ABSTRACT
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) increases mortality rates in older adults and those with comorbidities. Individuals with certain comorbidities may have a poor immune response and require early booster vaccines. We aimed to assess the immune response after two doses of ChAdOx1 nCoV-19 vaccine, at 84-day intervals, in participants with the following comorbidities; diabetes mellitus; obesity; cardiovascular disease; chronic kidney disease; rheumatological disease; cirrhosis; hematological disease; hematological malignancy; or solid malignancy. The study was conducted at Chulabhorn Hospital in Thailand, with healthy healthcare workers serving as the control group. Of the 769 participants, 352 were in the healthy cohort and 417 were in the comorbidity cohort, all received at least one dose of vaccine. Anti-RBD total antibody levels were evaluated on Day 0, Day 84, and Day 112. The results at Day 112 (4 weeks after the second dose) showed that individuals with comorbidities had a poor immune response compared to healthy individuals, especially those with hematological malignancy and solid malignancy. The geometric mean concentration (GMC) of anti-RBD antibody in the comorbidity cohort was significantly lower than that in the healthy cohort: 433.66 BAU/ml (95% CI 334.62–562.01) versus 1096.14 BAU/ml (95% CI 1010.26–1189.33), respectively. The geometric mean ratio (GMR) between the two cohorts was 0.40 (95% CI 0.30–0.52, p < .001). This study concluded that individuals with comorbidities, particularly hematological and solid malignancies, had poor immune responses and may require an early booster vaccine to prevent infection and death.
Introduction
The pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) affected individuals worldwide, causing coronavirus disease 2019 (COVID-19). Individuals with COVID-19 had a wide range of clinical characteristics, from asymptomatic to severe and life-threatening conditions.Citation1 Although the majority of individuals with COVID-19 had mild symptoms, approximately 14% had severe disease, and 5% had critical conditions, and 2–3% died. Patients with old age or comorbidity diseases had an increased risk of death.Citation2,Citation3 A meta-analysis of 42 studies, including 423,117 patients with COVID-19, revealed that those with death events were associated with old age, male gender, smoking status, obesity, cardiovascular disease, diabetes, and hypertension.Citation4 A very high mortality rate of 31.2% was observed in patients with hematological malignancies.Citation5 Patients with cancer who have recently received treatment also have an increased risk of death.Citation6 Individuals with chronic kidney disease (CKD), liver disease, stroke, dementia, and rheumatological disease also have an increased risk of death after SARS-CoV-2 infection.Citation7 In addition, it has been observed that immunocompromised individuals tend to have an increased duration of SARS-CoV-2 shedding and infectivity compared to immunocompetent individuals. This finding underscores the importance of understanding the implications of immunocompromised status in the context of COVID-19 transmission and highlights the need for special considerations in protecting this vulnerable population.Citation8
COVID-19 vaccines can prevent SARS-CoV-2 infection and decrease the risk of severe disease after infection. Most COVID-19 vaccines elicit both humoral and cell-mediated immunity, although inactivated COVID vaccines seem to elicit less cell-mediated immune response.Citation9 ChAdOx1 nCoV-19 (ChAdOx1) is an adenoviral vector vaccine developed by Oxford University. A clinical phase 3 study demonstrated that the ChAdOx1 vaccine had a high efficacy of up to 74.0% after two doses of vaccines.Citation10 A real-world study also supports this finding, with a vaccine effectiveness of 83% found by Sritipsukho et al.Citation11
Antibody against receptor-binding domain (RBD) of the spike (S) protein of SARS-CoV-2 could prevent viral infection. Furthermore, there was evidence that vaccine efficacy correlated with the titer of anti-RBD and anti-S protein antibodies.Citation12 However, Individuals with some comorbidity impaired these immune responses.Citation13
There is limited information in comparison of immunogenicity between healthy individuals with different comorbidity individuals. This study aimed to investigate whether immunogenicity differs between individuals with comorbidities and healthy individuals. Despite limited information on the topic, our study aims to fill this knowledge gap and provide valuable insights into the immune response to COVID-19 vaccination in individuals with comorbidities.
Materials and methods
Study design
We conducted a single-center, open-label prospective cohort study at Chulabhorn Hospital (a tertiary care setting) in Chulabhorn Royal Academy, Bangkok, Thailand, from June 2021 to July 2021. The objective of this study was to compare the humoral immune response in participants with comorbidity disease and healthcare workers without comorbidity. The study protocol, consent form, and case records form were reviewed and approved by the Chulabhorn Ethics Committee (reference number: 056/2564). Written informed consent was obtained from all participants before enrollment. In addition, this study was registered at thaiclinicaltrials.org (TCTR20211228003) and was conducted in compliance with the International Conference for Harmonization Good Clinical Practice Guideline.
Participants
Eligible participants for this study were individuals aged 18 years or older. Participants in the comorbidity disease cohort had one of the following underlying diseases: (1) diabetes mellitus; (2) obesity (defined as body mass index ≥30 kg/m2); (3) cardiovascular disease; 94) chronic kidney disease (CKD; defined as glomerular filtration rate less than 60 mL/min); (5) rheumatological disease; (6) cirrhosis; (7) hematological disease (benign disease); (8) hematological malignancy; or (9) solid malignancy. The control participants were healthcare workers in our institute who had no comorbidity disease. Healthcare workers with comorbidity disease were assigned to the comorbidity disease cohort. Participants for our study were recruited through notices posted at our institute’s specialist clinics (outpatient department). Posters detailing the study were distributed to the diabetes clinic, cardiovascular disease clinic, kidney disease clinic, rheumatological disease clinic, hematological disease clinic, and oncology clinic. All consecutive participants who expressed interest in the study were screened for eligibility criteria. The same recruitment process was used for healthcare workers. The participants were excluded if they had a known previous SARS-CoV-2 infection or seropositive status at baseline, a known history of vaccine allergy, or were pregnant or lactating.
