ABSTRACT
Human papillomavirus (HPV) infection is the primary cause of cervical cancer and its precursor lesions. The overall prevalence of HPV genotypes in Changzhou has previously been reported. However, the distribution of multiple HPV infections and their roles in cervical injury have less been investigated. We aimed to assess the prevalence of multiple HPV infections among the people in Changzhou. Furthermore, we analyzed whether multiple HPV infections comprising the top five prevalent HPVs were more associated with abnormalities in E6 and E7 (E6/E7) mRNA, liquid-based cytology, and cervical histopathology than a single infection. In the current study, HPV 16, 52, 58, 53, and 81 were the top five prevalent HPV types, both in single and multiple infections. Compared to a single infection, multiple infections containing HPV 16/52/58 were closely linked to positivity for E6/E7 mRNA. In addition to HPV 16, multiple infections containing the remaining top four HPVs conferred a significant advantage on atypical squamous cells of undermined significance or worse in comparison to a single infection. Furthermore, women with multiple infections containing the top five prevalent HPV types were more likely to develop cervical intraepithelial neoplasia grade II or worse than those with a single HPV infection. Our results demonstrate the superiority of multiple HPV infections containing the top five prevalent HPV types in cervical disease progression, which should be closely monitored. These findings are conducive for formulating regional preventive strategies for cervical cancer screening and vaccination in Changzhou.
Introduction
Cervical carcinoma, one of the most common genital tract tumors, has the fourth highest mortality rate among women worldwide.Citation1 It is mainly attributed to persistent infection with human papillomaviruses (HPVs), which are small, non-enveloped, double-stranded DNA viruses.Citation2 To date, more than 200 different HPV genotypes have been identified and nearly 40 among them infect the genital tract.Citation3 Several sexually active individuals acquire at least one genital HPV infection at some point during their lifetime. All HPVs associated with cervical cancer belong to the alpha genus.Citation4 As defined by the International Agency for Research on Cancer (IARC) monograph, they are further subdivided into high-risk HPVs (HR-HPVs, Group 1, HPV 16/18/31/33/35/39/45/51/52/56/58/59); probable HR-HPVs (Group 2A, HPV 68); possible HR-HPVs (Group 2B, HPV 26/30/34/53/66/67/69/70/73/82/85/97); and low-risk HPVs (LR-HPVs, Group 3, HPV 6/11).Citation5 The LR-HPV prevalent rate is higher in men than in women.Citation6 No clear age pattern is detected in men unlike in women.Citation7 Furthermore, the distribution of HPV types shows diverse regional and racial variations. In China, HPV 16, 18, 52, 58, and 33 are the most prevalent types.Citation8
Clinically, a single HPV type is frequently encountered in the vast majority of cases; however, multiple HPV types also account for a proportion of infections in women.Citation9 The superiority of multiple HPV infections over a single HPV infection with regards to causing disease progression is debated. The interactions of co-infected HPV types display a synergistic effect on the progression to high-grade squamous intraepithelial lesions (HSIL) and cervical squamous cell carcinomas.Citation10 On the contrary, recent findings suggest that cooperative interactions may be weakened by eliciting HPV intergenotypic competition and subsequent immune responses, thus conferring protection from SIL.Citation11
HPV infection alone is insufficient for cervical carcinogenesis. The biological functions of HPVs are linked to the oncoproteins E6 and E7, the HPV-mediated drives.Citation12 The dysregulation of vital oncoproteins results in prolonging proliferation and delaying differentiation, ultimately providing an environment for virus replication.Citation13 Interestingly, further studies have demonstrated that E6 and E7 synergistically work during tumor formation. E7 induces early carcinogenesis by targeting the tumor suppressor protein pRb and related proteins p107 and p130 to maintain an S-phase-like state in differentiating keratinocytes. E6 accelerates progression toward deterioration by promoting the degradation of the tumor-suppressor protein P53 to overcome cellular apoptotic processes.Citation4,Citation14
According to the natural history of HPV, our immune system can clear about 90% of new HPV acquisitions within 2 years.Citation15 The development of HPV-related cytological changes occurs once HPV infection fails to be cleared in time. Therefore, the need for HPV vaccination cannot be overstated. At present, three HPV vaccines have been licensed and are available: bivalent vaccine (HPV 16/18), the quadrivalent vaccine (HR-HPV 16/18 and LR-HPV 6/11), and the non-valent vaccine (HPV 16/18/31/33/45/52/58 and LR-HPV 6/11). Numerous clinical trials have documented that each drug exhibits significant efficacy and ensures safety.Citation16
Our study aimed to identify the characteristics of multiple HPV infections in female patients in Changzhou, China, including HPV infection rate, HPV genotype distribution, and the associations between E6/E7 mRNAs, cervical intraepithelial neoplasia (CIN) and HPV infection, compared to a single HPV infection. Investigating the epidemiology of multiple HPV infections will help improve the risk stratification of HPV-positive women in cervical screening programs and formulate regional prevention strategies for cervical cancer screening and vaccination based on each population.
