ABSTRACT
Using a test-negative case–control design, we aim to estimate influenza vaccine effectiveness (VE) against medically attended laboratory-confirmed influenza in Portugal in 2022/2023 season. Between week 41/2022 and week 14/2023, data on 592 patients with influenza-like illness aged 18 or more years old were collected by the national sentinel influenza surveillance system in primary care settings. Of those, 218 were positive for influenza and 374 were negative controls. We estimated seasonal influenza VE as (1-odds ratio)*100% of being vaccinated in laboratory-confirmed influenza cases vs. negative controls using logistic regression model adjusted for age group, sex, presence of chronic conditions, and month of symptoms onset. The seasonal VE was 59.3% (95% confidence interval (CI): 27.3 to 77.3) against any laboratory-confirmed influenza and not statistically significant 44.5% (95% CI: −5.6 to 70.8) against influenza A (H3N2). In the 2022/2023 season, characterized by early and low influenza activity and predominant A (H3N2) circulation, vaccination provided a moderate protection against medically attended laboratory-confirmed influenza in primary care.
Introduction
Vaccination is a key public health intervention to mitigate influenza-associated illnesses and their complications. 2022/2023 influenza vaccination campaign in Portugal started on week 36/2022, targeting those with a high risk of exposure and post-infection severe outcomes. They included individuals aged 65 or more years old, pregnant women, individuals with underlying chronic conditions, health professionals and other care providers, and those living in long-term care facilities.Citation1 Seasonal influenza vaccines available in the 2022/2023 season included inactivated quadrivalent vaccines Fluarix Tetra® and Vaxigrip Tetra®. The high-dose quadrivalent vaccine Efluelda® was available for residents in long-term care facilities. The vaccine was offered free of charge to the target population and co-inoculated at vaccination centers in parallel with the COVID-19 vaccine seasonal booster dose. By week 17/2023, 2 388 129 doses of influenza vaccine were administered in Portugal and vaccine coverage reached 75% among those aged 65 or more years old.Citation2
2022/2023 influenza season was characterized by a predominance of influenza A (H3N2) (77.9%), and sporadic circulation of influenza A (H1N1) pdm09 (12.7%) and influenza B viruses (9.3%).Citation3
Additionally, the epidemic influenza season occurred between week 42/2022 and week 01/2023 being the earliest seasonal influenza epidemic registered in Portugal. Epidemic peak occurred around week 46/2022, had low intensity, and mainly affected children and young adults. The number of influenza patients admitted to ICU was lower than in other seasons, and there was no excess mortality during the 2022/2023 influenza season.Citation2 Circulating viruses were genetically and antigenically similar to vaccine strains.
Within the established Portuguese national immunization program governance model, the National Health Institute Doutor Ricardo Jorge is responsible for virological surveillance of vaccine-preventable diseases and implementation of vaccine effectiveness and impact studies,Citation4 including influenza vaccine effectiveness studies.Citation5 Influenza vaccine effectiveness estimates are important to inform national public health policy and may contribute to upcoming decisions for vaccine strain selection for the next season at the international level.
This study aims to estimate the seasonal influenza vaccine effectiveness in preventing laboratory-confirmed influenza in adult patients attending primary care, using data from the national influenza surveillance system in 2022/2023 season (week 41/2022 to week 14/2023).
Materials and methods
A test-negative case – control study was developedCitation5,Citation6 relying on data collected by the national sentinel influenza surveillance system, which includes 76 primary health-care units on Portugal Mainland. Each sentinel general practitioner systematically recruited up to five Influenza-Like Illness (ILI) patients per week from whom nasopharyngeal swabs, clinical and epidemiological information were collected. Patients were recruited according to the European ILI case definition, i.e., with sudden onset of at least one systemic (fever or feverishness, malaise, headache, myalgia) and one respiratory symptom (cough, sore throat, shortness of breath). All data were collected during the general practitioner’s appointment either by asking the patients or through a health registry consultation.
