ABSTRACT
This study aimed to evaluate the immunogenicity non-inferiority and safety of the quadrivalent inactivated split-virion influenza vaccine in participants ≥ 3 years old. A total of 3,328 participants were enrolled. Participants 3–8 years old were administered one or two doses of the investigational vaccine or one dose of the control vaccine, whereas the other participants were administered only one dose of the investigational or control vaccine. The immunogenicity and occurrence of adverse events (AEs) after 30 days of full-course vaccination and serious adverse events (SAEs) within 6 months after full-course vaccination were assessed. The sero-conversion rates (SCRs) of anti-H1N1, H3N2, B(Y), and B(V) antibodies in the test group were 74.64%, 87.40%, 82.66%, and 78.89%, respectively, and their geometric mean titers were 1:250.13, 1:394.54, 1:200.84, and 1:94.91, respectively, which were non-inferior to those in the control group. The SCRs and sero-protection rates in the two-dose group of participants 3–8 years old were greater than those in the one-dose group. The incidences of total AEs and adverse reactions in the test group were 31.6% and 21.7%, respectively, which were close to those in the control group. In the two-dose group, the incidence of adverse reactions was considerably lower in the second dose (5.5%) than in the first dose (14.7%). Most AEs were grade 1 in severity, and no SAEs were recorded. The investigational vaccine had immunogenicity non-inferior to the control vaccine, and two doses were more effective than one dose in participants 3–8 years old, with a good overall safety.
Trial registration: CTR20200715.
Plain Language Summary
People in China are frequently infected by influenza viruses in specific seasons, causing a large burden of disease. Influenza viruses have distinct phenotypes depending on the season. Therefore, vaccines that can effectively prevent the infection of various influenza virus phenotypes need to be developed. The quadrivalent inactivated split-virion influenza vaccine is effective against four influenza virus phenotypes. In this trial, the immunogenicity and safety of the quadrivalent inactivated split-virion influenza vaccine (investigational vaccine) developed by Dalian Aleph Biomedical Co., Ltd. were evaluated. A total of 3,328 participants ≥ 3 years old were included. Participants 3–8 years old were further divided based on the presence or absence of a history of influenza vaccination. Those participants without a vaccination history were administered one or two doses of the investigational vaccine or one dose of a marketed quadrivalent influenza vaccine (control vaccine), and those participants with a vaccination history were administered one dose of the investigational or control vaccine. This study showed for the first time that the immunogenicity and safety of the investigational vaccine were not inferior to those of the control vaccine and that the two-dose procedure induced a good immune effect in the 3–8-year-old group. In conclusion, administration of the investigational vaccine can prevent seasonal influenza in populations aged ≥ 3 years.
Introduction
Human influenza viruses, including influenza A (H1N1 and H3N2) and influenza B (Victoria B (V) and Yamagata B (Y)), cause acute respiratory infectious diseases in humans.Citation1,Citation2 The predominant influenza B strain transmitted in humans approximately 40 years ago was B (Y), but the B (V) strain emerged.Citation3 Afterward, mutations in B (V) and B (Y) caused an annual incidence of relevant strains of 5%–10% in adults worldwide.Citation4 Approximately 3–5 million severe influenza-associated cases and 290,000–650,000 influenza-associated deaths are recorded worldwide each year.Citation5 In addition, the influenza-associated mortality in China was 6.11–18.7/100,000.Citation6
Influenza vaccination is the most effective means of preventing influenza virus infections and their serious complications. Vaccination not only protects vaccine recipients but also indirectly protects unvaccinated individuals in contact with vaccinated ones.Citation7,Citation8 However, when the influenza vaccine-targeted strain does not match the epidemic strain, the protective effect of the vaccine decreases.Citation8,Citation9 Therefore, an influenza vaccine containing antigens of influenza strains of multiple lineages needs to be developed.Citation3 The quadrivalent inactivated split-virion influenza vaccine contains two A lineage and two B lineage strain antigens, which effectively overcome the low vaccine effectiveness caused by influenza B virus mismatch and enhance the protective effect of the vaccine against seasonal influenza without compromising its safety.Citation10,Citation11 For example, a previous study compared the efficacies of the trivalent inactivated influenza vaccine (IIV3) and the quadrivalent inactivated influenza vaccine (IIV4) against influenza B in the US during 2011–2012 and 2016–2017.Citation12 This study showed that the overall efficacy of IIV4 for any influenza B virus strain was 53% (95% confidence interval [CI], 45–59), whereas that of IIV3 was 45% (95% CI, 34–54), and both vaccines had similar safety. In addition, a multi-season immunogenicity and safety analysis of IIV4 in children in the Northern and Southern Hemispheres showed that IIV4 is 50.98% (97% CI, 37.36%–61.86%) effective against influenza A and B and 68.40% effective against vaccine-like strain influenza (97% CI, 47.07%–81.92%), with no significant difference in safety between IIV4 and the placebo and IIV3.Citation13
However, whether the IIV4 (investigational vaccine) of Dalian Aleph Biomedical Co., Ltd. could prevent seasonal influenza in participants ≥3 years old remains unclear. Thus, this clinical trial assessed the non-inferiority of the investigational vaccine by comparing its safety and anti-seasonal influenza immunogenicity with the IIV4 (as the control commercial vaccine) of Hualan Biological Bacterin Inc. in individuals aged ≥3 years. This study provides additional data support for the clinical use of IIV4 in populations aged ≥3 years.