Vaccine and procedures
The ChAdOx1 vaccine was an adenoviral vector vaccine developed by Oxford University. The standard dose of the ChAdOx1 vaccine contained 5 × 1010 viral particles.
After enrollment, all participants received two ChAdOx1 vaccines at 12-week intervals. The vaccine was injected into the deltoid muscle. The vaccines used in this study were approved by Thailand’s regulatory agency, manufactured, and packaged in accordance with Good Manufacturing Practices guidelines.
Immunogenicity assessment
Blood samples were collected from the participants on Day 0 (before the first dose), Day 84 (before the second dose), and Day 112 (four weeks after the second dose). The humoral immune response was assessed using the binding antibody assay (Elecsys-S, Roche Diagnostics, Mannheim, Germany). The test kit measured the concentration of the anti-receptor binding domain of the spike protein of SARS-CoV-2 (anti-RBD) using the double-antigen sandwich principle. Levels of 0.8 U/ml or more were defined as seropositive. The Elecsys-S Unit had to be converted to binding antibody units (BAU) using Elecsys-S U = 0.972 × BAU, as per the World Health Organization’s recommendation.Citation14 The anti-RBD binding antibody exhibited a strong correlation (r = 0.86) with the neutralizing antibody.Citation15 In this study, we defined an anti-RBD antibody level of 133 BAU/ml or higher as a high level.Citation16
Outcomes
The primary outcome was humoral immune response on Day 112 (four weeks after the second dose of the ChAdOx1 vaccine). The secondary outcomes were the humoral immune response at Day 84 (before the second dose), safety profile, and factors contributing to the immune response.
Statistical analysis
Based on a study by Hoque et al., it was found that the mean concentration of anti-RBD antibodies was 10.38 optical density ratio (ODR) with a 2.0 standard deviation (SD) in participants without comorbidities and 9.93 (2.0) ODR in participants with comorbidities, at 2 weeks post the second dose of the ChAdOx1.Citation17 A sample size of 374 participants in each cohort was estimated to achieve a power of 80%, a significance level of 0.05 (two-sided), and a 20% dropout rate.
The mean concentration of anti-RBD was demonstrated as the geometric mean concentration (GMC) with a 95% confidence interval (CI). A comparison of the GMC of each group was shown as a geometric mean ratio (GMR) and 95% CI. Other summary statistics were demonstrated as the median with interquartile range (IQR) or mean with SD, as applicable. A comparison of the anti-RBD concentration between the comorbidity and healthy cohorts was performed using multiple linear regression analysis. Factors contributing to the humoral immune response were evaluated using multivariable analysis. The model began with univariable analysis, and statistically significant factors from the univariable analysis were then included in the multivariable analysis model. Several factors were included, such as age, sex, and type of comorbidity disease. In addition, we used a linear mixed model to analyze the repeated measures of anti-RBD antibodies on Day 0, 84, and 112, with within-individual correlations being modeled using the random intercept. A model was fitted via restricted maximum likelihood (REML) and included the following categorical covariates as fixed effects: time after the first vaccination (month), the cohort (healthy/comorbidity) × month interaction, age (<70, ≥70 years), gender, and BMI (<30, ≥30 kg/m2). As there were no differences in baseline anti-RBD antibody levels, the main effect of cohort was not included in the final models. Statistical analyses were performed using STATA version 17 (StataCorp LLC., College Station, TX, USA) and GraphPad Prism version 9 (GraphPad Software, San Diego, CA, USA). A p-value of < .05 was considered statistically significant.
Result
Patient characteristics
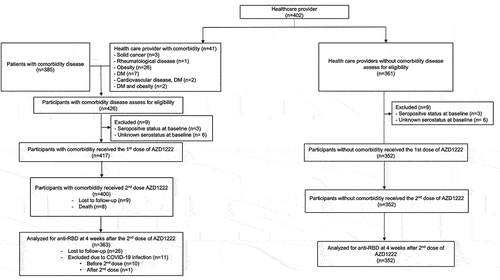
Between June 8, 2021 and July 12, 2021, 787 participants were assessed for eligibility, including 402 healthcare providers and 385 patients. Of the healthcare providers, 41 with comorbidity disease were assigned to the comorbidity cohort. Nine participants in each cohort were excluded due to seropositive or unknown serostatus at baseline. Finally, 352 participants in the healthy cohort and 417 in the comorbidity cohort received the first dose of the ChAdOx1. The summary of the participants’ flow is demonstrated in . The details of participants with comorbidity, categorized by specific diseases, are presented in Table S4-S11.
The median age of participants in the comorbidity cohort was 65 years old (IQR, 60–72), which was higher than the healthy cohort, 36 (29–49) years old. Females were 53.2% (193/363) in the comorbidity cohort and 72.2% (205/352) in the healthy cohort. The participants’ demographic data are summarized in .
Table 1. Characteristics of study participants.
