ABSTRACT
Recently, several novel medications, such as Ustekinumab, Infliximab, and Vedolizumab, have emerged as potential options for inflammatory bowel disease(IBD) management. Despite achieving some effects in clinical applications, these therapies are still plagued by inadequate response rates and adverse side effects. With rapid progress in immunological research, therapeutic vaccines are gaining traction as an alternative. These vaccines aim to activate the body’s immune system to generate specific antibodies, thereby offering a potential avenue for treating IBD. The efficacy and safety of vaccines, coupled with their potential to mitigate the financial and healthcare burden associated with disease treatment, render therapeutic vaccines a more favorable approach for managing patients with IBD. In this review, we critically examine the existing literature pertaining to therapeutic vaccines for IBD, aiming to offer researchers a comprehensive understanding of their applications and prospects and stimulate novel vaccine development by presenting innovative ideas in this field.
Introduction
Inflammatory bowel disease (IBD) is a group of chronic nonspecific inflammatory bowel diseases, including ulcerative colitis (UC) and Crohn ‘s disease (CD). For a long time, IBD has been mainly prevalent in Western countries such as North America, Europe, and Oceania, and its incidence has been increasing year by year. However, in recent years, IBD has also begun to show a gradual upward trend in Asia, South Africa and other places. Taking China as an example, from 1990 to 2019, the age-standardized incidence rate per 100,000 people increased from 1.47 to 3.01. Considering that nearly three-quarters of the world ‘s population lives in developing countries, although the incidence of these regions is relatively low, it may cause serious harm to the country in the long run.Citation1–4 The etiology of IBD is still unclear, but it is believed to be related to the interaction of multiple factors, such as genetics, environment, immunity and intestinal flora.
The current clinical treatment of IBD is mainly through drug therapy to control symptoms, if necessary, surgical treatment can also be used. The drugs commonly used in the treatment of IBD mainly include aminosalicylic acids, corticosteroids (CS), immunomodulators and biological agents. The emergence of biological agents provides a better treatment for refractory IBD and completely changes the therapeutic effect of IBD.Citation5,Citation6 In addition, some studies have shown that diet and psychology also seem to be beneficial for the treatment of IBD.Citation7,Citation8 However, due to the complexity of IBD, both biological therapy and surgical therapy have certain defects. Although biotherapy is effective in a variety of indicators of IBD, there are still a considerable number of patients who do not respond or lose response over time, which means that patients need to increase the dose or repeated treatment.Citation9 A large number of studies have shown that biological agents may also cause a series of infusion reactions, such as measles, nausea and vomiting, dyspnea, etc., which need to be stopped immediately.Citation10,Citation11 In addition, biological products commonly used in clinical practice are drugs with high treatment costs, which require long-term repeated use by patients. This has brought an increasingly heavy burden to the world to a certain extent, and it is also a major challenge for health care systems around the world. For some patients with ineffective drug treatment or serious complications, surgical treatment can be taken. IBD is a chronic wasting disease, which can cause sequelae such as anemia, malnutrition and weakened immunity, and increase the risk of postoperative adverse outcomes. Although laparoscopic surgery has progressed, the complexity and challenge of surgery still limit its wide application, and there is still a risk of recurrence after surgery. Some patients may still need to switch to open surgery.Citation12 And research shows that, the risk of postoperative anxiety and depressive symptoms is increased in IBD patients.Citation13 In addition, short bowel syndrome and intestinal failure may also occur due to the frequent bowel resection.Citation14 Therefore, it is urgent to further study and explore new strategies for IBD treatment.
In recent years, research on IBD therapeutic vaccines has emerged and attracted more and more researchers’ attention. As a potential therapeutic method, vaccines mainly enhance resistance by stimulating the human immune system, inducing specific humoral and cellular immune responses. It has been reported that the vaccine is generally safe and has strong supporting evidence.Citation15 Vaccines not only protect vaccinators from disease, promote normal life and work, and improve economic productivity, but also increase the workload in the field of health care and improve service quality.Citation16 Studies have pointed out that vaccines may positively affect antibiotic-resistant infections, so it is necessary to pay more attention to vaccine development and application to solve the problem of antibiotic resistance.Citation17 According to its mechanism of action, vaccines can be divided into therapeutic vaccines and preventive vaccines. Preventive vaccines are used to help establish immunity to certain diseases and strengthen the immune barrier of patients. Different from prophylactic vaccines, therapeutic vaccines can induce specific immune responses in diseased individuals and eliminate pathogens or abnormal cells for the treatment of clinical patients. The targeting of therapeutic vaccines can help patients recover more comprehensively, reduce repeated medications and surgeries, reduce treatment costs and burdens, and provide long-term protection. At present, therapeutic vaccines have been widely studied and used in cancer, autoimmune diseases and infectious diseases, and are expected to be further developed in the field of IBD.Citation18,Citation19
In conclusion, the development of therapeutic vaccines for IBD may be a better choice to alleviate intestinal inflammation and improve the clinical symptoms of patients, and has good development potential in the future. This review aims to summarize the main pathogenesis of IBD, and to find and read the relevant literature on IBD therapeutic vaccines from the perspectives of cytokines, intestinal flora and angiogenesis, and to review the main research progress.
Materials and methods
A comprehensive search was conducted in PubMed and Web of Science databases using the keywords of “Inflammatory Bowel Disease*” AND “Vaccine* OR Vaccination*” AND “therapy* OR treat* OR cure* OR remedy*.” The results included all relevant clinical trials, original articles, retrospective studies, and systematic reviews. Both animal and human studies were considered, while unfinished or unpublished publications were excluded. All authors carefully screened the titles and abstracts of the retrieved papers to assess their eligibility for inclusion in the review. Additionally, all references were examined to gather background information and identify potentially pertinent articles.
