 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Dengue is caused by a mosquito-transmitted flavivirus. The disease is now endemic to many tropical and subtropical regions, manifesting as approximately 96 million symptomatic cases of dengue each year. Clinical trials have shown TAK-003 (Qdenga®), a live attenuated dengue tetravalent vaccine, to be well-tolerated, immunogenic, and efficacious in adults with no prior exposure to dengue virus infection living in non-endemic regions, as well as in adults and children living in dengue-endemic areas. This open-label, single-arm phase 3 trial (NCT03771963) was conducted in two dengue non-endemic areas of the USA, and it evaluated the immunogenicity and safety of naturally-aged TAK-003 administered to adult participants. Overall, the immunogenicity data from this trial are consistent with those reported from other TAK-003 phase 2 and 3 trials, and the safety data are consistent with the broader integrated safety data analysis. The data show that naturally-aged TAK-003 had a well-tolerated reactogenicity and adverse events profile when administered in the second half of its clinical 24-month shelf-life and that it still elicited an immune response that persisted up to 6 months after the second dose against all four dengue serotypes, with no important safety risks identified during the trial.
Introduction
Dengue is caused by infection with the dengue virus (DENV), a flavivirus with a ribonucleic acid genome that occurs as four recognized DENV serotypes, DENV-1, DENV-2, DENV-3, or DENV-4.Citation1 The virus is transmitted to humans via bites from virus-infected Aedes aegypti or Aedes albopictus mosquitoes. Approximately 96 million cases of dengue manifest clinically every year, with 500,000 requiring hospitalization.Citation1,Citation2 Illness owing to infection by any of the four co-circulating DENV serotypes in endemic regions can result in a range of symptoms, from asymptomatic disease to debilitating but transient dengue fever, life-threatening dengue hemorrhagic fever, and dengue shock syndrome.Citation3–5
Infection with one DENV serotype produces life-long homologous immunity but may increase the risk of severe disease following a secondary heterotypic infection:Citation6,Citation7 81% of DENV-diagnosed children in one study presented with a secondary DENV infection and, of these, 53% had dengue hemorrhagic fever.Citation7 Vaccine development has, therefore, focused on tetravalent vaccines that generate protective responses against the four serotypes.Citation8,Citation9 Trials with an alternative licensed dengue vaccine, CYD-TDV (Dengvaxia®), revealed a high CYD-TDV efficacy for baseline seropositive recipients at the time of vaccination, but a much lower efficacy in those who were baseline seronegative.Citation10,Citation11 Moreover, the reduced efficacy for seronegative recipients disproportionately affected young-aged children. Owing to CYD-TDV vaccination increasing the risk of hospitalization in baseline seronegative individuals, it is licensed for administration only to baseline dengue seropositive individuals.Citation12 Consequently, two laboratory tests are required to confirm prior dengue exposure before CYD-TDV is administered as three doses 6-months apart (i.e., at months 0, 6, and 12).Citation13 This presents an arduous challenge both in terms of accomplishing the laboratory tests and the potential for incomplete dosing compliance before full protection is conferred. There is, therefore, a significant unmet clinical need for an effective dengue vaccine that can be used to protect both seronegative and seropositive individuals who are at risk from DENV infection.Citation14
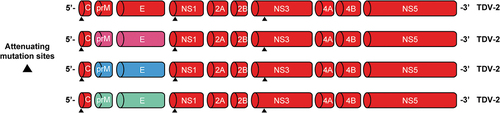
TAK-003 (Qdenga®) is a live attenuated dengue tetravalent vaccine that comprises four DENV strains: an attenuated dengue serotype 2 strain (DENV-2) and three recombinant strains comprising the DENV-2 backbone and the respective pre-membrane and envelope genes of DENV-1, DENV-3, and DENV-4 serotypesCitation2 (). Phase 1 and Phase 2 clinical trials have shown TAK-003 to be well tolerated and immunogenic in healthy adults with no prior exposure to DENV infection living in dengue non-endemic regions as well as in adults and children living in dengue-endemic areas such as Asia and Latin America.Citation15–18 Furthermore, TAK-003 is efficacious in preventing dengue in children and adolescents in endemic countries.Citation19,Citation20 To date, TAK-003 has been licensed for prevention against dengue in the European Union, Great Britain, Brazil, Argentina, Thailand, and Indonesia, among other countries.Citation21
Figure 1. Genetic structure of the four TAK-003 vaccine strains. Abbreviations: C, capsid; E, envelope; NS, nonstructural protein; prM, premembrane; TDV-1/2/3/4, dengue serotype 1/2/3/4 strain. Reproduced with permission from Patel et al.Citation42.
This open-label, single-arm phase 3 trial (DEN-307; NCT03771963) evaluated the immunogenicity and safety of naturally-aged TAK-003, administered as two doses 3 months apart, after storage at 2°C to 8°C for 14–20 months (i.e., more than half of its clinical 24-month shelf-life). The objective for this trial was to quantify the neutralizing antibody response that is elicited following the administration of TAK-003 during the latter half of its 2-year shelf-life.