Immunogenicity
The humoral immune response at 1 month after 2nd ChAdOx1 vaccination
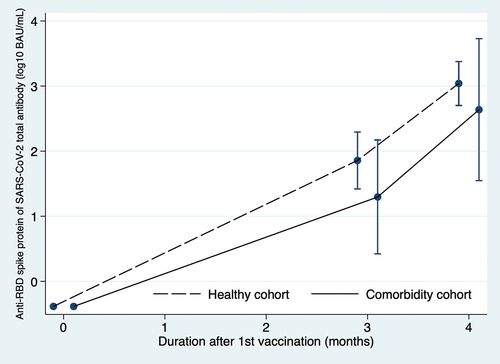
The GMC of anti-RBD antibody in the comorbidity cohort was significantly lower than that in the healthy cohort, 433.66 BAU/ml (95% CI 334.62–562.01) and 1096.14 BAU/ml, (95% CI 1010.26–1189.33), respectively. The GMR between these two cohorts was 0.40 (95%CI 0.30–0.52, p < .001) ( and ). The proportion of participants who had a high anti-RBD antibody level was also lower in the comorbidity cohort compared to the healthy cohort (82.4% vs. 99.4%, p < .001) ().
Figure 2. The geometric mean concentration of anti-RBD antibodies in the comorbidity cohort and healthy cohort at 3 months after 1st vaccination and 1 month after 2nd vaccination (4 months).
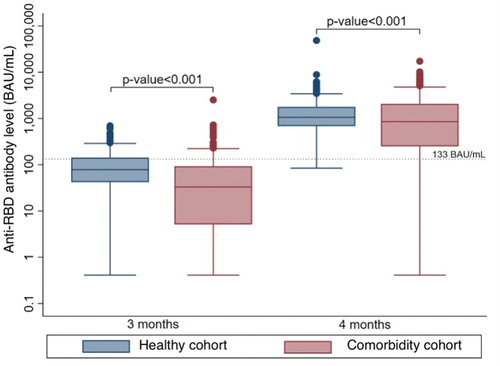
Table 2. The geometric mean concentration of anti-RBD antibody concentration at 3 months after 1st dose of ChAdOx1 nCoV-19 vaccine and 1 month after 2nd dose of ChAdOx1 nCoV-19 vaccine.
Table 3. The proportion of participants who had high anti-RBD antibody levels (antibody level ≥ 133 BAU/ml) at 3 months after 1st dose of ChAdOx1 nCoV-19 vaccine and 1 month after 2nd dose of ChAdOx1 nCoV-19 vaccine.
Multivariable analysis for anti-RBD antibody level (multiple linear regression model) showed that hematological malignancy and solid malignancy correlated with impairment of the immune response. In comparison to the healthy cohort, the GMRs of hematological malignancy and solid malignancy were 0.01 (95%CI 0.01–0.03, p < .001) and 0.57 (95%CI 0.36–0.90, p = .016), respectively. The hematological malignancy cohort had the lowest anti-RBD antibody level (GMC = 9.30 BAU/ml, 95%CI 2.86–30.13). The difference in anti-RBD antibody levels between participants with or without comorbidity, including rheumatological disease, cirrhosis, and diabetes, was found to be statistically insignificant. This effect was consistent among participants with diabetes, whether they had good glycemic control (GMC = 711.61 BAU/ml) or poor glycemic control (GMC = 605.83 BAU/ml) (p = .725) ().
Table 4. Univariable and multivariable analysis of factors associated with anti-RBD antibody at 1 month after 2nd dose ChAdOx1 nCoV-19 vaccine.
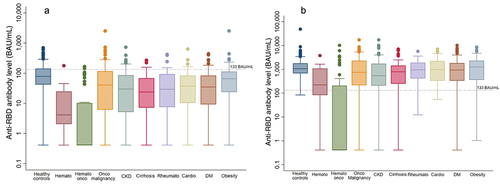
Multivariate analysis for a high level of anti-RBD antibody (multiple logistic regression model) showed that elderly patients (>70 years of age), patients with hematological disease, hematological malignancy, and solid cancer had a significantly lower proportion of participants who achieved a high level of anti-RBD antibody ( and Table S1).
Figure 3. Subgroup analysis of anti-RBD antibody level at 3 months after 1st dose of ChAdOx1 nCoV-19 vaccine and 1 month after 2nd dose of ChAdOx1 nCoV-19 vaccine. demonstrates anti-SARS-CoV2 IgG Ab level at 3 months after 1st dose of the ChAdOx1 nCoV-19 vaccine. demonstrates anti-SARS-CoV2 IgG Ab level at 1 month after 2nd dose of the ChAdOx1 nCoV-19 vaccine. BAU: binding antibody unit. Hemato: hematological disease (benign). Hemato onco (hematological malignancy). Onco malignancy: solid cancer. CKD: chronic kidney disease. Rheumato: rheumatological disease. Cardio: cardiovascular disease. DM: diabetes mellitus.
The result at 3 months after 1st ChAdOx1 nCoV-19 vaccination
At 3 months after the first ChAdOx1 nCoV-19 vaccination, the GMC of anti-RBD antibody in patients with comorbidity was significantly lower than in healthy participants (19.78 BAU/ml, 95% CI 15.99–24.48 vs. 71.92 BAU/ml, 95% CI 64.05–80.76). The geometric ratio was 0.28 (95% CI, 0.22–0.35), p < .001) ( and ).
The proportion of participants who had a high anti-RBD antibody level was also lower in the comorbidity cohort compared to the healthy cohort (28.0% vs. 15.3%, p < .001) ().
Multivariable analysis for anti-RBD antibody level (multiple linear regression model) found that participants with age ≥70 years, male gender, hematological disease, hematological malignancy, and diabetes mellitus correlated with an impairment of the immune response, as shown in Table S2. Participants with the male gender, hematological malignancy correlated with a lower proportion of a high-level anti-RBD (Table S3).
