ABSTRACT
With the development of the SARS-CoV-2 pandemic, there have been doubts about the necessity of vaccine boosters for healthy adults. However, due to the lack of relevant evidence, current research is unable to provide reliable medical advice for COVID-19 boost in healthy adults. We conducted a retrospective observational study to evaluate the effectiveness of different COVID-19 vaccination regimens by investigating the SARS-CoV-2 infection status of healthy donors in Southeast China. From December 2022 to February 2023, 737 healthy adult blood donors were analyzed. Results showed that any COVID-19 vaccine boosts reduced the risk of Omicron BA.5.2/BF.7 infection compared to only receiving prime vaccination (rVE = 16%, 95%CI = 4, 27%). The second boost further enhanced vaccine effectiveness compared to the received first booster (rVE = 39%, 95%CI = 16, 55%). Through retrospective observation of healthy adults during the BA.5.2/BF.7 surge in China, we found that boost vaccinations significantly reduce the risk of SARS-CoV-2 infection and disease. Findings show healthy adults benefit from boost vaccinations, even if not at high-risk for severe COVID-19.
KEYWORDS:
Introduction
As the SARS-CoV-2 pandemic continues to progress, there has been a gradual shift in understanding regarding COVID-19 vaccines. Although multiple studies have demonstrated the significant effectiveness of COVID-19 vaccines for both prime and boost doses, particularly for high-risk populations such as the elderly, in reducing the risk of severe disease and death,Citation1–6 controversy has arisen with the emergence of the Omicron variant toward the end of 2021, as the risks of severe disease and death have gradually decreased.Citation7 This has led to questions as to whether boost shots should only be focused on high-risk groups, or whether healthy adults still need them.
Unfortunately, previous vaccine effectiveness evaluation studies, especially those targeting Chinese populations,Citation8,Citation9 have mostly focused on the effectiveness against severe illnesses or high-risk populations, and it is difficult to provide credible medical advice on COVID-19 vaccine administration for healthy adults. Furthermore, as of the end of 2022, eight COVID-19 vaccines have been approved in mainland China,Citation10 including an influenza vector nasal spray vaccine (Wantai),Citation11 intramuscular and aerosolised adenovirus vector vaccines (CanSino),Citation12 and five recombinant protein vaccines (Zhifei,Citation13 Livzon,Citation14 Westvac,Citation15 Clover,Citation16 and SionCellTechCitation17), which have been used as the second COVID-19 boost. But there is currently no real-world evidence available to evaluate the effectiveness of the second boost shot in China after the adjustment of the COVID-19 policy at the 2022 end.
To update existing data and provide medical recommendations for COVID-19 vaccination in healthy adults, and evaluate the effectiveness of different COVID-19 vaccination regimens in preventing infection and reducing symptoms, this study investigated the incidence of COVID-19 infection among healthy adult blood donors during the SARS-CoV-2 infection surge from late 2022 to early 2023 in China, using a retrospective observational study design.
Methods
Study design and data sources
We conducted a retrospective observational study based on blood donors at Xiamen Blood Service between the end of December 2022 and February 2023. To this end, we collected information on participants’ characteristics, vaccination status, and SARS-CoV-2 infection through the use of questionnaires administered under investigators’ supervision. This study was approved by the Ethics Committees of Xiamen Blood Service (20210908). Written informed consent was obtained from all participants.
All volunteers who participated in the study were required to meet the National Health Commission of China’s health criteria for blood donation, and their blood samples had to test negative for HBV, HIV, HCV, Treponema pallidum, and HTLV in laboratory tests (as previously describedCitation18). Additionally, we excluded individuals who meet the following criteria: 1) have history of SARS-CoV-2 vaccination or inability to provide valid vaccination information; 2) have credible evidence of SARS-CoV-2 infection for individuals who claim to be infected (as evidenced by SARS-CoV-2 nucleic acid or antigen test results, or COVID-19-related symptoms and clear epidemiological contact with infected individuals with positive nucleic acid or antigen tests); 3) inability to provide valid disease information for individuals infected with SARS-CoV-2. Volunteers who have been infected with SARS-CoV-2 are only permitted to donate blood after one week of becoming virology negative. None of the volunteers had experienced SARS-CoV-2 infection before this surge.