Materials and methods
Study subjects
This retrospective study was designed to explore HPV prevalence in 12,887 women visiting the Department of Gynecology of the Changzhou Hospital of Traditional Chinese Medicine between June 2018 and February 2022. The participants were stratified into six subgroups based on their ages: ≤20 years, 21–30 years, 31–40 years, 41–50 years, 51–60 years, and ≥61 years. Of the 1752 HPV positive individuals, 1126 cases accepted cervical cytology, 187 underwent HPV E6/E7 mRNA testing, and 379 took colposcopic biopsy. This study was approved by the Hospital Ethics Committee of the Changzhou Hospital of Traditional Chinese Medicine.
HPV DNA test
Cervical specimens from 12,887 women were collected using a sterile cytobrush and then detected by HPV 21X Genotyping Kit (Jiangsu BioPerfectus Technology, Taizhou, China) from 21 HPV genotypes (HPV 06/11/16/18/26/31/33/35/39/45/51/52/53/56/58/59/66/68/73/81/82). The 21 HPVs were mainly classified based on the IARC Monograph 100b, while HPV 81 was not included. In the present study, HPV 81 was categorized as an LR-HPV. According to the manufacturer’s instructions, we amplified HPV L1 gene DNAs using PCR primers and the corresponding TaqMan probes following the extraction of HPV genomic DNAs from cervicovaginal cytology samples. All PCR programs were conducted using the SLAN-96S Real-Time PCR Instrument (Shanghai Hongshi) with the following settings:50°C for 5 min, 95°C for 10 min, and then 45 cycles of denaturation at 95°C for 10 s and the final step was 58°C for 40 s. Human TOP3 served as an endogenous marker to confirm successful DNA extraction.
Liquid based cytology
A disposable plastic cervical swab was used to scrape samples of shed cervical cells after exposure of the cervix. Samples were immediately stored in a BD SurePath collection vial (Becton Dickinson and Company, Franklin Lakes, NJ, USA). The Bethesda System was used to report the results. Samples were assigned into negative for intraepithelial lesion or malignancy (NILM), atypical squamous cells of undetermined significance (ASCUS), inflammation, low-grade squamous intraepithelial lesion (LSIL, mild intraepithelial cell morphological abnormality), high-grade squamous intraepithelial lesion (HSIL, moderate to severe dysplasia), and carcinoma. Liquid-based cytology results of ASCUS or worse were considered abnormal.
HPV E6/E7 mRNA test
Fourteen HPV types (HPV 16/18/31/33/35/39/45/51/52/56/58/59/66/68) were detected using the Aptima HPV E6/E7 mRNA assay (Aptima; Hologic Inc., San Diego, CA, USA) on the Panther platform according to the manufacturer’s instructions. The test involved three steps: target capture, target amplification, and detection of the amplification products.Citation17 In detail, upon collection, specimens were lysed into cells to release mRNAs. The target HPV mRNAs were isolated from the specimens using capture oligomers linked to magnetic particles and then amplified. The final results were interpreted based on the analyte signal-to-cutoff ratio (S/CO). IC was added to each reaction to monitor the capture, amplification, and detection steps of the assay.