A linear mixed model showed that the geometric mean concentration in the comorbidity cohort was significantly lower than the healthy cohort, p < .001 ().
Discussion
Our study demonstrated that the immune response to the ChAdOx1 vaccine in patients with comorbidity was significantly lower than in healthy controls after the first and second doses of vaccination. This finding is consistent with the proportion of participants who achieved a high anti-RBD level. Participants with hematological malignancies and solid cancer had significantly lower immune responses than other groups one month after the second dose of vaccination. In addition, participants with old age, male gender, hematological disease, hematological malignancy, and diabetes mellitus had significantly lower immune responses than other groups three months after the first dose of vaccination.
After COVID-19 vaccination, the titer of neutralizing antibodies and binding antibodies to the spike protein of SARS-CoV-2 were correlated with vaccine efficacy.Citation12,Citation18 Our study finding was similar to previous studies investigating the immune response after COVID-19 vaccination in solid and hematological malignancies.
The CAPTURE trial evaluated the immune response in 585 participants with malignancies, including both solid and hematological malignancies. These participants received two doses of either AZD1222 (n = 430, 73.5%) or BNT162b2 (n = 153, 26.2%) vaccines with a 12-week interval between doses. The results demonstrated that participants with solid cancer had a higher neutralizing antibody titer compared to those with hematological malignancies. Furthermore, the seroconversion rate was also higher in the participants with solid cancer than in hematological malignancies. This suggests that solid cancer patients may have a stronger immune response to the vaccine. However, when compared to healthy individuals, participants with solid cancer only had numerically lower seroconversion rates after the second vaccination (92% vs. 100% for wild type). On the other hand, participants with hematological malignancies had numerically lower seroconversion rates (73% vs. 100%).Citation19 Participants with solid malignancy also had a lower immune response to the mRNA vaccine, BNT162b2, as observed in the study by Ligumsky et al. The study evaluated the immune response 6 weeks after two doses of BNT162b2 and found that the anti-spike protein IgG level in participants with solid malignancy was significantly lower than in healthy individuals, with values of 931 AU/mL vs. 2817 AU/mL, p = .003.Citation20
The study by Lim, S. H. et al. (UK PROSECO study) further supports this idea, as it showed that half of the patients with lymphoma who received anti-cancer treatment had undetectable antibody levels after two doses of ChAdOx1 or BNT162b2 vaccines.Citation21 Similarly, a study by Maneikis K. et al. found that participants with hematological malignancies had a lower humoral immune response than healthcare workers in the same age group after two doses of the BNT162b2 vaccine.Citation22
The main reason for these weaker immune responses in individuals with hematological malignancies is thought to be due to the presence of antibodies to the B cell-specific surface antigen CD20 (anti-CD20) and the malignancies themselves.Citation19,Citation21 Teeyapun N. et al. also demonstrated that participants with solid cancer had lower humoral immune responses than healthy individuals (224.5 BAU/ml vs. 877.1 BAU/ml, p < .0001),Citation23 likely due to the effects of systemic chemotherapy.
To counter this, a recombinant zoster vaccine study showed that a more robust immune response can be achieved if the vaccine is administered 1 week before chemotherapy rather than during chemotherapy.Citation24 Additionally, a cohort study of cancer participants showed that vaccine effectiveness (VE) of COVID-19 vaccines was higher (85, 95%CI 29–100) in participants who received systemic therapy for more than 6 months compared to those who received it within 3 months (54, 95%CI 28–72) before vaccination.Citation25 This suggests that timing of vaccination in relation to chemotherapy can play a role in the immune response.
There is limited information available on the immune response after COVID-19 vaccination in participants with hematological conditions other than malignancy. A study by Rotterdam J. et al. reported that not all participants with hematological conditions other than malignancy achieved seroconversion after two doses of COVID-19 vaccination; 11.4% (4/35) of participants remained seronegative.Citation26 This suggests that individuals with hematological conditions other than malignancy may have a weaker immune response to the vaccine. The reason for this is thought to be due to the corticosteroid treatment that these individuals often receive.
For participants with diabetes, our study findings were correlated with the study of Sourij C. et al., which found that only half of the participants with diabetes had seroconversion after the first dose of the COVID-19 vaccine, mostly BNT162b2. However, the concentrations of anti-RBD antibodies were not found to be different between diabetes and healthy participants after the second vaccination.Citation27 This suggests that while diabetes may affect the immune response to the first dose of the vaccine, it does not appear to have a significant impact on the response to the second dose.
For participants of old age, our finding was similar to the finding of Ramasamy M.N. et al. The anti-RBD antibody concentration was lower in older adults than young adults after the first ChAdOx1 vaccination. However, the concentrations were not found to be different after the second vaccination.Citation28 This suggests that while age may affect the immune response to the first dose, it does not appear to have a significant impact on the response to the second dose.
Our finding that male participants had a lower anti-RBD concentration after the first ChAdOx1 vaccination is similar to our previous finding in healthcare workers.Citation29 This suggests that gender may also play a role in the immune response to the COVID-19 vaccine, but it does not appear to have a significant impact on the response to the second dose.
Overall, this research suggests that different factors such as diabetes, age and gender may affect the immune response to COVID-19 vaccination, but it does not appear to have a significant impact on the response to the second dose.
