ABSTRACT
SARS-CoV-2 anti-spike IgG production and protection from severe respiratory illness should be explored in greater depth after COVID-19 booster vaccination. This longitudinal observational retrospective study investigated the anti-spike IgG response elicited by the first, second and booster doses of BNT162b2 mRNA vaccine in healthcare workers (HCW) at San Martino IRCCS Policlinico Hospital (Genoa) up to the 12th month. Sequential blood sampling was performed at T0 (prior to vaccination), T1 (21 days after the 1st dose of vaccine), T2, T3, T4, T5, T6 (7 days and 1, 3, 6 and 9 months after the 2nd dose, respectively), T7 and T8 (1 and 3 months after a booster dose). A SARS-CoV-2 IgG panel (Bio-Rad, Marnes-la-Coquette, France) was used to determine levels of receptor-binding domain (RBD), spike-1 (S1), spike-2 and nucleocapsid structural proteins of SARS-CoV-2. In the 51 HCWs evaluated, seroprevalence was 96% (49/51) at T1 and 100% (51/51) from T2 to T5 for RBD and S1. At T6, only one HCW was negative. T2 [RBD = 2945 (IQR:1693–5364); S1 = 1574 (IQR:833–3256) U/mL], and T7 [RBD = 8204 (IQR:4129–11,912); S1 = 4124 (IQR:2124–6326) U/mL] were characterized by the highest antibody values. Significant humoral increases in RBD and S1 were documented at T7 and T8 compared to T2 and T4, respectively (p-value < .001). Following vaccination with BNT162b2 and a booster dose in the 9th month, naïve and healthy subjects show high antibody titers up to 12 months and a protective humoral response against COVID-19 disease lasting up to 20 months after the last booster.
Introduction
Since December 2019, nearly 6.9 million deaths due to severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) have been reported.Citation1 In Italy, the vaccination campaign against Coronavirus Disease 2019 (COVID-19) started on December 27 2020 with the Pfizer BioNTech vaccine (BNT162b2 mRNA COVID-19 vaccine – Comirnaty). According to the Centers for Disease Control and Prevention (CDC), COVID-19 vaccination was initially reserved for healthcare workers (HCW).Citation2 To date, about 65.3% of the world’s population have been fully vaccinated (Online Access of WHO Coronavirus Dashboard on August 7, 2023).Citation1
BNT162b2 was the first COVID-19 vaccine to be approved by the European Medicines Agency for subjects aged 16 years and over.Citation3 Subsequently, its use was extended to 12- to 15-year-olds; recently, the CDC recommends Pfizer-BioNTech vaccination for all subjects aged 6 months and older, in order to increase protection against COVID-19.Citation2,Citation4 BNT162b2 is a single-stranded messenger RNA (mRNA) encapsulated in lipid nanoparticles. The RNA encodes the surface spike protein of SARS-CoV-2, which, after translation, is presented on the cell surface by antigen presenting cells (i.e. dendritic cells), thereby stimulating T and B lymphocytes, the final aim being to produce anti-spike protein antibodies.Citation5 This vaccine is well tolerated and very effective in preventing COVID-19 respiratory illness.Citation6
The longevity of immunity to SARS-CoV-2 has been investigated by evaluating antibody levels or cellular immunity. Cellular immunity, in particular SARS-CoV-2 B and T cell, was found to reach a peak 3–6 months after infection and to persist for up to 15 months; T cells decreased from months 6 to 15.Citation7 Similarly, SARS-CoV-2 antibodies have been detected for up to 15.6 months in infected patients.Citation8 On the other hand, the follow-up of COVID-19 passive immunization has been explored for short periods, at most up to 8 months. The Pfizer-BioNTech vaccine has been shown to induce an antibody response able to neutralize all tested SARS-CoV-2 variants for up to 7.3 months after the second dose.Citation9–13
High antibody levels have been correlated with an optimal T cell response on measuring the interferon-γ production of T-cells in response to SARS-CoV-2 antigens.Citation11 Furthermore, the immune response against SARS-CoV-2 has shown a positive serum antibody response for up to 6 months, not only in healthy individuals, e.g. HCWs, but also in sick patients, e.g. patients with immune-mediated inflammatory diseases, chronic renal diseases (dialyzed or transplanted), rheumatic/musculoskeletal diseases and HIV infection, although they display significantly lower anti-spike antibody levels than healthy subjects.Citation12,Citation14–16
Finally, the new SARS-CoV-2 Omicron variant (B.1.1.529) has proved to be better able to elude neutralizing antibodies derived from vaccination than previous variants, including all Omicron lineages from BA.1 to BA.5.Citation17–19
We investigated the immunogenicity of BNT162b2 in HCWs up to 9 months after administration of the second dose and up to 3 months after the third dose by measuring serum anti-spike IgG levels. The first objective of this study was to evaluate the levels and the longevity of antibody responses elicited by BNT162b2; the second was to assess the incidence of COVID-19 infections during follow-up; the third was to investigate the safety of the vaccine by recording and analyzing minor and major adverse events.