Materials and methods
Study design
In this study, a randomized, double-blind, similar vaccine-positive-controlled, non-inferiority trial was designed to evaluate the immunogenicity and safety of IIV4 (Dalian Aleph Biomedical Co., Ltd, China) in populations aged ≥3 years (China Clinical Trial ID: CTR20200715). The study was conducted from July 16, 2020 (date of enrollment of the first participant) to July 20, 2021 (date of the last visit of the last participant), with data collected at the Guizhou Center for Disease Control and Prevention. No major change to the test method was made from the start of the study, the endpoint indicators were not changed, and no interim analyses, interruptions, or discontinuations were involved. The contract research organization of this trial was the Simoon Record Beijing Co., Ltd., and the testing institution for serum antibodies was the National Institutes for Food and Drug Control. The data manager and statistical analyst were from Beijing Key Tech Statistical Consulting Co., Ltd.
Study population
The eligibility criteria for participants were as follows: (1) participants ≥3 years old with a legal identity certificate available, (2) participants or their guardians voluntarily agreed to participate in the study and signed an informed consent form, and (3) participants can understand the study procedures and participate in all planned visits. The exclusion criteria were as follows: (1) participants who had received any influenza vaccine in the current epidemic season (2019–2020) before enrollment or had planned to receive any influenza vaccine during the study; (2) participants in the 3–8-year-old two-dose group who had received any influenza vaccine in any epidemic season before enrollment; (3) participants who had developed the influenza disease within the past 3 months; (4) participants with a history of severe allergy to any component of the investigational vaccine and any history of vaccine- or drug-related serious adverse reactions; (5) participants who had received other inactivated vaccines within 7 days or attenuated live vaccines within 14 days before enrollment; (6) participants who had received immunosuppressive agents or other immunomodulatory drugs within 3 months before enrollment; (7) participants who were diagnosed with immunodeficiency; (8) participants who had received blood or blood-related products within 6 months before enrollment; (9) participants with a history or a family history of convulsions, epilepsy, encephalopathy, and psychosis; and (10) participants who had any conditions that might interfere with the assessment of study objectives as considered by the investigator.
Randomization
A stratified block randomization method was used in this study, with age as the stratification factor. The participants in the 3–8-year-old group were stratified whether or not they had previously received any influenza vaccine. Those participants who had not previously received any influenza vaccine were randomly assigned to the one-dose, two-dose, and control groups in a 2:2:1 ratio, and those participants who had previously received an influenza vaccine were randomly assigned to the one-dose and control groups in a 2:1 ratio. The participants in the 9–59-year-old group and ≥ 60-year-old group were randomized in a 2:1 ratio to the test and control groups. Randomization assignment numbers (i.e., study number and vaccine number) were generated by the randomization statistician using SAS statistical software. The study numbers were used to identify all procedures that were completed after participant randomization.
Blinding
The blinding method was applied in the 3–8-year-old one-dose group, 9–59-year-old group, and ≥ 60-year-old group. The blinding personnel completed the vaccine labeling and written records as per the randomization number. Emergency letters were prepared through an online unblinding system during blinding. Each letter contained an unblinding randomization password that corresponded to a vaccine number. In addition, both vaccines were repackaged with the same appearance but with different labels.
The two vaccines used in this clinical trial were repackaged to have the same appearance. When the syringes of the investigational and control vaccines were labeled with different colors, several measures were taken to ensure blindness during the study. First, each dispenser box was sealed, and a loose label was placed in the box during blinding to the vaccine. Before vaccination, the vaccinators checked whether the security seal of the vaccine was intact. If the seal was broken, the participant was considered unblinded. Second, the vaccinators were specially assigned and not allowed to participate in other works in this trial. If the external package was opened, the vaccine was considered to have been used. The vaccinators signed a confidentiality agreement and promise not to disclose any blinded information to other personnel, including other investigators and participants. Third, the vaccines were assigned strictly according to the order of enrollment, and the vaccinators were not involved in the assignment of study numbers. Fourth, after opening the inner package in a special room, the vaccinators affixed the loose label to cover the original label of the vaccine on the syringe to ensure that the original label was not visible and then passed it to another vaccinator to vaccinate the participant. Fifth, the vaccinators restored the empty containers/boxes of used vaccines to the original boxes and timely counted and transferred them to the vaccine administrator (signing the confidentiality agreement) for sealing after the completion of the work on the same day. After the clinical trial, the containers/boxes were uniformly transferred by the study site to the medical waste disposal company or the sponsor for destruction, and the destruction record was retained. Last, serum sample testing was performed in a blinded manner.
Study vaccine
The investigational vaccine was the IIV4 manufactured by Dalian Aleph Biomedical Co., Ltd. with a strength of 0.5 mL/vial and shelf life until December 4, 2020. The active ingredients of the investigational vaccine were 15 μg each of H1N1, H3N2, B(V), and B(Y) influenza virus strain hemagglutinin per 0.5 mL. The control IIV4 vaccine with a strength of 0.5 mL/vial and shelf life until September 14, 2020 was purchased from Hualan Biological Bacterin Inc. The active ingredients of the control vaccine were consistent with those of the investigational vaccine.