Participants with CKD in our study had a lower anti-RBD antibody concentration than participants without CKD in univariable analysis. However, after adjusting for other factors in multivariable analysis, there was no statistically significant difference. This suggests that while CKD may affect the immune response to the COVID-19 vaccine, it may not have a significant impact when taking other factors into account. Our findings are similar to those of Sanders J.F. et al. who found that after two doses of mRNA-1273 COVID-19 vaccination, participants with severely impaired kidney function (eGFR <30 mL/min/1.73 m2) had a spike S1-specific IgG antibody level that was not significantly different compared to participants without kidney disease (eGFR >45 mL/min/1.73 m2), 2405 vs. 3186 BAU/mL, p value = 0.06. However, participants on hemodialysis or with kidney transplant recipients had a lower immune response significantly with the spike S1-specific IgG antibody level of 1650 BAU/mL (95%CI, 698–3024) and 25 BAU/mL (95%CI, 3–416), respectively.Citation30 This suggests that advanced stages of CKD or kidney replacement therapies may have a more significant impact on the immune response to the COVID-19 vaccine.
The results of our study in participants with cirrhosis were similar to those found by Ruether D.F. et al. This study evaluated the immune response in 48 participants with cirrhosis, 138 patients with liver transplant recipients and 52 healthy controls. These participants received two doses of COVID-19 vaccines, mainly BNT162b2 and mRNA1273 (204/238, 85.7%). They found that patients with liver cirrhosis had humoral immune responses that were not significantly different from healthy control participants. All patients with liver cirrhosis had seroconversion at 1 month after vaccination, though a small proportion had a low positive response. In contrast, participants with a post-liver transplant had a significantly different humoral immune response from healthy control and liver cirrhosis participants. Up to half of the patients with post-liver transplant had a negative or weak humoral immune response.Citation31
Our study findings for participants with rheumatological disease differ from those reported by Boekel L. et al. This study evaluated the immune response in 632 participants with autoimmune disease and 289 healthy controls. These participants received two doses of COVID-19 vaccines, which included the AZD1222 vaccine (343/921, 55.9%), BNT162b2 (349/921, 37.9%), and CX-024414 (Moderna) (55/921, 6.0%).According to their study, patients with rheumatological disease had a lower anti-RBD antibody level and lower seroconversion rate after the first dose of the COVID-19 vaccine than healthy control participants. They also found that treatment with methotrexate was associated with a weaker immune response to the first dose of the vaccine.Citation32 However, the main difference between our study and theirs lies in the methotrexate dosage administered to the patients. In our study, the patients received a lower median methotrexate dose of 2.5 mg/week (IQR, 0–7 mg/week), whereas more than half of the participants in the Boekel study received methotrexate doses greater than 15 mg/week. Additionally, it is noteworthy that 32% (202/632) of the participants in the Boekel study received biological agents, while none of our patients were administered such agents. Our study also differs from the one reported by Furer L. et al., which evaluated the immune response after two doses of the BNT162b2 vaccine in participants with autoimmune inflammatory rheumatic diseases. Their study included 686 participants with autoimmune disease and 121 healthy controls. According to their findings, patients with autoimmune disease exhibited a lower anti-RBD antibody level and a reduced seroconversion rate compared to healthy control participants. The anti-spike protein IgG titers were 132.9 and 218.6 BAU/ml for autoimmune patients and healthy participants, respectively, with a p-value < .001. The main difference between our study and the one conducted by Furer et al. lies in the type of study vaccine used. Additionally, in their study, 12.7% (87/686) of the participants received anti-CD20 agents, and 2.3% (16/686) received Abatacept. In contrast, none of the participants in our study received these agents.Citation33
For participants with overweight and obesity, our findings are consistent with previous studies which have shown no difference in vaccine efficacy or effectiveness between obesity and healthy-weight participants.Citation34 For example, Piernas C. et al. found that the vaccine effectiveness in overweight and obese participants was similar in magnitude to that of people with a healthy weight. They found that after 14 days or more after two doses of the COVID-19 vaccine, including ChAdOx1, BNT162b2, and mRNA1273, the odds ratio (OR) for hospital admission was 0.34 (95%CI, 0.32–0.36) for a healthy weight, 0.32 (95%CI, 0.30–0.34) for overweight, and 0.32 (95%CI, 0.32–0.36) for obesity participants.Citation35 Similarly, Polack F.P. et al. found that 7 days or more after two doses of BNT162b2 vaccines, the vaccine efficacy was 95.4% in participants with obesity and 94.8% in healthy weight.Citation36 Furthermore, Baden L.R. et al. found that the overall vaccine efficacy at 14 days or more after two doses of mRNA1273 vaccines was 94.1%. When vaccine efficacy was calculated separately for only participants with obesity, the vaccine efficacy was 95.8%.Citation37 These studies suggest that being overweight or obese does not have a significant impact on the efficacy of the COVID-19 vaccine.
For participants with chronic heart disease, our results differ from the findings of Naruse H., who found that participants with cardiovascular comorbidity had a lower immune response after two doses of the BNT162b2 vaccine. The median level of anti-RBD in the cardiovascular cohort was 137.2 U/mL compared with 176.2 U/mL in the healthcare workers’ cohort. After adjusting for age, gender, and other comorbidities, this level differed significantly. It’s worth noting that the study population in our study and Naruse H. study have some differences. For example, the participants in Naruse H.‘s study were older than those in our study, with a median age of 74 years (IQR, 68–77) compared with 66 years (IQR, 60–72) in our study.Citation38
Our study provides compelling evidence supporting the notion that extending the vaccine interval duration between the first and second doses significantly enhances the immune response, thereby improving vaccine efficacy. This effect was observed not only in healthy individualsCitation39 but also in patients with comorbidities. Specifically, our study showed that participants with rheumatological diseases, diabetes, and cirrhosis exhibited immune responses comparable to participants without those diseases when the vaccine interval was extended.