To classify the vaccination regimens, we relied on the Chinese SARS-CoV-2 vaccination strategy. And prime vaccination includes the following: 1) two doses of inactivated vaccine (Sinovac, Sinopharm, and Biokangtai); 2) one dose of adenovirus vector vaccine (CanSino); and 3) three doses of ZF2001 recombinant protein vaccine (Zhifei). We considered the participants who received first and second booster shots, in addition to the prime vaccination, as the first boost group and second boost group, respectively. Furthermore, as the initiation of the second boost vaccination in China was close to the timing of the surge, we considered only those who had received the booster dose at least one week before the date of infection or investigation as having completed the vaccination regimens.
Outcomes and symptoms
The study outcomes and symptoms were collected through questionnaires administered under the supervision of investigators. The study utilized several outcomes to assess the effectiveness of the different vaccination regimens, including any SARS-CoV-2 infection, symptomatic infection, infection with a symptom duration of at least five days, and infection with fever. It is worth noting that SARS-CoV-2 infection was defined earlier in the study as requiring nucleic acid or antigen test results, or COVID-19-related symptoms and clear epidemiological contact with infected individuals with positive nucleic acid or antigen tests. Symptomatic infectionCitation19 was defined as SARS-CoV-2 infected participants presenting with one or more symptoms. While infections with a symptom duration of at least five days were defined as symptomatic infections with a perceived symptomatic duration of five days or more. Infection with fever was defined as symptomatic infection with a body temperature greater than 37.2°CCitation20 at least once during the disease.
Moreover, we also collected data on specific symptoms experienced by SARS-CoV-2 infected participants, including sore throat, cough, nasal congestion and runny nose, smell or taste disorders, fever (body temperature greater than 37.2°C), dizziness, headache, fatigue, myalgia, joint pain, and gastrointestinal symptoms. It is important to note that fever was further divided into two categories based on temperature: >38.1°C (moderate fever) and >39.1°C (hyperpyrexia).
Statistical analysis
The baseline characteristics of the study participants were described using number and percentage or median and interquartile range as appropriate. The estimated adjusted hazard ratio (HR) was calculated using Poisson regression models. Vaccine effectiveness (VE) was calculated as 1-HR and given as a percentage. To account for potential confounding variables, the models were adjusted for age, gender, blood type, and education level for the assessed outcomes and age, gender, and blood type for the assessed symptoms. The statistical analyses were conducted using R software (version 4.2.2).
Results
During BA.5.2/BF.7 (BA.5.2.48 and BF.7.14 were predominant) surge in China from late December 2022 to February 2023Citation21 (), 737 healthy adult blood donors aged 18–59 years in Southeast China were enrolled in the analysis (Figure S1). The participants had a median age of 37 years and were predominantly male (75%). The participants were classified according to their COVID-19 vaccination regimens, with 57 receiving only the prime vaccination, 627 receiving the first boost on the prime regimen, and 53 receiving the second boost. The groups had similar demographic characteristics (). The participants in each group received their vaccinations following the COVID-19 vaccination campaign in China,Citation22 as shown in . The median interval between the last dose and the infection surge was approximately 18 months (520 days) and 12 months (379 days) for those who had received only the prime vaccination and the first booster, respectively. The observation time (median: 18 days) was shorter for individuals who received the second booster as its administration nearly coincided with the infection wave onset.
Figure 1. The daily COVID-19 cases, vaccine doses, and SARS-CoV-2 lineages prevalence in China.
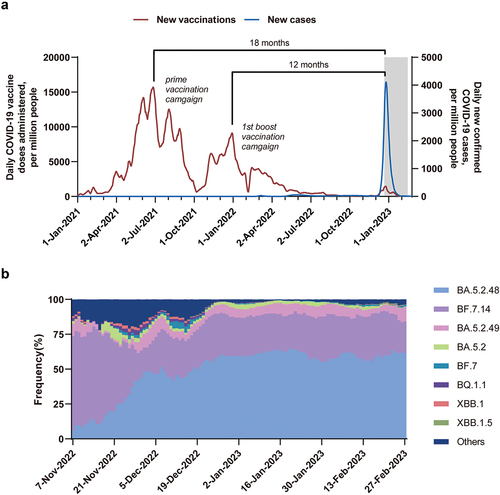
Table 1. Characteristics of the participants.