Materials and methods
Trial design
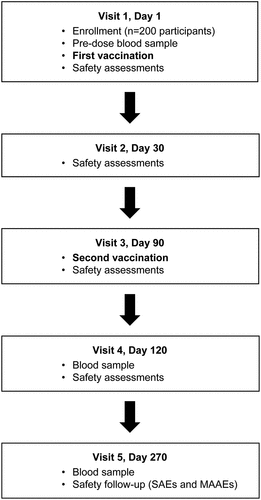
A single trial group of 200 healthy adult participants was enrolled at two centers (Anaheim, CA, and Hutchinson, KS) in the USA. Participants received a subcutaneous injection of naturally-aged (14–20 months in storage at 2°C to 8°C) TAK-003 on Day 1 and on Day 90 (). Trial duration was approximately 270 days (9 months) for each participant. The first trial participant received the first TAK-003 dose on 28 March 2019 and the last participant received the second TAK-003 dose on 9 September 2019, corresponding to 14–20 months of a single batch of TAK-003 vaccine aging during storage at 2°C to 8°C.
Figure 2. Trial visits schematic. Abbreviations: MAAEs, medically attended adverse events; SAEs, serious adverse events. Safety assessments were as follows: solicited local events (recorded up to 30 minutes, and up to 7 days after each TAK-003 dose), solicited systemic events (recorded up to 30 minutes, and up to 14 days after each TAK-003 dose), unsolicited events (recorded up to 28 days after each TAK-003 dose), and MAAEs and SAEs recorded for the entire duration of trial. Solicited local AEs comprised injection site pain, injection site erythema, and injection site swelling. Solicited systemic AEs comprised asthenia, headache, malaise, myalgia, and fever. MAAEs were defined as AEs leading to an unscheduled visit to, or by, a healthcare professional including visits to an emergency department, but not fulfilling seriousness criteria.
Participants
Eligible participants were enrolled if they were 18–60 years old at the time of entry into the trial, male or female, and in good health as determined by medical history, physical examination (including vital signs), and the clinical judgment of the investigator, and, if they could comply with trial procedures for the duration of the trial. The trial design did not stratify participant enrollment for any given variable; all participants were enrolled over a 7-month period as per study eligibility criteria.
The trial excluded participation from individuals with hypersensitivity to any TAK-003 formulation component, impaired or altered immune function, planned or ongoing pregnancy or breastfeeding, or previous vaccination against dengue fever. Detailed exclusion criteria are provided in Table S1.
Any participant was eligible to enroll voluntarily in this trial without prejudice to their baseline dengue serostatus. Baseline serostatus for each participant was ascertained subsequently from a blood sample obtained immediately prior to the first TAK-003 dose.
Participants for this trial were solicited from a database/register at each respective site and supplemented by recruitment via Independent Review Board (IRB)-approved advertisements on social media. Written informed consent was obtained from each participant before enrollment; this IRB-approved trial was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonization tripartite guidelines for good clinical practice.Citation22,Citation23 IRB oversight for this study (tracking number: 20190261) was conducted by the Copernicus Group Independent Review Board, 5000 Centre Green Way Suite 200, Cary, NC 27513, USA.
Immunogenicity evaluation
Immunogenicity assessments comprised the measurement of dengue neutralizing antibodies by microneutralization test 50% (MNT50) for each of the four dengue serotypes using blood samples collected pre-vaccination on Day 1, and, at 1 month (Day 120) and 6 months (Day 270) after the second TAK-003 dose.
Safety evaluation
Solicited local adverse events (AEs), comprising injection site pain, injection site erythema, and injection site swelling, and, solicited systemic AEs comprising fever, headache, asthenia, malaise, and myalgia, were assessed in the clinic for 30 min immediately after each TAK-003 dose. Solicited local and systemic AEs were further recorded for 7 days (day of vaccination +6 days) and 14 days (day of vaccination +13 days), respectively, following each TAK-003 dose. Diary cards were distributed to all participants to record the solicited local and systemic AEs.
Unsolicited AEs assessed for 28 days (day of vaccination +27 days) following each TAK-003 dose were recorded for all participants. Serious AEs (SAEs), medically attended AEs (MAAEs), AEs leading to withdrawal from a second dose of TAK-003, and discontinuation from trial were documented for the entire trial duration. MAAEs were defined as AEs leading to an unscheduled visit to, or by, a health-care professional, including visits to an emergency department but not fulfilling seriousness criteria.
The severity unsolicited AEs was categorized by investigators as mild, moderate, or severe, based on trial protocol definitions (Table S2). In the instances where severity was not recorded for an unsolicited AE, it was imputed as severe for the purposes of data analysis. All solicited local AEs were considered related to TAK-003 administration. The investigators assessed, where applicable, the causal relationship between TAK-003 vaccine/trial procedures and solicited systemic AEs and/or unsolicited AEs; attribution to TAK-003 was binary: either it was related or not related, and both were defined as follows:
Related: there is suspicion that there is a relationship between the trial vaccine and the AE (without determining the extent of probability); there is a reasonable possibility that the trial vaccine contributed to the AE.
Not Related: there is no suspicion that there is a relationship between the trial vaccine and the AE; there are other more likely causes, and the administration of the trial vaccine is not suspected to have contributed to the AE.
Trial objectives
The primary trial objective was to describe, at 1 month (day 120) post-second dose, the neutralizing antibody response against each dengue serotype following administration of a naturally-aged batch of TAK-003.
Secondary objectives were to describe the seroconversion rates, for all dengue serotypes, at 1 month and at 6 months (day 270) post-second dose, and to also describe the neutralizing antibody response for each dengue serotypes at 9 months (Day 270). Seropositive status was defined as a reciprocal neutralizing titer ≥10 against at least one dengue virus serotype at baseline.