Cervical histopathology examinations
A cervical biopsy specimen was collected from the suspicious site. Without visible acetowhite lesions, biopsies were performed at the three, six, nine, and 12 o’clock positions on the cervix. All biopsies were processed using standard histopathological methods and were diagnosed by two certified pathologists. The results of cervical histopathological examinations were assigned into five groups: inflammation, CIN grades I, II, III and carcinoma, according to the latest classification (the 4th WHO Women’s Reproductive System Tumor Classification).Citation18 Among the five groups, inflammation and CIN I were referred as ≤CIN I, while the other three groups were referred to as ≥CIN II.
Statistics
All data were statistically analyzed using GraphPad Prism 9.0 (GraphPad Software, Inc., San Diego, CA, USA). The chi-square test was used to compare clinicopathological parameters. The Fisher’s exact test was applied when the expected frequencies were < 5. P values < 05 were considered to be statistically significant. The adjusted p-value was used by the Bonferroni correction for multiple comparisons.
Results
HPV prevalence
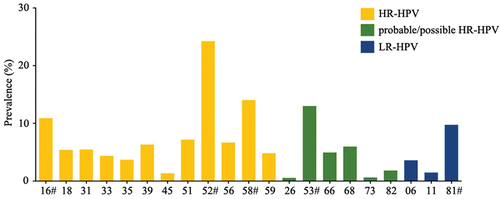
Of the 12,887 specimens, 1752 women were positive for HPV, with an overall HPV infection rate of 13.60% (1752/12887). shows the distribution of HPV infections. The most common type was HR-HPV 52 (24.26%, 425/1752), followed by HR-HPV 58 (14.04%, 246/1752), probable/possible HR-HPV 53 (13.01%, 228/1752), HR-HPV 16 (10.90%, 191/1752) and LR-HPV 81 (9.76%, 171/1752) (, Table S1). Thus, HPV 16, 52, 53, 58, and 81 were regarded as the top five prevalent HPV types in patients visiting our hospital, which were subsequently highlighted in our study.
Age distribution of the top five prevalent HPVs
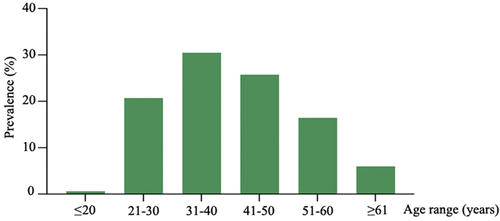
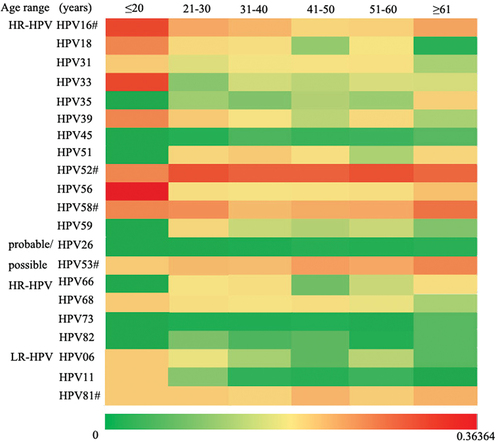
We further analyzed the age distribution of HPV infections in HPV-positive patients. 1752 subjects were further categorized into six age groups. The women aged 31–40 years accounted for the highest proportion (30.48%, 534/1752), followed by women aged 41–50 years (25.74%, 451/1752), 21–30 years (20.72%, 363/1752), 51–60 years (16.44%, 288/1752), ≥61 years (5.99%, 105/1752), and ≤20 years (0.63%, 11/1752) (, Table S1). For the top five prevalent HPV types, HR-HPV 52 was the most dominant type in the remaining groups excluding the ≤20 years group. The prevalence of HR-HPVs 16 and 58 exhibited a U sharped pattern, indicating that the infection rate peaked in ≤ 20 and ≥61 years group. The probable/possible HR-HPV 53 presented itself as a slow increase from the ≤ 20 to 41–50 years groups, then maintained a stable level in the 51–60 and ≥61 years groups. A similar trend emerged in the LR-HPV 81 infection data (). The specific data are presented in the supplementary data (Table S2).