Sample size
In this trial, a step-down strategy was applied to sequentially conduct the non-inferiority test of sero-conversion rate (SCR) and geometric mean titer (GMT) in the one-dose group of participants ≥3 years old and the lower 95% CI test of SCR and sero-protection rate (SPR) in the test groups of participants 3–59 and ≥60 years old. The overall test level was unilateral α = 0.025, and the power was 97.5% for each test. In addition, with a dropout rate of approximately 15%, 1,460 and 730 participants 3–59 years old were included in the test and control groups, respectively, and 400 and 200 participants ≥60 years old were included in the test and control groups, respectively. Moreover, 540 participants 3–8 years old who had not previously received any influenza vaccine were included. In summary, the total sample size was 3,330 participants.
Study population, grouping, and vaccination
A total of 3,330 participants ≥3 years old were enrolled in the study. Among the 3–8-year-old participants who had not previously received any influenza vaccine, 540 received one or two doses of the investigational vaccine, and 270 received one dose of the control vaccine. Among the 3–8-year-old participants who had previously received an influenza vaccine, 120 and 60 participants were included in the test and control groups, respectively. Eight hundred and 400 participants 9–59 years old were included in the test and control groups, respectively. Four hundred and 200 participants ≥60 years old were included in the test and control groups, respectively.
The participants in the 3–8-year-old two-dose group were administered one dose of the investigational vaccine on days 0 and 28, whereas the other participants were administered one dose of the investigational or control vaccine on day 0 to observe the immunogenicity and safety of the investigational vaccine.
Immunogenicity endpoints
Primary endpoints (30 days after receiving the first dose of the vaccine):
Non-inferiority comparison between groups: The lower two-sided 95% CIs of SCR differences in quadrivalent vaccine hemagglutination inhibition (HI) antibodies (investigational vaccine – control vaccine) were all ≥ 10%, and the lower two-sided 95% CIs of the GMT ratios (investigational vaccine/control vaccine) were all ≥ 0.67.
Evaluation on the lower 95% CI test of SCR and SPR in the in the test group: In the participants 3–59 years old, the lower two-sided 95% CIs of SCRs were ≥ 40%, and the lower 95% CIs of SPRs were ≥ 70%; in the participants ≥60 years old, the lower 95% CIs of SCRs were all ≥ 30%, and the lower 95% CIs of SPRs were all ≥ 60%. This was set based on the “Absolute Criteria” in the “2.2 Immunogenicity Evaluation Criteria” in the “Technical Guidelines for Clinical Studies of Seasonal Influenza Virus Vaccines (Draft for Comments)” by the Center for Drug Evaluation, National Medical Products Administration.Citation14
Secondary endpoints: To evaluate the SCRs, SPRs, GMTs, and fold increases in GMT (GMIs) in the participants from the different age groups (3–8, 9–59, 3–59, and ≥60 years) 30 days after full-course immunization.
Exploratory endpoints: To explore the immunogenicity of different immunizations (one dose vs. two doses) in the participants 3–8 years old.
The SCR was defined as the percentage of participants with pre-immunization HI antibody titers < 1:10 and post-immunization HI antibody titers ≥ 1:40 or pre-immunization HI antibody titers ≥ 1:10 and post-immunization HI antibody titers increased ≥ fourfold. The SPR was defined as the percentage of participants with post-immunization HI antibody titers ≥ 1:40.
Safety endpoints
Solicited adverse events (AEs) and unsolicited AEs were collected 0–7 days after each dose of vaccination and within 0–28/30 days after each dose of vaccination, respectively. Information on all serious adverse events (SAEs) was gathered within 6 months after full-course vaccination.
Solicited AEs: local AEs: pain, pruritus, redness, swelling, rashes, and induration. Systemic AEs: fever, fatigue/asthenia, headache, anorexia, muscle pain, arthralgia, nausea, vomiting, diarrhea, abdominal pain, and acute allergic reactions.
The severity of all AEs was assessed in reference to the “Guidelines for the Grading Criteria of Adverse Events in the Clinical Trials of Preventive Vaccines” issued by the National Medical Products Administration on December 31, 2019.
Statistical analysis
The safety set (SS) included all participants who received at least one dose of the investigational vaccine. For participants who received the wrong vaccine number, a safety assessment was conducted in accordance with the “all participants as treated (ASaT)” principle to follow the actual vaccination group of the participants. The full analysis set (FAS) included all participants who completed at least one dose of vaccination, completed pre-immunization blood sampling, and had acceptable antibody titers. Participants with vaccination errors were subjected to immunogenicity evaluation by randomization grouping in accordance with the intention-to-treat principle. The per protocol set (PPS) included all participants who complied with the inclusion criteria but did not meet the exclusion criteria, received vaccination as specified in the protocol after randomization, completed pre-immunization and post-immunization blood sampling for immunogenicity evaluation, and had acceptable antibody titers.
The FAS was used for primary and secondary immunogenicity analyses, PPS for exploratory immunogenicity analysis, and SS for safety analysis. An immunogenicity analysis was also performed in the subgroups of age, pre-immunization antibody-negative population (antibody titers < 1:10) or pre-immunization antibody-positive population (antibody titers ≥ 1:10), and presence or absence of influenza vaccination history.