TAK-003 tolerability was evaluated following each vaccination dose by assessing solicited local AEs, solicited systemic AEs, unsolicited AEs, MAAEs, and SAEs.
Statistics
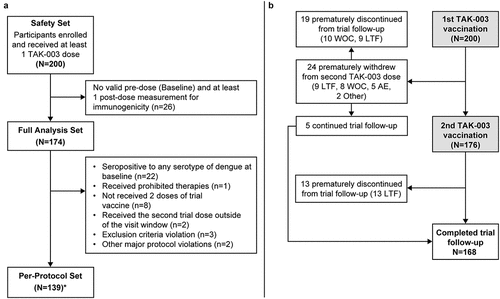
Descriptive statistics, including 95% confidence intervals (CIs), were provided for the primary and secondary immunogenicity endpoints; safety set, full analysis set (FAS), and per-protocol set (PPS) were generated. Safety Set comprised all enrolled participants who received at least one dose of TAK-003 (). FAS comprised all enrolled participants who received at least one dose of TAK-003 and for whom a valid pre-dose (baseline) measurement and at least one valid post-dose measurement were available for immunogenicity assessments. PPS comprised all participants from the FAS who had no major protocol violations, but excluding participants who were baseline seropositive for any serotype of dengue virus at baseline.
Figure 3. (a) Participant allocation to safety, full analysis and per-protocol sets. *Any participant not included in the per-protocol set if he/she experienced one or more trial protocol deviation(s) and (b) Participant disposition for the DEN-307 clinical trial. Abbreviations: AE, adverse event; LTF, lost to follow up; WOC, withdrawal of consent.
Results
Trial population and baseline characteristics
A total 200 participants were enrolled in this trial; of whom, 176 (88.0%) received two doses of TAK-003 (). Twenty-four (12.0%) participants did not receive their second TAK-003 dose: the majority either being lost to follow-up or withdrawing consent. The individual reasons for consent withdrawal were personal, and none was medically related.
A total 168 (84.0%) participants completed the trial, with 32 (16.0%) participants discontinued from the trial owing to them being lost to follow-up (n = 22) or withdrawing consent (n = 10).
Participant baseline demographic characteristics were generally consistent across trial analysis groups (). On average, the participants were 40–41 years old, approximately half were female participants, the majority (>70%) were white, and their mean BMI was approximately 27.5 kg/m2. Some 14.1% of participants were dengue seropositive at baseline (safety set).
Table 1. Participant demographic and baseline characteristics.
Immunogenicity
Seroconversion at Day 120 was evident for >97% of participants for all dengue serotypes (). By Day 270, there were ~12% fewer DENV-3 and ~13% fewer DENV-4 seropositive participants, whereas for DENV-1 and DENV-2 it remained relatively unchanged ().
Figure 4. (a) Percentage (95% CI) of participants who were seropositive against each DENV serotype at days 120 and 270 and (b) Percentage (95% CI) of trivalent and tetravalent baseline seropositive participants (per-protocol set). Baseline seropositive defined as a reciprocal neutralizing antibody titer ≥ 10 to 1 dengue virus serotypes. Baseline seronegative defined as titer < 10 to all dengue serotypes. Abbreviations: CI, confidence interval; DENV, dengue virus.
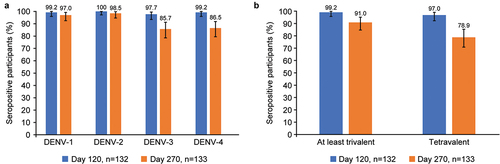
Trivalent seroconversion was observed in 99.2% of participants, and tetravalent seroconversion was seen in 97.0% of participants at Day 120 (). By Day 270, trivalent and tetravalent seroconversion rates had decreased to 91.0% and 78.9%, respectively ().
All participants included in PPS were seronegative for dengue at baseline. TAK-003 elicited dengue neutralizing antibody responses, as described by geometric mean titers (GMTs), against each of the four dengue serotypes at Day 120 (). For each serotype, GMTs at Day 120 were in the following order from greatest to least: DENV-2, DENV-1, DENV-3, and DENV-4.
Figure 5. Geometric mean titers (95% confidence interval) of neutralizing antibodies measured by MNT50 for each dengue serotype (per-protocol set). Abbreviations: DENV, dengue virus; MNT50, microneutralization test 50%.
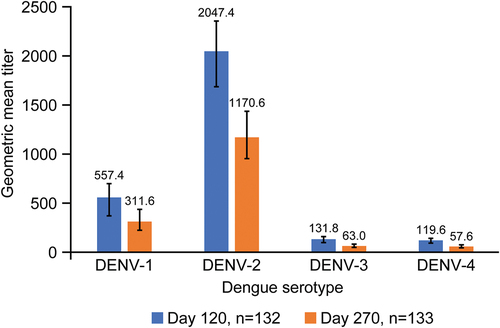
The immune response elicited following two doses of TAK-003 was persistent though somewhat attenuated (). Between Day 120 and Day 270, GMTs decreased by 44.1%, 42.8%, 52.2%, and 51.8% for DENV-1, DENV-2, DENV-3, and DENV-4, respectively, and, at Day 270 remained highest for DENV-2 (1170.6) and lowest for DENV-4 (57.6).