Comparison between single and multiple infections of the top five prevalent HPVs
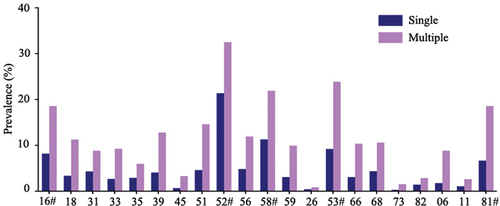
Single HPV infection was observed to be the most common pattern (74.20%, 1300/1752). The prevalence of multiple HPV infections was relatively low (25.80%, 452/1752) (Table S3). Sixteen of the 21 HPV types were more frequently detected in the multiple infections than the single infection, including 11 HR-HPV types (HPV 16/18/31/33/39/45/51/52/56/58/59), 3 probable/possible HR-HPV types (HPV 53/66/68) and 2 LR-HPV types (HPV 6/81) (Table S3). Interestingly, the top five prevalent HPV types also accounted for the highest rates in the top five for both single and multiple infections. HR-HPV 52 (21.38%, 278/1300) was the most frequently detected single infection, followed by HR-HPV58 (11.31%, 147/1300), probable/possible HR-HPV 53 (9.23%, 120/1300), HR-HPV 16 (8.23%, 107/1300), and LR-HPV 81 (6.69%, 87/1300). However, in multiple infections, the top five prevalent HPV types were ranked as follows: HR-HPV 52 (32.52%, 147/452), probable/possible HR-HPV 53 (23.89%, 108/452), HR-HPV 58 (21.90%, 99/452), HR-HPV 16 (18.58%, 84/452) and LR-HPV 81 (18.58%, 84/452) ().
Cytology of the top five prevalent HPVs in single and multiple HPV infections
Patients subjected to HPV DNA screening are generally recommended to undergo liquid-based cytological examinations simultaneously. Among the 1752 HPV-positive patients, 1126 received liquid-based cytology detection. Patients with multiple HPV infections were more prone to cytological abnormalities than those with a single infection (p < 0001). The relationship between cytology and the top five most prevalent HPV types was further analyzed. HR-HPV 52 (p = .0415) and 58 (p = .0322), probable/possible HR-HPV 53 (p < .0001), and LR-HPV 81 (p = .0210) were significantly more associated with cytological abnormalities in multiple HPV infections when compared with single HPV infections. A corresponding finding was shown in HR-HPV 16 infection, although it was not statistically significant ().
Table 1. Cytology of the top five prevalent HPVs in single and multiple HPV infections.
E6/E7 mRNA expression of the top five prevalent HPVs in single and multiple HPV infections
In view of the potential of the E6/E7 mRNA test for detection in the triage of patients with ASCUS lesions, it is usually recommended to detect in ASCUS or worse patients.Citation19 In our study, only 187 patients with ASCUS or worse lesions were willing to accept the E6/E7 mRNA test. Our results showed that 60.27% for E6/E7 mRNA was in single HPV infections, whereas 75.61% for E6/E7 mRNA was in multiple HPV infections. HR-HPV types 16, 52, and 58 were more frequently associated with E6/E7 mRNA positivity in multiple infections than in single infections (). However, the difference was not statistically significant.
Table 2. E6/E7 mRNA expression of the top five prevalent HPVs in single and multiple HPV infections.