Results
Major pathogenesis of IBD
So far, the etiology of IBD is not clear. It may be related to the interaction of multiple factors, among which intestinal flora disorder and intestinal immune imbalance are the key to its pathogenesis.
It has been proposed that the effects of ecosystem changes in the gut microbiota may play a key role in the mechanisms of disease development, such as UC.Citation20 When intestinal homeostasis is disrupted, the host immune system is over-activated, leading to intestinal inflammation, and the proportion of harmful bacteria in the gut is increased, which disrupts intestinal epithelial cells and increases intestinal permeability, leading to immune dysfunction.Citation21,Citation22 At the same time, epithelial cells and damaged intestinal mucosa that have been damaged by microorganisms are more susceptible to bacteria, stimulating the pathogenicity of many commensal bacteria, causing a number of compensatory immune responses in the gut, and aggravating inflammation.Citation23
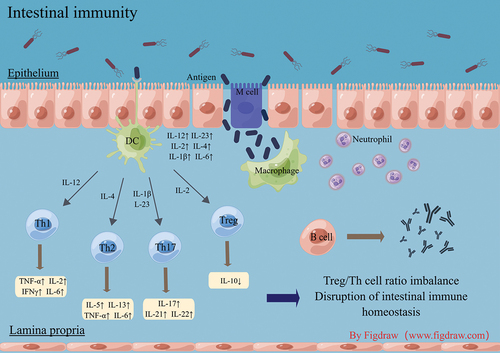
In addition to the effects of intestinal flora dysbiosis on the gut of IBD patients, immune imbalances in the gut can also cause excessive inflammation and lead to intestinal damage. The immune response to infections in IBD patients is complex, involving multiple interconnected interactions similar to a network ().
In people with IBD, things like environmental factors or infections can damage and change the protective lining of the intestines. This can make the intestines more permeable, letting harmful substances pass through. Once the pathogenic antigens enter the intestinal mucosa, macrophages are first activated to play a protective role for the organism and directly engulf the pathogenic antigens. It has been shown that CD14 macrophages predominate in the inflamed mucosa of CD patients and that this class of macrophages in the intestinal mucosa of IBD patients is capable of producing high levels of IL-12 and IL-23 in response to microbial stimulation, thereby promoting inflammation.Citation24 In addition, TNF-α-producing macrophages have also been shown to reduce epithelial barrier resistance by disrupting the structure and function of tight junctions, thereby disrupting intestinal barrier function, increasing pathogen invasion, and exacerbating the inflammatory response.Citation25 Once macrophages cannot continue to fully defend against pathogens, they recruit neutrophils through their surface messenger proteins to form a bactericidal network. When the inflammatory response reaches a certain level, dendritic cells can sense the presence of pathogenic antigens, secrete cytokines such as IL-12, IL-2, and IL-6, and present antigens to T and B cells across the intestinal lamina propria to complete the body’s next immune response.
Therapeutic vaccines of IBD
Vaccines targeting cytokines
Cytokines are important messengers that oversee and control the body’s immune and inflammatory reactions. Significant changes in the expression levels of cytokines, including TNF-α, IL-1β, IL-10, IL-6, IL-8, and IL-12, can be found in the intestinal mucosa of IBD patients.Citation26 These cytokines can cause damage and apoptosis of intestinal mucosal epithelial cells, resulting in impaired intestinal barrier function and allowing intestinal flora to easily cross the intestinal mucosa into the bloodstream and cause systemic inflammatory responses.Citation27 In addition, cytokines can affect the differentiation and function of immune cells, causing the immune system to attack its own tissues and exacerbate IBD. Because of this link between cytokines and IBD, the use of vaccines to modulate the abnormal expression of endogenous cytokines has emerged as a new therapeutic strategy for the long-term management of the disease. The main cytokine therapeutic vaccines currently under investigation are the TNF-α vaccine and the interleukin vaccine.
IBD and TNF vaccine
Tumor necrosis factor-α (TNF-α) is mainly secreted by mononuclear macrophages, Th1/Th2 cells, increases the expression of cytokines such as IL-1β, IL-6, and IL-33, activates the JNK signaling pathway, and therefore plays an important role in the pathogenesis of IBD.Citation28 TNF-α is one of the major effectors of inflammation in IBD and can regulate the transcription of TJs proteins to alter intestinal permeability and induce apoptosis in intestinal epithelial cells.Citation29 Chong He et al. found that induction of TNF-α can promote intestinal mucosal inflammation in IBD patients.Citation30 Therefore, researchers have proposed that antibodies targeting TNF-α can be used to treat IBD, and good results have been achieved in clinical work.Citation31,Citation32 And current clinical practice guidelines also recommend anti-TNF-α drugs for induction and maintenance of remission in patients with moderately to severely active IBD.Citation33
However, recent studies have shown that anti-TNF-α monoclonal antibodies require repeated long-term use, are costly to treat, and are prone to hypersensitivity reactions.Citation34 Using this medication is connected to a higher chance of patients with IBD getting various harmful infections. So, before starting TNF-α inhibitor therapy, patients must be screened for potential reactivation of infections such as tuberculosis and hepatitis B and the risk of opportunistic infections such as EBV, cytomegalovirus, and Clostridium difficile infections.Citation35 In contrast, kinin vaccines against endogenous TNF-α mainly produce anti-specific antibodies through active immunization, which can limit the incidence of treatment failure.Citation36 At present, a large number of studies have shown that TNF therapeutic vaccines have a significant effect in the treatment of arthritis and are expected to enter the clinic as soon as possible.Citation37–39 It is speculated that such vaccines may become a promising strategy for the treatment of autoimmune diseases.