Effectiveness of vaccination regimens in preventing SARS-CoV-2 infection
Out of all participants, 504 were found to be infected with SARS-CoV-2. The infected individuals accounted for 81%, 69%, and 43% in the prime, first, and second boost vaccination groups, respectively. Most of the reported infections were symptomatic, with 79%, 64%, and 40% of participants in the aforementioned groups having symptomatic infections, respectively. The disease’s severity was relatively mild among the individuals, and no hospitalizations were reported. To compare the vaccination regimens’ effectiveness in preventing illness further, we selected two other outcomes: duration of symptoms greater than or equal to 5 days and infection with fever, which accounted for 32% and 76% of total infected persons, respectively. In the prime vaccination, first, and second boost vaccination groups, the proportion of the aforementioned outcomes was 37%, 21%, and 11%; 58%, 53%, and 26%, respectively ().
Table 2. Incidence of outcomes and symptoms in participants receiving different vaccination regimens.
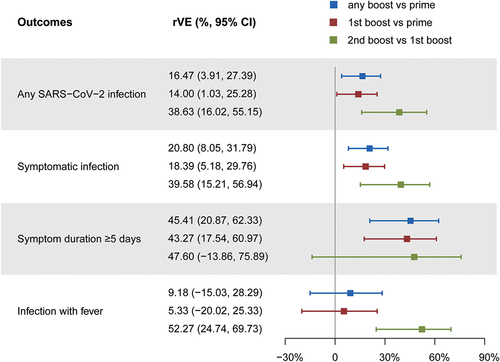
We used a stepwise comparison to evaluate the effectiveness of different vaccination regimens, which were grouped into three categories: a comparison between those who received any number of boosts and those who received only the prime vaccination, a comparison between those who received the first boost and those who received only the prime vaccination, and comparison between those who received the second and the first boost. Receiving boost shots provides significant protection against SARS-CoV-2 infection during the surge () for healthy adults. Participants who received any number of boosts had a significantly lower risk of any SARS-CoV-2 infection, symptomatic infection, and infection with symptoms duration ≥5 days, compared to those who received only prime immunization, with relative vaccine effectiveness (rVE) of 16% (95% CI: 4, 27%), 21% (95% CI: 8, 32%), and 45% (95% CI: 21, 62%), respectively. As participants who received the first boost accounted for a large proportion of the boost group, they showed similar results to any boost group in comparison to the prime vaccination group, with rVEs of 14% (95% CI: 1, 25%), 18 (95% CI: 5, 30%), and 30% (95% CI: 18, 61%), respectively. More notably, it was found that receiving a second boost could further significantly reduce the risk of SARS-CoV-2-related outcomes on top of the first boost. For those who received the second boost, the rVE for any SARS-CoV-2 infection was 39% (95% CI: 16, 55%) compared to participants who only received the first boost, and the rVEs for symptomatic infection and infection with fever were 40% (95% CI: 15, 57%) and 52% (95% CI: 25, 70%), respectively.
Figure 2. Effectiveness of various vaccination regimens in preventing different SARS-CoV-2 infections.
Effectiveness of vaccination regimens in reducing symptoms among symptomatic infection
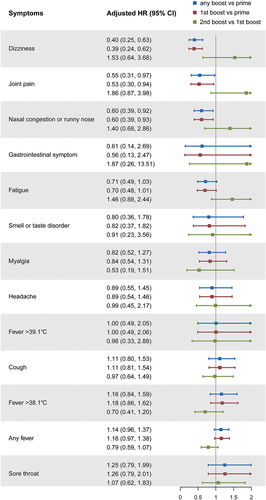
The study further compared the differences in symptom occurrence among participants with symptomatic infections under different vaccination regimens. Among the monitored symptoms, the fever had the highest incidence, with 81% of symptomatic infections reporting it, and 63% of those with fever having a temperature above 38.1°C. Subsequently, the main symptoms reported included cough (53%), sore throat (36%), fatigue (32%), muscle pain (28%), and headache (25%). The incidence of disorder of taste or smell was relatively low, with only 11% of symptomatic infections reporting this symptom ().