Samples from all recruited ILI patients were analyzed at the National Reference Laboratory for Influenza and other respiratory viruses for the detection of influenza (type), SARS-CoV-2, and RSV, using a commercial RT-PCR assay Allplex™ SARS-CoV-2/FluA/FluB/RSV (ASFR, Seegene Technologies Inc; Seoul, South Korea). Subtyping of influenza A (H3N2 and H1pdm09) and determination of the lineage of influenza B (Victoria and Yamagata) were performed by adapting in-house RT-PCR. Genetic characterization of influenza virus was performed based on the subunit 1 of hemagglutinin (HA1). Those samples that presented CT value < 25 were selected to be sequenced by NGS (Illumina) methodology. Sequences were analyzed using the INSaFLU pipeline (https://insaflu.insa.pt/), and phylogenetic trees were built using Nextstrain (https://nextstrain.org/).
Cases were defined as ILI patients with positive RT-PCR results for influenza. Controls were defined as those with laboratory-negative results for influenza. Both cases and controls were considered vaccinated for influenza if the influenza vaccine uptake in the 2022/2023 season occurred 14 days before the symptoms onset.
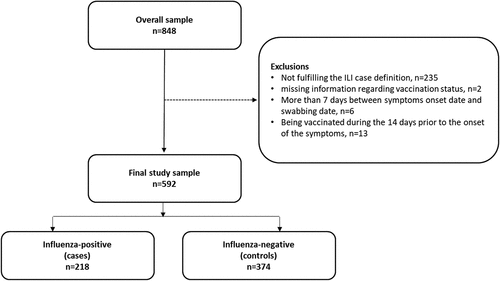
The following exclusion criteria were established: i) not fulfilling the ILI case definition; ii) being vaccinated during the 14 days prior to the onset of the symptoms; iii) controls recruited before the first influenza case; iv) missing information regarding vaccination status and date of symptoms onset; v) more than 7 days between symptoms onset date and swabbing date.
Absolute and relative frequencies were used to describe the study participants. Characteristics of influenza-positive ILI patients (cases) were compared to influenza-negative controls using the chi-square test. Influenza vaccine effectiveness was estimated as VE = (1- OR) *100%, where OR stands for odds ratio of being vaccinated in RT-PCR influenza-positive cases vs influenza-negative controls, obtained through a logistic regression model adjusted for age group, sex, and presence of chronic conditions (at least one among diabetes, asthma, or other chronic respiratory diseases, cancer, chronic renal disease, hypertension or other chronic cardiovascular disease, obesity, chronic hepatic disease, neuromuscular disease, and immunodeficiency), and month of symptoms onset. Vaccine effectiveness was estimated against all influenza and A (H3N2) subtypes, predominant in the 2022/2023 early season. The significance level was set at 5%.
The study was conducted in compliance with the Helsinki Declaration and national data protection legal and ethical requirements. Data were collected under the surveillance protocol, which has specific approvals from the Ethical Committee and the Data Protection Officer. The study protocol was updated in 2022/2023 and approved by the Ethics Committee of the INSA on 2022, December, 14th, and the INSA data protection officer on 2023, January, 26th. The requirement for written informed consent was waived by the Ethics Committee. Oral informed consent was taken from participants. The study guideline, distributed to the Sentinel General Practitioners included the detailed procedure to inform patients about the study´s objectives and implications and ask for their consent.
Results
Between week 41/2022 and week 14/2023, a total of 848 patients aged 18 or more years old were notified by the national sentinel influenza surveillance system. Of those, 592 had information on clinical symptoms compatible with the ILI case definition and vaccination status and were included in the analysis ().
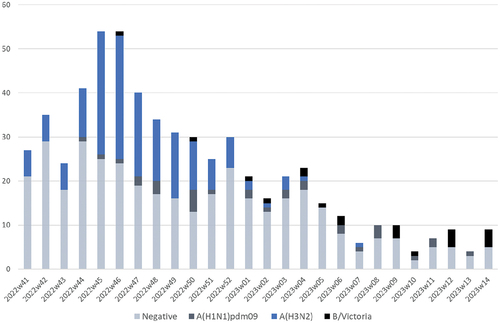
The weekly distribution of ILI patients (influenza-positive cases and influenza-negative controls) included in the analysis is shown in . Overall, of laboratory-confirmed influenza infections, 76.2% (n = 166) were A (H3N2), 14.2% (n = 31) were A (H1N1) pdm09 and 9.6% (n = 21) were influenza B (Victoria lineage). Among influenza-negative ILI controls, SARS-CoV-2, was the most prevalent virus identified (20.3%) followed by Rhinovirus (17.1%). In addition, during the 2022/2023 season, in 5.6% of the influenza-negative controls multiple infections were detected (Table S1).