It has been shown that a therapeutic vaccine against human TNF-α can be developed using TNF-α kinase (TNF-K), and by administering this vaccine to patients, the body can induce its own production of neutralizing antibodies against high titers of TNF-α to reduce the excessive inflammatory response in the gut of IBD patients and alleviate the associated clinical symptoms.Citation18 Additionally, researchers suggest creating multi-antigenic polypeptides (MAP) to make powerful antibodies and vaccines against specific peptides. These vaccines can be tailored for various multi-branched peptides by using the main chain of lysine from the a or e group. This forms the base, and the outer surface of this synthetic peptide carries multiple copies of the antigen.Citation40 Jun Z et al. found that TNF-α as an auto-component alone could not induce specific humoral immune responses, whereas the MAP strategy could significantly enhance its immunogenicity and induce specific polyclonal antibodies against TNF-α.Citation34 Yan S et al. also found in their study that the designed eight-branched MAP vaccine possessed good immunogenicity and could The eight-branched MAP vaccine was also found to possess good immunogenicity and could exert significant anti-UC effects in the organism.Citation40 Yang Wan and his team have found in unpublished studies that self-made TNF-α protein vaccine can overcome self-tolerance, induce specific neutralizing antibodies against TNF-α, and protect mice from acute and chronic colitis. These results may prove that therapeutic vaccines against TNF-α have a certain alleviating effect on the symptoms of IBD patients and have a certain safety profile, showing good prospects in the field of IBD treatment.
IBD and interleukin vaccine
Interleukins are a major component of the immune system and play an important role in the inflammatory response. Different types of interleukins have different biological functions to regulate immunity, promote or suppress inflammatory responses, and participate in cell proliferation and differentiation.Citation26 Therefore, depending on the different functions of different cytokines, the external administration of specific intervention factors to increase or decrease their levels in the gut of IBD patients can modulate the severity of the inflammatory response and thus modify the clinical symptoms of patients. Currently, the most commonly used drugs in the clinic against interleukins are human monoclonal antibodies with specific targeting effects, such as mepolizumab against IL-5, ixekizumab against IL-17A, and ustekinumab against IL-12 and IL-23.Citation41–43 However, some of these drugs are known to have short half-lives, require repeated, regular dosing, and are expensive, so other more viable alternatives need to be explored. In recent years, the efficacy and safety of interleukin therapeutic vaccines have been demonstrated in other areas. Some researchers have observed anti-inflammatory IL-1β or IL-23 peptide vaccines in their own experimental models of arthritis and have shown that anti-IL-1β vaccines can be used to treat type 2 diabetes.Citation18,Citation19 Therefore, the discovery of a therapeutic vaccine against intestinal overexpression of interleukins in patients with IBD to help the body produce its own counterpart antibodies to reduce the excessive inflammatory response is clearly a potentially better option and could provide long-term efficacy and reduce adverse effects.Citation44
In their study, Qingdong G et al. successfully developed an IL-12/IL-23p40 peptide-based vaccine that induced relatively long-lasting anti-IL-12, IL-23, and p40 antibodies, and through experiments concluded that administration of the IL-12/IL-23p40 vaccine was effective in ameliorating trinitrobenzene sulfonic acid (TNBS)-induced active intestinal inflammation and fibrosis.Citation45 The following year, they successfully developed a therapeutic anti-IL-18 vaccine that was shown to partially block IL-18-induced IFN-γ secretion and ameliorate colitis symptoms in mice in vivo.Citation44 Meanwhile, studies have shown that IL-1 vaccines are also indicated for the treatment of inflammatory diseases, and vaccination trials are ongoing.Citation46 Thus, interleukin vaccination for therapeutic effect in IBD patients with excessive intestinal inflammatory response may be an interesting direction for further research.
Vaccines targeting intestinal flora
The intestinal flora is a group of microbial communities that grow and multiply in the human gut and interact with it to perform a variety of important physiological functions, such as nutrient metabolism and immune regulation. Dysbiosis of the intestinal flora is closely associated with the development of many intestinal diseases, such as colitis, IBD, and even intestinal tumors. In IBD patients with intestinal dysbiosis, removal of the affected bowel segment is usually the first line of treatment in the absence of vaccines or other effective pharmacological treatments. However, even after 2/3 of the bowel segment has been removed, the remaining healthy bowel is usually re-involved, leading to disease recurrence.Citation47 Therefore, there is a need to develop an alternative therapy for this disordered flora infection. In recent years, researchers have begun to explore the use of gut microbiota vaccines for the treatment of IBD, i.e. the use of vaccines to stimulate the gut to produce antibodies that act with greater specificity and targeting on the relevant pathogenic microorganisms.
Mycobacterium avium subspecies paratuberculosis (MAP) is a dairy-transmitted pathogen and a major cause of disease progression in IBD.Citation47 Although there is controversy regarding the possible causal relationship between CD and MAP,Citation48 several clinical trials have shown that selective anti-MAP drugs have been evaluated as a potential treatment for CD and found to be beneficial in terms of final outcome. In a study by Agrawal G et al. a patient with CD could achieve profound mucosal healing after anti-MAP treatment alone.Citation49,Citation50 All these studies suggest that anti-MAP therapeutic vaccines are effective in patients with IBD and may be more widely used in the future. Failure of other therapeutic measures or poor patient response to other modalities are indications for anti-MAP therapy, especially in patients with IBD who have met the criteria for bowel resection; contraindications to anti-MAP therapy mainly include conditions such as allergy to any of the components or leukopenia.Citation48 Several studies have suggested that mucosal-derived Escherichia coli (E. coli) strains are involved in the pathogenesis of IBD, particularly the adherent invasive E. coli (AIEC) pathogen, suggesting that anti-E. coli vaccines may also ameliorate intestinal inflammation.Citation51,Citation52 One study found that a transgenic enterotoxin-producing E. coli vaccine could induce a significant immune response in the gut, improving E. coli flora dysbiosis and alleviating symptoms, and this vaccine was shown to be safe.Citation53
Flagellin, the major structural protein of the bacterial flagellum, is a potent immune activator and antigen that may play a role in patients with IBD.Citation54 It has been shown that flagellin can activate pro-inflammatory gene expression via TLR5 and NLRC4 inflammatory vesicles, which in turn leads to an enhanced inflammatory response, increased mucosal permeability, and worsened symptoms in the intestine of patients. Researchers led by Hao Q. Tran discovered that mice immunized with flagellin showed improvements in colitis-related features like epithelial cell damage, inflammation, and narrowed gut passages.Citation55 This suggests that antibodies produced naturally against flagellin could be used to treat chronic inflammatory diseases. Therefore, using flagellin vaccination to stimulate these specific antibodies might be an effective approach to address intestinal inflammation in IBD. Thus, flagellin, a structural protein of the pathogen, is found to be more effective in alleviating the main symptoms of IBD patients by inducing specific antibodies against flagellin through vaccination with the appropriate therapeutic vaccine.
