ABSTRACT
Varicella-Zoster Virus (VZV) belongs to the family Herpesviridae. Herpes zoster (HZ) is caused by reactivation of latent VZV. It is associated with risk factors such as immunosenescence, immunosuppressive pathologies and pharmacological treatments. Patients with these risk factors are more likely to be hospitalized. Increases in HZ hospitalizations have been reported in many countries in recent years. The objective of this study is to estimate hospitalization rates, mortality rates and costs due to HZ during the worst years of the COVID-19 pandemic in Spain (2020–2021). This is a descriptive study based on an analysis of information from the Minimum Basic Dataset and coded according to the Spanish version of the 10th International Classification of Diseases (ICD-10-CM). Hospitalization, mortality and case-fatality rates, and median length of hospitalization were calculated.. The hospitalization rate was 14.4 cases per 100,000 inhabitants, and the mortality rate was 1.3 cases per 100,000 inhabitants. Both increased considerably with age. In this time period, 92.3% of the registered cases were people over 50 years of age. Nevertheless, during the COVID-19 pandemic period, hospitalization rate decreased and the mortality rate increased from previous years. HZ hospitalization and mortality rates are relevant issues in the public health of older people. It is highly recommended to evaluate new vaccination strategies against VZV to include the HZ vaccine for health care for elderly people, as well as to reduce the disease burden and associated risk factors. The estimation of HZ disease hospitalization costs were €100,433,904.
Introduction
Varicella-Zoster virus (VZV) belongs to the Herpesviridae family. Primary infection with VZV causes an illness, often a mild form, known as varicella (chickenpox). Chickenpox is most likely to affect preschool and school-age children between the ages of 2 and 10.Citation1 The infection manifests as a generalized, pruritic, maculopapular and vesicular rash in unvaccinated persons The clinical manifestation of varicella among vaccinated persons is usually modified, with fewer skin lesions, mostly maculopapular, and milder presentation.Citation2
This virus establishes latency in the dorsal root ganglia of cutaneous nerves and cranial sensory nerves for long periods of time after this primary infection. Herpes zoster (HZ) or shingles is caused by reactivation of latent VZV.Citation3
Herpes zoster can occur at any age and usually produces a very painful, self-limited vesicular eruption, normally on one side of the body with a dermatomal distribution. The main risk factor is a decrease in the cellular immune response associated with other risk factors such as immunosenescence, immunosuppressive pathologies (diabetes mellitus, rheumatoid arthritis, COPD, chronic kidney disease, mood disorders)Citation4–7 and drug treatments.Citation3,Citation6–8 Patients with these risk factors are more likely to be hospitalized. The most common complication is known as postherpetic neuralgia (PHN), which is defined as persistent pain associated with the HZ for three or more months from the onset of an HZ episode and after the rash has cleared.Citation4,Citation9
The incidence of chickenpox has decreased in Spain since 2016 as more kids receive the vaccine. Spain has a universal varicella vaccine program for infants with two doses for all autonomous communities.Citation10 Nevertheless, despite the high vaccination coverage achieved against chickenpox and the decline in disease incidence, the burden of disease, due to HZ, has progressively increased each year worldwide.Citation11
The main cause is the aging of the world population, regardless of geographic location. A systematic literature review published in 2021Citation12 showed an annual incidence between 5,23 5.23 and 10,9 cases per 1,000 person-years and a cumulative incidence between 2.9 and 19.5 cases per 1000 population worldwide In the epidemiological data available for European countries, the annual incidence of HZ was 2–4.6 cases per 1,000 population.Citation13 Over 30% of adults worldwide are at risk of acquiring HZ throughout their lives, and 50% of these individuals are >80 years of age.Citation11,Citation14,Citation15 In regard to PHN, it is estimated that 18% of HZ patients will develop PHN at any age, and the risk increases to 33% in patients 85 years of age or older.Citation16
In Spain, 27642 hospitalizations were registered between 2016 and 2019. Ninety percent of hospitalized patients were over 50 years, of age and 45.8% were over 80 years of age. A total of 51.7% of the cases were in women, and 59.9% were related to a complicated HZ.Citation17 For the same period of time, the hospitalization rate for HZ was 17.7 per 100,000 inhabitants (95% CI: 17.53–17.95), the mortality rate was 1.2 deaths per 100,000 inhabitants (95% CI: 1.15–1.25) and the case fatality rate was 6.8% (95% CI: 6.45–7.05).Citation15 Despite low mortality rates and case fatality rates, HZ generates a significant disease burden for patients and consumes resources for health systems.
