ABSTRACT
The incidence and mortality rates of renal cell carcinoma (RCC) have been increasing annually due to obesity and environmental pollution. Although immunotherapy of RCC has been studied for decades, few comprehensive bibliometric analyses exist on the treatment. Therefore, the purpose of this bibliometric analysis was to identify scientific achievements of the global research on RCC immunotherapy from 2003 to 2022 and discuss research trends. Data were retrieved from the Clarivate Web of Science Core Collection using a set retrieval strategy. The Bibliometrics tool Cite Space 6.2 R2 (Chaomei Chen, Drexel University) was used to analyze 4,841 articles. The USA had the most publications (n = 1,864); Harvard University was identified as the leading institution (n = 264); and Dr. Toni K. Choueiri, was the most productive researcher in the field (n = 55). Keyword analysis showed that nivolumab, immune checkpoint inhibitors, tumor microenvironment, everolimus, cabozantinib, resistance, pembrolizumab and ipilimumab were the main hotspots and frontier directions of RCC. By analyzing the results of bibliometrics, national and international researchers can better understand the current research status of RCC immunotherapy and identify new directions for future research. However, the analysis also identified pockets of insularity, highlighting a need for greater collaboration and cooperation among researchers to advance the field of RCC immunotherapy.
Introduction
Renal cell carcinoma (RCC) includes various malignancies caused by nephrons,Citation1 and accounts for about 90% of all renal malignancies.Citation2 As the most common form of kidney cancer, RCC accounts for nearly 3% of all cancers globally.Citation3 Unfortunately, incidences of RCC have been gradually increasing recently due to factors such as population aging, obesity, and environmental pollution.Citation4 While different countries and regions, worldwide report the incidence of RCC in men is significantly higher than that in women, about twice as high,Citation5 the overall incidence of RCC is usually regional dependent, with North America and the Czech Republic demonstrating a high incidence of the disease.Citation6 According to the American Cancer Society (2022), it is estimated that approximately 79,000 new cases of kidney cancer will be diagnosed in the USA in 2022, and nearly 13,920 people will die from kidney cancer.Citation4 Approximately 20%-30% of patients with RCC are diagnosed at the metastatic stage, and 20% relapse after initial treatment.Citation7
Current research indicates the most important treatment for RCC is surgical resection, which can be divided into partial resection or radical resection depending on the tumor size.Citation8 In the cytokine era, cytoreductive nephrectomy is recommended for patients with good partial resection,Citation9 which is still a second-line option for patients with local symptoms of the primary tumor or near complete response to systemic therapy.Citation8
Later, vascular endothelial growth factor (VEGF) targeting drugs, such as bevacizumab, sunitinib and pazopanib, significantly improved progression-free survival (PFS) of kidney cancer patients,Citation10–12 and subsequently produced second-and third-generation targeted drugs.Citation13
Over the decades, the therapeutic strategies of RCCs have evolved from the first nonspecific immune approaches (the cytokine era) to targeted therapies targeting vascular endothelial growth factor (VEGF). With the emergence of PD-1 inhibitors, PD-L1 inhibitors and CTLA4 inhibitors, new therapeutic methods and concepts continue to emerge.
The new era of immunotherapy drugs,Citation14 significantly prolongs the overall survival rate (OS) of patients with advanced RCC.Citation15 Although these treatments have effectively improved patients’ survival rates, their success is limited by resistance to the drug and other adverse reactions.Citation15 Therefore, there is an urgent need to develop new treatment strategies to overcome this problem and improve the success rate of treatment in patients with RCC. With the introduction of next-generation VEGF targeting therapy and immunotherapy, the therapeutic landscape of RCC is changing in favor of combination therapy with targeted drugs and immunotherapy. This is an attractive strategy for use in combination with checkpoint inhibitors.Citation16
Indeed, the last decade has seen tremendous in immunotherapy for RCCs. However, these studies have not been systematically measured. While bibliometric analysis has been applied in multiple fields such as urological surgery, and metastatic castration-resistant prostate cancer, only a few bibliometrics studies have focused on RCCs. It is undeniable that there is currently a hot research analysis on immunotherapy for renal cell carcinoma from 2002 to 2021,Citation17 but many articles on immunotherapy for renal cell carcinoma have been published in 2022, including new clinical trial results. The previous article were not included. In order to avoid duplication and balance new hotspots, we chose the period 2003–2022.