Thus, therapeutic vaccines targeting the gut microbiome are safe and effective for the treatment of IBD, represent a promising new option for the treatment of IBD and are expected to be widely used in the future. However, research on gut microbiota vaccines for IBD is still in its early stages. Future research is needed to further explore the design and type of vaccine, the impact of vaccine-host interactions on improving the function of the dysfunctional gut microbiome, and to find more therapeutic vaccines that effectively target other pathogens.
Angiogenic vaccines
In the intestine, angiogenesis plays important physiological functions, such as regulating immune response, promoting tissue repair and maintaining homeostasis.Citation56 Angiogenesis can also maintain the inflammatory state by providing oxygen and nutrients, while helping immune cell migration, triggering excessive inflammatory response.Citation57,Citation58 A number of studies have shown that angiogenesis may be one of the causes of the incidence and persistence of IBD patients, so anti-angiogenesis therapy may become a major research direction in the future in the field of IBD treatment.Citation59,Citation60
VEGF is a key factor in angiogenesis. Several studies have shown increased VEGF expression in the intestine of patients with active IBD compared to the healthy population.Citation58,Citation61 It has been shown that in a dextran sodium sulfate (DSS)-induced colitis model, there is an increase in vascular density and an exacerbation of the typical histopathological manifestations of inflammation, resulting in histological damage to the intestinal mucosa.Citation62 Drugs that block VEGF-A signal transduction may reduce intestinal inflammation in IBD patients.Citation63 Studies indicate that the primary drugs aimed at VEGF or its related pathways are monoclonal antibodies and small molecule receptors, however, these treatments have short-lived effects and are not fully effective for the current IBD patients.Citation64 This highlights the pressing need to explore alternative therapies with better practicality. Elina S et al. found that EMMPRIN protein can induce VEGF expression, which is pro-angiogenic, and that in a DSS-induced colitis model, anti-EMMPRIN protein vaccination can target damaged bowel segments and reduce angiogenesis to alleviate excessive inflammatory responses.Citation65 In addition, angiopoietin has a key function in angiogenesis, and its aberrant activation may depend on the surrounding inflammatory environment. It has been proposed as a factor in maintaining pathological angiogenesis during the development of IBD and as a target for antiangiogenic therapeutic vaccination in the future.Citation66 There are few studies on therapeutic vaccines for IBD angiogenesis, but this type of vaccination can reduce the severity of the disease and has great potential for future development in the field of IBD treatment. Its safety and efficacy deserve further exploration and investigation.
Parasite vaccine and IBD
In recent years, harnessing the immunomodulatory effects of worms and their products for the treatment of allergic diseases, autoimmune diseases, and IBD has become a popular direction of research. Many studies have shown that worms have a powerful immunomodulatory ability to reduce the intensity of the inflammatory response and improve the clinical symptoms of autoimmune diseases by producing a large number of immunomodulatory molecules.Citation67,Citation68
Summers RW et al. found that vaccination of patients with IBD with porcine whipworm eggs downregulated abnormal intestinal inflammation and was safe.Citation69 However, a meta-analysis of the treatment of IBD with porcine whipworm eggs found no statistical benefit, while Jürgen et al. also found no evidence of a beneficial effect of porcine whipworm therapy in inducing symptomatic relief in patients with active IBD in a randomized, double-blind, placebo-controlled trial.Citation70,Citation71 In 2019, Capron M et al. found that P28 glutathione S-transferase (P28GST), a protein derived from Schistosoma haematobium, could be modified into a therapeutic vaccine that effectively alleviated the intestinal inflammatory response and was also shown to be well tolerated in patients with CD.Citation72 Previous studies have also supported the conclusion that the P28GST protein can down-regulate intestinal inflammation.Citation73
Overall, these studies indicate that vaccines targeting parasites could offer improved therapeutic benefits for IBD patients by partially relieving associated clinical symptoms. These vaccines have demonstrated good safety records, suggesting significant potential for IBD treatment advancement. Exploring other innovative approaches might be considered in the future.
Discussion
In recent years, the global incidence of IBD has continued to rise. However, there are many limitations in the current clinical methods used to combat IBD, including high treatment costs, potential infusion reactions, and other surgery-related sequelae. Therefore, it is necessary to seek safer and more effective treatment methods, which is essential to improve the therapeutic effect of IBD and improve the quality of life of patients. In this context, IBD therapeutic vaccine has attracted much attention as an emerging treatment method. This vaccine mainly plays a therapeutic role by regulating intestinal immune response and reducing excessive inflammation. The treatment cost is low, the safety is high,Citation74 the curative effect is remarkable, and it can also provide patients with more targeted treatment, which is of great value in clinical and economic aspects. Current research shows that for patients with autoimmune diseases, therapeutic vaccines can achieve long-term clinical improvement through specific immunotherapy, avoiding the use of other immunosuppressants.Citation75–77 In view of IBD as a typical autoimmune disease,Citation78 this makes therapeutic vaccines have significant potential in the field of IBD, or will become an important treatment strategy in this field. At present, IBD therapeutic vaccines include anti-TNF-α vaccine, anti-VEGF vaccine, anti-IL-12/IL-23p40 peptide vaccine, anti-MAP vaccine and E. coli vaccine (). Studies have shown that these vaccine types can improve intestinal inflammation and show good development potential. The anti-inflammatory effects of some vaccines have been confirmed in clinical trials in other disease fields.Citation18,Citation19
Table 1. Summary of IBD therapeutic vaccines.