Although there are several treatments to limit the rash, relieve pain and prevent the onset of PHN, none of them have proven to be effective in all cases.Citation18 Current therapeutic treatment options for HZ focus on preventing the spread of the disease and the sequelae of PHN after recovery. Therefore, vaccination is considered consolidated as the best method of primary prevention and protection against severe disease; and is capable of reducing hospitalizations, mortality and the appearance of PHN.Citation19–24
In March 2021, the Consejo Interterritorial del Sistema Nacional de Salud (CISNS) recommended HZ vaccination for all adults ≥18 years of age with hematopoietic stem cell, transplantation (HSCT), solid organ transplantation (SOT), hematologic tumors and solid tumors undergoing chemotherapy as well as for individuals infected with human immunodeficiency virus (HIV) and patients with rheumatoid arthritis (RA) receiving anti-JAK therapy. In addition, the HZ vaccine was included in the systematic vaccination schedule for people aged 65 and 80 years (cohorts born in 1942 and 1957 respectively) [19, 29]. Vaccination against HZ will be incorporated in all autonomous communities before the end of 2024.
Currently, there are two licensed HZ vaccines approved by the European Medicines Agency (EMA). Zostavax® (ZVL) is a live-attenuated VZV vaccine, and Shingrix® (RZV) is an adjuvanted recombinant VZV vaccine. The federal government’s Advisory Committee on Immunization Practices (ACIP) recommended the recombinant and adjuvanted HZ vaccine (Shingrix) over a live-attenuated HZ vaccine (Zostavax) for adults 50 years of age and older with chronic medical conditions and for immunocompromised individuals in 2018.Citation25
The main objective of this study was to estimate the epidemiological aspects of HZ infection and the burden of disease (cost, mortality, morbidity) during the worst years of the COVID-19 pandemic in Spain (2020–2021).
Methods
Study design and data source
A retrospective descriptive, population-based study was performed to assess the burden of disease in admissions for HZ from January 2020 through December 2021. All hospital discharges with HZ codified as primary or secondary diagnosis were included in this study. The available hospital dataset was collected from the Minimum Basic DataSet (MBDS) published annually by the Spanish Ministry of Health and codified according to the 10th International Classification of Disease (ICD-10-ES) (codes from B02 to B02.9) (the Spanish version). We collected several variables for administrative, demographic and medical information such as date of birth, sex, postal code of residence, autonomous community of residency, main and secondary diagnosis, outcome (discharge date/death) and comorbidities for the whole population studied.
We selected and codified those comorbidities for which vaccination has been recommended by the Public Health Commission since 2018 in Spain: hematopoietic stem cell transplantation, solid organ transplantation, rheumatoid arthritis, hematologic tumors, solid tumors and HIV in people aged 18 years and over.Citation15 The resident population in Spain was estimated by the Office for National Statistics on July 1, 2020, stratified by age and sex.
HZ cases were classified as complicated or uncomplicated. Complicated HZ included: encephalitis, meningitis or other nervous system complications, ophthalmic affection, disseminated HZ and other unspecified complications.
The patient information was deidentified and anonymized prior to the statistical analysis.
Statistical analysis
The global number of hospitalizations examined was expressed as absolute frequencies (n) per year and period. Hospitalization rate (HR) (number of admissions diagnosed) and mortality rate (MR) (number of deaths at hospital) were expressed per 100,000 population by age group, sex and year. The case-fatality rate (CFR) (number of deaths at hospital/number of hospitalizations) expressed as a percentage of deaths was also calculated. On the other hand, median length of stay (50th percentile, as length of stay did not follow a normal distribution) and comorbidities (by age groups and sex) were also calculated for complicated and uncomplicated cases in Spain. The total and average costs due to HZ were also estimated by subtracting the day of admission from the day of discharge and considering the total cost, and the diagnostic cost group.
The median hospital stay lengths were calculated. Costs for hospitalizations were computed using days spent in hospital, diagnoses, hospital discharges and the total cost.
Five age groups were categorized: <50, 50–59, 60–69, 70–79 and >80 years. For the comorbidities analysis, we used three groups (<65, 65–79 and >80 years) based on the recommendations for vaccination made by the Spanish Ministry of Health.
To assess for significant differences in the hospitalization and mortality rates in all age groups Poisson regression was used. To assess for significant differences in the case-fatality rate by age group Logistic regression was used. Variables used for adjustment in both Logistic and Poisson models included: year, autonomous community of residence and sex. Chi-square test was used to compare the presence of complications by group of age Median hospital stay lengths were compared between groups using Kruskal-Wallis tests.
All rates were calculated with 95% confidence intervals (CIs). For statistical comparison, a p value of 0.05 was considered significant. Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS/PASW, version 28).