Bibliometric analysis involves the use of citation data from databases to evaluate published studies. The process entails the systematic studies and visualization of the knowledge structure and development trend of a scientific field through qualitative and quantitative analysis of the cooperation, co-occurrence, or co-citation of publications.Citation18,Citation19 It is a powerful tool to survey research progress on diverse topics, and their contributors, and assess future research trends. Accordingly, this paper reviews the literature on RCC using bibliometric analysis software. The purpose of this study is to identify characteristics of these articles to provide value to the development of immunotherapy research in RCC, and highlight trends to enhance the research and treatment of patients.
Methods and materials
Database and study criteria
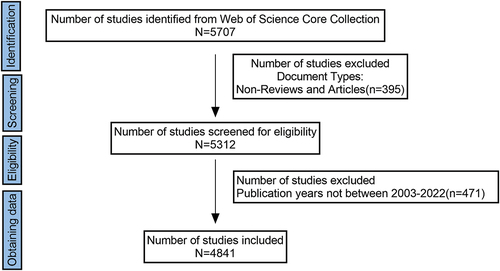
The Clarivate Web of Science Core Collection (WoSCC) is a best-in-class database of over 10,000 high-quality journals and the most commonly used database in bibliometrics research. In this study, only WoSCC’s Science Citation Index extension database was searched. To avoid data bias, a literature search of original articles and reviews was conducted independently on April 10, 2023, for papers published from 2003 to 2022. The search terms were as follows: #1.TS = ((renal) OR (kidney) OR (nephroid)) AND TS = ((cancer*) OR (tumor*) OR (tumour*) OR (oncology) OR (neoplasm*) OR (carcinoma*)); # 2. TS = ((immunotherapy) OR (immunotherapies) OR (immunotherapeutic)); Final data sources #1AND #2, from 2003 to 2022; Types of literature: articles and reviews. The research flow chart is shown in .
Visualization and statistical analysis
After acquiring a clustering network of 4,841 articles, bibliometrics analysis software (CiteSpace 6.2.R2) was used to analyze the overall information, trends, and emerging hotspots of RCC immunotherapy in the past 20 years, including authors, countries, institutions, reference journals, references, reference authors and keywords. Data extraction and analysis were managed separately to ensure data accuracy and reliability.
Microsoft Office Excel 2019 (Microsoft, Redmond, WA, USA) was the primary software used to analyze the data and visualize the number of publications and total citations from 2003 to 2022. Collaboration between countries or regions was demonstrated using the Bibliometrix package in R software (version 4.2.1) and the online bibliometric analysis platform (http://bibliometric.com/). Visualization was carried out mainly through CiteSpace 6.2 R2 (Leiden University, Leiden, Netherlands), which identified authors, countries, institutions, and keyword co-occurrence networks. In the graphics generated by the software, each dot represented an element, including author, country, institution and keyword, and the size of the element was represented by the size of the dot. In addition, the connections between points represented co-occurrence or co-citation relationships, while the number of interconnections increased the thickness of cooperation, which represented the strength of co-occurrence or co-citation. In the co-citation graph, different points represented different co-citation literatures/journals/authors, and the size of points was proportional to the citation times of publications. The lines between the points represented the co-citation relationship. Different colored dots and lines represented different clusters of galaxies or years. The parameters of CiteSpace6.2R2 were set as follows: Time slice (2003–2022), Year (4), Time-slicing (2003–2033), Year per slice (4), Term source (all selected), Selection criteria (top 100) and pruning (Pathfinder, Pruning sliced networks and Pruning the merged network). The size of each dot in the graph indicated frequency or was referenced. The thickness and color of the ring on each point indicated the number of occurrences or references and the corresponding time period.
Journal impact factors(IF) were derived from the 2021 Journal Citation Reports (JCR). Informed consent and ethical approval were not required for this study as the analysis comprised secondary data.