Safety
Solicited local adverse events
Within the 30 min after the first TAK-003 dose, two (1.0%) participants experienced mild injection site pain, and, following the second dose, five (2.8%) participants experienced two events of mild pain, two events of mild erythema, and one event of mild swelling ().
Table 2. TAK-003 overview of adverse events.
Solicited local AEs within 7 days were reported by 69 (35.9%) participants after the first vaccination and by 54 (32.3%) after the second vaccination (). The majority of these events were of mild intensity with only one (0.5%) participant with severe injection site pain after the first dose and two (1.2%) others who also reported it as severe after the second dose (). Overall, injection site pain was the most frequently reported solicited local AE after either vaccine dose, followed by injection site erythema ().
Table 3. Summary of solicited local adverse events within 7 days post vaccination (safety set).
Most solicited local AEs occurred within the first 3 days after vaccination and the most common day of onset of pain after any vaccination was on the day of vaccination, whereas erythema and swelling were evident on the next day after vaccination. The mean duration for local AEs after any vaccination ranged between 3 days (pain) and 3.6 days (swelling).
Prolonged solicited local AEs (that is, those that persisted beyond the 7-day diary period) were observed for eight (4.0%) and two (1.1%) participants after the first and second vaccination, respectively (); all events subsequently resolved within 14 days.
Solicited systemic adverse events
Participants experienced fewer solicited systemic AEs after the second TAK-003 dose (34.1%) than after the first dose (42.4%), and correspondingly fewer AEs after the second dose were considered attributable to the vaccine ().
Headache was the most frequently reported event followed by myalgia, malaise, and asthenia (). No participant experienced any fever ≥39°C ().
Table 4. Summary of solicited systemic adverse events within 14 days post vaccination (safety set).
Most of the solicited systemic events experienced were either mild or moderate after either dose of TAK-003: five (2.6%) participants experienced severe AEs after the first dose and three (1.8%) after the second dose ().
The majority of solicited systemic AEs occurred within the first 7 days after vaccination; the most common day of onset for headache, myalgia, malaise, and asthenia was on the day of vaccination. The mean duration of solicited systemic AEs after vaccination ranged between 3.0 days (myalgia) and 4.2 days (fever).
Prolonged solicited systemic AEs (that is, those that persisted beyond the 14-day diary period) were reported by one (0.5%) participant after the first vaccination, and two (1.1%) participants after the second vaccination (); all events subsequently resolved within 20 days.
Unsolicited AEs
Thirty-eight (19.0%) participants experienced a total 59 unsolicited events, none of which was severe; 50 (84.7%) events were mild and 9 (15.3%) were moderate in intensity ().
A total 16 events reported by 11 participants (5.5%) were considered attributable to TAK-003 administration, with the majority (14 events in 9 participants) reported after the first dose (). All events considered attributable to TAK-003 after the first dose were: ocular hyperemia, abdominal discomfort, diarrhea, injection site events (bruising, pain, pruritus, induration), pain, headache, pharyngeal pain, pruritus, and rash. Those considered attributable to TAK-003 after the second dose were injection bruising and injection site induration. The majority of TAK-003 related unsolicited AEs were mild in intensity, and no event was rated as severe.
The most frequently reported unsolicited AEs, by preferred term (PT), were abdominal discomfort and upper respiratory tract infection, each reported in three (1.5%) participants in total (). There were no individual unsolicited AEs, by PT, that were reported by >4 (>2.0%) participants. Other AEs reported within 28 days of dose were singly occurring events, all of which were of mild or moderate intensity. All unsolicited AEs resolved, and none led to withdrawal from administration of the second TAK-003 dose or to participant discontinuation from the trial.
Table 5. Summary of unsolicited adverse events up to 28 days post vaccination, by preferred term (safety set).
Medically attended AEs
MAAEs (146 events in total) after any vaccination were experienced by 52 (26.0%) participants: all events were either mild or moderate in intensity (). The most common MAAEs, by PT, were acute sinusitis (4.5% of participants), bronchitis (2.0%), and dermatitis contact (2.0%), and they were the only ones reported with a frequency of ≥2% (). Other MAAEs reported were singly occurring events, none of which was a severe event. All MAAEs resolved.
Table 6. Summary of medically attended adverse events (MAAEs) post vaccination up to the end of study (safety set).
Two MAAEs for one (0.5%) participant were the only ones attributed to TAK-003 and were observed after the first dose: abdominal discomfort and oropharyngeal pain. Both events were moderate in intensity, resolved, and did not result in discontinuation from the trial or withdrawal from the second TAK-003 dose.
TAK-003 withdrawal and/or participant discontinuation from the trial
None of the AEs leading to TAK-003 withdrawal and/or discontinuation from trial was attributed to TAK-003 or to trial procedures.
A total 24 participants (12.0%) prematurely discontinued the planned TAK-003 dose regimen (). Five (3.0%) participants who experienced 7 AEs after the first vaccine dose withdrew second TAK-003 dose administration. One event (mild back pain) failed to resolve and resulted in both withdrawal before the second dose of TAK-003 and discontinuation from the trial. Other events leading to withdrawal before second TAK-003 dose were moderate hypertension (not resolved), moderate hepatic failure (SAE, resolved), and mild events of tachycardia (not resolved), back pain (not resolved), urticaria (resolved), and abortion induced (resolved).