Materials and methods
Study design and participants
We carried out a longitudinal observational retrospective study among HCWs at Hygiene Unit of San Martino Policlinico Hospital – IRCCS (Genoa, Italy) to evaluate anti-spike IgG levels after BNT162b2 vaccination in accordance with the National COVID-19 vaccination plan.Citation20 HCWs aged 18 years and older, naive to SARS-CoV-2 and with extensive serological follow-up were eligible to participate in the study.
For reasons of serological surveillance, HCWs underwent several different serology tests during the study period, given the high risk of SARS-CoV-2 exposure when they were assigned to intensive treatment or critical care units. In the context of this prevention protocol, 51 subjects naïve to SARS-CoV-2 were selected from among 5000 HCWs, as they had undergone sequential serological SARS-CoV-2 tests at planned time-points of interest to the study. summarizes these time-points: T0 (prior to vaccination), T1 (21 days after 1st dose of vaccination), T2, T3, T4, T5, T6 (7 days after 2nd dose of vaccination and 1, 3, 6, 9 months after full vaccination, respectively), T7 and T8 (1 and 3 months after a booster dose). A total of 459 SARS-CoV-2 antibody assays were performed in the Medicine Laboratory of our hospital from December 27, 2020, to February 18th, 2022. All time-points of all serological tests have been included with a temporal range of 4–7 days from the sampling.
Table 1. Sampling times of BNT162b2 HCWs serological specimens.
BNT162b2 was injected as recommended by the manufacturers, BioNTech and Pfizer.Citation3–5,Citation21
At the time of the first dose, all HCWs received a questionnaire concerning their main clinical and socio-demographic data. After vaccination, they were invited to communicate any minor adverse events by means of the hospital intranet system, and to immediately report any major events through direct contact with the medical staff of the Hygiene Unit.
The study was conducted in accordance with the guidelines of the Declaration of Helsinki. Ethical review and approval were waived for this study, since it was based on routine SARS-CoV-2 serological surveillance of healthcare workers at San Martino Policlinico Hospital – IRCCS for Oncology and Neurosciences (Genoa, Italy).
Pre-analytical and analytical procedures
All HCWs underwent peripheral venous blood sampling in their unit of affiliation. Serum was separated and either promptly analyzed or stored at 2–8°C in the biorepository until processing and for up to 2 weeks. Analytical procedures were performed at the Medicine Laboratory Unit of our hospital by means of the SARS-CoV-2 IgG panel (Bio-Rad, Marnes-la-Coquette, France), which is routinely used in our hospital to detect IgG antibodies against the Spike and nucleocapsid (N) protein of SARS-CoV-2.
Serological testing
The SARS-CoV-2 IgG panel is analyzed on BioPlex 2200 by means of Luminex xMAP technology, which uses Luminex magnetic beads for the semiquantitative and qualitative detection of four different IgG antibodies against the receptor-binding domain (RBD), spike 1 (S1), spike 2 (S2), and N structural proteins of SARS-CoV-2. The semi-quantitation amplitude of these four target antibodies ranges from 1 to 100 U/mL; the automatic dilution (1:8, 1:16 or 1:32) of the instrument enables the detection interval to be increased up to 3200 U/mL. Manual dilutions were performed to quantify antibody values above 3200 U/mL. As reported by the manufacturer, in the clinical scenario, this multiplex immuno-assay is characterized by ≥99.9% specificity and 96.3% sensitivity ≥15 days after symptom onset. Total antibody levels are calculated as U/mL. According to the manufacturer’s recommendations, IgG anti-RBD, -S1 and -S2 are positive above 10 U/mL; values of anti-N IgG above 24 U/mL are considered reactive for SARS-CoV-2 infection.
COVID-19 testing
Molecular assay
The diagnosis of SARS-CoV-2 infection was performed on nasopharyngeal swabs from HCWs by means of the Allplex SARS‐CoV‐2 Assay (Seegene Inc.; Seoul, South Korea) according to the manufacturer’s instructions. Real-time reverse transcription polymerase-chain reaction (RT-PCR) targets E, RdRp/S and N genes and runs on the CFX96 instrument (Bio‐Rad Laboratories, USA) in accordance with an extraction‐free method.Citation22 The amplification of two viral targets with cycle threshold (Ct) value <40 defines a positive result. As recommended by the CDC, COVID-19 testing was carried out on HCWs with suspected respiratory symptoms, exposure to COVID-19 without symptoms, before contact with a subject with comorbidities, who could be exposed to severe COVID-19 disease and for screening program based on hospital affiliation.Citation2
Rapid antigen test
A lateral flow immunochromatographic test (JOYSBIO’s coronavirus antigen test kit) was used in order to reach a rapid diagnosis of SARS-CoV-2 infection. This approach is used to screen asymptomatic subjects in particular settings (e.g. nursing homes, dormitories, homeless shelters, etc.) and for surveillance testing, according to the manufacturer’s instructions.Citation2
Data collection and statistical analysis
Demographic, clinical and laboratory data were collected. Continuous variables were expressed as medians with interquartile range (IQR) or means with standard deviation (SD), and categorical variables were reported as numbers and percentages. In order to compare RBD and S1 IgG levels at different post-vaccination times, a Wilcoxon signed-rank test was applied. Multiple linear regression analysis for sex, age, comorbidities and adverse effects was performed in order to explore potential factors affecting the anti-RBD and -S1 IgG antibody response.