Results
General situation description
Analysis of annual publications and citation trends
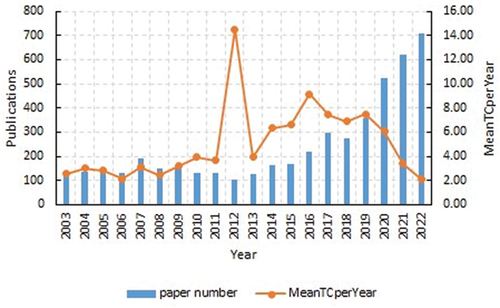
From 2003 to 2022, WOSCC published 4,841 papers related to RCC immunotherapy, including 3,429 ARTICLES (70.83%) and 1,412 REVIEWS (29.17%). This literature originated from 62 countries/regions and 5,273 institutions. The annual publication volume of RCC immunotherapy during 2003–2022 is shown in . This was divided into two periods, namely a period of sustained stability (2003–2012) and a period of rapid growth (2012–2022). Before 2012, the growth of publications was relatively slow, but after 2012, the growth rate increased, and 2019 sew a second acceleration period. The number of papers on RCC immunotherapy in 2022 was the highest in the last decade, reaching 4,841 by 2022, with a significant continuous increase. The corresponding annual citation volume increased annually from 2003 to 2012, and showed an upward zigzag trend. ()
Co-authorship: countries, institutions, and authors
shows the geographic distribution of RCC immunotherapy research; 62 countries made significant contributions to RCC immunotherapy research (, ). The top five countries for paper production were the USA (1,864), the People’s Republic of China (815), Italy (497), Germany (482) and France (390). Regarding centrality, the top five countries were Jordan (1.09), the Czech Republic (0.94), Serbia (0.8), Russia (0.61), and Finland (0.36). The cooperative relationship between research institutions is shown in . As shown in , the most prolific institutions for publications were Harvard University (264 publications), followed by the University of Texas (247), Udice-French Research University (233), UNICANCER (191), and the University of California System (190). The Harvard Research Institute dominated with the most published papers among all research institutions. However, the institution was not highly centralized and rarely cooperated with other research institutes.
Figure 3. (a) Geographic distribution map based on the total publications of different countries/regions. (b) Co-authorship between countries. (c) Co-authorship between institutions.
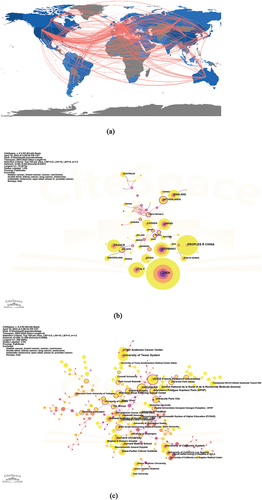
Table 1. Top 10 co-authorship: countries, institutions, and authors.
Table 2. The top 10 countries with high centrality value.
The collaboration between authors is shown in . shows that Dr. Toni K Choueiri from Dana Farber Cancer Institute, Boston, USA, ranked first with 55 papers. Professor David F Mcdermott ranked second with 49 papers, followed by Dr. Matteo Santoni(47 papers), Dr. Francesco Massari (45 papers) and Professor Camillo Porta (36 papers). Among them, Dr.Choueiri also ranked the highest in centrality, contributing immensely to the advancement and development of research in this field.
Co-citation: journals, authors, and references
This study reviewed the progress of RCC immunotherapy through literature analysis. Co-cited literature or document coupling is the frequency with which papers were cited by two other publications simultaneously, and co-cited by the author/journal from the co-cited literature. Co-citation analysis demonstrates the development and evolution of a certain discipline, by classifying the literature in clusters to find research hot spots. The most cited literature was analyzed, and CiteSpace 6.2R2 software was used to visualize the literature co-citation network. summarizes the top 10 co-cited literatures. As can be seen, Nivolumab versus Everolimus in advanced renal cell carcinoma authored by Dr. Robert J Motzer and colleagues (2015) and published in the NEW ENGL J MED was a pivotal article. Dr. Motzer’s had the highest centrality, indicating a significant influence in the field with 527 citations. (see )
Table 3. Top 10 references with highest citations.