A total 32 (16%) participants discontinued from the trial (), of whom 10 withdrew consent to participate further in the trial. Eight participants withdrew consent because of non-medical reasons (e.g., relocation to another state) and the other two participants because of AEs: one withdrew consent 91 days after the first dose (no second TAK-003 dose) because of ongoing mild back pain (not TAK-003-related), and the other who withdrew consent 115 days after the first dose (no second TAK-003 dose) after diagnosis of moderate hepatic failure that was an SAE not attributable to TAK-003.
SAEs
Five (2.5%) participants experienced five SAEs. Two events were reported between the first and second vaccinations: moderate hepatic failure and severe osteoarthritis. Three other SAEs of moderate intensity (bradycardia, inguinal hernia, and sepsis) were reported in the 6-month period after the second vaccination. Sepsis was reported 2 days following the second TAK-003 dose, the event was considered not related to TAK-003 administration. All SAEs resolved and were assessed by the investigator as unrelated to vaccine administration or to trial procedures.
No deaths were reported in this trial.
Discussion
This open-label, single-arm phase 3 trial evaluated the immunogenicity and safety of naturally-aged TAK-003 in adult participants administered two doses 3 months apart during the latter half (14–20 months) of its clinical 24-month shelf-life. It was considered reasonable to omit a comparator placebo group in this trial at the time of its conception because the emerging cumulative safety data from preceding and ongoing TAK-003 trials had provided some confidence in TAK-003’s safety profile.
The emergence of SARS-CoV-2 virus and COVID-19 disease pandemic during the trial did not affect its conduct or completion, participant safety or data integrity: the last participant visit occurred 2 days after the COVID-19 pandemic was declared on 11 March 2020.Citation24
Sixty-one (31.5%) participants in this trial were excluded from the PPS immunogenicity analysis, and this is partly attributable to a sizable proportion (28 [14.1%]) of participants who were baseline dengue seropositive. This was not considered unusual as it is similar to the 15.3% adult baseline seropositive participants noted in a predecessor TAK-003 trial, DEN-304 (NCT03423173), which was also exclusively USA-based.Citation25 It is posited that the baseline seropositive participants probably acquired dengue infection inadvertently while previously traveling to dengue endemic areas. Three times as many participants at the Anaheim site, in the present trial DEN-307, compared with the Hutchinson site, were baseline seropositive to anti-DENV neutralizing antibodies. All four DENV serotypes were associated with the baseline-positive serostatus, and, although positive serostatus with DENV-1 and DENV-2 was predominant, the neutralizing antibody titers were low: <65 for DENV-1, ≤85 for DENV-2, between 12 and 38 for DENV-3, and, between 12 and 50 for DENV-4.
The results from this trial affirmed that TAK-003 elicited neutralizing antibodies against each of the four DENV serotypes in dengue seronegative adults, with the highest titers induced against DENV-2, and persistence of immune responses against all four serotypes up to 6 months after the second dose although, a decline in neutralization titers ranging from 43% to 52% was observed for all serotypes over the same period. Presently, there is no established correlate of protection for DENV unlike that of flavivirus vaccines such as yellow fever virusCitation26,Citation27 and Japanese encephalitis.Citation28 Without an established correlate of protection, changes in neutralizing antibody titers do not portend a direction of effect on vaccine efficacy. Seroconversion rates for all dengue serotypes in the present study were robust and persistent (>85%) through to 6 months after the second dose. This persistency of neutralizing antibody response and maintenance of high seroconversion rates up to 6 months after the scheduled two doses, coupled with higher GMTs for the DENV-2 serotype than those for DENV-1, DENV-3, and DENV-4 is a pattern that is consistent with the data observed in the pivotal TAK-003 efficacy trial, DEN-301,Citation19,Citation20,Citation29 and other Phase 2Citation30–32 and 3 TAK-003 trials.Citation25,Citation33–35
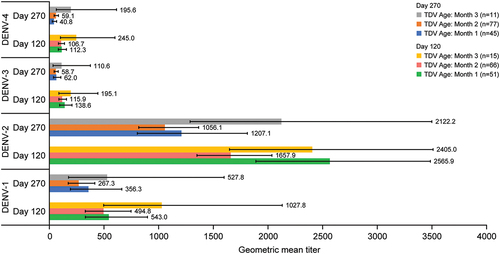
There was no formal hypothesis testing in this trial. Nevertheless, an informal alignment of data from this trial shows that the immune response elicited from baseline seronegative participants, as described by GMTs, is of the same order of magnitude as those observed in two TAK-003 trials (DEN-301 and DEN-304) that involved its administration without predefined aging at long-term storage conditions (). In comparison with trial DEN-307 where TAK-003 had “aged” 14–20 months by the time the last participant was administered with the second dose, the DEN-301 trial (NCT02747927) involved >20,000 pediatric (4–16 years old) participants, for whom the vaccine material had been targeted for potency to simulate material that had been held for a period approaching the shelf-life of the product, whereas trial DEN-304 (NCT03423173) involved >900 adult (18–60 years old) participants, for whom the vaccine material had “aged” 7.5–11 months.Citation25 As evident from (see also Figure S1), the immune response elicited with the TAK-003 vaccine batch in trial DEN-307 against all four dengue serotypes in baseline dengue seronegative participants does not differ substantially from that elicited with the TAK-003 administered in trials DEN-301 and DEN-304. TAK-003 aging over time does not materially affect its immunogenic properties across the four serotypes and is further illustrated from the month-by-month GMTs () and titer (Figure S2) data for trial DEN-307 baseline seronegative participants during the course of their enrollment and dosing over the 14–20 months interval.