The bilateral 95% CIs of SCRs and SPRs were calculated using the Clopper – Pearson method, and the difference between groups was statistically tested using the Chi-square test/Fisher exact probability method. The logarithm-transformed antibody GMTs were statistically analyzed using analysis of variance and paired t-tests, and the post-immunization GMTs corrected by post-immunization least squares mean, the corrected GMT ratio, and 95% CIs of the test and control groups were calculated.
AEs and SAEs were coded using MedDRA (version 24.1), and the difference between groups was statistically compared using the Fisher exact probability method with P < .05 indicating statistically significant difference.
Statistical significance was set at P < .05, and all statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Serological methods
All serum samples were collected by the National Institutes for Food and Drug Control in strict accordance with regulations and laboratory manuals, using the micro-hemagglutination inhibition test to detect the HI antibodies.
Results
Study population
In total, 3,328 participants were enrolled in the study and analyzed by initial grouping. A total of 2,788 participants ≥3 years old (except for the 3–8-year-old two-dose group) were included. Among them, 2,698 participants completed the trial (1,793 in the one-dose group and 905 in the control group). However, 90 participants dropped out of the trial, among whom 66 refused blood sampling, 25 left the region of the study site, 2 withdrew their informed consent (not caused by AEs), 1 experienced a SAE (1 participant in the test group, not related to vaccination), 1 violated the protocol, and 2 died (1 each in the test and control groups, both unrelated to vaccination) (, Appendix ). A total of 540 participants were enrolled in the 3–8-year-old two-dose group, among whom 453 completed the trial and 87 dropped out of the trial, including 5 due to AEs, 1 due to withdrawal of informed consent (not caused by AEs), 1 due to a SAE (not related to vaccination), and 2 due to protocol violation ().
Figure 1. Participant disposition.
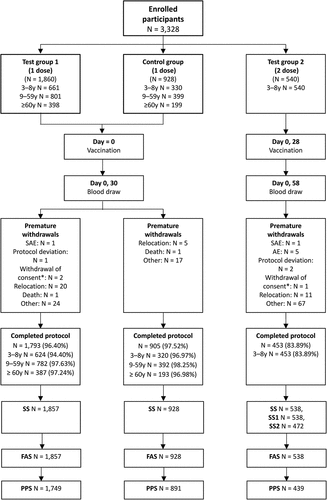
All participants ≥3 years old (1,857 from the test group and 928 from the control group, except for those in the two-dose group) were included in the FAS. The PPS included 439 participants in the 3–8-year-old two-dose group ().
The ages, sexes, heights, and body weights of all participants ≥3 years old and participants from the different age groups (except 3–8 years) were balanced between the test and control groups (, Appendix ).
Table 1. Demographic data and baseline clinical characteristics of study participants (FAS).
Immunogenicity
Major immunogenicity results (based on FAS)
After one dose of the investigational vaccine, the SCRs of the anti-H1N1, H3N2, B(Y), and B(V) antibodies of the participants aged ≥3 years in the test group were 74.64%, 87.40%, 82.66%, and 78.89%, respectively. The lower 95% CIs of SCR differences of the various serotype antibodies were greater than −10% (). In addition, the GMTs corresponding to the H1N1, H3N2, B(Y), and B(V) serotypes in the test group were 1:250.13, 1:394.54, 1:200.84, and 1:94.91, respectively, and the GMT ratios and lower 95% CIs were greater than 0.67 (). These results indicated that the immunogenicity of the investigational vaccine was non-inferior to that of the control vaccine.
Figure 2. Immunogenicity in all populations aged ≥3 years with pre-immunization antibody titers < 1:10 and pre-immunization antibody titers ≥ 1:10 (one-dose group) (FAS).
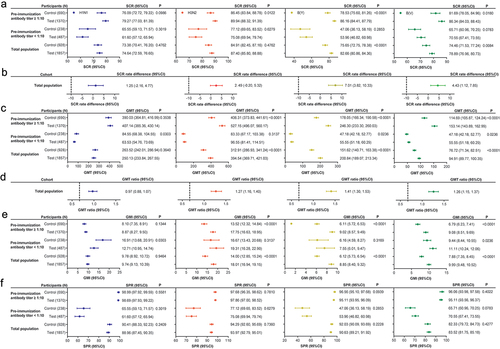
In the participants 3–59 years old, the SCRs of the anti-H1N1, H3N2, B(Y), and B(V) antibodies were 73.41%, 86.98%, 81.91%, and 78.62%, respectively, with lower 95% CIs > 40%, and their SPRs were 88.01%, 93.21%, 89.44%, and 82.73%, respectively, with lower 95% CIs > 70%. In the participants ≥60 years old, the SCRs of the anti-H1N1, H3N2, B(Y), and B(V) antibodies were 79.15%, 88.94%, 85.43%, and 79.90%, respectively, with lower 95% CIs > 30%, and their SPRs were 92.46%, 96.73%, 94.97%, and 86.43%, respectively, with lower 95% CIs > 60% (Appendix ). The lower 95% CIs of the SCRs and SPRs of the participants from the different age groups in the test group meet the requirements for the primary endpoint indicators.