The reference co-citation network diagram was composed of 467 nodes and was divided into 19 subclusters. Both the modulus Q and the average profile S were greater than 0.9; the clustering results were significant and the homogeneity was good. Citation outbreaks were used to show the change in popularity and importance in the field over time. Simultaneously, the time axis view of co-cited literature intuitively showed the changing trend of research topics over time (). It was observed that #0 dendritic cells, # 10 allogeneic stem cell transplantation, # 11carbonic anhydrase ix, #13 nephrectomy, and #14 chemotherapy were early subjects of study in this field. The cluster #5 immune checkpoint inhibitor, and #12 nivolumab are located at the far right end of this line and are currently a new research focus.
Figure 4. (a) A timeline view for co-cited references associated with RCC immunotherapy. (color version of figure is available online.) (b) CiteSpace visualization map of top 25 references with the strongest citation bursts from 2003 to 2022.
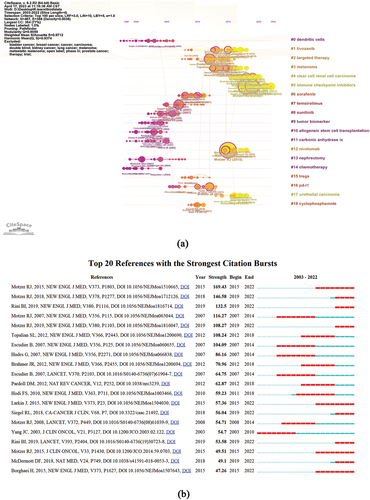
Reference bursts show the change in popularity and importance over time in the field. Based on the results in , the publication by Dr. James C and his colleagues(2003) showed the earliest reference outbreak. Dr Robert J Motzer and colleague’s emerged as a strong reference based on this paper showed the outcome of randomized controlled trials(RCT) on PD-1 inhibitors in the clinic. Among the top 10 most cited journals (), J Clin Oncol (3,350 articles) ranked first. New Engl J Med (3,337) was second, followed by Clin Cancer Res (2,903), Cancer Res (2,478), and Lancet (1,961). lists the 10 most cited authors in RCC immunotherapy studies. Dr. Robert J Motzer and others (2,087 records) ranked first, followed by Dr. BI Rini and others (1,155 records), Dr. Bernard Escudier (974 records), Dr. Toni K Choueiri (874 records), and Professor David F McDermott (713 records).
Table 4. Top 10 journals and authors with highest citations.
Keyword analysis
Citescape obtained a co-occurrence map of key words in RCC immunotherapy studies (). The top 20 keywords are shown in . The top 10 keywords were RCC (1,585 records), immunotherapy (963 records), expression (617 records), survival (615 records), interferon alpha (557 records), sunitinib (485 records), nivolumab (391 records), T-cells (368 records), targeted therapy (338 records), and dendritic cells (307 records). It can be observed that “immunotherapy,” “nivolumab,” “targeted therapy,” “immune checkpoint inhibitor,” “ipilimumab,” and “combination” were the common key words in recent years. In other words, these keywords seem to represent the current research frontier. Citescape 6.2R2 keyword clustering network knowledge map clustered keyword as the item. The value of clustering module Q was 0.865, and the value of average clustering contour S was 0.973, so the clustering was reasonable and convincing. The timeline view of keywords intuitively showed the changing trend of research topics over time (). It was observed that #3 interleukin-2, # 4TKI inhibitor, #8 dendritic cells, and #10 tumor infiltrating lymphocytes were early research subjects in this field. Cluster #0 nivolumab, #2 tumor microenvironment, and #14 everoliums were located at the far right of this line and are new research hotspots in this field.
Figure 5. (a) Keywords co-occurrence map of publications on RCC immunotherapy. (b) A timeline view for keywords associated with RCC immunotherapy. T (c) CiteSpace visualization map of top 25 keywords with the strongest citation bursts of publications in the field of RCC immunotherapy from 2003 to 2022.

Table 5. Top 20 keywords in terms of records.