The Ethics Investigation Committee Board (Rey Juan Carlos University) ruled that no formal ethics approval was required for this project.
Results
A total of 14,042 hospitalizations related to HZ were reported during the study period (2020–2021) in Spain. A significant (p < .001) increase of 11.9% of cases was observed between the two years. Hospitalizations were recorded in all age groups; the <50 years group represented only 7.7% of the hospitalizations, while the population aged 50 and over represented 92.35% of all cases. Of these, 45.8% were >80 years (). By sex, 52.2% of the cases occurred in women. A total of 62.6% of hospitalized cases were related to complicated HZ ().
Table 1. Cases, hospitalization rate, mortality rate due to herpes zoster (2020–2021): total and by age group, sex and presence or not of complications.
The total hospitalization rate (HR) due to HZ was 14.4 per 100,000 inhabitants (CI 95% 14.6–15.0). In 2020, it was 14 per 100,000 inhabitants (CI 95% 13.7–14.3), and in 2021, it was 15.8 per 100,000 inhabitants (CI 95% 15.46–16.0) (). This represents a significant (p < .001) increase of 12%. The age group that reported the highest HR was >80 years with 112.1 per 100,000 inhabitants (CI 95% 109.7–114.4). By sex, HR was significantly higher among men for all age groups ().
A total of 1,189 deaths related to HZ were registered among hospitalized patients (). An increase in cases of 4.6% was observed between both years. Deaths were recorded in all age groups; patients >50 years represented 98.6% of deaths. Of these, 65% corresponded to the age group of >80 years. By sex, 50.8% of deaths occurred in females. A total of 61% of deaths were related to complicated HZ ().
The mortality rate (MR) due to HZ was 1.3 deaths per 100,000 inhabitants (CI 95% 1.2–1.3). In 2020, it was 1.2 deaths per 100,000 inhabitants (CI 95% 1.1–1.3), and in 2021, it was 1.3 per 100,000 inhabitants (CI 95% 1.2–1.4) (). This represents a no significant (p = .064) increase of 4%. The age group that reported the highest MR was >80 years, with 13.5 deaths per 100,000 inhabitants (CI 95% 12.7–14.3). By sex, the MR was only 0.8% higher among men ().
The case fatality rate (CFR) was 8.5% (95% CI 8.0–8.9). In 2020 it was 8.8% (95% CI 8.1–9.5), and in 2021, it was 8.2% (95% CI 7.6–8.8) (). This represents a no significant (p = 0,109) decrease of 6.9%. The highest CFR was reported by the > 80 age group at 12.0% (95% CI 11.2–12.8). By sex, the CFR was 5.8% higher among men ().
Table 2. Case fatality rate (CFR) and median length (days/IQR) due to herpes zoster (2020–2021): total and by age group, sex and presence of complications.
The median length of stay was 7 days (IQR 4–13) for all patients included in the study. No significant differences were observed by sex or age group in length of stay in hospitals ().
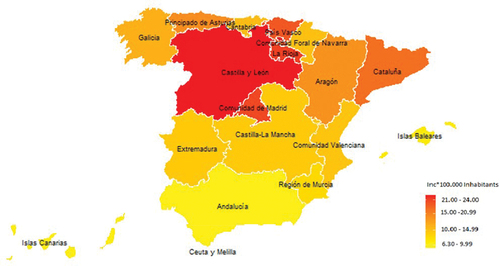
The analysis of geographical distribution in HR among the Spanish Autonomous Communities (AACC) showed a decreasing gradient of HR from north to south. The AACC that reported the highest hospitalization rates were: the Autonomous Community of Castilla y León (23.68 hospitalizations per 100,000 inhabitants), followed by La Rioja (22.1 per 100,000 inhabitants), the Community of Madrid (21.8 per 100,000 inhabitants) and the Basque Country (20.8 per 100,000 inhabitants). These four autonomous communities accounted for 36.4% of the total hospitalized cases. In contrast, the autonomous cities of Ceuta and Melilla reported the lowest rates (6 and 6.6 hospitalizations per 100,000 inhabitants, respectively). The Autonomous Community of the Canary Islands also reported one of the lowest rates (2.7 per 100,000 inhabitants) ().
Among the different comorbidities associated with HZ, the most common cause was solid tumors, with 5867 cases (41.8% of all registered hospitalizations). A total of 3,488 cases (59.5%) corresponded to a complicated HZ. The second most common comorbidity was hematological tumors, with 2,422 cases (17.3% of all registered hospitalizations), of which 1,375 cases (56.8%) corresponded to a complicated HZ. All comorbidities were more frequent among men, except for rheumatoid arthritis (RA), which was more frequent among women. Regardless of sex and their classification as complicated or uncomplicated, the number of total deaths registered during the study period, showed a clear increase with age for both years analyzed (). Only 7.8% of hospitalizations had a COVID-19 diagnosis in the study.