In addition to the above-mentioned therapeutic vaccine types, there are many other vaccine therapeutic targets with great development potential that deserve further exploration. For example, a study in the field of gut microbiota found that a recombinant lipoprotein-based vaccine against C. difficile infection can improve intestinal inflammation.Citation79 In addition, for the immune imbalance in the intestine, a study on anti-TGF-β1 vaccines found that the weight of vaccinated mice was increased and colon inflammation was also improved, indicating that vaccines against TGF-β1 cytokines can also play a role in IBD treatment.Citation80 At the same time, inflammatory factors such as IL-1, IL-17 and IL-4 are closely related to the pathogenesis of IBD.In the next five years, they may become potential therapeutic vaccine targets. The in-depth excavation and expansion of these targets will bring new exploration directions in the field of IBD.
However, most of the current research on IBD therapeutic vaccines is still in its infancy, and the clinical scientific research related to it is relatively limited, and it has not really entered the stage of clinical trials. At the same time, the safety and efficacy of these therapeutic vaccines are still not fully supported by experimental data, and there are still a series of problems and doubts in this field. Even some research hypotheses may be wrong and need further verification and in-depth discussion. In the selection of appropriate therapeutic vaccine targets, for IBD patients, it is necessary to have an in-depth understanding of the multiple biological mechanisms of the disease, while fully considering the individual differences between different patients. This work is quite complex, and the role of the vaccine is difficult to be quickly lifted. Therefore, further research on therapeutic vaccines in the future requires interdisciplinary collaboration and large-scale clinical trials.
Conclusion
There are various methods for the treatment of IBD, but each method has certain limitations. Therefore, there is an urgent need to adopt comprehensive solutions, including both interventions to prevent IBD and innovative treatments. In recent years, with the deepening of research, therapeutic vaccines have gradually attracted people’s attention. At present, significant progress has been made in the fields of tumors and autoimmune diseases, and great potential and prospects have also been shown in the treatment of IBD. The next step is to further verify the safety and effectiveness of therapeutic vaccines through clinical trials, explore more vaccine targets, develop more personalized treatment plans for IBD patients, and improve clinical treatment effects. We hope that future efforts can promote the vigorous development of IBD therapeutic vaccines and open up new possibilities.
Author contributions
Yafei Liu and Fei Liao provided the idea for the study, Yafei Liu conducted the literature search. Yafei Liu wrote the original draft and contributed critical reviews, Fei Liao made revisions. All authors read and approved the final version of the manuscript and agreed with its submission for publication.
Grant support
All the figures in the manuscript were drawn in Figdraw.
Acknowledgments
The authors would like to thank the editors and the anonymous reviewers for their valuable comments and suggestions to improve the quality of the paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alatab S, Sepanlou SG, Ikuta K, Vahedi H, Bisignano C, Safiri S, Sadeghi A, Nixon MR, Abdoli A, Abolhassani H, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):17–9. doi:10.1016/S2468-1253(19)30333-4.
- Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769–78. doi:10.1016/S0140-6736(17)32448-0.
- Zhang Y, Liu J, Han X, Jiang H, Zhang L, Hu J, Shi L, Li J. Long-term trends in the burden of inflammatory bowel disease in China over three decades: a joinpoint regression and age-period-cohort analysis based on GBD 2019. Front Public Health. 2022;10:994619. doi:10.3389/fpubh.2022.994619.
- Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18(1):56–66. doi:10.1038/s41575-020-00360-x.
- Cai Z, Wang S, Li J. Treatment of inflammatory bowel disease: a comprehensive review. Front Med. 2021;8:765474. doi:10.3389/fmed.2021.765474.
- Mao R, Hu P-J. The future of IBD therapy: where are we and where should we go next? Digest Dis. 2016;34(1–2):175–9. doi:10.1159/000443135.
- Jiang Y, Jarr K, Layton C, Gardner CD, Ashouri JF, Abreu MT, Sinha SR. Therapeutic implications of diet in inflammatory bowel disease and related immune-mediated inflammatory diseases. Nutrients. 2021;13(3):890. doi:10.3390/nu13030890.
- Mikocka-Walus A, Prady SL, Pollok J, Esterman AJ, Gordon AL, Knowles S, Andrews JM. Adjuvant therapy with antidepressants for the management of inflammatory bowel disease. Cochrane Database Syst Rev. 2019;4(4):CD012680. doi:10.1002/14651858.CD012680.pub2.
- Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Official Am J Gastroentero. 2011;106(4):685. doi:10.1038/ajg.2011.103.
- Lichtenstein L, Ron Y, Kivity S, Ben-Horin S, Israeli E, Fraser GM, Dotan I, Chowers Y, Confino-Cohen R, Weiss B. Infliximab-related infusion reactions: systematic review. J Crohns Colitis. 2015;9(9):806–15. doi:10.1093/ecco-jcc/jjv096.
- Conrad MA, Stein RE, Maxwell EC, Albenberg L, Baldassano RN, Dawany N, Grossman AB, Mamula P, Piccoli DA, Kelsen JR. Vedolizumab therapy in severe pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2016;22(10):2425–31. doi:10.1097/MIB.0000000000000918.
- Holder-Murray J, Marsicovetere P, Holubar SD. Minimally invasive surgery for inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(6):1443–58. doi:10.1097/MIB.0000000000000316.
- Zangenberg MS, El-Hussuna A. Psychiatric morbidity after surgery for inflammatory bowel disease: a systematic review. World J Gastroenterol. 2017;23(48):8651–9. doi:10.3748/wjg.v23.i48.8651.