Mutant words referred to keywords that appeared more frequently or were used more frequently in a short period. This test usually included intensity and age distribution, which reflected the research frontier and development trend over time. CiteSpace also detected the top 25 keywords with the strongest citation outbreak (). When coupled with the keywords with the highest frequency in , by 2021, The keywords with persistent citations associated with RCC immunotherapy were “nivolumab,” “immune checkpoint inhibitor,” “tumor microenvironment,” “everolimus,” “ipilimumab,” “cabozantinib,” “pembrolizumab,” and “long-term safety.” These key keywords were the focus because of their role in identifying the frontiers of RCC immunotherapy research.
Discussion
Analysis of the top ten most popular journals showed that 100% (10/10) had an IF > 10. These were J Clin Oncol (IF 2021 = 50.739), New Engl J Med (IF 2021 = 176.082), Clin Cancer Res (IF 2021 = 13.801), Cancer Res (IF 2021 = 13.312), Lancet (IF 2022 = 202.731), Ann Oncol (IF 2021 = 51.769), Nature (IF 2021 = 69.504), Lancet Oncol (IF 2021 = 54.433), P Natl Acad Sci Usa (IF 2021 = 12.779), and Cience (IF 2021 = 63.832). To summarize, the papers in the field of RCC immunotherapy were mainly published in high-IF journals. These papers were of great significance to the field of RCC immunotherapy research and allowed researchers to better grasp the extent of the research progress made in this field. Compared with the previous article, we not only listed the top 10 journals with the most published articles, but also indicated their influencing factors, making the content more detailed.
Cooperation between countries played an important role in the progress of immunotherapy research in RCCs. The top 10 countries constituted six in Europe, two in North America, and two in Asia (). The USA had more than twice as many publications as China, which stood in second place. Harvard University had the highest value of immunotherapy centrality of RCC. As a result, the USA dominated the global issue of RCC immunotherapy. The research showed trends in collaboration between different countries and institutions. However, there was still a lack of in-depth cooperation between different agencies/countries. These are consistent with the previous article indicating that the United States has made the greatest contribution to RCC immunotherapy research and needs to increase cooperation and communication between countries. However, we found that France and China seem to have become emerging stars in immunotherapy research. Unicancer was a key player in cancer research at the Federation of French comprehensive cancer centers. This group of 20 private, nonprofit medical organizations specialized in oncology, and ranked among the top five in terms of the number of publications. Unicancer also ranked among the top institutions in terms of centrality, thus combining innovation and collaboration. Regarding the number of articles, China was identified among the top two producers, which is a phenomenal achievement for a developing country. The ranking showcases China’s contributions to the research of RCC immunotherapy in the past 20 years.
The most frequently co-cited literature was Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal Cell Carcinoma, published by Dr. Robert Motzer and colleagues in 2018. This paper was a phase 3 RCT of Nivolumab plus Ipilimumab (N+I) versus Sunitinib for advanced RCC. The results showed that the overall survival rate, objective response rate, and progression-free survival in the N+I group were better than those in the Sunitinib group, and the probability of adverse events was also lower than that in the control group. The latest results of this trial were reported in 2020 with a 4-year follow-up.Citation20,Citation21 The second co-citation was published in 2015, i.e., Nivolumab versus Everolimus in advanced RCC by Dr. Robert J Motzer and colleagues in 2015. This was a randomized, open-label Phase 3 study that compared nivolumab and everolimus in the treatment of advanced RCC. The results showed that nivolumab had a longer overall survival and fewer Grades 3 or 4 adverse events than everolimus. Long-term follow-up results of this trial were reported in 2020Citation22,and similar conclusions supported the long-term benefits of nivolumab monotherapy in previously treated RCC patients.
The third co-cited article was Pembrolizumab plus Axitinib versus Sunitinib for advanced RCC published by Dr. BI Rini and others in 2019. This was a Phase 1b RCT of immunotherapy combined with targeted therapy (i.e., pembrolizumab combined with axitinib) in patients with advanced RCC. The researchers found that target therapy had significantly longer overall survival and progression-free survival than sunitinib, and a higher objective response rate. These benefits were independent of and dissociated with programmed death ligand 1 expression. Unfortunately, the incidence of Grade 3 or higher adverse events was higher in the pembrolizumab + axitinib group than in the control group (sunitinib). The phase 3 trial of this experiment was reported in 2020,Citation23 and the conclusion mimicked the Phase 1b study. In a health-related quality of life analysis of this trial reported in 2022Citation24,patient-reported outcome scales showed no difference in outcomes between the pembrolizumab+ axitinib and sunitinib groups, except for the TTcD of FKSI-DRS.