However, the differences in the incidence of most symptoms were not significant among the different vaccination regimens (). We found that compared to the prime vaccination, participants who received any boost had a significantly reduced risk of dizziness, joint pain, and nasal congestion or runny nose, with HRs of 0.40 (95% CI: 0.25, 0.63), 0.55 (95% CI: 0.31, 0.97), and 0.60 (95% CI: 0.39, 0.92), respectively. However, there was no significant difference in symptom occurrence between the second boost and the first boost.
Figure 3. Effectiveness of various vaccination regimens in reducing symptoms.
Discussion
This study retrospectively observed the infection and symptom occurrence of healthy adult participants during the BA.5.2/BF.7 infection surge from the end of late 2022 to early 2023 in China and evaluated the effectiveness of different COVID-19 vaccination regimens in preventing SARS-CoV-2 infection and reducing symptoms. Since the interval between the final vaccination and the surge in participants receiving the prime vaccination and the first boost is consistent with Chinese two COVID-19 vaccination campaigns (), this study has a certain degree of representativeness and can reflect the differences in SARS-CoV-2 infection and symptom occurrence among groups receiving different vaccination regimens in different vaccination campaigns.
We found that boost doses, particularly the second boost, significantly reduced the risk of infection among healthy adults. Compared with the prime vaccination, boosts reduced the risk of SARS-CoV-2 infection by approximately 16% and the risk of symptomatic infection by approximately 21%, and can reduce the risk of infections with symptoms duration ≥5 days by about 45%. Importantly, similar to previous studies,Citation23,Citation24 receiving a second boost compared to the first boost can reduce the risk of SARS-CoV-2 infection and symptomatic infection by about 40%, and the risk of infection with fever by about 50%. The adoption of a heterologous second boost strategy in China is posited as one potential explanation for the enhanced protective effectiveness of this vaccination regimen. These results indicate that even for healthy adult populations not at high risk of severe COVID-19, boost doses, especially the second boost, can effectively reduce the risk of SARS-CoV-2 infection and disease.
During our analysis of symptoms in symptomatic individuals infected during the surge, we found that fever was the most common symptom, occurring in over 80% of patients. Furthermore, the majority of fever patients (63%) had a temperature of ≥38.1°C, with almost 20% having a temperature of ≥39.1°C. However, we did not observe a significant difference in the incidence of most symptoms when comparing different vaccination regimens. The vaccine’s immune protection may be more effective in preventing infection and disease, but individual differences may play a greater role in determining the occurrence of symptoms in individuals with mild breakthrough infections. Additionally, the limitation of sample size may have also influenced the comparison to some extent, which may have resulted in some point estimates of symptom incidence being even higher in participants who received the second boost than in those who only received the first boost. Of course, this could also be owing to participants who received the second boost being more concerned about their health than the general population.
Some limitations should be noted for our study. First, a retrospective questionnaire was used to collect the infection history in this study, which may introduce recalling bias. Moreover, due to the limited availability of SARS-CoV-2 testing during the surge period, the reported infections may more likely refer to symptomatic cases. Additionally, the limited sample size may have impacted the participants’ representativeness, which may have influenced the result to some extent, and also cannot provide further evaluation of the effectiveness of different COVID-19 vaccines.
In conclusion, our study during the BA.5.2/BF.7 surge in China found that booster vaccinations can significantly reduce the risk of SARS-CoV-2 infection. The second booster shot showed even greater effectiveness compared to the first booster. These findings provide partial evidence on whether healthy adults should continue receiving booster vaccinations, as the benefits remain significant even for non-high-risk individuals for severe COVID-19 disease.
Author contributions
Z.H.H., Y.C.Z., S.H.O., Y.L.Z., and Q.Y. conceptualized and designed the study. Y.C.Z., S.H.O., S.C.Z., J.H.W., Z.H.H., M.L.L., H.L.G., J.J.C., and J.H.Y. enrolled the participants and collected the data. Z.H.H. analyzed and interpreted the data. Z.H.H. drafted the article. Y.C.Z., S.H.O., Y.L.Z., and Q.Y. critically revised important intellectual content. All authors critically reviewed the manuscript and approved the final version.