Secondary immunogenicity results (based on FAS)
After the administration of one dose of the investigational vaccine to participants with pre-immunization antibody titers < 1:10 in the test group, the SCRs (same as SPRs) of the anti-H1N1, H3N2, B(Y), and B(V) antibodies in the test group after one dose of vaccine were 61.60%, 75.08%, 53.96%, and 70.55%, respectively, and their GMTs were 1:63.53, 1:96.55, 1:37.73, and 1:55.55, respectively. The SCRs (the same as SPRs) and GMTs of all serotype antibodies, except for the anti-H1N1 antibodies, were not lower in the test group than in the control group (). In addition, the SCRs and GMTs of the participants from the different age groups were not lower than those in the control group (Appendix ).
After the administration of one dose of the investigational vaccine to participants with pre-immunization antibody titers ≥ 1:10, the SCRs with fourfold increases of the anti-H1N1, H3N2, B(Y), and B(V) antibodies were 79.27%, 89.94%, 86.16%, and 86.34%, respectively, their SPRs were 98.69%, 97.86%, 95.11%, and 95.11%, respectively, and their GMTs were 1:407.14, 1:527.15, 1:246.30, and 1:153.14, respectively; these values which were not lower in the test group than in the control group (). The SPRs and GMTs of the participants from the different age groups were also not lower than those in the control group (Appendix ).
Exploratory immunogenicity results (participants 3–8 years old, based on PPS)
After the administration of one dose of the investigational vaccine in the two-dose group of participants 3–8 years old, the SCRs of the anti-H1N1, H3N2, B(Y), and B(V) antibodies were 87.70%, 94.99%, 88.61%, and 88.84%, respectively, their SPRs were 95.22%, 96.81%, 93.17%, and 91.34%, respectively, and their GMTs were 1:365.38, 1:598.94, 1:152.36, and 1:90.91, respectively, regardless of their pre-immunization influenza vaccination history. In addition, the SCRs and SPRs of the various serotype antibodies were higher in the two-dose group than in the one-dose group, and the GMTs of the anti-H1N1 and anti-H3N2 antibodies were higher in the two-dose group than in the one-dose group (Appendix ). Meanwhile, the SCRs and GMTs of the various serotype antibodies were significantly higher in the two-dose group than in the one-dose group of participants with pre-immunization antibody titers < 1:10 (Appendix ).
According to the stratified analysis of influenza vaccination history, the SCRs, SPRs, and GMTs of the various serotype antibodies were significantly higher in the two-dose group than in the one-dose group of participants without a history of vaccination. (Appendix ). The participants with a history of vaccination showed significantly higher SPRs, SCRs of the anti-B antibodies, and GMTs than those participants without any vaccination history (Appendix ).
Safety
Safety results in participants ≥3 years old
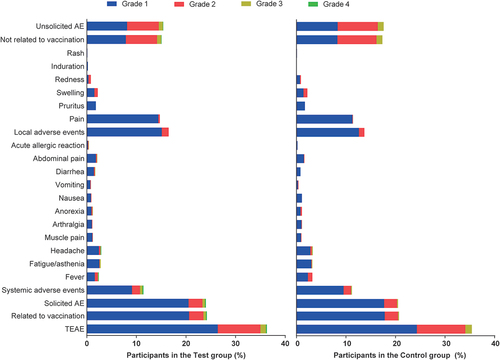
The incidence and severity of AEs after 1 dose of the vaccine in the participants ≥3 years old are presented in . The overall AE incidence rates in the test and control groups were 31.6% and 29.9%, respectively, and the incidence rates of adverse reactions were 21.7% and 18.9%, respectively, with no significant difference between the groups. In terms of severity, most AEs were grade 1, and the grade ≥ 3 adverse reactions were all systemic AEs with incidence rates of 0.6% and 0.2% in the test and control groups, respectively. No vaccination-related SAEs were observed.
Safety results in participants 3–8 years old
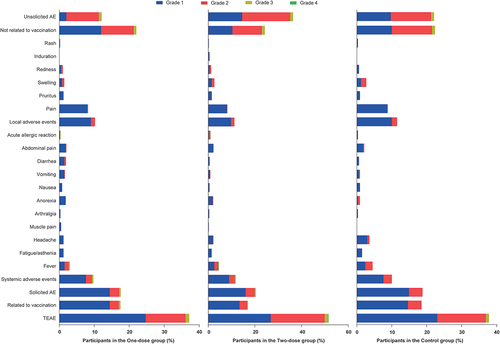
The incidence and severity of AEs after vaccination in the participants 3–8 years old are presented in and Appendix , respectively. The incidence rates of adverse reactions were comparable among the one-dose test group (15.8%), two-dose test group (14.7%), and control group (15.8%). Most AEs were still grade 1, and the incidence rates of grade ≥ 3 adverse reactions were 0.5%, 0.4%, and 0.0%, respectively. No vaccination-related SAEs occurred.
Discussion
In this study, the immunogenicity and safety of the investigational vaccine were evaluated in a randomized, double-blind, positive-controlled phase III clinical trial. The study indicated that in healthy populations ≥3 years of age, the investigation vaccine was not inferior to the control vaccine in terms of immunogenicity, and its safety was not significantly different from that of the control vaccine.