The fourth most commonly cited paper was published in 2019 again by Dr. Robert J Motzer and colleagues. A Phase 1b RCT of immunotherapy in combination with targeted therapy (nivolumb plus axitinib) in patients with advanced RCC showed significantly longer progression-free survival compared with sunitinib, but with comparable rates of adverse risk events. This trial was updated in 2022,Citation25 and showed that nivolumab plus axitinib showed good efficacy in all age groups, including patients ≥75 years of age. Follow-up work is ongoing in this area.
We focused on analyzing the co-cited literatures and provided a detailed interpretation of the top 4 co-cited literature, of which 3 (75%) were new articles from 2022. It was concluded that the current research hotspot for advanced RCC is combination therapy trials, such as dual immunotherapy, immunotherapy combined with targeted drug therapy, which was not well reflected in the previous article and is also an advantage and highlight of this article.This suggested a new trend in RCC immunotherapy.
The timeline showed the transition from the first nonspecific immune approaches (the cytokine era) to targeting vascular endothelial growth factor (VEGF) and now into the era of new immunotherapeutic agents. This was also confirmed in the cited outbreak literature. Compared to the previous article, we have increased our interpretation of the timeline and increased the novelty of the article.
The key-words reflected the core theme and main content of the articles. Therefore, the content is sufficiently valid to describe the research hotspot. shows that interferon alpha, dendritic cells, interleukin 2, survival, and expression were key words used in the early stages of RCC immunotherapy. Keywords such as immunotherapy, immune checkpoint inhibitors, atezolizumab, ipilimumab, everolimus, and pembrolizumab emerged recently, and seem to represent the current research frontier. Among them, atezolizumab, ipilimumab, and pembrolizumab have not received sufficient attention in previous articles, but this is a rising star in immunotherapy.
Treatment strategies of people for cancer have shifted from using simple physical and chemical methods such as surgical resection, radiotherapy, and chemotherapy to developing targeted drugs based on the mechanisms of cancer occurrence and development to fight against cancer. In recent years, significant progress has been made in understanding how cancer escapes the immune system, and cancer immunotherapy has emerged.Citation26 Tumor cells generally evade recognition and clearance by the immune system through four mechanisms: 1) downregulating surface antigen expression to reduce immunogenicity; 2) Upregulation of surface immune checkpoints inhibits t cell activity; 3) Recruiting suppressive immune cells to form an inhibitory immune microenvironment; 4) The acidic and toxic metabolites that release immune cell activity,Citation27 among which the main immunotherapy is the use of immune checkpoint inhibitors. Compared with previous radiotherapy, chemotherapy, and targeted drug therapy, it has achieved exciting benefits while reducing damage to normal cells, but it also inevitably produces corresponding adverse reactions. Currently, immunotherapy is combined with probiotics to enhance efficacy while reducing adverse reactions. There are also immunotherapy combined with radiotherapy and chemotherapy to enhance anti-tumor efficacy, such as PD-1 inhibitors/PD-L1 inhibitors combined with stereotactic therapy for non-small cell lung cancer,Citation28
After surgery and Targeted therapy, the treatment of renal cell carcinoma has entered the era of immunotherapy, showing exciting clinical value and strong therapeutic potential in clinical trials.Immunotherapy studies on RCC mainly focused on pd-1 inhibitors, PD-L1 inhibitors, and combination drugs. In the future these aspects will continue to be important research hotspots. Furthermore, studies on the immune microenvironment have alerted researchers to its important role in immunotherapy. Improving the effectiveness of immunotherapy by regulating immune microcircuits has become increasingly important in the treatment of RCC. Hence, there is high predictability that the study of the tumor microenvironment will be more active in the future. Overall, RCC immunotherapy is anticipated to become a research hotspot and should be monitored closely.