- Fuglestad MA, Thompson JS. Inflammatory bowel disease and short bowel syndrome. Surg Clin North Am. 2019;99(6):1209–21. doi:10.1016/j.suc.2019.08.010.
- Dudley MZ, Halsey NA, Omer SB, Orenstein WA, O’Leary ST, Limaye RJ, Salmon DA. The state of vaccine safety science: systematic reviews of the evidence. Lancet Infect Dis. 2020;20(5):e80–e9. doi:10.1016/S1473-3099(20)30130-4.
- Doherty M, Buchy P, Standaert B, Giaquinto C, Prado-Cohrs D. Vaccine impact: benefits for human health. Vaccine. 2016;34(52):6707–14. doi:10.1016/j.vaccine.2016.10.025.
- Klugman KP, Black S. Impact of existing vaccines in reducing antibiotic resistance: primary and secondary effects. Proc Natl Acad Sci USA. 2018;115(51):12896–901. doi:10.1073/pnas.1721095115.
- Semerano L, Assier E, Boissier M-C. Anti-cytokine vaccination: a new biotherapy of autoimmunity? Autoimmun Rev. 2012;11(11):785–6. doi:10.1016/j.autrev.2012.02.003.
- Spohn G, Schori C, Keller I, Sladko K, Sina C, Guler R, Schwarz K, Johansen P, Jennings GT, Bachmann MF. Preclinical efficacy and safety of an anti-IL-1β vaccine for the treatment of type 2 diabetes. Mol Ther Methods Clin Dev. 2014;1. doi:10.1038/mtm.2014.48.
- Matsuoka K. Fecal microbiota transplantation for ulcerative colitis. Immunol Med. 2021;44(1):30–4. doi:10.1080/25785826.2020.1792040.
- Lobionda S, Sittipo P, Kwon HY, Lee YK. The role of gut microbiota in intestinal inflammation with respect to diet and extrinsic stressors. Microorganisms. 2019;7(8):271. doi:10.3390/microorganisms7080271.
- Shen Z-H, Zhu C-X, Quan Y-S, Yang Z-Y, Wu S, Luo W-W, Tan B, Wang X-Y. Relationship between intestinal microbiota and ulcerative colitis: mechanisms and clinical application of probiotics and fecal microbiota transplantation. WJG. 2018;24(1):5–14. doi:10.3748/wjg.v24.i1.5.
- Chen Y, Cui W, Li X, Yang H. Interaction between commensal bacteria, immune response and the intestinal barrier in inflammatory bowel disease. Front Immunol. 2021 April 18;12. doi:10.3389/fimmu.2021.761981.
- Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. 2019;2019:1–16. doi:10.1155/2019/7247238.
- Lissner D, Schumann M, Batra A, Kredel LI, Kühl AA, Erben U, May C, Schulzke JD, Siegmund B. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm Bowel Dis. 2015 Jun;21(6):1297–305. doi:10.1097/MIB.0000000000000384.
- Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014 May;14(5):329–42. doi:10.1038/nri3661.
- Agirman G, Yu KB, Hsiao EY. Signaling inflammation across the gut-brain axis. Science. 2021 Nov 26;374(6571):1087–92. doi:10.1126/science.abi6087.
- Lee SH, Eun Kwon J, Cho M-L. Immunological pathogenesis of inflammatory bowel disease. Intest Res. 2018;16(1):26. doi:10.5217/ir.2018.16.1.26.
- Michielan A, D’Incà R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm. 2015;2015:1–0. doi:10.1155/2015/628157.
- He C, Shi Y, Wu R, Sun M, Fang L, Wu W, Liu C, Tang M, Li Z, Wang P, et al. miR-301a promotes intestinal mucosal inflammation through induction of IL-17A and TNF-α in IBD. Gut. 2016;65(12):1938–50. doi:10.1136/gutjnl-2015-309389.
- Pouillon L, Bossuyt P, Peyrin-Biroulet L. Considerations, challenges and future of anti-TNF therapy in treating inflammatory bowel disease. Expert Opin Biol Ther. 2016;16(10):1277–90. doi:10.1080/14712598.2016.1203897.
- Magro F, Portela F. Management of inflammatory bowel disease with infliximab and other anti-tumor necrosis factor alpha therapies. BioDrugs. 2010;24(Suppl 1):3–14. doi:10.2165/11586290-000000000-00000.
- Vulliemoz M, Brand S, Juillerat P, Mottet C, Ben-Horin S, Michetti P. On behalf of Swiss IBDnet, an official working group of the Swiss society of Gastroenterology. TNF-Alpha blockers in inflammatory bowel diseases: practical recommendations and a user’s guide: an update. Digestion. 2020;101 Suppl 1:16–26. doi:10.1159/000506898.
- Zhang J, Cui Y, Wu Y, Wang H, Ke J. Prediction and identification of B-cell epitopes for tumor necrosis factor-α. Mol Med Rep. 2017;16(3):3439–44. doi:10.3892/mmr.2017.7007.
- Murdaca G, Negrini S, Pellecchio M, Greco M, Schiavi C, Giusti F, Puppo F. Update upon the infection risk in patients receiving TNF alpha inhibitors. Expert Opin Drug Saf. 2019;18(3):219–29. doi:10.1080/14740338.2019.1577817.
- Le Buanec H, Delavallée L, Bessis N, Paturance S, Bizzini B, Gallo R, Zagury D, Boissier M-C. Tnfα kinoid vaccination-induced neutralizing antibodies to TNFα protect mice from autologous TNFα-driven chronic and acute inflammation. Proc Natl Acad Sci USA. 2006;103(51):19442–7. doi:10.1073/pnas.0604827103.
- Collison J. Rheumatoid arthritis: paving the way for TNF vaccines. Nat Rev Rheumatol. 2016;12(12):692. doi:10.1038/nrrheum.2016.171.