The participants in this study developed a strong serological response after receiving one dose of the investigational vaccine. The minimum lower 95% CIs of SCR differences of the various serotype antibodies and the minimum GMT ratios in the participants ≥3 years old were −2.16% and 0.88, respectively, which meet the relative criteria for the non-inferiority evaluated by the immunogenicity based on the “Technical Guidelines for Clinical Studies of Seasonal Influenza Virus Vaccines (Draft for Comments).”Citation14 In addition, the lower 95% CIs of SCRs and SPRs were ≥ 40% and ≥ 70%, respectively, in the participants 3–59 years old while ≥ 30% and ≥ 60%, respectively, in the participants ≥60 years old, all of which meet the absolute criteria of the immunogenicity evaluation criteria.Citation15 The immunogenicity non-inferiority results described above were similar to those of other clinical trials of IIV4.Citation15–17 The investigational vaccine in this study achieved high SPRs, which corresponded to a high level of clinical protection against influenza B in populations aged ≥3 years.Citation18 Thus, the investigational vaccine could serve as a candidate seasonal quadrivalent influenza vaccine.
Assessment of secondary immunogenicity showed that the SCRs and SPRs in the test group of participants ≥3 years old with pre-immunization antibody titers < 1:10 or ≥ 1:10 were not lower than those in the control group. This result indicates that the investigational vaccine was applicable to populations with different antibody titers. Interestingly, the SCRs and SPRs of the various serotype antibodies in all participants 3–8 years old and in those with pre-immunization antibody titers < 1:10 were lower than those in the participants of other age groups. This result is similar to that reported by Stephanie et al.,Citation19 which may be caused by the low antibody titers in participants 3–8 years old at the time of vaccination. These findings indicated that the immunogenicity of IIV4 in individuals 3–8 years old may be affected by their pre-immunization antibody titers.
Given their limited previous exposure to vaccines and viruses, children who are 6 months to 8 years old and have not received any influenza vaccine should receive two doses of influenza vaccine to generate sufficient protective antibody titers.Citation20 However, only the one-dose procedure is currently available for all influenza vaccines marketed in Mainland China for populations aged ≥3 years. Thus, in the present study, the immunogenicity levels of the participants 3–8 years old with or without a history of influenza vaccination were compared after one dose of IIV4, and the effects of different doses of the investigational vaccine on the immunogenicity of participants 3–8 years old were explored. The SCRs and GMTs of the anti-B(Y) and -B(V) antibodies and the SPRs of the various serotype antibodies in the participants with a vaccination history were higher than those in the participants without a vaccination history. In addition, the GMTs of all serotype antibodies, except for the anti-H3N2 antibodies, and the SCRs, SPRs, and GMIs of all serotype antibodies in the two-dose group were either higher than or showed no significant differences from those in the one-dose group. Moreover, among the participants with pre-immunization antibody titers < 1:10, the SCRs, GMTs, and GMIs of the various serotype antibodies were significantly higher in the two-dose group than in the one-dose group. These results indicated that the two-dose procedure of the investigational vaccine provided better protective effects on the participants 3–8 years old.
Safety is another important indicator of vaccine quality. In a phase III study by Treanor et al.,Citation21 the incidence of adverse reactions was 43.8% in adults after IIV4 vaccination. Kim et al.Citation22 found a 44.5% overall incidence of adverse reactions in participants ≥65 years old after IIV4 vaccination. Chang et al.Citation23 reported 43.1% and 41.4% incidence rates of local and systemic AEs, respectively, in participants 3–17 years old after IIV4 vaccination. In the present study, the incidence rates of adverse reactions, systemic, and local AEs were 21.7%, 10.0%, and 15.6%, respectively, in the participants ≥3 years old after the administration of the investigational vaccine. Moreover, most AEs in the participants who received the investigational vaccine were grade 1 in severity. Although the incidences of fever, fatigue/asthenia, headache, acute allergic reaction (urticaria), anorexia, abdominal pain, and diarrhea ≥grade 3 were higher in the test group than in the control group, the difference in the overall AE incidence between the test and control groups was not statistically significant. Moreover, these ≥grade 3 AEs developed 0–7 days after vaccination and mostly resolved within 2 weeks after the corresponding medical interventions. No vaccination-related SAEs were observed in this study. These findings indicated that the investigational vaccine was safe and well tolerated. For participants 3–8 years old, the overall AE incidence after two doses of the investigational vaccine was 40.0%, which was higher than that in the one-dose group (31.9%). However, the overall AE incidence after the first dose of the investigational vaccine in the participants 3–8 years old was 30.3%, which was close to that in the one-dose group (31.9%). Moreover, the incidence of solicited AEs after two doses of the investigational vaccine in the participants 3–8 years old was 17.5%, which was lower than the 62.4% reported in the literature.Citation19 These results indicated the safety of two doses of the investigational vaccine in participants 3–8 years old, and the incidence of adverse reactions did not show an increasing trend with the number of doses. Thus, safety assessment of infant immunization with the investigational vaccine could be conducted in subsequent trials.