Not unexpectedly, this study has some limitations. 1) The aim was to present the current status of clinical immunotherapy for RCC, by analyzing papers published between 2003 and 2022 that are directly related to this topic. Therefore, papers outside the stated range, and papers that were not considered original research or reviews were excluded. While the abstracts of some basic or clinical publications included keywords were not directly related to the topic. Therefore, they were excluded during the search strategy to ensure the inclusion of papers that fit the research criteria. 2) Literature retrieval was based only on the core data set of the Wo S, so papers not included in this database were omitted leading to possible selection bias and analysis errors. 3) Since it was impossible to read every paper based on the large number of papers in the database only the top-ranked articles were perused. Consequently, it was difficult to identify them quantitatively. 4) Finally, this study focused on clinical research, so important basic research may have been overlooked and clinical aspects such as basic immunology were not discussed.
Conclusion
In summary, this bibliometric analysis identified the course of RCC immunotherapy from 2003 to 2022, and predicted future research hotspots. The analysis highlighted the USA as having the most high-quality publications, with China a close second. Despite being forerunners in this research field, there is a need to strengthen cooperation and collaboration between countries and institutions. Immune checkpoint inhibitors were the most important research hotspot, and mainly included aspects such as combined drug therapy. Based on the research approach researchers can better grasp the research status of RCC immunotherapy and determine new direction for future research. However, the analysis shows a need for more extensive collaboration among research institutions to help guide scientific research activities and optimize cooperation, thus promoting development and progress in the field.
Author contributions
Haiyan Zhu, Xin Wang, and Shihao Lu have contributed to the study of concepts. Haiyan Zhu and Xin Wang analyzed the data, and Haiyan Zhu and Shihao Lu wrote a manuscript. All authors have contributed to this article and approved the submitted version.
Author’s statement
This study was conducted without any commercial or financial relationships, which may be interpreted as potential conflicts of interest.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The original data supporting the conclusion of this article will be provided by the author without any reservations.
Additional information
Funding
References
- Wolf MM, Kimryn Rathmell W, Beckermann KE. Modeling clear cell renal cell carcinoma and therapeutic implications. Oncogene. 2020;39(17):3413–11. doi:10.1038/s41388-020-1234-3.
- Gray RE, Harris GT. Renal cell carcinoma: diagnosis and management. Am Fam Physician. 2019;99:179–84.
- Siddiqi A, Rani M, Bansal P, Rizvi MMA. Renal cell carcinoma management: a step to nano-chemoprevention. Life Sci. 2022;308:120922. doi:10.1016/j.lfs.2022.120922.
- Bukavina L, Bensalah K, Bray F, Carlo M, Challacombe B, Karam JA, Kassouf W, Mitchell T, Montironi R, O’Brien T, et al. Epidemiology of renal cell carcinoma: 2022 update. Eur Urol. 2022;82(5):529–42. doi: 10.1016/j.eururo.2022.08.019.
- Scelo G, Larose TL. Epidemiology and risk factors for kidney cancer. J Clin Oncol. 2018;36(36):Jco2018791905. doi:10.1200/jco.2018.79.1905.
- Singh D. Current updates and future perspectives on the management of renal cell carcinoma. Life Sci. 2021;264:118632. doi:10.1016/j.lfs.2020.118632.
- Wiechno P, Kucharz J, Sadowska M, Michalski W, Sikora-Kupis B, Jonska-Gmyrek J, Poniatowska G, Nietupski K, Ossolinski K, Demkow T, et al. Contemporary treatment of metastatic renal cell carcinoma. Med Oncol. 2018;35(12):156. doi: 10.1007/s12032-018-1217-1.
- Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, Grünwald V, Gillessen S, Horwich A. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(5):706–20. doi:10.1093/annonc/mdz056.
- Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171(3):1071–6. doi:10.1097/01.ju.0000110610.61545.ae.
- McDermott DF, George DJ. Bevacizumab as a treatment option in advanced renal cell carcinoma: an analysis and interpretation of clinical trial data. Cancer Treat Rev. 2010;36(3):216–23. doi:10.1016/j.ctrv.2009.12.003.
- Oudard S, Beuselinck B, Decoene J, Albers P. Sunitinib for the treatment of metastatic renal cell carcinoma. Cancer Treat Rev. 2011;37(3):178–84. doi:10.1016/j.ctrv.2010.08.005.