Figure 6. Baseline dengue seronegative participant geometric mean titers (95% confidence interval) at day 120 and day 270 after TAK-003 administration in trials DEN-301,Citation19,Citation20,Citation29 DEN-304Citation25 and DEN-307. Abbreviation: DENV, dengue virus.
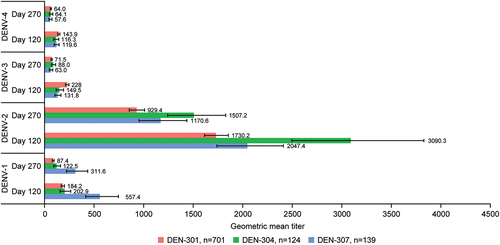
Figure 7. Baseline dengue seronegative participant geometric mean titers (95% confidence interval) at day 120 and day 270 following administration of TAK-003 in trial DEN-307. Abbreviation: DENV, dengue virus.
TAK-003 was well tolerated with respect to reactogenicity (solicited local and systemic AEs), with the second dose eliciting fewer events than the first dose. The unsolicited AEs and MAAEs encountered in this trial were reflective of the age range of adult population enrolled, although the incidences of the most frequently reported unsolicited AEs here were considerably lower than those reported as background rates and frequently associated with comorbidities.Citation36–41 No important safety risks were identified from this trial: the safety data from this trial generally mirror that of the broader population in the TAK-003 clinical development database.Citation42
This trial demonstrated that the immune response that was elicited and persisted up to 6 months after the second dose of TAK-003 is acceptable when the product is stored as directed and when it is administered in the second half of its clinical 24-month shelf-life. The safety profile following administration of a naturally-aged TAK-003 product is consistent with that from other phase 2 and 3 trials with TAK-003, and no important safety risks were identified.
Data sharing
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results of the completed studies, will be made available within 3 months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization. Data requests should follow the process described in the Data Sharing section on https://clinicaltrials.takeda.com/and https://vivli.org/ourmember/takeda/.
Supplementary Materials
Download PDF (450.5 KB)Acknowledgments
This trial was funded by Takeda. Support in writing and editing this manuscript was provided by Nathaniel Holman, PhD (Excel Medical Affairs), and funded by Takeda. The authors interpreted the data independently.
The authors wish to thank the DEN-307 trial participants, trial investigators (Dr Ruhlman, Hutchinson, KS, USA, and Dr Winkle, Anaheim, CA, USA) and their respective staff for their support in conducting this TAK-003 clinical trial.
Disclosure statement
S.S.P., A.F., F.N., and C.G.-T. are employees of Takeda and own stock/stock options in Takeda. I.L. was an employee of Takeda when this trial was conducted and completed. S.S.P., A.F., F.N., and C.G.-T. report support from Takeda for attending meetings and/or travel, and stock options from Takeda.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2254964.
Additional information
Funding
References
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–12. doi:10.1038/nature12060.
- López-Medina E, Biswal S, Saez-Llorens X, Borja-Tabora C, Bravo L, Sirivichayakul C, Vargas LM, Alera MT, Velásquez H, Reynales H, et al. Efficacy of a dengue vaccine candidate (TAK-003) in healthy children and adolescents 2 years after vaccination. J Infect Dis. 2022;225(9):1521–32. doi:10.1093/infdis/jiaa761.
- Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10(2):100–3. doi:10.1016/s0966-842x(01)02288-0.
- Halstead SB, Deen J. The future of dengue vaccines. Lancet. 2002;360(9341):1243–5. doi:10.1016/s0140-6736(02)11276-1.
- World Health Organization. Dengue and severe dengue. 2023 [accessed 2023 Sep 6]. http://www.who.int/mediacentre/factsheets/fs117/en/.
- Endy TP, Nisalak A, Chunsuttitwat S, Vaughn DW, Green S, Ennis FA, Rothman AL, Libraty DH. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J Infect Dis. 2004;189(6):990–1000. doi:10.1086/382280.
- Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181(1):2–9. doi:10.1086/315215.
- Hou J, Ye W, Chen J. Current development and challenges of tetravalent live-attenuated dengue vaccines. Front Immunol. 2022;13:840104. doi:10.3389/fimmu.2022.840104.
- Huang CY, Butrapet S, Tsuchiya KR, Bhamarapravati N, Gubler DJ, Kinney RM. Dengue 2 PDK-53 virus as a chimeric carrier for dengue tetravalent vaccine development. J Virol. 2003;77(21):11436–47. doi:10.1128/jvi.77.21.11436-11447.2003.
- Capeding MR, Tran NH, Hadinegoro SR, Ismail HI, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R, et al. Clinical efficacy and safety of a novel dengue tetravalent vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014;384(9951):1358–65. doi:10.1016/s0140-6736(14)61060-6.
- Villar L, Dayan GH, Arredondo-García JL, Rivera DM, Cunha R, Deseda C, Reynales H, Costa MS, Morales-Ramírez JO, Carrasquilla G, et al. Efficacy of a dengue tetravalent vaccine in children in Latin America. N Engl J Med. 2015;372(2):113–23. doi:10.1056/NEJMoa1411037.