Histology of the top five prevalent HPVs in single and multiple HPV infections
Women who were HPV E6/E7 mRNA-positive and/or had abnormal cytological results (grade higher than ASCUS) were referred for colposcopy and biopsy. A total of 379 patients underwent colposcopy and biopsy. To determine the link between HPV prevalence and cervical histopathological lesions, we grouped HPV-positive women into ≤CIN I and ≥CIN II groups. As shown in , the proportion of single HPV infections was 83.65% for ≤CIN I and 16.35% for ≥CIN II, whereas the proportion of multiple HPV infections was 81.03% for ≤CIN I and 18.97% for ≥CIN II. The positive rate of multiple infection containing HR-HPV 16 was higher than the single HR-HPV 16 infection among women of both ≤CIN I and ≥CIN II status, although no significant differences were observed. Similar results were displayed in HR-HPV52, 58, probable/possible HR-HPV53, and LR-HPV81 infections.
Table 3. Histology of the top five prevalent HPVs in single and multiple HPV infections.
Discussion
It has been well-established that HPV infection is involved in the gradual changes of cervical squamous epithelium from mild to HISL, eventually leading to cancerous lesions.Citation20 HPV DNA-based screening effectively reduces the incidence and mortality of cervical carcinogenesis.Citation21 Thus, establishing a database of HPV prevalence and distribution in our local region is necessary. We revealed that HR-HPV 16, 52, 58, probable/possible HR-HPV 53 and LR-HPV81 were the top five prevalent HPV types in Changzhou, Jiangsu, China, which was in congruence with previous studies conducted in three other cities (Nanjing, Xuzhou, and Taizhou) in Jiangsu Province.Citation22,Citation23 This may be attributed to similarities in the geographic and socioeconomic characteristics of these cities.
HPV is considered the most common sexually transmitted virus. Age, as an independent factor, is strongly correlated with HPV prevalence.Citation24 We observed that women aged 31–40 years accounted for the highest proportion of HPV-positive patients, followed by women aged 41–50, and then 21–30 years. However, another study showed the contrary outcome that the two peaks of overall HPV occurred in ≤ 20 and >50 years groups due to the immature immune system of younger and the immunologic disorders resulted from hormone decrease of older women.Citation25 The reason for these inconsistencies may be partly explained by the fact that women aged 31–40 years have more active sexual behavior and may have unstable sexual partners.Citation26
Single infections have been proven to be more frequent than multiple infections.Citation27 This is in agreement with our results. Moreover, the top five most prevalent HPV types in Changzhou ranked among the top five in both single and multiple infections. HPV infection is frequently transient.Citation28 Several co-factors, such as coal tar induced by smoking or cooking, hormonal contraceptives, and genetic alterations, establish a status under which HPV infection persistently occurs, which is a prerequisite for the development of cancer. Another key event in HPV-mediated carcinogenesis is insertion of the HPV sequence into the host genome. This process involves downregulation of the viral oncogenes E6 and E7, which are expressed and retained in HPV-positive cancer cells. They act as functional cooperatives during tumor formation.Citation29,Citation30 A plenty of researches have suggested that the E6/E7 mRNA test may provide prognostic and diagnostic value in cervical cancer screening.Citation31,Citation32 In our study, positivity for E6/E7 mRNA was higher in multiple HPV infections than in single infections.
Precancerous lesions occur as a result of the host’s failure to inhibit HPV replication and abnormal cell proliferation.Citation33 Our analysis of cervical cytology demonstrated that multiple infections distinctly showed a stronger relation to cytological abnormalities (ASCUS or worse) than a single infection. Histologically, CINs are graded according to the severity of the cervical injury and CIN I is referred to as a low-grade squamous intraepithelial lesion that can be repaired by the host’s immune system. CIN II and III are classified as high-grade precancerous lesions. More than 30% regress spontaneously, and cervical ablation is the standard procedure for CIN II/III treatment.Citation34 Our data showed that multiple HPV infections were more likely to trigger high-grade cervical lesions than a single HPV infection. However, this result was not statistically significant. A likely explanation could be that the sample size of each group was insufficient, which resulted in the statistical limitations of our study. Taken together, these results suggest that multiple infections are prone to have high carcinogenic potential. This supports the hypothesis that HPV intergenotypic cooperation aggravates disease progression.Citation11,Citation35 A possible mechanism is that multiple HPV infections may increase the viral load and changes in immune status, leading to persistent HPV infection.Citation36,Citation37
HPV vaccination has a substantial effect on the prevention of HPV infection.Citation16,Citation38 Based on our epidemiological investigation, only the non-valent vaccine of the three current vaccines covers three of the top five prevalent HPVs types (HPV 16/52/58) in Changzhou. Therefore, it is appropriate to recommend a non-valent HPV vaccine for women living in Changzhou. Probable/possible HR-HPV53 and LR-HPV81 are the two remaining common HPV genotypes that should be the focus of future vaccine development. The more targeted the vaccination is to the geographical distribution characteristics of HPV, the better the preventive effect.