Data analyses were carried out by means of GraphPad Prism, version 8.0 (GraphPad Software, San Diego, CA) and jamovi, version 2.3 (computer software, https://www.jamovi.org). P values ≤ .05 were considered statistically significant.
Results
All participants received three doses; none was infected with SARS-CoV-2 between the first and the third vaccine dose. The median age of the HCWs was 49 (IQR: 35–57) years and females were slightly predominant (60.8%). The HCWs enrolled in the study were mostly nurses (12, 23.5%), laboratory staff (10, 19.6%) and medical staff (10, 19.6%). Hypertension (3/51, 5.8%) and hypercholesterolemia (2/51, 3.9%) were the only comorbidities reported. summarizes the main demographic and clinical features of our study group.
Table 2. Demographic and clinical characteristics of participants.
Anti-RBD and -S1 IgG response
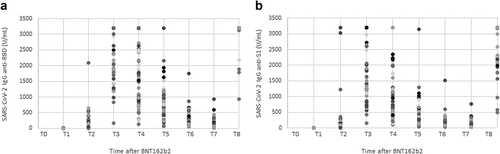
Seroprevalence for RBD and S1 was 0% (0/51) at T0, 96% (49/51) at T1 and 100% (51/51) from T2 to T5. At T6, only one HCW had become negative on anti-RBD and -S1 antibody detection. Median values of RBD [100 (IQR: 51–188) vs 2945 (IQR: 1693–5364) U/mL] and S1[IQR: 79 (30.7–131) vs 1574 (IQR: 833–3256) U/mL] increased markedly from T1 to T2. According to clinical trials, T2 and T7 are the recommended time-points for the evaluation of vaccine effectiveness.Citation6,Citation23 T2 (data reported above) and T7, with median values of RBD = 8204 (IQR: 4129–11912) U/mL and S1 = 4124 (IQR: 2124–6326) U/mL displayed the highest antibody values. At T2, only 3.9% (2/51) of HCWs had a detectable value of anti-RBD antibodies <1000 U/mL; while a value <1000 U/mL of anti-S1 antibodies was observed in 27.9% (14/51). In addition, at T7, 25.5% (13/51) and 11.8% (6/51) of HCWs had anti-RBD and -S1 IgG levels >5000 U/mL, respectively. From T3 to T6, overall antibody levels lowered gradually, decreasing 20-fold (IQR: 18–23) and 19-fold (IQR: 17–22) for RBD and S1, respectively. The trend of median for RBD and S1 antibodies is depicted graphically in . To illustrate the trends of each immunological response, a scatter plot of RBD and S1 antibody values has been included in .
Figure 1. Temporal trends and differences of SARS-CoV-2 IgG anti-RBD (panel a) and -S1 (panel b) antibodies elicited by BNT162b2 mRNA vaccine. Serum antibody values are reported as medians (U/ml).
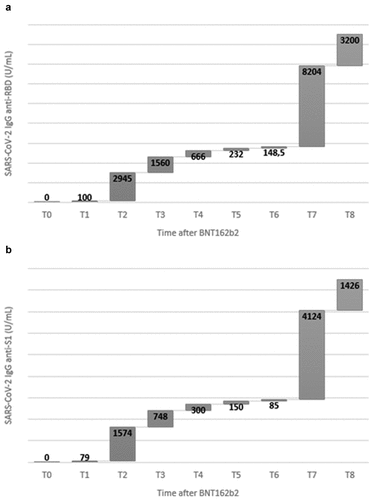
Figure 2. Scatterplot of humoral immune responses to BNT162b2 mRNA vaccine in SARS-CoV-2 naïve healthcare workers. A series of SARS-CoV-2 IgG anti-RBD (panel a) and -S1 (panel b) data were plotted at different time points in the post-vaccination period. Serum antibody levels are reported as absolute values ranging from 1 to 3200 U/mL. Levels of anti-RBD and anti-S1 ≥ 3200 U/mL were reported as 3200 U/mL, the upper limit of detection. This graph does not include the results of manual dilutions.