Figure 2. Weekly distribution of laboratory-confirmed influenza-positive ILI cases and influenza-negative controls, Portugal, 2022/2023 season.
A total of 214 influenza viruses were genetically characterized. The characterized influenza A (H3N2) was clustered in three different clades: 3C.2a1b.2a.2b (2b), 3C.2a1b.2a.2a.1b (2a.1b), 3C.2a1b.2a.2a.3a.1 (2a.3a.1). The first one was the predominant clade circulating in Portugal during 2022/2023 season (Figure S1). The clades 6B.1A.5a.2a (5a.2a) and 6B.1A.5a.2a.1 (5a.2a.1) were identified in similar proportions among the characterized influenza A (H1N1) pdm09 virus. Regarding influenza B/Victoria, all the characterized viruses were clustered in clade V1A.3a.2.
The distribution of lab-confirmed influenza-positive ILI cases by sex, age group, and presence of chronic conditions was statistically different from the negative controls (). Influenza-positive cases were younger than influenza-negative controls and had a higher proportion of males (38.1% vs. 25.9%). Chronic conditions were more frequent among controls (32.6% vs.20.6%). Variation by month of symptoms onset was significant between cases and controls, with a higher number of cases was detected earlier in the season (November, 44.5% vs.26.5%).
Table 1. Characteristics of laboratory-confirmed influenza cases and influenza-negative controls, Portugal, week 41/2022-week 14/2023.
Influenza vaccine effectiveness in the adult population (18+ years old) was 59.3% (27.3 to 77.3) against laboratory-confirmed influenza attended in primary care (both A and B) and 44.5% (−5.6 to 70.8) against A (H3N2) subtype, predominant in Portugal in 2022/23 season ().
Table 2. Interim influenza vaccine effectiveness against any influenza and A (H3N2) influenza, Portugal, week 41/2022-week 14/2023.
Discussion
Using data collected by the Portuguese influenza sentinel surveillance network, we estimated 2022/2023 end-of-season vaccine effectiveness against laboratory-confirmed influenza in a primary care setting for the adult population ranging from 44.5% for A (H3N2) subtype to 59.3% for any influenza.
Estimates of VE obtained in this study were similar to early 2022/2023 season estimates in Canada (58–59%) and the USA (54–60%),Citation7,Citation8 countries also characterized by predominant A (H3N2) circulation, although our estimates against influenza A (H3 N2) were not statistically significant, probably due to lower sample size.
When comparing to other seasons, dominated by A (H3N2), 2022/2023 seasonal A (H3N2) VE obtained in Portugal was higher compared to the end of 2021/2022 season VE reported by I-MOVE network in EuropeCitation6 and Flu VE Network in the USA.Citation9
Genetic analysis has shown that the circulating strains in Portugal were grouped into three different clades for influenza A (H3N2), two different clades for influenza A (H1N1) pdm09 and one unique clade for influenza B/Victoria. Despite some genetic diversity, influenza-characterized viruses were antigenically similar to the 2022/2023 vaccine strains. Antigenic results also indicated that circulating influenza A (H3N2) and B/Victoria were antigenically similar to the vaccine strains. Moreover, influenza circulation occurred early in the season, very close to the vaccination campaign when the population had higher levels of vaccine-induced protection,Citation10 which could explain the higher VE estimates when compared to previous late seasons. It is also interesting to note that this influenza season, had lower intensity and impact on ICU hospitalization and in mortality than observed in previous A (H3N2) influenza seasons
Among the study strengths we should mention test-negative case–control design, which is frequently used in influenza vaccine effectiveness studies as it has the advantage of control for selection bias related to the outcome and minimizes bias by health-seeking behavior. All laboratory analyses were performed at the National Reference Laboratory for Influenza and other respiratory viruses using high-sensitivity and high-specificity RT-PCR assays, so we expect the misclassification of outcome is expected to be residual. Information on vaccination status was collected by the general practitioners and was retrieved from health registries, so we expect the measure of the main exposure also to be accurate.