In conclusion, we demonstrated the top five prevalent HPVs in Changzhou, ranking them in both single and multiple HPV infections. In terms of the cytological results, multiple HPV infections, other than HR-HPV16, were more likely to induce cervical damage than single HPV infections. However, our study has some limitations. First, this was a retrospective, cross-sectional study conducted at a single institution. Furthermore, the specific mechanism of cervical disease exacerbation under the synergistic action of multiple HPVs has not been described. Subsequent studies will need to be conducted at multiple regional and medical centers. More in vitro and in vivo experiments are warranted to confirm the effects of multiple HPV infections suggested in this study.
Supplemental Material
Download PDF (454.6 KB)Acknowledgments
We appreciate the assistance of the pathologists and gynecologists at our hospital in the preparation of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2245723.
Additional information
Funding
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–7. doi:10.3322/caac.20107.
- Zhang C, Huang C, Zheng X, Pan D. Prevalence of human papillomavirus among Wenzhou women diagnosed with cervical intraepithelial neoplasia and cervical cancer. Infect Agent Cancer. 2018;13(1):37. doi:10.1186/s13027-018-0211-8.
- Zou K, Huang Y, Li Z. Prevention and treatment of human papillomavirus in men benefits both men and women. Front Cell Infect Microbiol. 2022;12:1077651. doi:10.3389/fcimb.2022.1077651.
- IARC. Monographs on the evaluation of carcinogenic risks to humans. Vol. 90, Human Papillomaviruses; Geneva, Switzerland: WHO; 2007.
- Cabibi D, Napolitano C, Giannone AG, Micciulla MC, Porcasi R, Lo Coco R, Bosco L, Vinciguerra M, Capra G. Predictive role of the p16 immunostaining pattern in atypical cervical biopsies with less common high risk HPV genotypes. Diagnostics (Basel). 2021;11(11):1947. doi:10.3390/diagnostics11111947.
- Rezaee Azhar I, Yaghoobi M, Mossalaeie MM, Kollaee Darabi A, Nejadeh AH, Jamshidi M, Ahani A, Karkhane Mahmoodi M, Ghalichi L, Shabanzadeh A, et al. Prevalence of human papilloma virus (HPV) genotypes between outpatients males and females referred to seven laboratories in Tehran, Iran. Infect Agent Cancer. 2022;17(1):7. doi:10.1186/s13027-022-00421-7.
- Giuliano AR, Tortolero-Luna G, Ferrer E, Burchell AN, de Sanjose S, Kjaer SK, Muñoz N, Schiffman M, Bosch FX. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine. 2008;26(10):K17–28. doi:10.1016/j.vaccine.2008.06.021.
- Li K, Li Q, Song L, Wang D, Yin R. The distribution and prevalence of human papillomavirus in women in mainland China. Cancer. 2019 Apr 1;125(7):1030–7. doi:10.1002/cncr.32003.
- Chaturvedi AK, Katki HA, Hildesheim A, Rodríguez AC, Quint W, Schiffman M, Van Doorn LJ, Porras C, Wacholder S, Gonzalez P, et al. Human papillomavirus infection with multiple types: pattern of coinfection and risk of cervical disease. J Infect Dis. 2011;203(7):910–20. doi: 10.1093/infdis/jiq139.