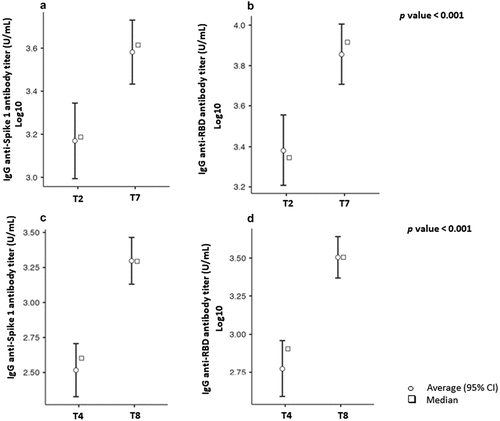
At T7, antibody levels were seen to have increased significantly in comparison with T2 (p value < .001); RBD and S1 IgG increased 3-fold (IQR: 2–5) and 2.5-fold (IQR: 1,25–4), respectively. Similarly, the humoral increase was preserved up to the final serological test. On comparing the antibody levels, both RBD and S1 IgG at T8 were 6 times higher than at T4 [RBD = 6 (IQR: 2–10) and S1 = 6 (IQR: 3–10)] (p value < .001). These data are shown in .
Figure 3. Comparison of humoral response following full vaccination with BNT162b2 at T2 and T4 and after the booster dose at T7 and T8. Average and median values, expressed as Log10 of RBD and S1 IgG 7 days after the 2nd dose of vaccine (T2) were compared with antibody levels 1 month after a booster dose (T7), as reported in panels a and b. Similarly, average (95% CI) and median values of RBD and S1 IgG 3 months after full vaccination (T4) were compared with antibody levels 3 months after the booster dose (T8), as reported in panels c and d.
Overall, S2 was produced at low level, constantly. At T1, 99.2% (46/51) of HCWs proved serologically positive for this antibody, which gradually decreased over the subsequent times; at T6, all HCWs were found to be negative. The highest values of anti-S2 antibodies were observed at T2, with 67 U/mL being the highest value detected.
Sex, age, comorbidities and adverse effects were not associated with high antibody levels at T2 and T7 (Linear regression, p > .05).
Prevention of SARS-CoV-2 infection with COVID-19 vaccine
Anti-N IgG were never detected in any participants from T0 to T7. This finding confirmed their naïve status and the immunological protection of BNT162b2 from COVID-19 disease in this study period. At T8, however, five HCWs (9.8%) were seen to have experienced a SARS-CoV-2 infection, with detection of anti-N IgG and confirmed molecular or antigen diagnosis. The use of molecular and antigen tests differed among our cases of viral infection, as from January 2022, the molecular assay in Italy was gradually replaced by the antigen assay; the use of these different methods of diagnosing SARS-CoV-2 was guided by the CDC recommendations.Citation2
Outside of the serological surveillance period until July 2023 (i.e. from March 2022 to July 2023), 26/51 (50.9%) HCWs had a diagnosis of SARS-CoV-2 infection, as revealed by molecular or antigen testing. In these participants, the median time from the booster dose to the positive result was 183.5 (IQR: 102.7–220.2) days. No moderate or severe symptomatic infection was observed; our early and late post-booster infected participants developed only mild symptoms (fever, cough, sore throat and headache).
Self-reported local and systemic reactogenicity effects
All participants enrolled in the study were instructed to self-report any local and/or systemic reactogenicity effects that occurred within 7 days after injection of the first and second doses of vaccine. Overall, at least one adverse effect was registered in 82.3% (42/51) and 70.6% (36/51) of participants after the first and the second dose, respectively. The most common clinical signs recorded after both injections were local pain at the injection site (74.5–58.8%), fatigue/malaise (21.5–27%) and muscle pain/joint pains (9.8–19.6%), as reported in . No data were collected after the booster doses due to reactogenic effects of the vaccine. However, overall, no medical care was required following the first, second and booster doses as well as no severe and/or long-term adverse events were documented in the study population.
Table 3. Local and systemic reactogenicity effects after the first and second doses of BNT162b2.
Discussion
This is the first long-term study to assess antibody response after the full vaccination and booster doses, to our knowledge. We investigated the humoral response to the BNT162b2 vaccine over one year in naïve and healthy HCWs. In this serological survey, no immunological breakthrough identified as non-response was registered.
BNT162b2 elicited an immunological response to the SARS-CoV-2 spike protein one week after the injection of the second dose until to 9 months after full vaccination, except for one patient that it was negative at 6 months post-full vaccination. Similar immunological reactivity was reported by Ponticelli et al. in a cohort of 444 HCWs after full vaccination without boosting dose and at 3 months follow-up.Citation23
Anti-RBD and -S1 IgG elicited by this mRNA vaccine persisted for all during serological follow-up up to 1 year after vaccination with a booster dose at 9 months.
Furthermore, it is providing 100% of protection against SARS-CoV-2 disease for up to 20 months after the last vaccine dose, that it is the booster.