- US Food and Drug Administration . Dengvaxia package insert. 2023 [accessed 2023 Sep 6 Sep]. https://www.fda.gov/media/124379/download.
- Vannice KS, Wilder-Smith A, Barrett ADT, Carrijo K, Cavaleri M, de Silva A, Durbin AP, Endy T, Harris E, Innis BL, et al. Clinical development and regulatory points for consideration for second-generation live attenuated dengue vaccines. Vaccine. 2018;36(24):3411–7. doi:10.1016/j.vaccine.2018.02.062.
- Lam HM, Phuong HT, Thao Vy NH, Le Thanh NT, Dung PN, Ngoc Muon TT, Van Vinh Chau N, Rodríguez-Barraquer I, Cummings DAT, Wills BA, et al. Serological inference of past primary and secondary dengue infection: implications for vaccination. J R Soc Interface. 2019;16(156):20190207. doi:10.1098/rsif.2019.0207.
- George SL, Wong MA, Dube TJ, Boroughs KL, Stovall JL, Luy BE, Haller AA, Osorio JE, Eggemeyer LM, Irby-Moore S, et al. Safety and immunogenicity of a live attenuated dengue tetravalent vaccine candidate in flavivirus-naive adults: a randomized, double-blinded phase 1 clinical trial. J Infect Dis. 2015;212(7):1032–41. doi:10.1093/infdis/jiv179.
- Osorio JE, Velez ID, Thomson C, Lopez L, Jimenez A, Haller AA, Silengo S, Scott J, Boroughs KL, Stovall JL, et al. Safety and immunogenicity of a recombinant live attenuated dengue tetravalent vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: a randomised, placebo-controlled, phase 1 study. Lancet Infect Dis. 2014;14(9):830–8. doi:10.1016/s1473-3099(14)70811-4.
- Rupp R, Luckasen GJ, Kirstein JL, Osorio JE, Santangelo JD, Raanan M, Smith MK, Wallace D, Gordon GS, Stinchcomb DT. Safety and immunogenicity of different doses and schedules of a live attenuated dengue tetravalent vaccine (TDV) in healthy adults: a phase 1b randomized study. Vaccine. 2015;33(46):6351–9. doi:10.1016/j.vaccine.2015.09.008.
- Sirivichayakul C, Barranco-Santana EA, Esquilin-Rivera I, Oh HM, Raanan M, Sariol CA, Shek LP, Simasathien S, Smith MK, Velez ID, et al. Safety and immunogenicity of a dengue tetravalent vaccine candidate in healthy children and adults in dengue-endemic regions: a randomized, placebo-controlled phase 2 study. J Infect Dis. 2016;213(10):1562–72. doi:10.1093/infdis/jiv762.
- Biswal S, Reynales H, Saez-Llorens X, Lopez P, Borja-Tabora C, Kosalaraksa P, Sirivichayakul C, Watanaveeradej V, Rivera L, Espinoza F, et al. Efficacy of a dengue tetravalent vaccine in healthy children and adolescents. N Engl J Med. 2019;381(21):2009–19. doi:10.1056/NEJMoa1903869.
- Biswal S, Borja-Tabora C, Martinez Vargas L, Velásquez H, Theresa Alera M, Sierra V, Johana Rodriguez-Arenales E, Yu D, Wickramasinghe VP, Duarte Moreira E Jr., et al. Efficacy of a dengue tetravalent vaccine in healthy children aged 4-16 years: a randomised, placebo-controlled, phase 3 trial. Lancet. 2020;395(10234):1423–33. doi:10.1016/s0140-6736(20)30414-1.
- European Medicines Agency. Qdenga summary of product characteristics. [accessed 2023 Sep 6]. https://www.ema.europa.eu/en/documents/product-information/qdenga-epar-product-information_en.pdf.
- European Medicines Agency. ICH E6 (R2) good clinical practice - scientific guideline. 2016 [accessed 2023 Sep 6]. https://www.ema.europa.eu/en/ich-e6-r2-good-clinical-practice.
- World Medical Association. Declaration of Helsinki. Ethical principles for medical research involving human subjects. 2022 [accessed 2023 Sep 6]. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/.
- World Health Organization. Rolling updates on coronavirus disease (COVID-19). 2020 [accessed 2023 Sep 6]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen.
- Tricou V, Winkle PJ, Tharenos LM, Rauscher M, Escudero I, Hoffman E, Lefevre I, Borskowski A, Wallace D. Consistency of immunogenicity in three consecutive lots of a dengue tetravalent vaccine candidate (TAK-003): a randomized placebo-controlled trial in US adults. Vaccine. In press.
- Mason RA, Tauraso NM, Spertzel RO, Ginn RK. Yellow fever vaccine: direct challenge of monkeys given graded doses of 17D vaccine. Appl Microbiol. 1973;25(4):539–44. doi:10.1128/am.25.4.539-544.1973.
- Julander JG, Trent DW, Monath TP. Immune correlates of protection against yellow fever determined by passive immunization and challenge in the hamster model. Vaccine. 2011;29(35):6008–16. doi:10.1016/j.vaccine.2011.06.034.