Table 3. Total hospitalizations due to herpes zoster: clinical characteristics by age group and presence of complications.
Total costs per hospitalizations due to HZ were calculated at €100,433,904. The annual expenditure of the health care system was €50,216,952, and the mean cost per patient was €7,152. Two thirds of the total cost were used on complicated cases ().
Table 4. Total and average costs per HZ total hospitalization (2020–2021).
Discussion
This study collects all HZ-related hospitalizations in Spain in 2020 and 2021, as well as their clinical characteristics and outcome. The number of HZ cases and the hospitalization, mortality and case fatality rates due to HZ have an increasing trend with age, being more frequent in the adult population over 50 years of age, increasing significantly with age to 112 cases per 100,000 population over 80 years of age. HZ was more frequent in women than in men in all age groups except 50–59 years between 2020 and 2021. The explanation for these figures lies in a decline in cell-mediated immunity, commonly associated with age, and its associated pathologies, which have a major impact on the effectiveness of the immune system, being the main risk factors for VZV reactivation.In the Hospital Morbidity Survey published by the Office for National Statistics in 2020 in Spain, the number of total hospital discharges decreased by 12.7% in 2020 compared to 2019. The main reason for discharge was cure or improvement in 88.1% of all cases.Citation26 Decreases were observed in from March to May and from October to December. All this coincides with the first two waves of COVID-19. Although the rate of hospitalization for diseases related to respiratory infections increased, the rates of the rest of the pathologies decreased. These data agree with the report published by the RAE-CMBD, which reports a total drop in discharges of 12%. The Hospital Morbidity Survey is not yet available for 2021.
However, several published studies have reported an association between SARS-CoV-2 and the reactivation of latent VZV during the acute or subacute phase of COVID-19.Citation27–32 This increase in the number of HZ cases reaches 15% in people aged 50 or over, according to a study published by Bhavsar A, et al. in 2022.Citation23 It has been hypothesized that COVID-19 infection could cause biological changes in the immune system, causing dysfunction of T cells and opportunistic reactivation of VZV. Although the association between HZ and COVID-19 is currently unknown, experts have raised the need for controlled studies to determine whether COVID-19 disease increases the risk of HZ.Citation23,Citation32 According to the results obtained in our study, we are not able to state whether increase in the number of HZ cases related to SARS-CoV-2 infectionCitation23,Citation31 has translated into an increase in the hospitalization rate. Nor can we state that the relationship between hospitalized patients with COVID-19 and HZ was statistically significant.
Number of HZ cases and rates occurred in all age groups, but it is especially striking from the age of 50 and older. The number of HZ cases in each age group analyzed was 2.5 times larger than that of the previous age group. Therefore, the probability of hospitalization in the over 80 years age group was 15 times greater than in the 50–59 years age group. This confirms immunosenescence as the main risk factor in the population.Citation17,Citation25 Regarding sex, the number of hospitalizations was higher among women than among men. This difference may not be real and may be because women request more consultations from the health system. Nevertheless, when calculating the hospitalization rate, the result is reversed to what we supposed the output would be (that is higher among men). Although sex does not appear to be a determining risk factor and has been described as an independent risk factor for VZV reactivation, the hospitalization rate was significantly higher in men during this period. However, these higher hospitalization rates have been frequently associated with comorbidities such as tumors, transplants or AIDS and a lower average life expectancy among men.Citation4 This is why HZ vaccination has been extensively recommended for at-risk groups, such as immunocompromised people.Citation33–35
The mortality rate increased by 5% during the years analyzed in relation to the former period (2016–2019). Deaths occurred in all age groups, especially in elderly individuals. By sex, the number of deaths was higher in men for all age groups except for those aged more than 80 years. Despite a decrease in the hospitalization rate, during the years analyzed, the mortality rate increased. This discrepancy between hospitalization rate and mortality rate does not correspond to the epidemiological reality or to the trend reported by previous studiesCitation11,Citation17,Citation26 but to factors related to the worst years of the pandemic. The fear of contagion from COVID-19 together with the confinement measures may be some of the causes that delayed visits to emergency rooms.