During the serological follow-up, we observed a regular decrease of anti-spike IgG levels. This has been documented in several other serological studies, even measuring neutralizing antibody titers and specific T-cell responses.Citation24–29 However, it was documented by Jalkanen et al. that this decrease had no effect on neutralizing capacity up to 6 months after vaccination against five SARS-CoV-2 variants.Citation24
These studies involved both small and large study groups, and follow-up was mostly conducted for 3–7 months. A long follow-up period has been described in a study by Coppeta et al.; a subgroup of naïve HCWs displayed detectable antibody levels for up to 253 days, as also reported in other similar cohort studies.Citation27,Citation30,Citation31 In addition, our study extended serological follow-up to 12 months, considering the booster dose after 9 months, and clinical follow-up to 20 months. To date, correlates of long-term protection for COVID-19 vaccines have been very poorly investigated. Indeed, there are no data as yet on the real-world vaccine efficacy against severe acute respiratory infections after 9 months of passive immune response and it is difficult to find subjects who have not had a booster dose in most of the serological surveys. On the basis of the CDC recommendations, everyone aged 6 years and older should be planned to receive one updated Pfizer-BioNTech or Moderna COVID-19 vaccine; children aged 6 months–5 years, people aged 65 years and older and moderately or severely immunocompromised patients have different recommendations.Citation2
Our findings demonstrate that the reactogenicity of the BNT162b2 booster dose was superior to the responses elicited by the initial vaccine doses at one- and three-months post-booster. Therefore, it is reasonable to assume that booster doses induce higher and more prolonged immunogenicity, as reported by other serological surveys, including those enrolling immunocompromised patients or non-responders.Citation32–36 Furthermore, as demonstrated elsewhere, the neutralization capacity levels and the cellular immune response following administration of boosting dose was also shown to be well sustained.Citation36
Overall, the proportion of testing positive for SARS-CoV-2 RNA/antigen or anti-N antibodies from the full vaccination until one month later of booster dose was 0%. Other serological surveys reported an incidence of SARS-CoV-2 infection for comparable category of study population between 0.26% and 5%.Citation37,Citation38 These studies were conducted on large sample size but for short-time of clinical follow-up about 3 and 4 months post-full vaccination. At 3 months from the booster dose, we observed the first SARS-CoV-2 infection cases in our population study, 5/51 (9.8%). The diagnosis of SARS-CoV-2 infection increased during 20 months later of virological follow-up; indeed, 26/51 (49.1%) of the study population from being diagnosed with SARS-CoV-2 infection with no or mild symptoms. However, it is also important to note that hospital workers are at increased risk of contracting SARS-CoV-2 compared to the general population.Citation39 Before long, additional data will be produced on the impact of the update dose in enhancing long-term protection and immunological memory.
Epidemiological surveillance by means of SARS-CoV-2 sequencing is a molecular procedure that involves monthly and weekly sampling carried out on ascertained clinical cases, as suggested by the Italian National Institute of Health (ISS).Citation40,Citation41 Specifically, like every regional Italian reference laboratory for SARS-CoV-2 diagnosis, we sequence a representative sample of newly reported COVID-19 cases on one day a month, which is fixed by the ISS. In addition, every week we select special clinical cases for the genomic identification of viral variants according to one of the following inclusion criteria: clinical cases with severe symptoms requiring hospitalization; intensive care unit patients; reinfections; immunocompromised subjects and travelers from countries at high risk owing to the circulation of emerging viral variants. Therefore, we could not include our infected study population in the planned sequencing libraries.
Notably, this serological survey was performed during the third wave of the SARS-CoV-2 pandemic, which was characterized by the circulation of several variants. From December 2020 to October 2022, variant B.1.1.7 Lineage (Alpha WHO), B.1.617.2-AY. Lineages (Delta WHO) and/or B.1.1.529 - BA.1 to BA.5 Lineages (Omicron WHO) were the circulating variants of concern. The third dose of BNT162b2 vaccine raised neutralization efficiency against the Omicron variant for more than 6 months after full vaccination.Citation42 However, the first SARS-CoV-2 infection reported among our study participants was registered 42 days after the booster dose, in January 2022, namely during the shift from the viral variant Delta to Omicron, as previously investigated in one of our brief communications on the rapid molecular diagnosis of Omicron and Delta variants.Citation41 Nevertheless, we can suppose that the viral infections documented in our HCWs from January to June 2022 were caused by the Omicron variant, as is revealed by the sequences submitted to GISAID by our affiliation during this interval time, and as we had already ascertained.Citation41 Indeed, Omicron escapes the neutralizing antibodies elicited by BNT162b2 more easily than other viral variants.Citation17
This study enabled us to investigate the kinetics and the persistence of antibodies against SARS-CoV-2 in response to BNT162b2 over one year of serological follow-up, to evaluate the clinical follow-up in terms of incidence of SARS-CoV-2 infections and the safety for long follow-up.
Different limitations must be recognized in this real-world study, namely the small sample size, no placebo control group, its single-center nature and the exclusion from enrollment of older patients, who are known to display a lower immune response to the BNT162b2 vaccine.Citation43 Another important limitation is that we did not perform the plaque reduction neutralization tests but our serological assay detects the neutralizing antibodies against the Spike and nucleocapsid (N) protein of SARS-CoV-2. Likewise, we did not assess the cell-mediated immune response to vaccine nor did we perform SARS-CoV-2 sequencing of cases of infection in our study population, as mentioned above. However, it was not possible to perform additional tests on our serum samples, as these were clinical samples and, above all, were stored at 2–8°C for up to 2 weeks. Similarly, SARS-CoV-2 sequencing of our infection cases because nasopharyngeal swabs were randomly collected at −20°C when viral load was high, otherwise they were discarded.