Ethical approval statement
This study was approved by the Ethics Committees of Xiamen Blood Service (20210908). Written informed consent was obtained from all participants.
Supplemental Material
Download PDF (284.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support this study are available from the corresponding authors upon request. The Date used for showing the daily COVID-19 cases and vaccine doses in China is available from the Our World in Data COVID-19 dataset (https://ourworldindata.org/coronavirus). The daily prevalence of SARS-CoV-2 PANGO lineages is available from GISAID (https://www.epicov.org).
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2246483.
Additional information
Funding
References
- Altarawneh HN, Chemaitelly H, Ayoub HH, Tang P, Hasan MR, Yassine HM, Al-Khatib HA, Smatti MK, Coyle P, Al-Kanaani Z, et al. Effects of previous infection and vaccination on symptomatic Omicron infections. N Engl J Med. 2022;387(1):21–8. doi:10.1056/NEJMoa2203965.
- Accorsi EK, Britton A, Shang N, Fleming-Dutra KE, Link-Gelles R, Smith ZR, Derado G, Miller J, Schrag SJ, Verani JR, et al. Effectiveness of homologous and heterologous covid-19 boosters against Omicron. N Engl J Med. 2022;386(25):2433–5. doi:10.1056/NEJMc2203165.
- Gray G, Collie S, Goga A, Garrett N, Champion J, Seocharan I, Bamford L, Moultrie H, Bekker L-G. Effectiveness of Ad26.COV2.S and BNT162b2 vaccines against Omicron variant in South Africa. N Engl J Med. 2022;386(23):2243–5. doi:10.1056/NEJMc2202061.
- Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O’Connell A-M, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–46. doi:10.1056/NEJMoa2119451.
- Cerqueira-Silva T, Katikireddi SV, de Araujo Oliveira V, Flores-Ortiz R, Júnior JB, Paixão ES, Robertson C, Penna GO, Werneck GL, Barreto ML, et al. Vaccine effectiveness of heterologous coronaVac plus BNT162b2 in Brazil. Nat Med. 2022;28(4):838–43. doi:10.1038/s41591-022-01701-w.
- Lin DY, Xu Y, Gu Y, Zeng D, Wheeler B, Young H, Sunny SK, Moore Z. Effectiveness of bivalent boosters against severe Omicron infection. N Engl J Med. 2023;388(8):764–6. doi:10.1056/NEJMc2215471.
- Lewnard JA, Hong VX, Patel MM, Kahn R, Lipsitch M, Tartof SY. Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in Southern California. Nat Med. 2022;28(9):1933–43. doi:10.1038/s41591-022-01887-z.
- McMenamin ME, Nealon J, Lin Y, Wong JY, Cheung JK, Lau EHY, Wu P, Leung GM, Cowling BJ. Vaccine effectiveness of one, two, and three doses of BNT162b2 and coronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis. 2022;22(10):1435–43. doi:10.1016/S1473-3099(22)00345-0.
- Wei Y, Jia KM, Zhao S, Hung CT, Mok CKP, Poon PKM, Man Leung EY, Wang MH, Yam CHK, Chow TY, et al. Estimation of vaccine effectiveness of coronaVac and BNT162b2 against severe outcomes over time among patients with SARS-CoV-2 Omicron. JAMA Netw Open. 2023;6(2):e2254777. doi:10.1001/jamanetworkopen.2022.54777.
- Notification on the issuance of the implementation plan for the second booster of COVID-19 vaccine Immunization: National health commission of China. 2022 [accessed 2023 Mar 30]. http://www.gov.cn/xinwen/2022-12/14/content_5731899.htm.
- Zhu F, Zhuang C, Chu K, Zhang L, Zhao H, Huang S, Su Y, Lin H, Yang C, Jiang H, et al. Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Respir Med. 2022;10(8):749–60. doi:10.1016/S2213-2600(22)00131-X.
- Li J-X, Hou L-H, Gou J-B, Yin Z-D, Wu S-P, Wang F-Z, Zhang Z, Peng Z-H, Zhu T, Shen H-B, et al. Safety, immunogenicity and protection of heterologous boost with an aerosolised Ad5-nCov after two-dose inactivated COVID-19 vaccines in adults: a multicentre, open-label phase 3 trial. Lancet Infect Dis. 2023. (Online First). doi:10.1016/S1473-3099(23)00350-X.
