV-2 Omicron BA.2.2 in China: a case-control study. Hum Vaccin Immunother. 2023;19(1):2194189. doi:10.1080/21645515.2023.2194189.
- Lamprino M, Sachinidis A, Stamoula E, Vavilis T, Papazisis G. COVID-19 vaccines adverse events: potential molecular mechanisms. Immunol Res. 2023;71(3):356–72. doi:10.1007/s12026-023-09357-5.
- Youn BY, Lee SY, Cho W, Bae KR, Ko SG, Cheon C. Global trends of nutrition in cancer research: a bibliometric and visualized analysis study over the past 10 years. Int J Environ Res Public Health. 2022;19(7):4165. doi:10.3390/ijerph19074165.
- Ma D, Yang B, Guan B, Song L, Liu Q, Fan Y, Zhao L, Wang T, Zhang Z, Gao Z, et al. A bibliometric analysis of pyroptosis from 2001 to 2021. Front Immunol. 2021;12:731933. doi:10.3389/fimmu.2021.731933.
- Cheng K, Guo Q, Yang W, Wang Y, Sun Z, Wu H. Mapping Knowledge landscapes and emerging trends of the links between bone metabolism and diabetes mellitus: a bibliometric analysis from 2000 to 2021. Front Public Health. 2022;10:918483. doi:10.3389/fpubh.2022.918483.
- Zhang J, Song L, Jia J, Tian W, Lai R, Zhang Z, Li J, Ju J, Xu H. Knowledge mapping of necroptosis from 2012 to 2021: a bibliometric analysis. Front Immunol. 2022;13:917155. doi:10.3389/fimmu.2022.917155.
- Akintunde TY, Chen S, Musa TH, Amoo FO, Adedeji A, Ibrahim E, Tassang AE, Musa IH, Musa HH. Tracking the progress in COVID-19 and vaccine safety research - a comprehensive bibliometric analysis of publications indexed in Scopus database. Hum Vaccin Immunother. 2021;17(11):3887–97. doi:10.1080/21645515.2021.1969851.
- Xiong HY, Zhang ZJ, Wang XQ. Bibliometric analysis of research on the comorbidity of pain and inflammation. Pain Res Manag. 2021;2021:6655211. doi:10.1155/2021/6655211.
- Maniu I, Costea R, Maniu G, Neamtu BM. Inflammatory biomarkers in febrile seizure: a comprehensive bibliometric, review and visualization analysis. Brain Sci. 2021;11(8):1077. doi:10.3390/brainsci11081077.
- Li X, Wichai N, Wang J, Liu X, Yan H, Wang Y, Luo M, Zhou S, Wang K, Li L, et al. Regulation of innate and adaptive immunity using herbal medicine: benefits for the COVID-19 vaccination. Acupunct Herb Med. 2022;2(3):196–206. doi:10.1097/hm9.0000000000000046.
- Chen Y, Cheng L, Lian R, Song Z, Tian J. COVID-19 vaccine research focusses on safety, efficacy, immunoinformatics, and vaccine production and delivery: a bibliometric analysis based on VOSviewer. Biosci Trends. 2021;15(2):64–73. doi:10.5582/bst.2021.01061.
- Musa HH, Musa TH. A systematic and thematic analysis of the top 100 cited articles on mRNA vaccine indexed in Scopus database. Hum Vaccin Immunother. 2022;18(6):2135927. doi:10.1080/21645515.2022.2135927.
- Yang KL, Jin XY, Gao Y, Xie J, Liu M, Zhang JH, Tian JH. Bibliometric analysis of researches on traditional Chinese medicine for coronavirus disease 2019 (COVID-19). Integr Med Res. 2020;9(3):100490. doi:10.1016/j.imr.2020.100490.
- Nazlı ŞB, Yığman F, Sevindik M, Deniz Özturan D. Psychological factors affecting COVID-19 vaccine hesitancy. Ir J Med Sci. 2022;191(1):71–80. doi:10.1007/s11845-021-02640-0.
- Sirikalyanpaiboon M, Ousirimaneechai K, Phannajit J, Pitisuttithum P, Jantarabenjakul W, Chaiteerakij R, Paitoonpong L. COVID-19 vaccine acceptance, hesitancy, and determinants among physicians in a university-based teaching hospital in Thailand. BMC Infect Dis. 2021;21(1):1174. doi:10.1186/s12879-021-06863-5.
- Dadras O, Mehraeen E, Karimi A, Tantuoyir MM, Afzalian A, Nazarian N, Mojdeganlou H, Mirzapour P, Shamsabadi A, Dashti M, et al. Safety and adverse events related to inactivated COVID-19 vaccines and Novavax; a systematic review. Arch Acad Emerg Med. 2022;10(1):e54. doi:10.22037/aaem.v10i1.1585.
- Zhou Z, Zhu Y, Chu M. Role of COVID-19 vaccines in SARS-CoV-2 variants. Front Immunol. 2022;13:898192. doi:10.3389/fimmu.2022.898192.
- Risk M, Shen C, Hayek SS, Holevinski L, Schiopu E, Freed G, Akin C, Zhao L. Comparative effectiveness of coronavirus disease 2019 (COVID-19) vaccines against the delta variant. Clin Infect Dis. 2022;75(1):e623–e9. doi:10.1093/cid/ciac106.
- Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28(2):202–21. doi:10.1016/j.cmi.2021.10.005.
- Wang Q, Iketani S, Li Z, Liu L, Guo Y, Huang Y, Bowen AD, Liu M, Wang M, Yu J, et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 2023;186(2):279–86.e8. doi:10.1016/j.cell.2022.12.018.
- Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O’Connell AM, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–46. doi:10.1056/nejmoa2119451.