There was an increase of 25.5% in the case fatality rate, which is a significant change compared to previous studies.Citation17 This suggests that only the most serious and complicated cases entered into the health system.Citation36,Citation37 The true impact that the SARS-CoV-2 pandemic will have on HZ disease is still unknown.Citation38–40
Hospitalization rates presented clear differences between autonomous communities from north to south, following the same pattern shown in previous studies.Citation17 This north-south gradient could be explained by sociodemographic differences between autonomous communities. In addition, life expectancy at 65 years and the median age of the population in Spain showed higher values in the northern region than in the southern region by sex in 2021. On the other hand, studies attributed a lower seroprevalence of VZV in the southern regions as a crucial factor in the decrease in hospitalizations.Citation41 Total cases, and hospitalization and mortality rates were considerably higher in the group classified as complicated HZ. In other words, complicated HZ caused more hospitalizations and deaths than uncomplicated HZ. This can be found for all age groups, though there were higher rates for groups over 60 years old. These results show that the most important risk factor for VZV reactivation is the age of the individual. By sex, hospitalization and mortality rates were higher for all male groups than female groups, except for patients over 80 years.
Analyzing complicated HZ cases, the most frequent pathologies were solid tumors, followed by hematological tumors. Both reported a greater number of cases classified as complicated HZ. When analyzing comorbidities by age, the largest number of hospitalization cases does not always belong to the oldest group. This can be due to lower survival probability and life expectancy in this kind of pathology.
There were no significant differences in median length of stay by age or sex. By age group, the median duration was shorter in patients <50 years than expected. Regarding the classification as complicated or uncomplicated HZ, no significant differences were observed in the median length.
This study has several limitations that should be mentioned. During study years, a global pandemic hit the world unexpectedly, and this could be the main limitation for hospitalization and mortality rates related to HZ. The decrease in the hospitalization rate is not related to the trend reported by previous studies.Citation11,Citation17,Citation26,Citation41 On the other hand, hospitalizations related to HZ in any diagnostic position were considered an added limitation. We did not know whether a patient was hospitalized for HZ or developed it during their hospital stay. Another important limitation when interpreting the MBDS data is its reliability, which depends on the quality of the discharge reported from hospitals and their codification process. Although we assumed certain misclassification errors, the use of MBDS records is also our main strength. MBDS is considered one of the highest quality and homogeneous data sources for statistical analyses for epidemiological research. Evaluating the burden of disease and costs produced by HZ, we decided to count the total number of hospitalizations, regardless of the number of patients. Therefore, the same patient may have been hospitalized on several occasions. The cost per hospitalization among uncomplicated HZ, according to data obtained, were unexpectedlyCitation42 higher than among complicated HZ. One possible explanation is that the median length of stay among uncomplicated HZ was slightly longer than among complicated HZ. Another limitation was recommending the systematization of vaccination from the age of 65 onward, based on its efficacy. The studies consultedCitation26 never had a median follow-up of patients greater than four years. The resident population in Spain was published by the National Institute of Statistics (NIS) as of July 1, 2020–21, and this is the information that was used. This information is considered homogeneous, precise and high-quality. The results of our study should be interpreted according to their limitations.
Conclusions
Vaccination is considered the best method way of primary prevention and protection against severe disease, and is capable of reducing hospitalizations, mortality, the appearance of PHN and costs.Citation43,Citation44 Vaccination will be included in all autonomous communities in Spain before the end of 2024.
Abbreviations
| ACIP | = | Advisory Committee on Immunization Practices |
| ANOVA | = | analysis of variance |
| CFR | = | case fatality rate |
| HR | = | hospitalization rate |
| ICD‑10‑CM | = | International Classification of Diseases 10th Revision, Clinical Modification |
| MBDS | = | Minimum Basic DataSet |
| MR | = | mortality rate |
| SSO | = | Spanish Statistical Office |
| PHN | = | postherpetic neuralgia |
| VZV | = | varicella-zoster virus |
| HZ | = | herpes zoster |
| ZVL | = | Zostavax® |
| RZV | = | Shingrix® |
| HSCT | = | hematopoietic stem cell transplantation |
| SOT | = | solid organ transplantation |
| HIV | = | human immunodeficiency virus |
| RA | = | rheumatoid arthritis |
Compliance with ethics guidelines
The patient information was anonymized and deidentified prior to the analysis in compliance with ethics guidelines. The study was performed in accordance with the Helsinki Declaration Infect Dis Ther 1964 and its later amendments. The Rey Juan Carlos University Ethics Committee Board ruled that no formal ethics approval was required for this study.
Medical writing/editorial assistance
American Journal Experts edited this manuscript for proper use of English language. This assistance was funded by the authors.
Acknowledgments
The authors thank the Subdireccion General del Instituto de Informacion Sanitaria for providing the information on which this study is based.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets analyzed during the current study are available from the corresponding author, Ruth Gil-Prieto, on reasonable request.