Our study has several limitations. The low intensity of influenza activity in the 2022/2023 season limited the study sample size. Due to the small sample size, we were not able to estimate influenza VE by age group and presence of chronic condition as well as against A (H1N1) pdm09 and B influenza subtypes and we acknowledge this limited the power to obtain significant estimates against influenza A (H3N2). Considering the differences in age, sex and chronic conditions between cases and controls, vaccine effectiveness estimates were adjusted by potential confounders, however some residual confounding might persist, as the surveillance system collects information on a limited number of confounders.
In conclusion, our results suggest that seasonal influenza vaccine effectiveness confers moderate protection against laboratory-confirmed influenza in a primary care setting.
Supplemental Material
Download MS Word (174.4 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2263219.
Additional information
Funding
References
- Direção-Geral da Saúde. Campanha de Vacinação Sazonal contra a Gripe: Outono-Inverno 2022-2023. Norma 007/2022 [Internet]. https://www.dgs.pt/normas-orientacoes-e-informacoes/normas-e-circulares-normativas/norma-n-0072022-de-02092022-pdf.aspx.
- Direção-Geral da Saúde. Relatório de Resposta Sazonal em Saúde — Vigilância e Monitorização. Relatório n.o 21, semana 17/2023. 2023. https://www.dgs.pt/em-destaque/relatorio-n-6-da-resposta-sazonal-em-saude-vigilancia-e-monitorizacao-pdf.aspx.
- Instituto Nacional de Saúde Doutor Ricardo Jorge. Boletim de vigilância epidemiológica da gripe e outros vírus respiratórios. https://www.insa.min-saude.pt/category/informacao-e-cultura-cientifica/publicacoes/atividade-gripal/.
- Portaria n.o 248/2017, de 4 de agosto | DRE [Internet]; [Accessed 2023 Jan 11]. https://dre.pt/dre/detalhe/portaria/248-2017-107951592.
- Machado A, Kislaya I, Nunes B, Rodrigues AP, Guiomar R. Moderate influenza vaccine effectiveness in a B mismatch season: preliminary results from the 2017/2018 season in Portugal. Pulmonology. 2018;24(4):260–5. doi:10.1016/j.pulmoe.2018.05.004.
- Kissling E, Pozo F, Martínez-Baz I, Buda S, Vilcu AM, Domegan L, Mazagatos C, Dijkstra F, Latorre-Margalef N, Kurečić Filipović S, et al. Influenza vaccine effectiveness against influenza a subtypes in Europe: results from the 2021–2022 I-MOVE primary care multicentre study. Influenza Other Respi Viruses [Internet]. 2023;17. https://pubmed.ncbi.nlm.nih.gov/36702797/.
- McLean HQ, Petrie JG, Hanson KE, Meece JK, Rolfes MA, Sylvester GC, Neumann G, Kawaoka Y, Belongia EA. Interim estimates of 2022–23 seasonal influenza vaccine effectiveness — Wisconsin, October 2022–February 2023. Morb Mortal Wkly Rep [Internet]. 2023;72:201–5. doi:10.15585/mmwr.mm7208a1.
- Skowronski DM, Chuang ES, Sabaiduc S, Kaweski SE, Kim S, Dickinson JA, Olsha R, Gubbay JB, Zelyas N, Charest H, et al. Vaccine effectiveness estimates from an early-season influenza A(H3N2) epidemic, including unique genetic diversity with reassortment, Canada, 2022/23. Eurosurveillance [Internet]. 2023;28:2300043. doi:10.2807/1560-7917.ES.2023.28.5.2300043.
- Price AM, Flannery B, Talbot HK, Grijalva CG, Wernli KJ, Phillips CH, Monto AS, Martin ET, Belongia EA, McLean HQ, et al. Influenza vaccine effectiveness against influenza A(H3N2)-related illness in the United States during the 2021-2022 influenza season. Clin Infect Dis [Internet]. 2022. https://pubmed.ncbi.nlm.nih.gov/36504336/.
- Young B, Sadarangani S, Jiang L, Wilder-Smith A, Chen MIC. Duration of influenza vaccine effectiveness: a systematic review, meta-analysis, and meta-regression of test-negative design case-control studies. J Infect Dis [Internet]. 2018;217(5):731–41. doi:10.1093/infdis/jix632.