The complexity of participants in different age groups was considered in this study, and multiple factors that affected vaccination results were excluded. However, some limitations still exist. First, immunogenicity is an indirect indicator for influenza prevention in clinical practice and cannot be considered identical to the efficacy results; therefore, the immune efficacy studies reflecting the actual clinical efficacy of the vaccine are warranted in the future. Second, the effects of multiple conditions were excluded from this study. Thus, caution should be exercised when extrapolating these findings to populations with these conditions. Moreover, assessing the efficacy of the investigational vaccine against various influenza strains through immunogenicity evaluation during a single epidemic season may be insufficient.
Conclusion
The investigational vaccine has non-inferior immunogenicity to the control vaccine and is as safe as the control vaccine in the population aged ≥3 years. Furthermore, the two-dose procedure of the investigational vaccine shows better immunogenicity and clinically acceptable safety in children 3–8 years old. This study shows for the first time that administration of the investigational vaccine can prevent seasonal influenza in populations aged ≥3 years. It also provides additional data to support the clinical use of IIV4 in populations aged ≥3 years.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The protocol is saved at Dalian Aleph Biomedical Co., Ltd. and is available from the corresponding authors if necessary.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Fitzner J, Qasmieh S, Mounts AW, Alexander B, Besselaar T, Briand S, Brown C, Clark S, Dueger E, Gross D, et al. Revision of clinical case definitions: influenza-like illness and severe acute respiratory infection. Bull World Health Organ. 2018 Feb 1;96(2):122–20. doi:10.2471/BLT.17.194514. [Epub 27 Nov 2017]. PMID: 29403115.
- Caini S, Kroneman M, Wiegers T, Guerche-Séblain C E, Paget J. Clinical characteristics and severity of influenza infections by virus type, subtype, and lineage: a systematic literature review. Influenza Other Respir Viruses. 2018 Nov;12(6):780–92. doi:10.1111/irv.12575. [Epub 20 Jul 2018]. PMID: 29858537.
- Jennings L, Huang QS, Barr I, Lee PI, Kim WJ, Buchy P, Sanicas M, Mungall BA, Chen J. Literature review of the epidemiology of influenza B disease in 15 countries in the Asia-Pacific region. Influenza Other Respir Viruses. 2018 May;12(3):383–411. doi:10.1111/irv.12522. [Epub 7 Mar 2018]. PMID: 29127742.
- French PMID. Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Rec. 2012;87(47):461–76. English.
- Li J, Chen Y, Wang X, Yu H. Influenza-associated disease burden in mainland China: a systematic review and meta-analysis. Sci Rep. 2021 Feb 3;11(1):2886. doi:10.1038/s41598-021-82161-z. PMID: 33536462.
- Li S, Liu SS, Zhu AQ, Cui JZ, Qin Y, Zheng JD, Feng LZ, Wang LP, Li ZJ. The mortality burden of influenza in China: a systematic review. Zhonghua Yu Fang Yi Xue Za Zhi. 2019 Oct 6;53(10):1049–55. Chinese. doi:10.3760/cma.j.issn.0253-9624.2019.10.018. PMID: 31607054.
- Ainslie KEC, Haber M, Orenstein WA. Challenges in estimating influenza vaccine effectiveness. Expert Rev Vaccines. 2019 Jun;18(6):615–28. doi:10.1080/14760584.2019.1622419. [Epub 31 May 2019]. PMID: 31116070.
- Lewnard JA, Cobey S. Immune history and influenza vaccine effectiveness. Vaccines (Basel). 2018 May 21;6(2):28. doi:10.3390/vaccines6020028. PMID: 29883414.
- Tenforde MW, Kondor RJG, Chung JR, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, Monto AS, Martin ET, Belongia EA, et al. Effect of antigenic drift on influenza vaccine effectiveness in the United States—2019–2020. Clinical Infectious Diseases. 2021 Dec 6;73(11):e4244–50–e4250. doi:10.1093/cid/ciaa1884. PMID: 33367650.
- Arefin MR, Masaki T, Kabir KMA, Tanimoto J. Interplay between cost and effectiveness in influenza vaccine uptake: a vaccination game approach. Proc Math Phys Eng Sci. 2019 Dec;475(2232):20190608. doi:10.1098/rspa.2019.0608. [Epub 18 Dec 2019]. PMID: 31892839.
- Centers for Disease Control and Prevention. Past seasons vaccine effectiveness estimates. [accessed 2021 Dec 7]. https://www.cdc.gov/flu/vaccines-work/past-seasons-estimates.html.
- Gaglani M, Vasudevan A, Raiyani C, Murthy K, Chen W, Reis M, Belongia EA, McLean HQ, Jackson ML, Jackson LA, et al. Effectiveness of trivalent and quadrivalent inactivated vaccines against influenza B in the United States, 2011–2012 to 2016–2017. Clin Infect Dis. 2021 Apr 8;72(7):1147–57. doi:10.1093/cid/ciaa102. PMID: 32006430.
- Pepin S, Dupuy M, Borja-Tabora CFC, Montellano M, Bravo L, Santos J, de Castro JA, Rivera-Medina DM, Cutland C, Ariza M, et al. Efficacy, immunogenicity, and safety of a quadrivalent inactivated influenza vaccine in children aged 6–35 months: a multi-season randomised placebo-controlled trial in the Northern and Southern hemispheres. Vaccine. 2019 Mar 22;37(13):1876–84. doi:10.1016/j.vaccine.2018.11.074. [Epub 14 Dec 2018]. PMID: 30558818.