- Zhang L, Wang J, Xu A, Zhong C, Lu W, Deng L, Li R. A rationally designed TNF-α epitope-scaffold Immunogen induces sustained antibody response and alleviates collagen-induced arthritis in mice. PloS One. 2016;11(9):e0163080. doi:10.1371/journal.pone.0163080.
- Durez P, Vandepapeliere P, Miranda P, Toncheva A, Berman A, Kehler T, Mociran E, Fautrel B, Mariette X, Dhellin O, et al. Therapeutic vaccination with TNF-Kinoid in TNF antagonist-resistant rheumatoid arthritis: a phase II randomized, controlled clinical trial. PloS One. 2014;9(12):e113465. doi:10.1371/journal.pone.0113465.
- Sun Y, Pan W, Zhang J, Cui Y, Wang H, Ru G, Chen L. Complex TNF-α B cell epitope MAP vaccine alleviates murine ulcerative colitis. Int J Mol Med. 2019 Sep;44(3):1106–16. doi:10.3892/ijmm.2019.4271.
- Almradi A, Hanzel J, Sedano R, Parker CE, Feagan BG, Ma C, Jairath V. Clinical trials of IL-12/IL-23 inhibitors in inflammatory bowel disease. BioDrugs. 2020;34(6):713–21. doi:10.1007/s40259-020-00451-w.
- Yanagibashi T, Satoh M, Nagai Y, Koike M, Takatsu K. Allergic diseases: from bench to clinic - contribution of the discovery of interleukin-5. Cytokine. 2017;98:59–70. doi:10.1016/j.cyto.2016.11.011.
- Hohenberger M, Cardwell LA, Oussedik E, Feldman SR. Interleukin-17 inhibition: role in psoriasis and inflammatory bowel disease. J Dermatolog Treat. 2018;29(1):13–18. doi:10.1080/09546634.2017.1329511.
- Guan Q, Warrington R, Moreno S, Qing G, Weiss C, Peng Z. Sustained suppression of IL-18 by employing a vaccine ameliorates intestinal inflammation in TNBS-induced murine colitis. Future Sci OA. 2019;5(7):FSO405. doi:10.2144/fsoa-2018-0125.
- Guan Q, Weiss CR, Wang S, Qing G, Yang X, Warrington RJ, Bernstein CN, Peng Z. Reversing ongoing chronic intestinal inflammation and fibrosis by sustained block of IL-12 and IL-23 using a vaccine in mice. Inflamm Bowel Dis. 2018;24(9):1941–52. doi:10.1093/ibd/izy142.
- Assier E, Bessis N, Zagury JF, Boissier MC. IL-1 vaccination is suitable for treating inflammatory diseases. Front Pharmacol. 2017 Jan 31;8:6. doi:10.3389/fphar.2017.00006.
- Srivastava V, Navabharath M, Gupta S, Singh SV, Ahmad S. Exploration of Solanum xanthocarpum Schrad. & Wendl. against mycobacterium avium subspecies paratuberculosis and assessment of its immunomodulatory and anti-inflammatory potential. Pharmaceuticals. 2022;15(11):1367. doi:10.3390/ph15111367.
- Agrawal G, Borody T, Turner R, Leis S, Campbell J. Combining infliximab, anti-MAP and hyperbaric oxygen therapy for resistant fistulizing Crohn’s disease. Future Sci OA. 2015;1(4):FSO77. doi:10.4155/fso.15.77.
- Aitken JM, Phan K, Bodman SE, Sharma S, Watt A, George PM, Agrawal G, Tie ABM. A mycobacterium species for Crohn’s disease? Pathology. 2021 Dec;53(7):818–23. doi:10.1016/j.pathol.2021.03.003.
- Agrawal G, Clancy A, Huynh R, Borody T. Profound remission in Crohn’s disease requiring no further treatment for 3-23 years: a case series. Gut Pathog. 2020;12:16. doi:10.1186/s13099-020-00355-8.
- Khan S, Imran A, Malik A, Chaudhary AA, Rub A, Jan AT, Syed JB, Rolfo C. Bacterial imbalance and gut pathologies: association and contribution of E. coli in inflammatory bowel disease. Crit Rev Clin Lab Sci. 2019;56(1):1–17. doi:10.1080/10408363.2018.1517144.
- Palmela C, Chevarin C, Xu Z, Torres J, Sevrin G, Hirten R, Barnich N, Ng SC, Colombel J-F. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut. 2018;67(3):574–87. doi:10.1136/gutjnl-2017-314903.
- Daley A, Randall R, Darsley M, Choudhry N, Thomas N, Sanderson IR, Croft NM, Kelly P. Genetically modified enterotoxigenic Escherichia coli vaccines induce mucosal immune responses without inflammation. Gut. 2007;56(11):1550–6. doi:10.1136/gut.2006.112805.
- Alexander KL, Zhao Q, Reif M, Rosenberg AF, Mannon PJ, Duck LW, Elson CO. Human microbiota flagellins drive adaptive immune responses in Crohn’s disease. Gastroenterology. 2021;161(2):522–35.e6. doi:10.1053/j.gastro.2021.03.064.
- Tran HQ, Ley RE, Gewirtz AT, Chassaing B. Flagellin-elicited adaptive immunity suppresses flagellated microbiota and vaccinates against chronic inflammatory diseases. Nat Commun. 2019;10(1):5650. doi:10.1038/s41467-019-13538-y.
- Rafii S, Butler JM, Ding B-S. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529(7586):316–25. doi:10.1038/nature17040.
- Jeong J-H, Ojha U, Lee YM. Pathological angiogenesis and inflammation in tissues. Arch Pharm Res. 2021;44(1):1–15. doi:10.1007/s12272-020-01287-2.
