ABSTRACT
Rotavirus remains a major cause of diarrhea among 5-y-old children, and vaccination is currently the most effective and economical measure. We conducted a randomized, double-blind, placebo-controlled phase II clinical trial designed to determine the dosage, immunogenicity, and safety profile of a novel hexavalent rotavirus vaccine. In total, 480 eligible healthy infants, who were 6–12 weeks of age at the time of randomization were randomly allocated (1:1:1) to receive 105.5 focus-forming unit (FFU) or 106.5FFU of vaccine or placebo on a 0, 28 and 56-d schedule. Blood samples were collected 28 d after the third dose to assess rotavirus immunoglobulin A (IgA) antibody levels. Adverse events (AEs) up to 28 d after each dose and serious adverse events (SAEs) up to 6 months after the third dose were recorded as safety measurements. The anti-rotavirus IgA seroconversion rate of the vaccine groups reached more than 70.00%, ranging from 74.63% to 76.87%. The postdose 3 (PD3) geometric mean concentrations (GMCs) of anti-rotavirus IgA among vaccine recipients ranged from 76.97 U/ml to 84.46 U/ml. At least one solicited AE was recorded in 114 infants (71.25%) in the high-dose vaccine group, 106 infants (66.25%) in the low-dose vaccine group and 104 infants (65.00%) in the placebo group. The most frequently solicited AE was fever. The novel oral hexavalent rotavirus vaccine was safe and immunogenic in infants support the conclusion to advance the candidate vaccine for phase 3 efficacy trials.
KEYWORDS:
Introduction
Rotavirus infection is the leading cause of acute gastroenteritis (AGE) worldwide in children under 5 y of age and causes dehydration of varying degrees.Citation1,Citation2 The WHO broadened its recommendation for rotavirus vaccination use in 2009 to cover all nations, with a focus on those with high rates of death from diarrhea.Citation3 The introduction of oral rotavirus vaccines into national immunization programs has led to substantial reductions in rotavirus and all-cause acute gastroenteritis, hospital admissions and mortality among children younger than 5 y.Citation4 In 2008, prior to the widespread implementation of rotavirus vaccines, rotavirus acute gastroenteritis (RAGE) led to estimated 453,000 deaths globally among children under 5 y of age.Citation5–7 Following the introduction of rotavirus vaccination, it is estimated that deaths due to RAGE dropped to 146,500 in 2015Citation8 and 128,500 in 2016.Citation9
As of November 2019, rotavirus vaccination was available or planned in 120 countries.Citation10 Although rotavirus vaccines are commercially available, they are mostly unaffordable in developing countries. Children in low-income and middle-income countries are far more likely to be infected earlier in life than those in high-income countries. Because access to urgent care can be limited or unavailable in rural, impoverished settings, these children are more likely to develop severe disease and die.Citation11,Citation12 However, in recent years, the predominant serotypes of epidemic rotavirus in China have been G1, G2, G3, G4, and G9.Citation13 Based on the bovine rotavirus UK Compton strain, we developed a human-bovine reassortant hexavalent rotavirus vaccine (HRV) containing G1, G2, G3, G4, G8, and G9 serotypes, which was shown to be safe and highly immunogenic in animals and was well tolerated in a phase 1 clinical trial.Citation14 The present study is a double-blinded, randomized controlled phase 2 clinical trial to assess the safety and immunogenicity of the hexavalent rotavirus vaccine.
Methods
Study design and participants
This phase 2 study was a single-center, randomized, double-blind, placebo-controlled clinical trial performed from June 2017, to July 2018 in Zhengding County, Hebei Province, China. This study was approved by the Institutional Review Board of the Hebei Center for Disease Control and Prevention. This study was conducted in accordance with good clinical practice, and the Declaration of Helsinki. Written informed consent was obtained from guardians before any study-related procedures. The clinical trial is registered at the National Medical Products Administration (NMPA) under number CRT20150878.
Healthy infants between 6 and 12 weeks of age were eligible for enrollment if they did not have a history of any confirmed or suspected immunosuppressive or immunodeficient condition, history of rotavirus gastroenteritis or intussusception (IS), or history of allergic disease. Vaccination was postponed in cases of acute febrile illness, vomiting, or diarrhea at the time of scheduled vaccination.
Eligible healthy infants aged 6–12 weeks were randomly assigned, in a 1:1:1 ratio, to receive three 2-ml oral doses of a high dose (l × 106.5 focus-forming unit (FFU)) of vaccine, low dose (l × 105.5 FFU) of vaccine or placebo on day 0, day 28, and day 56.
Randomization and blinding
Participants were randomly assigned (1:1:1) to receive a high-dose level, low-dose level of vaccine, or placebo only. The randomization codes were generated by the randomization statistician by means of block randomization using SAS software (version 9.2). A randomization code was assigned to each subject in sequence in the order of enrollment, and then the participants received the study vaccine labeled with the same code. Trial participants, study site staff, the data management team, and statisticians who performed the analysis remained blinded to the treatment assignment throughout this study.
Vaccines
The investigational vaccine (Wuhan Biological Products Research Institute Co. Ltd., Hubei, China) used in this study was a live attenuated, hexavalent rotavirus vaccine consisting of a mixture of bovine-human mono-reassortant viruses expressing the human VP7 genes encoding G1, G2, G3, G4, G8, and G9 on the genetic background of the bovine rotavirus UK Compton strain. A comprehensive description of the investigational products using indigenous manufacturing technology at the Wuhan National Biological Institute of China is described elsewhere.Citation14 Two vaccine dose levels were assessed (low dose: 1 × 105.5 FFU, high dose: 1 × 106.5 FFU), according to the results of a phase 1 clinical trial. Each dose of the vaccine consisted of 2 ml. The placebo contained the same constituents as the vaccine but without the RVs.