Mehta et al. investigated the immune response after two doses of AZD1222 vaccines in participants with autoimmune Rheumatic Diseases and found that those who received the long interval vaccination (10–12 weeks) exhibited a more robust immune response compared to the short interval group (4 weeks) (anti-RBD antibody titer 1310.6 vs. 736 IU/mL, p < .001).Citation40
Similarly, Goel et al. reported that participants with cirrhosis who received the long interval vaccination (7–12 weeks) also showed a significantly stronger immune response compared to the short interval group (≤6 weeks) (anti-spike antibody titer 2613 vs. 6365 U/mL, p = .027).Citation41
These findings underscore the potential benefits of optimizing vaccine dosing intervals, especially for vulnerable populations with underlying health conditions.
Furthermore, Marfella et al. found that participants with diabetes receiving mRNA-BNT162b2, mRNA-1273, and ChAdOx1 vaccines had a lower immune response, particularly those with poor glycemic control, compared to participants without diabetes. The vaccine interval between the first and second dose in the ChAdOx1 group was 32 ± 3 days.Citation42
One of the key strengths of our study is that we recruited participants with a wide range of comorbidities, including solid cancer, hematological malignancy, benign hematological disease, diabetes mellitus, CKD, cirrhosis, rheumatological disease, obesity, and cardiovascular disease. This allows us to compare the immune response between each comorbidity group and healthy participants, as well as make indirect comparisons between different comorbidity groups. This provides a comprehensive understanding of the impact of various comorbidities on the immune response to the COVID-19 vaccine.
Despite its strengths, our study also had some limitations. One limitation is that it was challenging to enroll control participants with similar baseline characteristics as the comorbidity participants. Therefore, we chose healthcare workers as the control group. However, the baseline characteristics of participants with comorbidities differed from those of the healthcare workers. The healthcare workers were generally younger, with a high proportion of females, which tend to have more robust immune responses after vaccination. Although we adjusted for these confounding factors in the multivariable analysis, the results may not be entirely accurate to interpret. Another limitation of our study is that some participants had more than one comorbidity, and we performed multivariable analysis separately for each comorbidity, considering each one as an individual factor. While we attempted to address the impact of each comorbidity, this approach may not be perfect due to potential correlations within individuals who have multiple comorbidities. Consequently, the interpretation of results for participants with overlapping comorbidities should be cautiously considered. Additionally, it’s essential to acknowledge that measuring antibody response is only one aspect of assessing the immune response. The T-cell function and innate immunity are not evaluated in our study. Additionally, a single-center study may affect the generalizability of our results. It would be beneficial to replicate our findings in larger, multi-center studies to establish the robustness of our conclusions. Furthermore, our study only covers a short-term follow-up of the immunogenicity, the long-term immunogenicity of the vaccine is unknown. Furthermore, our study was conducted during the early phase of the COVID-19 pandemic, and the results may not be relevant for new variants of SARS-CoV-2. Additionally, the type of comorbidities and disease severity may impact the study results. Our research revealed heterogeneity within and between each comorbidity group, which could potentially influence the outcomes. Lastly, it is important to note that our study did not collect information about adverse effects associated with the COVID-19 vaccines. The reason for this limitation was the inclusion of a large number of participants from various specialist clinics, which resulted in limited manpower to collect and document such valuable information.
Overall, the study provides important information about the immune response to COVID-19 vaccination in participants with different comorbidities, however, it’s important to note that the results should be interpreted with caution and need to be confirmed in future studies with larger sample sizes and different populations.
Supplemental Material
Download PDF (225.6 KB)Supplemental Material
Download PDF (364.2 KB)Supplemental Material
Download PDF (301.2 KB)Supplemental Material
Download PDF (124.4 KB)Supplemental Material
Download PDF (106.7 KB)Supplemental Material
Download PDF (109.7 KB)Supplemental Material
Download PDF (112.7 KB)Supplemental Material
Download PDF (110.6 KB)Supplemental Material
Download PDF (106.7 KB)Supplemental Material
Download PDF (175.5 KB)Supplemental Material
Download PDF (121.6 KB)Acknowledgments
We would like to express our gratitude to the clinical research management unit for their expert management of this project. We also extend our appreciation to the central laboratory of Chulabhorn Hospital for their outstanding laboratory testing and analysis. This study was supported by funding from Chulabhorn Royal Academy, Thailand, which we gratefully acknowledge. Additionally, we would like to thank the physicians, nurses, and hospital staff who were involved in the vaccine administration process for their valuable contributions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2251850.
Additional information
Funding
References
- WHO. Coronavirus disease (COVID-19). 2022. https://www.who.int/health-topics/coronavirus#tab=tab_1.
- Cao Y, Hiyoshi A, Montgomery S. COVID-19 case-fatality rate and demographic and socioeconomic influencers: worldwide spatial regression analysis based on country-level data. BMJ Open. 2020;10(11):e043560. doi:10.1136/bmjopen-2020-043560.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–11. doi:10.1001/jama.2020.2648.
- Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21(1):855. doi:10.1186/s12879-021-06536-3.
- Pagano L, Salmanton-Garcia J, Marchesi F, Busca A, Corradini P, Hoenigl M, Klimko N, Koehler P, Pagliuca A, Passamonti F, et al. COVID-19 infection in adult patients with hematological malignancies: a European hematology association survey (EPICOVIDEHA). J Hematol Oncol. 2021;14(1):168. doi:10.1186/s13045-021-01177-0.