- De Brot L, Pellegrini B, Moretti ST, Carraro DM, Soares FA, Rocha RM, Baiocchi G, da Cunha IW, de Andrade VP. Infections with multiple high-risk HPV types are associated with high-grade and persistent low-grade intraepithelial lesions of the cervix. Cancer Cytopathol. 2017;125(2):138–43. doi:10.1002/cncy.21789.
- Salazar KL, Zhou HS, Xu J, Peterson LE, Schwartz MR, Mody DR, Ge Y. Multiple human papilloma virus infections and their impact on the development of high-risk cervical lesions. Acta Cytol. 2015;59(5):391–8. doi:10.1159/000442512.
- Hoppe-Seyler K, Bossler F, Braun JA, Herrmann AL, Hoppe-Seyler F. The HPV E6/E7 oncogenes: key factors for viral carcinogenesis and therapeutic targets. Trends Microbiol. 2018 Feb;26(2):158–68. doi:10.1016/j.tim.2017.07.007.
- Pal A, Kundu R. Human papillomavirus E6 and E7: the cervical cancer hallmarks and targets for therapy. Front Microbiol. 2020;10:3116. doi:10.3389/fmicb.2019.03116.
- Derbie A, Mekonnen D, Woldeamanuel Y, Van Ostade X, Abebe T. HPV E6/E7 mRNA test for the detection of high grade cervical intraepithelial neoplasia (CIN2+): a systematic review. Infect Agent Cancer. 2020;15:9. doi:10.1186/s13027-020-0278-x.
- Denis F, Hanz S, Alain S. Clearance, persistence and recurrence of HPV infection. Gynecol Obstet Fertil. 2008;36(4):430–40. doi:10.1016/j.gyobfe.2008.02.008.
- Capra G, Giovannelli L, Matranga D, Bellavia C, Guarneri MF, Fasciana T, Scaduto G, Firenze A, Vassiliadis A, Perino A. Potential impact of a nonavalent HPV vaccine on HPV related low-and high-grade cervical intraepithelial lesions: a referral hospital-based study in Sicily. Hum Vaccin Immunother. 2017;13(8):1839–43. doi:10.1080/21645515.2017.1319026.
- Dong B, Sun P, Ruan G, Huang W, Mao X, Kang Y, Pan D, Lin F. Type-specific high-risk human papillomavirus viral load as a viable triage indicator for high-grade squamous intraepithelial lesion: a nested case- control study. Cancer Manag Res. 2018;10:4839–51. doi:10.2147/CMAR.S179724.
- Andersen B, Njor SH, Jensen AMS, Johansen T, Jeppesen U, Svanholm H. HrHPV testing vs liquid-based cytology in cervical cancer screening among women aged 50 and older: a prospective study. Int J Gynecol Cancer. 2020;30(11):1678–83. doi:10.1136/ijgc-2020-001457.
- Asciutto KC, Forslund O, Borgfeldt C. Prevalence of high-risk HPV in postmenopausal women with benign cervical cytology-a population-based cohort study. Anticancer Res. 2018;38(7):4221–8. doi:10.21873/anticanres.12718.
- Yuan Y, Cai X, Shen F, Ma F. HPV post-infection microenvironment and cervical cancer. Cancer Lett. 2021;497:243–54. doi:10.1016/j.canlet.2020.10.034.
- Desai S, Zhu MJ, Lapidos-Salaiz I. Cervical cancer prevention: human papillomavirus testing as primary screening. Cancer. 2022;128(5):939–43. doi:10.1002/cncr.34006.
- Zhang C, Cheng W, Liu Q, Guan Q, Zhang Q. Distribution of human papillomavirus infection: a population-based study of cervical samples from Jiangsu Province. Virol J. 2019;16(1):67. doi:10.1186/s12985-019-1175-z.
- Geng Y, Liu L. Human papillomavirus genotypes and infection among women in Changzhou, China. Hum Vaccin Immunother. 2019;15(7–8):1884–8. doi:10.1080/21645515.2019.1611159.
- Zang L, Hu Y. Risk factors associated with HPV persistence after conization in high-grade squamous intraepithelial lesion. Arch Gynecol Obstet. 2021;304(6):1409–16. doi:10.1007/s00404-021-06217-1.