- NMPA. Technical guidelines for clinical studies of seasonal influenza virus vaccines. 2021. [accessed 2023 Jun 25]. https://www.cde.org.cn/main/news/viewInfoCommon/237d5f7de6bcfcd08037dcce873794f3.
- Chu K, Xu K, Tang R, Tian X, Hu J, Yang T, Li C, Hu Y, Zeng G. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine: a randomized, double-blind, controlled phase III study in healthy population aged ≥3 years. Vaccine. 2020 Aug 18;38(37):5940–6. doi:10.1016/j.vaccine.2020.06.071. [Epub 27 Jul 2020]. PMID: 32732142.
- Huang X, Fan T, Li L, Nian X, Zhang J, Gao X, Zhao W, Chen W, Zhang Z, Yao Z, et al. Safety and immunogenicity of a quadrivalent, inactivated, split-virion influenza vaccine (IIV4-W) in healthy people aged 3–60 years: a phase III randomized clinical noninferiority trial. Human Vaccines & Immunotherapeutics. 2022 Jun 17;18(5):2079924. doi:10.1080/21645515.2022.2079924. [Epub ahead of print]. PMID: 35714276.
- Tinoco JC, Pavia-Ruz N, Cruz-Valdez A, Aranza Doniz C, Chandrasekaran V, Dewé W, Liu A, Innis BL, Jain VK. Immunogenicity, reactogenicity, and safety of inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine in healthy adults aged ≥18 years: a phase III, randomized trial. Vaccine. 2014 Mar 14;32(13):1480–7. doi:10.1016/j.vaccine.2014.01.022. [Epub 28 Jan 2014]. PMID: 24486352.
- Airey J, Albano FR, Sawlwin DC, Jones AG, Formica N, Matassa V, Leong J. Immunogenicity and safety of a quadrivalent inactivated influenza virus vaccine compared with a comparator quadrivalent inactivated influenza vaccine in a pediatric population: a phase 3, randomized noninferiority study. Vaccine. 2017 May 9;35(20):2745–52. doi:10.1016/j.vaccine.2017.03.028. [Epub 5 Apr 2017]. PMID: 28390934.
- Pepin S, Szymanski H, Rochín Kobashi IA, Villagomez Martinez S, González Zamora JF, Brzostek J, Huang LM, Chiu CH, Chen PY, Ahonen A, et al. Safety and immunogenicity of an intramuscular quadrivalent influenza vaccine in children 3 to 8 y of age: a phase III randomized controlled study. Hum Vaccin Immunother. 2016 Dec;12(12):3072–8. doi:10.1080/21645515.2016.1212143. [Epub 26 Aug 2016]. PMID: 27565435.
- National Immunization Advisory Committee (NIAC) Technical Working Group (TWG), Influenza Vaccination TWG. Technical guidelines for seasonal influenza vaccination in China (2021–2022). Zhonghua Liu Xing Bing Xue Za Zhi. 2021 Oct 10;42(10):1722–49. Chinese. doi:10.3760/cma.j.cn112338-20210913-00732. PMID: 34814607.
- Treanor JT, Albano FR, Sawlwin DC, Graves Jones A, Airey J, Formica N, Matassa V, Leong J. Immunogenicity and safety of a quadrivalent inactivated influenza vaccine compared with two trivalent inactivated influenza vaccines containing alternate B strains in adults: a phase 3, randomized noninferiority study. Vaccine. 2017 Apr 4;35(15):1856–64. doi:10.1016/j.vaccine.2017.02.066. [Epub 13 Mar 2017]. PMID: 28302411.
- Kim TH, Choi JH, Park SH, Yoo JH, Lee DG, Choi SM, Kim YR, Lee MS, Choo EJ, Choi HJ. Safety and immunogenicity of a seasonal quadrivalent influenza vaccine (GC3110A) in healthy participants aged ≥ 65 years. Vaccine. 2021 Jun 16;39(27):3621–5. doi:10.1016/j.vaccine.2021.05.001. [Epub 13 May 2021]. PMID: 33992436.
- Chang CY, Cho CY, Lai CC, Lu CY, Chang LY, Hung MC, Huang LM, Wu KG. Immunogenicity and safety of a quadrivalent inactivated influenza vaccine in healthy subjects aged 3 to 17 years old: a phase III, open label, single-arm study. Vaccine. 2020 May 8;38(22):3839–46. doi:10.1016/j.vaccine.2020.03.048. [Epub 10 Apr 2020]. PMID: 32284272.
Appendix
Table A1. List of dropouts.
Table A2. Demographic data and baseline clinical characteristics of participants by age group (FAS).
Table A3. Comparison of non-inferiority in various age groups between the investigational and control vaccines (one-dose group) (FAS).
Table A4. Immunogenicity in participants in various age groups with pre-immunization antibody titers < 1:10 and pre-immunization antibody titers ≥ 1:10 (one-dose group) (FAS).
Table A5. Immunogenicity in participants 3–8 years old (PPS).
Table A6. Immunogenicity in participants 3–8 years old with and without vaccination history in the one-dose group (PPS).
Table A7. Total adverse events after different immunization procedures in participants 3–8 years old.