Additional information
Funding
References
- Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, Grose C, Hambleton S, Kennedy PG, Oxman MN, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015 Jul 2;1:15016. doi:10.1038/nrdp.2015.16. PMID: 27188665; PMCID: PMC5381807.
- Dooling K, Marin M, Gershon AA. Clinical manifestations of Varicella: disease is largely forgotten, but it’s not gone. J Infect Dis. 2022 Oct 21;226(Suppl 4):S380–8. doi:10.1093/infdis/jiac390. PMID: 36265857; PMCID: PMC10205892.
- Harbecke R, Jensen NJ, Depledge DP, Johnson GR, Ashbaugh ME, Schmid DS, Breuer J, Levin MJ, Oxman MN. Recurrent herpes zoster in the shingles prevention study: are second episodes caused by the same varicella-zoster virus strain? Vaccine. 2020 Jan 10;38(2):150–7. doi:10.1016/j.vaccine.2019.10.038.
- Esteban-Vasallo MD, Domínguez-Berjón MF, Gil de Miguel Á, Astray-Mochales J, Blanco-Ancos LM, Gil-Prieto R. Characteristics of herpes zoster-associated hospitalizations in Madrid (SPAIN) before vaccine availability. J Infect. 2016 Jan;72(1):70–9. doi:10.1016/j.jinf.2015.10.003.
- Aldaz P, Díaz JA, Loayssa JR, Dronda MJ, Osacáriz M, Castilla J. Incidencia de herpes zóster en pacientes diabéticos. Anales Sis San Navarra [Internet]. Abr [citado 2023 Mayo 15]. 2013;36(1):57–62. doi:10.4321/S1137-66272013000100006.
- Forbes HJ, Bhaskaran K, Thomas SL, Smeeth L, Clayton T, Langan SM. Quantification of risk factors for herpes zoster: population based case-control study. BMJ. 2014 May 13;348:g2911. doi:10.1136/bmj.g2911.
- Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin Proc. 2017 Dec;92(12):1806–21. doi:10.1016/j.mayocp.2017.10.009.
- UK Health Security Agency.Shingles (herpes zoster): the green book, chapter 28a. Published 12 July 2013. Last updated 23 August 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1012943/Green_book_of_immunisation_28a_Shingles.pdf.
- Amicizia D, Domnich A, Arata L, Zoli D, Zotti CM, Cacello E, Gualano MR, Gasparini R, Panatto D. The role of age-sex interaction in the development of post-herpetic neuralgia. Hum Vaccin Immunother. 2017 Feb;13(2):376–8. doi:10.1080/21645515.2017.1264799.
- Centro Nacional de Epidemiología. Instituto de Salud Carlos III. Resultados de la vigilancia Epidemiológica de las enfermedades transmisibles. Informe anual. Años 2017-2018. Madrid, 2020. https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/INFORMES%20RENAVE/RENAVE_Informe_anual__2017-2018.pdf.
- Masa-Calles J, López-Perea N, Vila Cordero B, Carmona R. Vigilancia y epidemiología del herpes zóster en España [Surveillance and epidemiology of Herpes Zoster in Spain.]. Rev Esp Salud Publica. 2021 Jun 25; 95:e202106088. Spanish
- van Oorschot D, Vroling H, Bunge E, Diaz-Decaro J, Curran D, Yawn B. A systematic literature review of herpes zoster incidence worldwide. Hum Vaccin Immunother. 2021 Jun 3;17(6):1714–32. doi:10.1080/21645515.2020.1847582.
- Pinchinat S, Cebrián-Cuenca AM, Bricout H, Johnson RW. Similar herpes zoster incidence across Europe: results from a systematic literature review. BMC Infect Dis. 2013 Apr 10;13(1):170. doi:10.1186/1471-2334-13-170.
- Arruti M, Piñeiro LD, Salicio Y, Cilla G, Goenaga MA, López de Munain A. Incidence of varicella zoster virus infections of the central nervous system in the elderly: a large tertiary hospital-based series (2007-2014). J Neurovirol. 2017 Jun;23(3):451–9. doi:10.1007/s13365-017-0519-y.
- Grupo de trabajo de vacunación frente a herpes zóster de la Ponencia de Programa y Registro de Vacunaciones. Comisión de Salud Pública del Consejo Interterritorial del Sistema Nacional de Salud. Ministerio de Sanidad, marzo 2021. https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/programasDeVacunacion/docs/HerpesZoster_RecomendacionesVacunacion.pdf.
- Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology. 2013 Sep 3; 81(10):928–30. doi:10.1212/WNL.0b013e3182a3516e.
- Corcuera-Munguia M, Gil-Prieto R, Garcia-Carretero R, Gil-de-Miguel A. Hospitalization burden related to herpes zoster infection in Spain (2016-2019). Infect Dis Ther. 2023 Jan;12(1):143–56. doi:10.1007/s40121-022-00717-6.
- Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ, Backonja M, Betts RF, Gershon AA, Haanpaa ML, McKendrick MW, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007 Jan 1;44(Suppl 1):S1–26. doi:10.1086/510206.
- Boutry C, Hastie A, Diez-Domingo J, Tinoco JC, Yu CJ, Andrews C, Beytout J, Caso C, Cheng HS, Cheong HJ, et al. The adjuvanted recombinant zoster vaccine confers long-term protection against herpes zoster: interim results of an extension study of the pivotal phase 3 clinical trials ZOE-50 and ZOE-70. Clin Infect Dis. 2022 Apr 28;74(8):1459–67. doi:10.1093/cid/ciab629.
- Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015 May 28;372(22):2087–96. doi:10.1056/NEJMoa1501184.
- Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Díez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barberà J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016 Sep 15;375(11):1019–32. doi:10.1056/NEJMoa1603800.
- Marra Y, Lalji F. Prevention of herpes zoster: a focus on the effectiveness and safety of herpes zoster vaccines. Viruses. 2022 Nov 29;14(12):2667. doi:10.3390/v14122667.
- Bhavsar A, Lonnet G, Wang C, Chatzikonstantinidou K, Parikh R, Brabant Y, Servotte N, Shi M, Widenmaier R, Aris E. Increased risk of herpes zoster in adults ≥50 years old diagnosed with COVID-19 in the United States. Open Forum Infect Dis. 2022 Mar 9;9(5):ofac118. doi:10.1093/ofid/ofac118.
- Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, Harpaz R. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018 Jan 26;67(3):103–8. doi: 10.15585/mmwr.mm6703a5.
- Barranco Quintana JL, García Cenoz M, Moza Moríñigo H, Onieva García MÁ, Rodríguez García J, Román Ortiz C (2022). Consenso de la SEMPSPGS sobre la vacunación frente a Herpes Zóster. Rev Esp Med Prev Salud Pública, 21–77. https://pesquisa.bvsalud.org/portal/resource/pt/ibc-212833
- Centro Nacional de Epidemiología, CIBERESP. Instituto de Salud Carlos III. Informe epidemiológico sobre la situación del Herpes Zóster en España, 1998-2018. Madrid, agosto 2020. https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/archivos%20A-Z/HERPES%20ZOSTER/Informe_HZ_Espa%C3%B1a_1998-2018.pdf.
- Fillat ÁC, Ramón González-Juanatey J. COVID-19. Las consecuencias sociales, sanitarias y cardiovasculares. Revista Española de Cardiología Suplementos. 2020;20:1. doi:10.1016/S1131-3587(20)30027-3.
- Risco Risco C, Herrador Z, Lopez-Perea N, Martínez-Urbistondo D, Villar Carrero RS D, Masa-Calles J. Epidemiology of herpes zoster in the pre-vaccination era: establishing the baseline for vaccination programme’s impact in Spain. Euro Surveill. 2023 Feb;28(8):2200390. doi:10.2807/1560-7917.ES.2023.28.8.2200390.
- Maia CMF, Marques NP, de Lucena EHG, de Rezende LF, Martelli DRB, Martelli-Júnior H. Increased number of herpes zoster cases in Brazil related to the COVID-19 pandemic. Int J Infect Dis. 2021 Mar;104:732–3. doi:10.1016/j.ijid.2021.02.033.
- Pourazizi M, Dehghani S, Abtahi-Naeini B. Herpes zoster oftálmico y COVID-19: ¿Complicación post-COVID-19 o coincidencia? [Herpes Zoster Ophthalmicus and COVID-19: A Post-COVID-19 Complication or a Coincidence? Actas Dermosifiliogr. 2022 Dec; 113:S16–S17. Spanish.
- Diez-Domingo J, Parikh R, Bhavsar AB, Cisneros E, McCormick N, Lecrenier N. Can COVID-19 increase the risk of herpes zoster? A narrative review. Dermatol Ther (Heidelb). 2021 Aug;11(4):1119–26. doi:10.1007/s13555-021-00549-1.
- Almutairi N, Almutairi AN, Almazyad M, Alwazzan S. Herpes zoster in the era of COVID 19: a prospective observational study to probe the association of herpes zoster with COVID-19 infection and vaccination. Dermatol Ther. 2022 Jul;35(7):e15521. doi:10.1111/dth.15521.