In conclusion, naïve and healthy subjects vaccinated with BNT162b2 and a booster dose in the 9th month, show high antibody titers up to 12 months and a protective humoral response against COVID-19 disease lasting up to 20 months after the last booster. Given both the greater escape ability of the Omicron variant and the decline in humoral response, an updated booster may be planned for HCWs, as recommended by the CDC, in order to prevent new infections and to reduce the risk of nosocomial transmission among hospitalized patients, thus improving the cost-effectiveness of SARS-CoV-2 management.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- World Health Organization. Coronavirus (COVID-19) dashboard. [accessed 2023 Aug 7]. https://covid19.who.int/.
- Centers for Disease Control and Prevention. COVID-19. [accessed 2023 Aug 7]. https://www.cdc.gov/coronavirus/2019-nCoV/index.html.
- Lamb YN. BNT162b2 mRNA COVID-19 vaccine: first approval. Drugs. 2021;81(4):495–8. doi:10.1007/s40265-021-01480-7. PMID: 33683637.
- Frenck RW Jr, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, Perez JL, Walter EB, Senders S, Bailey R, et al. Safety, immunogenicity, and efficacy of the BNT162b2 covid-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239–50. doi:10.1056/NEJMoa2107456. PMID: 34043894.
- BNT162b2 vaccine for prevention of COVID-19. Aust Prescr. 2021;44(1):57–8. doi:10.18773/austprescr.2021.008. PMID: 33911334.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi:10.1056/NEJMoa2034577. PMID: 33301246.
- Marcotte H, Piralla A, Zuo F, Du L, Cassaniti I, Wan H, Kumagai-Braesh M, Andréll J, Percivalle E, Sammartino JC, et al. Immunity to SARS-CoV-2 up to 15 months after infection. iScience. 2022;25(2):103743. doi:10.1016/j.isci.2022.103743. PMID: 35018336.
- Zhang S, Xu K, Li C, Zhou L, Kong X, Peng J, Zhu F, Bao C, Jin H, Gao Q, et al. Long-term kinetics of SARS-CoV-2 antibodies and impact of inactivated vaccine on SARS-CoV-2 antibodies based on a COVID-19 patients cohort. Front Immunol. 2022;13:829665. doi:10.3389/fimmu.2022.829665. PMID: 35154152.
- Valleriani F, Mancuso E, Vincifori G, Teodori L, Di Marcantonio L, Spedicato M, Leone A, Savini G, Morelli D, Bonfini B, et al. Neutralization of SARS-CoV-2 variants by serum from BNT162b2 vaccine recipients. Viruses. 2021;13(10):2011. doi:10.3390/v13102011. PMID: 34696441.
- Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–73. doi:10.1056/NEJMoa2110345. PMID: 34525277.
- Tychala A, Meletis G, Katsimpourlia E, Gkeka I, Dimitriadou R, Sidiropoulou E, Skoura L. Evaluation of the QuantiFERON SARS-CoV-2 assay to assess cellular immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in individuals with low and high humoral response. Hum Vaccin Immunother. 2021;17:5148–9. doi:10.1080/21645515.2021.1991710. PMID: 34714711.
- Firinu D, Perra A, Campagna M, Littera R, Fenu G, Meloni F, Cipri S, Sedda F, Conti M, Miglianti M, et al. Evaluation of antibody response to BNT162b2 mRNA COVID-19 vaccine in patients affected by immune-mediated inflammatory diseases up to 5 months after vaccination. Clin Exp Med. 2022;22(3):477–85. doi:10.1007/s10238-021-00771-3. PMID: 34741188.
- Wei J, Pouwels KB, Stoesser N, Matthews PC, Diamond I, Studley R, Rourke E, Cook D, Bell JI, Newton JN, et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat Med. 2022;28(5):1072–82. doi:10.1038/s41591-022-01721-6. PMID: 35165453.
- Bouwmans P, Messchendorp AL, Sanders JS, Hilbrands L, Reinders MEJ, Vart P, Bemelman FJ, Abrahams AC, van den Dorpel MA, Ten Dam MA, et al. Long-term efficacy and safety of SARS-CoV-2 vaccination in patients with chronic kidney disease, on dialysis or after kidney transplantation: a national prospective observational cohort study. BMC Nephrol. 2022;23(1):55. doi:10.1186/s12882-022-02680-3. PMID: 35123437.
- Frey S, Chiang TP, Connolly CM, Teles M, Alejo JL, Boyarsky BJ, Christopher-Stine L, Werbel WA, Massie AB, Segev DL, et al. Antibody durability 6 months after two doses of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal disease. Lancet Rheumatol. 2022;4(4):e241–e3. doi:10.1016/S2665-9913(21)00417-3. PMID: 35072108.