- Dai L, Gao L, Tao L, Hadinegoro SR, Erkin M, Ying Z, He P, Girsang RT, Vergara H, Akram J, et al. Efficacy and safety of the RBD-Dimer–based covid-19 vaccine ZF2001 in adults. N Engl J Med. 2022;386(22):2097–111. doi:10.1056/NEJMoa2202261.
- Wang XY, Mahmood SF, Jin F, Cheah WK, Ahmad M, Sohail MA, Ahmad W, Suppan VK, Sayeed MA, Luxmi S, et al. Efficacy of heterologous boosting against SARS-CoV-2 using a recombinant interferon-armed fusion protein vaccine (V-01): a randomized, double-blind and placebo-controlled phase III trial. Emerg Microbes Infect. 2022;11(1):1910–19. doi:10.1080/22221751.2022.2088406.
- Meng FY, Gao F, Jia SY, Wu X-H, Li J-X, Guo X-L, Zhang J-L, Cui B-P, Wu Z-M, Wei M-W, et al. Safety and immunogenicity of a recombinant COVID-19 vaccine (Sf9 cells) in healthy population aged 18 years or older: two single-center, randomised, double-blind, placebo-controlled, phase 1 and phase 2 trials. Sig Transduct Target Ther. 2021;6(1):271. doi:10.1038/s41392-021-00692-3.
- Bravo L, Smolenov I, Han HH, Li P, Hosain R, Rockhold F, Clemens SAC, Roa C, Borja-Tabora C, Quinsaat A, et al. Efficacy of the adjuvanted subunit protein COVID-19 vaccine, SCB-2019: a phase 2 and 3 multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2022;399(10323):461–72. doi:10.1016/S0140-6736(22)00055-1.
- Hannawi S, Saifeldin L, Abuquta A, Alamadi A, Mahmoud SA, Li J, Chen Y, Xie L. Safety and immunogenicity of a bivalent SARS-CoV-2 protein booster vaccine, SCTV01C in adults previously vaccinated with inactivated vaccine: a randomized, double-blind, placebo-controlled phase 1/2 clinical trial. J Infect. 2023;86(2):154–225. doi:10.1016/j.jinf.2022.12.003.
- Ou S, Huang Z, Lan M, Ye J, Chen J, Guo H, Xiao J, Zhuang S, Wu J, Yang C, et al. The duration and breadth of antibody responses to 3-dose of inactivated COVID-19 vaccinations in healthy blood donors: an observational study. Front Immunol. 2022;13. doi:10.3389/fimmu.2022.1027924.
- Notice on the issuance of the diagnosis and treatment protocol for COVID-19 (trial version 9): National health commission of the people’s republic of China. 2022. http://www.gov.cn/zhengce/zhengceku/2022-03/15/content_5679257.htm.
- Wan X, Lu X. Diagnostics. 9th ed. Beijing, China: People’s Medical Publishing House; 2018.
- Shu Y, McCauley J. GISAID: global initiative on sharing all influenza data - from vision to reality. Euro Surveill. 2017;22(13). doi:10.2807/1560-7917.ES.2017.22.13.30494.
- Coronavirus pandemic (COVID-19) [Internet]. OurWorldindata.Org. 2020. https://ourworldindata.org/coronavirus.
- Ranzani OT, Hitchings MDT, de Melo RL, de França GVA, Fernandes CDFR, Lind ML, Torres MSS, Tsuha DH, David LCS, Said RFC, et al. Effectiveness of an inactivated covid-19 vaccine with homologous and heterologous boosters against Omicron in Brazil. Nat Commun. 2022;13(1):5536. doi:10.1038/s41467-022-33169-0.
- Cerqueira-Silva T, de Araujo Oliveira V, Paixão ES, Júnior JB, Penna GO, Werneck GL, Pearce N, Barreto ML, Boaventura VS, Barral-Netto M, et al. Duration of protection of coronaVac plus heterologous BNT162b2 booster in the Omicron period in Brazil. Nat Commun. 2022;13(1):4154. doi:10.1038/s41467-022-31839-7.