- Chavez-MacGregor M, Lei X, Zhao H, Scheet P, Giordano SH. Evaluation of COVID-19 mortality and adverse outcomes in US patients with or without cancer. JAMA Oncol. 2022;8(1):69–78. doi:10.1001/jamaoncol.2021.5148.
- Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–6. doi:10.1038/s41586-020-2521-4.
- Rahmani A, Dini G, Leso V, Montecucco A, Kusznir Vitturi B, Iavicoli I, Durando P. Duration of SARS-CoV-2 shedding and infectivity in the working age population: a systematic review and meta-analysis. Med Lav. 2022;113(2):e2022014. doi:10.23749/mdl.v113i2.12724.
- Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21(8):475–84. doi:10.1038/s41577-021-00578-z.
- Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, Neuzil KM, Hahn W, Hunt J, Mulligan MJ, et al. Phase 3 safety and efficacy of AZD1222 (ChAdox1 nCoV-19) covid-19 vaccine. N Engl J Med. 2021;385(25):2348–60. doi:10.1056/NEJMoa2105290.
- Sritipsukho P, Khawcharoenporn T, Siribumrungwong B, Damronglerd P, Suwantarat N, Satdhabudha A, Chaiyakulsil C, Sinlapamongkolkul P, Tangsathapornpong A, Bunjoungmanee P, et al. Comparing real-life effectiveness of various COVID-19 vaccine regimens during the delta variant-dominant pandemic: a test-negative case-control study. Emerg Microbes Infect. 2022;11(1):585–92. doi:10.1080/22221751.2022.2037398.
- Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, Dold C, Fuskova M, Gilbert SC, Hirsch I, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27(11):2032–40. doi:10.1038/s41591-021-01540-1.
- Rincon-Arevalo H, Choi M, Stefanski AL, Halleck F, Weber U, Szelinski F, Jahrsdörfer B, Schrezenmeier H, Ludwig C, Sattler A, et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6(60):eabj1031. doi:10.1126/sciimmunol.abj1031.
- Kristiansen PA, Page M, Bernasconi V, Mattiuzzo G, Dull P, Makar K, Plotkin S, Knezevic I. WHO International standard for anti-SARS-CoV-2 immunoglobulin. Lancet. 2021;397(10282):1347–8. doi:10.1016/S0140-6736(21)00527-4.
- Fu J, Shen X, Anderson M, Stec M, Petratos T, Cloherty G, Montefiori DC, Landay A, Moy JN. Correlation of binding and neutralizing antibodies against SARS-CoV-2 omicron variant in infection-naïve and convalescent BNT162b2 recipients. Vaccines (Basel). 2022;10(11):1904. doi:10.3390/vaccines10111904.
- Resman Rus K, Korva M, Knap N, Avsic Zupanc T, Poljak M. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J Clin Virol. 2021;139:104820. doi:10.1016/j.jcv.2021.104820.
- Hoque A, Barshan AD, Chowdhury FUH, Fardous J, Hasan MJ, Khan MAS, Kabir A. Antibody response to ChAdOx1-nCoV-19 vaccine among recipients in Bangladesh: a prospective observational study. Infect Drug Resist. 2021;14:5491–500. doi:10.2147/IDR.S335414.
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–11. doi:10.1038/s41591-021-01377-8.
- Fendler A, Shepherd STC, Au L, Wilkinson KA, Wu M, Byrne F, Rzeniewicz K, Gordon W, Shum B, Gerard CL, et al. Adaptive immunity and neutralizing antibodies against SARS-CoV-2 variants of concern following vaccination in patients with cancer: the CAPTURE study. Nat Cancer. 2021;2(12):1321–37. doi:10.1038/s43018-021-00275-9.
- Ligumsky H, Safadi E, Etan T, Vaknin N, Waller M, Croll A, Nikolaevski-Berlin A, Greenberg I, Halperin T, Wasserman A, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine among actively treated cancer patients. J Natl Cancer Inst. 2022;114(2):203–9. doi:10.1093/jnci/djab174.
- Lim SH, Stuart B, Joseph-Pietras D, Johnson M, Campbell N, Kelly A, Jeffrey D, Turaj AH, Rolfvondenbaumen K, Galloway C, et al. Immune responses against SARS-CoV-2 variants after two and three doses of vaccine in B-cell malignancies: UK PROSECO study. Nat Cancer. 2022;3(5):552–64. doi:10.1038/s43018-022-00364-3.
- Maneikis K, Sablauskas K, Ringeleviciute U, Vaitekenaite V, Cekauskiene R, Kryzauskaite L, Naumovas D, Banys V, Pečeliūnas V, Beinortas T, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8(8):e583–e92. doi:10.1016/S2352-3026(21)00169-1.
- Teeyapun N, Luangdilok S, Pakvisal N, Sainamthip P, Mingmalairak S, Poovorawan N, Sitthideatphaiboon P, Parinyanitikul N, Sriuranpong V, Namkanisorn T, et al. Immunogenicity of ChAdOx1-nCoV-19 vaccine in solid malignancy patients by treatment regimen versus healthy controls: a prospective, multicenter observational study. EClinicalMedicine. 2022;52:101608. doi:10.1016/j.eclinm.2022.101608.
- Vink P, Delgado Mingorance I, Maximiano Alonso C, Rubio-Viqueira B, Jung KH, Rodriguez Moreno JF, Grande E, Marrupe Gonzalez D, Lowndes S, Puente J, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: a randomized trial. Cancer. 2019;125(8):1301–12. doi:10.1002/cncr.31909.