- Pedrazzoli P, Lasagna A, Cassaniti I, Ferrari A, Bergami F, Silvestris N, Sapuppo E, Di Maio M, Cinieri S, Baldanti F. Vaccination for herpes zoster in patients with solid tumors: a position paper on the behalf of the Associazione Italiana di Oncologia Medica (AIOM). ESMO Open. 2022 Aug;7(4):100548. doi:10.1016/j.esmoop.2022.100548. Epub 2022 Jul 16. PMID: 35853350; PMCID: PMC9434335.
- Curran D, Patterson BJ, Carrico J, Salem A, La EM, Lorenc S, Hicks KA, Poston S, Carpenter CF. Public health impact of recombinant zoster vaccine for prevention of herpes zoster in US adults immunocompromised due to cancer. Hum Vaccin Immunother. 2023 Dec 31;19(1):2167907. doi:10.1080/21645515.2023.2167907. Epub 2023 Mar 7. PMID: 36880669; PMCID: PMC10038038.
- Kiderlen TR, Trostdorf K, Delmastro N, Salomon A, de Wit M, Reinwald M. Herpes zoster vaccination rates in hematological and oncological patients-stock taking 2 years after market approval. Healthcare (Basel). 2022 Aug 12;10(8):1524. doi:10.3390/healthcare10081524. PMID: 36011181; PMCID: PMC9408327.
- Moynihan R, Sanders S, Michaleff ZA, Scott AM, Clark J, To EJ, Jones M, Kitchener E, Fox M, Johansson M, et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open. 2021 Mar 16;11(3):e045343. doi:10.1136/bmjopen-2020-045343.
- Cura-González I D, Polentinos-Castro E, Fontán-Vela M, López-Rodríguez JA, Martín-Fernández J. ¿qué hemos dejado de atender por la COVID-19? Diagnósticos perdidos y seguimientos demorados. Informe SESPAS 2022 [what have we missed because of COVID-19? Missed diagnoses and delayed follow-ups. SESPAS report 2022. Gac Sanit. 2022;36(Suppl 1):S36–S43. Spanish. doi:10.1016/j.gaceta.2022.03.003.
- Achilleos S, Quattrocchi A, Gabel J, Heraclides A, Kolokotroni O, Constantinou C, Pagola Ugarte M, Nicolaou N, Rodriguez-Llanes JM, Bennett CM, et al. Excess all-cause mortality and COVID-19-related mortality: a temporal analysis in 22 countries, from January until August 2020. Int J Epidemiol. 2022 Feb 18;51(1):35–53. doi:10.1093/ije/dyab123.
- Ortiz-Cardenas M, Solis-Taquire L, Osada-Liy J. Discontinuidad en el tratamiento de los pacientes con enfermedades crónicas durante la pandemia por la COVID-19. Rev Cubana Med [Internet]. 2020 [citado 15 May 2023];60(3).
- Huenchuan S, La pandemia por COVID-19 y su relación con las enfermedades no transmisibles y la protección social en salud (LC/MEX/TS.2021/18). Ciudad de México, Comisión Económica para América Latina y el Caribe (CEPAL). 2021.
- Gil A, Gil R, Alvaro A, San Martín M, González A. Burden of herpes zoster requiring hospitalization in Spain during a seven-year period (1998-2004). BMC Infect Dis. 2009 May 7;9:55. doi:10.1186/1471-2334-9-55.
- Bongers LD, Navarini A, Berger CT, Mueller SM. Complications and cost estimations in herpes zoster - a retrospective analysis at a Swiss tertiary dermatology clinic. Swiss Med Wkly. 2021 Dec 28;151:w30081. doi:10.4414/smw.2021.w30081. PMID: 34964592.
- Strezova A, Diez-Domingo J, Al Shawafi K, Tinoco JC, Shi M, Pirrotta P, Mwakingwe-Omari A, Zoster-049 Study Group. Long-term protection against herpes zoster by the adjuvanted recombinant zoster vaccine: interim Efficacy, immunogenicity, and safety results up to 10 years after initial vaccination. Open Forum Infect Dis. 2022 Oct 23;9(10):ofac485. doi:10.1093/ofid/ofac485.
- Giannelos N, Ng C, Curran D. Cost-effectiveness of the recombinant zoster vaccine (RZV) against herpes zoster: an updated critical review. Hum Vaccin Immunother. 2023 Dec 31;19(1):2168952. doi:10.1080/21645515.2023.2168952. Epub 2023 Mar 14. PMID: 36916240; PMCID: PMC10054181.