- Macías J, Fernández-Fuertes M, Oliver N, Corma-Gómez A, Real LM, Pineda JA. Lower probability of persistence of total anti-SARS-CoV-2 antibodies after COVID-19 among people living with HIV. Clin Microbiol Infect. 2022;28:755–6. doi:10.1016/j.cmi.2022.01.028. PMID: 35150883.
- Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, San JE, Cromer D, Scheepers C, Amoak DG, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602(7898):654–6. doi:10.1038/s41586-021-04387-1. PMID: 35016196.
- Yu J, Collier AY, Rowe M, Mardas F, Ventura JD, Wan H, Miller J, Powers O, Chung B, Siamatu M, et al. Comparable neutralization of the SARS-CoV-2 Omicron BA.1 and BA.2 variants. medRxiv. 2022 (in press). doi:10.1101/2022.02.06.22270533. PMID: 35169817.
- Cheng SS, Mok CK, Li JK, Ng SS, Lam BH, Jeevan T, Kandeil A, Pekosz A, Chan KC, Tsang LC, et al. Plaque-neutralizing antibody to BA.2.12.1, BA.4 and BA.5 in individuals with three doses of BioNTech or CoronaVac vaccines, natural infection and breakthrough infection. J Clin Virol. 2022;156:105273. doi:10.1016/j.jcv.2022.105273. PMID: 36081282.
- Istituto Superiore di Sanità; Epicentro - Epidemiology for Public Health. National COVID-19 vaccination plan. [accessed 2023 Mar 26]. https://www.epicentro.iss.it/en/vaccines/covid-19-vaccination-plan.
- Agenzia Italiana del Farmaco. Comirnaty-BioNTech/Pfizer. [accessed 26 Mar 2023]. https://www.aifa.gov.it/comirnaty.
- Domnich A, De Pace V, Pennati BM, Caligiuri P, Varesano S, Bruzzone B, Orsi A. Evaluation of extraction-free RT-qPCR methods for SARS-CoV-2 diagnostics. Arch Virol. 2021;166(10):2825–8. doi:10.1007/s00705-021-05165-0. PMID: 34302551.
- Ponticelli D, Madotto F, Conti S, Antonazzo IC, Vitale A, Della Ragione G, Romano ML, Borrelli M, Schiavone B, Polosa R, et al. Response to BNT162b2 mRNA COVID-19 vaccine among healthcare workers in Italy: a 3-month follow-up. Intern Emerg Med. 2022;17(2):481–6. doi:10.1007/s11739-021-02857-y. PMID: 34637084.
- Jalkanen P, Kolehmainen P, Haveri A, Huttunen M, Laine L, Österlund P, Tähtinen PA, Ivaska L, Maljanen S, Reinholm A, et al. Vaccine-induced antibody responses against SARS-CoV-2 variants-of-concern six months after the BNT162b2 COVID-19 mRNA vaccination. Microbiol Spectr. 2022;10(2):e0225221. doi:10.1128/spectrum.02252-21. PMID: 35262410.
- Olariu TR, Ursoniu S, Marincu I, Lupu MA. Dynamics of antibody response to BNT162b2 mRNA COVID-19 vaccine: a 7-month follow-up study. Medicina (Kaunas). 2021;57(12):1330. doi:10.3390/medicina57121330. PMID: X.
- Infantino M, Manfredi M, Stacchini L, Cosma C, Grossi V, Lari B, Russo E, Amedei A, Benucci M, Veneziani F, et al. The role of neutralizing antibodies by sVNT after two doses of BNT162b2 mRNA vaccine in a cohort of Italian healthcare workers. Clin Chem Lab Med. 2022;60(6):934–40. doi:10.3390/medicina57121330. PMID: 34946275.
- Coppeta L, Ferrari C, Somma G, Mazza A, D’Ancona U, Marcuccilli F, Grelli S, Aurilio MT, Pietroiusti A, Magrini A, et al. Reduced titers of circulating anti-SARS-CoV-2 antibodies and risk of COVID-19 infection in healthcare workers during the nine months after immunization with the BNT162b2 mRNA vaccine. Vaccines (Basel). 2022;10(2):141. doi:10.3390/vaccines10020141. PMID: 35214600.
- Cocomazzi G, Piazzolla V, Squillante MM, Antinucci S, Giambra V, Giuliani F, Maiorana A, Serra N, Mangia A. Early serological response to BNT162b2 mRNA vaccine in healthcare workers. Vaccines (Basel). 2021;9(8):913. doi:10.3390/vaccines10020141. PMID: 35214600.
- Bonnet B, Chabrolles H, Archimbaud C, Brebion A, Cosme J, Dutheil F, Lambert C, Junda M, Mirand A, Ollier A, et al. Decline of humoral and cellular immune responses against SARS-CoV-2 6 months after full BNT162b2 vaccination in hospital healthcare workers. Front Immunol. 2022;13:842912. doi:10.3389/fimmu.2022.842912. PMID: 35309363.