- Van Gessel Y, Klade CS, Putnak R, Formica A, Krasaesub S, Spruth M, Cena B, Tungtaeng A, Gettayacamin M, Dewasthaly S. Correlation of protection against Japanese encephalitis virus and JE vaccine (IXIARO(®)) induced neutralizing antibody titers. Vaccine. 2011;29(35):5925–31. doi:10.1016/j.vaccine.2011.06.062.
- Rivera L, Biswal S, Sáez-Llorens X, Reynales H, López-Medina E, Borja-Tabora C, Bravo L, Sirivichayakul C, Kosalaraksa P, Martinez Vargas L, et al. Three-year efficacy and safety of takeda’s dengue vaccine candidate (TAK-003). Clin Infect Dis. 2022;75(1):107–17. doi:10.1093/cid/ciab864.
- Sáez-Llorens X, Tricou V, Yu D, Rivera L, Jimeno J, Villarreal AC, Dato E, Mazara S, Vargas M, Brose M, et al. Immunogenicity and safety of one versus two doses of dengue tetravalent vaccine in healthy children aged 2-17 years in Asia and Latin America: 18-month interim data from a phase 2, randomised, placebo-controlled study. Lancet Infect Dis. 2018;18(2):162–70. doi:10.1016/s1473-3099(17)30632-1.
- Sáez-Llorens X, Tricou V, Yu D, Rivera L, Tuboi S, Garbes P, Borkowski A, Wallace D. Safety and immunogenicity of one versus two doses of Takeda’s dengue tetravalent vaccine in children in Asia and Latin America: interim results from a phase 2, randomised, placebo-controlled study. Lancet Infect Dis. 2017;17(6):615–25. doi:10.1016/s1473-3099(17)30166-4.
- Tricou V, Sáez-Llorens X, Yu D, Rivera L, Jimeno J, Villarreal AC, Dato E, Saldaña de Suman O, Montenegro N, DeAntonio R, et al. Safety and immunogenicity of a dengue tetravalent vaccine in children aged 2-17 years: a randomised, placebo-controlled, phase 2 trial. Lancet. 2020;395(10234):1434–43. doi:10.1016/s0140-6736(20)30556-0.
- Tricou V, Eyre S, Ramjee M, Collini P, Mojares Z, Loeliger E, Mandaric S, Rauscher M, Brose M, Lefevre I, et al. A randomized phase 3 trial of the immunogenicity and safety of coadministration of a live-attenuated dengue tetravalent vaccine (TAK-003) and an inactivated hepatitis a (HAV) virus vaccine in a dengue non-endemic country. Vaccine. 2023;41(7):1398–407. doi:10.1016/j.vaccine.2023.01.007.
- Tricou V, Essink B, Ervin JE, Turner M, Escudero I, Rauscher M, Brose M, Lefevre I, Borkowski A, Wallace D, et al. Immunogenicity and safety of concomitant and sequential administration of yellow fever YF-17D vaccine and dengue tetravalent vaccine candidate TAK-003: a phase 3 randomized, controlled study. PLoS Negl Trop Dis. 2023;17(3):e0011124. doi:10.1371/journal.pntd.0011124.
- Biswal S, Mendez Galvan JF, Macias Parra M, Galan-Herrera JF, Carrascal Rodriguez MB, Rodriguez Bueno EP, Brose M, Rauscher M, LeFevre I, Wallace D, et al. Immunogenicity and safety of a dengue tetravalent vaccine in dengue-naïve adolescents in Mexico city. Rev Panam Salud Publica. 2021;45:e67. doi:10.26633/rpsp.2021.67.
- Agréus L, Svärdsudd K, Nyrén O, Tibblin G. Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology. 1995;109(3):671–80. doi:10.1016/0016-5085(95)90373-9.
- Almario CV, Ballal ML, Chey WD, Nordstrom C, Khanna D, Spiegel BMR. Burden of gastrointestinal symptoms in the United States: results of a nationally representative survey of over 71,000 Americans. Am J Gastroenterol. 2018;113(11):1701–10. doi:10.1038/s41395-018-0256-8.
- Anger JT, Dallas KB, Bresee C, De Hoedt AM, Barbour KE, Hoggatt KJ, Goodman MT, Kim J, Freedland SJ. National prevalence of IC/BPS in women and men utilizing veterans health administration data. Front Pain Res (Lausanne). 2022;3:925834. doi:10.3389/fpain.2022.925834.
- Jin X, Ren J, Li R, Gao Y, Zhang H, Li J, Zhang J, Wang X, Wang G. Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019. EClinicalMedicine. 2021;37:100986. doi:10.1016/j.eclinm.2021.100986.
- Lyons MM, Bhatt NY, Pack AI, Magalang UJ. Global burden of sleep-disordered breathing and its implications. Respirology. 2020;25(7):690–702. doi:10.1111/resp.13838.
- Xue Y, Bao W, Zhou J, Zhao QL, Hong SZ, Ren J, Yang BC, Wang P, Yin B, Chu CC, et al. Global burden, incidence and disability-adjusted life-years for dermatitis: a systematic analysis combined with socioeconomic development status, 1990-2019. Front Cell Infect Microbiol. 2022;12:861053. doi:10.3389/fcimb.2022.861053.
- Patel SS, Rauscher M, Kudela M, Pang H. Clinical safety experience of TAK-003 for dengue fever: a new tetravalent live attenuated vaccine candidate. Clin Infect Dis. 2023;76(3):e1350–e9. doi:10.1093/cid/ciac418.