- Ward JE, Stadler WM. Pazopanib in renal cell carcinoma. Clin Cancer Res. 2010;16(24):5923–7. doi:10.1158/1078-0432.Ccr-10-0728.
- Rini BI, Pal SK, Escudier BJ, Atkins MB, Hutson TE, Porta C, Verzoni E, Needle MN, McDermott DF. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2020;21(1):95–104. doi: 10.1016/s1470-2045(19)30735-1.
- Barata PC, Rini BI. Treatment of renal cell carcinoma: current status and future directions. CA Cancer J Clin. 2017;67(6):507–24. doi:10.3322/caac.21411.
- Braun DA, Bakouny Z, Hirsch L, Flippot R, Van Allen EM, Wu CJ, Choueiri TK. Beyond conventional immune-checkpoint inhibition — novel immunotherapies for renal cell carcinoma. Nat Rev Clin Oncol. 2021;18(4):199–214. doi:10.1038/s41571-020-00455-z.
- Navani V, Heng DYC. Treatment selection in first-line metastatic renal cell carcinoma-the contemporary treatment paradigm in the age of combination therapy: a review. JAMA Oncol. 2022;8(2):292–9. doi:10.1001/jamaoncol.2021.4337.
- Liu K, Zhao S, Li J, Zheng Y, Wu H, Kong J, Shen Z. Knowledge mapping and research hotspots of immunotherapy in renal cell carcinoma: a text-mining study from 2002 to 2021. Front Immunol. 2022;13:969217. doi:10.3389/fimmu.2022.969217.
- Wang H, Shi J, Shi S, Bo R, Zhang X, Hu Y. Bibliometric analysis on the progress of chronic heart failure. Curr Probl Cardiol. 2022;47(9):101213. doi:10.1016/j.cpcardiol.2022.101213.
- Ahmad P, Slots J. A bibliometric analysis of periodontology. Periodontol 2000. 2021;85(1):237–40. doi:10.1111/prd.12376.
- Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthélémy P, Porta C, Powles T, Donskov F, George S, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5(6):e001079. doi: 10.1136/esmoopen-2020-001079.
- Motzer RJ, Escudier B, McDermott DF, Arén Frontera O, Melichar B, Powles T, Donskov F, Plimack ER, Barthélémy P, Hammers HJ, et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J Immunother Cancer. 2020;8(2). doi:10.1136/jitc-2020-000891.
- Motzer RJ, Escudier B, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Plimack ER, Procopio G, McDermott DF, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer. 2020;126(18):4156–67. doi: 10.1002/cncr.33033.
- Powles T, Plimack ER, Soulières D, Waddell T, Stus V, Gafanov R, Nosov D, Pouliot F, Melichar B, Vynnychenko I, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563–73. doi: 10.1016/s1470-2045(20)30436-8.
- Bedke J, Rini BI, Plimack ER, Stus V, Gafanov R, Waddell T, Nosov D, Pouliot F, Soulières D, Melichar B, et al. Health-related quality of life analysis from KEYNOTE-426: pembrolizumab plus axitinib versus sunitinib for advanced renal cell carcinoma. Eur Urol. 2022;82(4):427–39. doi: 10.1016/j.eururo.2022.06.009.
- Tomita Y, Motzer RJ, Choueiri TK, Rini BI, Miyake H, Uemura H, Albiges L, Fujii Y, Umeyama Y, Wang J, et al. Efficacy and safety of avelumab plus axitinib in elderly patients with advanced renal cell carcinoma: extended follow-up results from JAVELIN renal 101. ESMO Open. 2022;7(2):100450. doi: 10.1016/j.esmoop.2022.100450.
- Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125(9):3335–7. doi:10.1172/jci83871.
- Liu C, Yang M, Zhang D, Chen M, Zhu D. Clinical cancer immunotherapy: current progress and prospects. Front Immunol. 2022;13:961805. doi:10.3389/fimmu.2022.961805.
- Chen Y, Gao M, Huang Z, Yu J, Meng X. SBRT combined with PD-1/PD-L1 inhibitors in NSCLC treatment: a focus on the mechanisms, advances, and future challenges. J Hematol Oncol. 2020;13(1):105. doi:10.1186/s13045-020-00940-z.