- Stellini R, Gianello R, Gomarasca W. Durability of anti-spike antibodies after vaccination with mRNA SARS-CoV-2 vaccine is longer in subjects with previous infection: could the booster dose be delayed? Infection. 2022;50(6):1573–7. doi:10.1007/s15010-022-01816-9. PMID: 35391649.
- Isgrò MA, Trillò G, Russo L, Tornesello AL, Buonaguro L, Tornesello ML, Miscio L, Normanno N, Bianchi AAM, Buonaguro FM, et al. Bimodal antibody-titer decline following BNT162b2 mRNA anti-SARS-CoV-2 vaccination in healthcare workers of the INT – IRCCS “Fondazione Pascale” cancer center (Naples, Italy). Infect Agent Cancer. 2022;17(1):40. doi:10.1186/s13027-022-00451-1. PMID: 35902961.
- Hussein K, Dabaja-Younis H, Szwarcwort-Cohen M, Almog R, Leiba R, Weissman A, Mekel M, Hyams G, Horowitz NA, Gepstein V, et al. Third BNT162b2 vaccine booster dose against SARS-CoV-2-induced antibody response among healthcare workers. Vaccines (Basel). 2022;10(10):1741. doi:10.3390/vaccines10101741. PMID: 36298606.
- Redjoul R, Le Bouter A, Beckerich F, Fourati S, Maury S. Antibody response after second BNT162b2 dose in allogeneic HSCT recipients. Lancet. 2021;398(10297):298–9. doi:10.1016/S0140-6736(21)01594-4. PMID: 34270933.
- Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–2. doi:10.1056/NEJMc2108861. PMID: 34161700.
- Falsey AR, Frenck RW Jr, Walsh EE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Bailey R, Swanson KA, Xu X, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med. 2021;385(17):1627–9. doi:10.1056/NEJMc2113468. PMID: 34525276.
- Desmecht S, Tashkeev A, El Moussaoui M, Marechal N, Perée H, Tokunaga Y, Fombellida-Lopez C, Polese B, Legrand C, Wéry M, et al. Kinetics and persistence of the cellular and humoral immune responses to BNT162b2 mRNA vaccine in SARS-CoV-2-naive and -experienced subjects: impact of booster dose and breakthrough infections. Front Immunol. 2022;13:863554. doi:10.3389/fimmu.2022.863554. PMID: 35711445.
- Pani A, Cento V, Vismara C, Campisi D, Di Ruscio F, Romandini A, Senatore M, Schenardi PA, Gagliardi OM, Giroldi S, et al. Results of the RENAISSANCE study: REsponse to BNT162b2 COVID-19 vacciNe—short- and long-term immune reSponse evAluation in health care workErs. Mayo Clinic Proc. 2021;96(12):2966–79. doi:10.1016/j.mayocp.2021.08.013. PMID: 34736776.
- Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J, Olsho LEW, Caban-Martinez AJ, Fowlkes AL, Lutrick K, et al. Prevention and attenuation of covid-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med. 2021;385(4):320–9. doi:10.1056/NEJMoa2107058. PMID: 34192428.
- Della Valle P, Fabbri M, Madotto F, Ferrara P, Cozzolino P, Calabretto E, D’Orso MI, Longhi E, Polosa R, Riva MA, et al. Occupational exposure in the Lombardy Region (Italy) to SARS-CoV-2 infection: results from the MUSTANG–OCCUPATION–COVID-19 study. Int J Environ Res Public Health. 2021;18(5):2567. doi:10.3390/ijerph18052567. PMID: 33806578.
- De Pace V, Bruzzone B, Orsi A, Ricucci V, Domnich A, Guarona G, Randazzo N, Stefanelli F, Battolla E, Dusi PA, et al. Comparative analysis of five multiplex RT-PCR assays in the screening of SARS-CoV-2 variants. Microorganisms. 2022;10(2):306. doi:10.3390/microorganisms10020306. PMID: 35208761.
- Ricucci V, De Pace V, Domnich A, Orsi A, Icardi G, Bruzzone B. Fast and presumptive diagnosis of SARS-CoV-2 Omicron variants vs Delta. BJSTR. 2022;42(3). doi:10.26717/BJSTR.2022.42.006793. PMID: Not applicable.
- Nemet I, Kliker L, Lustig Y, Zuckerman N, Erster O, Cohen C, Kreiss Y, Alroy-Preis S, Regev-Yochay G, Mendelson E, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 Omicron infection. N Engl J Med. 2022;386(5):492–4. doi:10.1056/NEJMc2119358. PMID: 34965337.
- Abu Jabal K, Ben-Amram H, Beiruti K, Batheesh Y, Sussan C, Zarka S, Edelstein M. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26(6):210009. doi:10.2807/1560-7917.ES.2021.26.6.2100096. PMID: 33573712.