- Wu JT, La J, Branch-Elliman W, Huhmann LB, Han SS, Parmigiani G, Tuck DP, Brophy MT, Do NV, Lin AY, et al. Association of COVID-19 vaccination with SARS-CoV-2 infection in patients with cancer: a US nationwide veterans affairs study. JAMA Oncol. 2022;8(2):281–6. doi:10.1001/jamaoncol.2021.5771.
- Rotterdam J, Thiaucourt M, Weiss C, Schwaab J, Reiter A, Kreil S, Steiner L, Fenchel S, Popp HD, Hofmann W-K, et al. Definition of factors associated with negative antibody response after COVID-19 vaccination in patients with hematological diseases. Ann Hematol. 2022;101(8):1825–34. doi:10.1007/s00277-022-04866-z.
- Sourij C, Tripolt NJ, Aziz F, Aberer F, Forstner P, Obermayer AM, Kojzar H, Kleinhappl B, Pferschy PN, Mader JK, et al. Humoral immune response to COVID-19 vaccination in diabetes is age-dependent but independent of type of diabetes and glycaemic control: the prospective COVAC-DM cohort study. Diabetes Obes Metab. 2022;24(5):849–58. doi:10.1111/dom.14643.
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, Voysey M, Aley PK, Angus B, Babbage G, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–93. doi:10.1016/S0140-6736(20)32466-1.
- Tawinprai K, Siripongboonsitti T, Porntharukchareon T, Dechates B, Monprach H, Sornsamdang G, Wittayasak K, Soonklang K, Mahanonda N. Persistence of immunogenicity, contributing factors of an immune response, and reactogenicities after a single dose of the ChAdOx1 (AZD1222) COVID-19 vaccine in the Thai population. Hum Vaccin Immunother. 2022;18(1):2035573. doi:10.1080/21645515.2022.2035573.
- Sanders JF, Bemelman FJ, Messchendorp AL, Baan CC, van Baarle D, van Binnendijk R, Diavatopoulos DA, Frölke SC, Geers D, GeurtsvanKessel CH, et al. The RECOVAC immune-response study: the immunogenicity, tolerability, and safety of COVID-19 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Transplantation. 2022;106(4):821–34. doi:10.1097/TP.0000000000003983.
- Ruether DF, Schaub GM, Duengelhoef PM, Haag F, Brehm TT, Fathi A, Wehmeyer M, Jahnke-Triankowski J, Mayer L, Hoffmann A, et al. SARS-CoV2-specific humoral and T-cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin Gastroenterol Hepatol. 2022;20(1):162–72 e9. doi:10.1016/j.cgh.2021.09.003.
- Boekel L, Steenhuis M, Hooijberg F, Besten YR, van Kempen ZLE, Kummer LY, van Dam KPJ, Stalman EW, Vogelzang EH, Cristianawati O, et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol. 2021;3(11):e778–e88. doi:10.1016/S2665-9913(21)00222-8.
- Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, Zisapel M, Elalouf O, Kaufman I, Meidan R, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80(10):1330–8. doi:10.1136/annrheumdis-2021-220647. Epub 2021 Jun 14. Erratum in: Ann Rheum Dis. 2022 Jul;81(7):e133.
- Butsch WS, Hajduk A, Cardel MI, Donahoo WT, Kyle TK, Stanford FC, Zeltser LM, Kotz CM, Jastreboff AM. COVID-19 vaccines are effective in people with obesity: a position statement from the obesity society. Obesity (Silver Spring). 2021;29(10):1575–9. doi:10.1002/oby.23251.
- Piernas C, Patone M, Astbury NM, Gao M, Sheikh A, Khunti K, Shankar-Hari M, Dixon S, Coupland C, Aveyard P, et al. Associations of BMI with COVID-19 vaccine uptake, vaccine effectiveness, and risk of severe COVID-19 outcomes after vaccination in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2022;10(8):571–80. doi:10.1016/S2213-8587(22)00158-9.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi:10.1056/NEJMoa2034577.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. doi:10.1056/NEJMoa2035389.
- Naruse H, Ito H, Izawa H, Sarai M, Ishii J, Sakaguchi E, Murakami R, Ando T, Fujigaki H, Saito K, et al. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine in patients with cardiovascular disease. J Clin Med. 2021;10(23):5498. doi:10.3390/jcm10235498.
- Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Oxford COVID Vaccine Trial Group, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–91. doi:10.1016/S0140-6736(21)00432-3.
- Mehta P, Paul A, Ahmed S, Cherian S, Panthak A, Benny J, Shenoy P. Effectiveness of delayed second dose of AZD1222 vaccine in patients with autoimmune rheumatic disease. Clin Rheumatol. 2022;41(11):3537–42. doi:10.1007/s10067-022-06247-3.
- Goel A, Verma A, Tiwari P, Katiyar H, Aggarwal A, Khetan D, Mayank, Kishore RVK, Kumar P, Singh TP, et al. Serological immune response following ChAdOx1 nCoV-19 vaccine (covishield®) in patients with liver cirrhosis. Vaccines (Basel). 2022;10(11):1837. doi:10.3390/vaccines10111837.
- Marfella R, D’Onofrio N, Sardu C, Scisciola L, Maggi P, Coppola N, Romano C, Messina V, Turriziani F, Siniscalchi M, et al. Does poor glycaemic control affect the immunogenicity of the COVID-19 vaccination in patients with type 2 diabetes: the CAVEAT study. Diabetes Obes Metab. 2022;24(1):160–5. doi:10.1111/dom.14547.