- Zhu X, Wang Y, Lv Z, Su J. Prevalence and genotype distribution of high-risk HPV infection among women in Beijing, China. J Med Virol. 2021;93(8):5103–9. doi:10.1002/jmv.27013.
- Li M, Du X, Lu M, Zhang W, Sun Z, Li L, Ye M, Fan W, Jiang S, Liu A, et al. Prevalence characteristics of single and multiple HPV infections in women with cervical cancer and precancerous lesions in Beijing, China. J Med Virol. 2019;91(3):473–81. doi:10.1002/jmv.25331.
- Xu J, Xia Z, Wang L, Yang B, Zhu Y, Zhu X, Xu L. Human papillomaviruses 16 and 58 are distributed widely among women living in Shanghai, China, with high-grade, squamous intraepithelial lesions. Epidemiol Infect. 2018;147:e42. doi:10.1017/S0950268818003011.
- Hu Z, Ma D. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. 2018;7(10):5217–36. doi:10.1002/cam4.1501.
- Gustinucci D, Giorgi Rossi P, Cesarini E, Broccolini M, Bulletti S, Carlani A, D’angelo V, D’amico MR, Di Dato E, Galeazzi P, et al. Use of cytology, E6/E7 mRNA, and p16INK4a-Ki-67 to define the management of human papillomavirus (HPV)-positive women in cervical cancer screening. Am J Clin Pathol. 2016;145(1):35–45. doi: 10.1093/ajcp/aqv019.
- Estêvão D, Costa NR, Gil da Costa RM, Medeiros R. Hallmarks of HPV carcinogenesis: the role of E6, E7 and E5 oncoproteins in cellular malignancy. Biochim Biophys Acta Gene Regul Mech. 2019;1862(2):153–62. doi:10.1016/j.bbagrm.2019.01.001.
- Giorgi Rossi P, Carozzi F, Ronco G, Allia E, Bisanzi S, Gillio-Tos A, De Marco L, Rizzolo R, Gustinucci D, Del Mistro A, et al. p16/ki67 and E6/E7 mRNA accuracy and prognostic Value in triaging HPV DNA-Positive women. J Natl Cancer Inst. 2021;113(3):292–300. doi:10.1093/jnci/djaa105.
- Gutiérrez-Hoya A, Soto-Cruz I. Role of the JAK/STAT pathway in cervical cancer: its relationship with HPV E6/E7 oncoproteins. Cells. 2020;9(10):2297. doi:10.3390/cells9102297.
- Okunade KS. Human papillomavirus and cervical cancer. J Obstet Gynaecol. 2020 Jul;40(5):602–8. doi:10.1080/01443615.2019.1634030.
- World Health Organization. WHO guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. PMID: 24716265. Bookshelf ID: NBK195239.
- Kim M, Park NJ, Jeong JY, Park JY. Multiple human papilloma virus (HPV) infections are associated with HSIL and persistent HPV infection status in Korean patients. Viruses. 2021;13(7):1342. doi:10.3390/v13071342.
- Oyervides-Muñoz MA, Pérez-Maya AA, Sánchez-Domínguez CN, Berlanga-Garza A, Antonio-Macedo M, Valdéz-Chapa LD, Cerda-Flores RM, Trevino V, Barrera-Saldaña HA, Garza-Rodríguez ML. Multiple HPV infections and viral load association in persistent cervical lesions in Mexican women. Viruses. 2020;12(4):380. doi:10.3390/v12040380.
- Della Fera AN, Warburton A, Coursey TL, Khurana S, McBride AA. Persistent human papillomavirus infection. Viruses. 2021;13(2):321. doi:10.3390/v13020321.
- Jentschke M, Kampers J, Becker J, Sibbertsen P, Hillemanns P. Prophylactic HPV vaccination after conization: a systematic review and meta-analysis. Vaccine. 2020;38(41):6402–9. doi:10.1016/j.vaccine.2020.07.055.