- Aguilar-Cazares D, Chavez-Dominguez R, Carlos-Reyes A, Lopez-Camarillo C, Hernadez de la Cruz ON, Lopez-Gonzalez JS. Contribution of angiogenesis to inflammation and cancer. Front Oncol. 2019;9:1399. doi:10.3389/fonc.2019.01399.
- Koutroubakis IE, Tsiolakidou G, Karmiris K, Kouroumalis EA. Role of angiogenesis in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12(6). doi:10.1097/00054725-200606000-00012.
- Danese S, Sans M, De La Motte C, Graziani C, West G, Phillips MH, Pola R, Rutella S, Willis J, Gasbarrini A, et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130(7):2060–73. doi:10.1053/j.gastro.2006.03.054.
- Sajib S, Zahra FT, Lionakis MS, German NA, Mikelis CM. Mechanisms of angiogenesis in microbe-regulated inflammatory and neoplastic conditions. Angiogenesis. 2018;21(1):1–14. doi:10.1007/s10456-017-9583-4.
- Gardlik R, Bartonova A, Celec P. Therapeutic DNA vaccination and RNA interference in inflammatory bowel disease. Int J Mol Med. 2013;32(2):492–6. doi:10.3892/ijmm.2013.1388.
- Scaldaferri F, Vetrano S, Sans M, Arena V, Straface G, Stigliano E, Repici A, Sturm A, Malesci A, Panes J, et al. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology. 2009;136(2):585–95.e5. doi:10.1053/j.gastro.2008.09.064.
- Rahat MA. Targeting angiogenesis with peptide vaccines. Front Immunol. 2019;10:1924. doi:10.3389/fimmu.2019.01924.
- Simanovich E, Brod V, Rahat MA. Active vaccination with EMMPRIN-Derived multiple antigenic peptide (161-MAP) reduces angiogenesis in a dextran sodium sulfate (DSS)-induced colitis model. Front Immunol. 2018;9:2919. doi:10.3389/fimmu.2018.02919.
- Linares PM, Chaparro M, Gisbert JP. Angiopoietins in inflammation and their implication in the development of inflammatory bowel disease. A review. J Crohns Colitis. 2014;8(3):183–90. doi:10.1016/j.crohns.2013.06.013.
- Arai T, Lopes F. Potential of human helminth therapy for resolution of inflammatory bowel disease: the future ahead. Exp Parasitol. 2022;232:108189. doi:10.1016/j.exppara.2021.108189.
- Abdoli A. Therapeutic potential of helminths and helminth-derived antigens for resolution of inflammation in inflammatory bowel disease. Arch Med Res. 2019;50(1):58–9. doi:10.1016/j.arcmed.2019.03.001.
- Summers RW, Elliott DE, Qadir K, Urban JF, Thompson R, Weinstock JV. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2003 Sep;98(9):2034–41. doi:10.1111/j.1572-0241.2003.07660.x.
- Huang X, Zeng L-R, Chen F-S, Zhu J-P, Zhu M-H. Trichuris suis ova therapy in inflammatory bowel disease: a meta-analysis. Medicine. 2018;97(34):e12087. doi:10.1097/MD.0000000000012087.
- Schölmerich J, Fellermann K, Seibold FW, Rogler G, Langhorst J, Howaldt S, Novacek G, Petersen AM, Bachmann O, Matthes H, et al. A randomised, Double-blind, placebo-controlled trial of Trichuris suis ova in active Crohn’s disease. J Crohn’s Colitis. 2017;11(4):390–9. doi:10.1093/ecco-jcc/jjw184.
- Capron M, Béghin L, Leclercq C, Labreuche J, Dendooven A, Standaert A, Delbeke M, Porcherie A, Nachury M, Boruchowicz A, et al. Safety of P28GST, a protein derived from a schistosome helminth Parasite, in patients with Crohn’s disease: a pilot study (ACROHNEM). J Clin Med. 2020;9(1):41. doi:10.3390/jcm9010041.
- Sarazin A, Dendooven A, Delbeke M, Gatault S, Pagny A, Standaert A, Rousseaux C, Desreumaux P, Dubuquoy L, Capron M, et al. Treatment with P28GST, a schistosome-derived enzyme, after acute colitis induction in mice: decrease of intestinal inflammation associated with a down regulation of Th1/Th17 responses. PloS One. 2018;13(12):e0209681. doi:10.1371/journal.pone.0209681.
- Löffler P. Review: vaccine myth-buster - cleaning up with prejudices and dangerous misinformation. Front Immunol. 2021;12:663280. doi:10.3389/fimmu.2021.663280.
- Yu X, Mai Y, Wei Y, Yu N, Gao T, Yang J. Therapeutic potential of tolerance-based peptide vaccines in autoimmune diseases. Int Immunopharmacol. 2023;116:109740. doi:10.1016/j.intimp.2023.109740.
- Sabatos-Peyton CA, Verhagen J, Wraith DC. Antigen-specific immunotherapy of autoimmune and allergic diseases. Curr Opin Immunol. 2010;22(5):609–15. doi:10.1016/j.coi.2010.08.006.
- Larche M, Wraith DCM. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat Med. 2005;11(4 Suppl):S69–S76. doi:10.1038/nm1226.
- Zhang Y-Z, Li Y-Y. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20(1):91–9. doi:10.3748/wjg.v20.i1.91.
- Huang J-H, Wu C-W, Lien S-P, Leng C-H, Hsiao K-N, Liu S-J, Chen H-W, Siu L-K, Chong P. Recombinant lipoprotein-based vaccine candidates against C. difficile infections. J Biomed Sci. 2015;22(1):65. doi:10.1186/s12929-015-0171-x.
- Ma Y, Guan Q, Bai A, Weiss CR, Hillman CL, Ma A, Zhou G, Qing G, Peng Z. Targeting TGF-β1 by employing a vaccine ameliorates fibrosis in a mouse model of chronic colitis. Inflamm Bowel Dis. 2010 Jun;16(6):1040–50. doi:10.1002/ibd.21167.