Assessment of immunogenicity
The seroconversion rates and anti-rotavirus immunoglobulin A (IgA) antibody geometric mean concentrations (GMCs) at 28 d after receiving the full course of vaccination were the primary endpoints. To assess the serum immune response, approximately 2.5–3.0 ml of blood was collected before the first dose and 28 d after the third dose. Serum samples were isolated and stored at −20°C until evaluation. The rotavirus IgA antibody GMC was measured by the National Institute of Food and Drug Control (NIFDC) with an in-house-developed enzyme-linked immunosorbent assay kit; the method has been described elsewhere.Citation14 The GMC was tested in the reference laboratory of the NIFDC, Beijing, China.
Assessment of safety
Participants were observed at the study site for at least 30 min after the administration of the vaccine to collect information on immediate adverse events. Thereafter, the subjects’ carers were given a thermometer and a diary card covering days 0–14 for safety follow-up. Parents or guardians of the participants were required to record any adverse events (e.g., fever, diarrhea, vomiting, anorexia, irritability/abnormal crying, drowsiness, and allergy) on the diary cards. On days 15 and 28, parents or guardians visited the study site for assessment by the study investigators who conducted face-to-face interviews to confirm subject safety. The incidence of solicited adverse events within 14 d, unsolicited adverse events within 28 d after each dose and serious adverse events (SAEs) reported during the 6 months after receiving the full course of vaccination were the secondary endpoints. The reported adverse events were graded according to the China National Medical Products Administration guidelines.Citation15 The causal relationship between adverse events and vaccination was determined by the investigators.
Statistical analysis
All analyses were performed using SAS version 9.2. Hypothesis testing was two-sided and differences resulting in p values of .05 or less were considered statistically significant. We used the Pearson chi-square or Fisher’s exact test for the analysis of categorical outcomes. We calculated GMCs and corresponding 95% confidence intervals (CIs) on the basis of the standard normal distribution of the log-transformed antibody titer. We used ANOVA to compare the log-transformed antibody concentrations. Seroconversion rates were defined as anti-rotavirus IgA antibody concentrations ≥20 U/ml in initially seronegative infants, and a fourfold increase in the concentration from baseline to postdose 3 (PD3).Citation14 An anti-rotavirus IgA antibody concentration <20 U/ml was calculated as 10 U/ml to be included in the GMC calculations.
Results
Demographic and other baseline characteristics
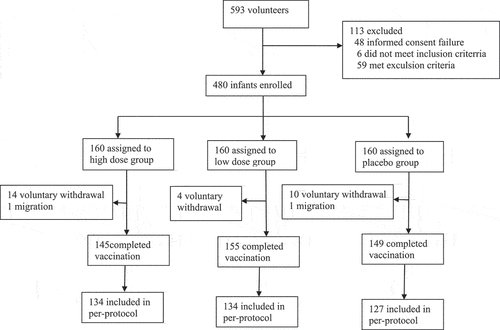
In total, 593 volunteers participated in interviews and physical examinations for this trial. Of these volunteers, 113 were excluded due to failure to sign the informed consent form, meeting the exclusion criteria or other reasons, and 480 subjects were enrolled in the trial (). Overall, 480 subjects (100.0%) received at least one dose of vaccine or placebo; 449 (93.54%) received three doses and were followed up for safety for 30 d after the third dose; and 395 (82.29%) were included in the per-protocol population for immunogenic evaluation on day 28 after the third dose. The demographic characteristics of subjects were similar across treatment groups in terms of sex, mean age, length, and height (). The mean age of the subjects at the time of entry was 8.66 ± 1.89 weeks.
Table 1. Summary of demographic characteristics.
Immunogenicity
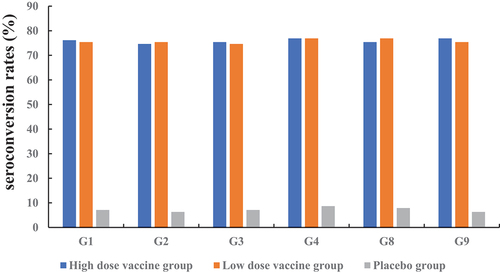
The anti-rotavirus IgA seroconversion rate of the vaccine groups reached more than 70.00%. The seroconversion rates ranged from 74.63% to 76.87% in the two vaccine groups. The seroconversion rates ranged from 74. 63% (G2) to 76.87% (G4 and G9) in the high-dose vaccine group and from 74.63% (G3) to 76.87% (G4 and G8) in the low-dose vaccine group. A significant difference was not observed between the low-dose and high-dose groups ().
The baseline GMCs of anti-rotavirus IgA were comparable between the groups. The GMC ranged from 79.25 U/ml (G4) to 84.46 U/ml (G1) in the high-dose vaccine group, and from 76.97 U/ml (G3) to 81.88 U/ml (G9) in the low-dose vaccine group, whereas it was approximately 10.0 U/ml in the placebo group. A significant difference was not observed between the low-dose and the high-dose vaccine group ().
Table 2. Summary of IgA response to serotypes G1, G2, G3, G4, G8, and G9 in the per-protocol immunogenicity population.
Vaccine safety
During the 28-d postvaccination follow-up period, AEs (solicited and unsolicited) occurred at a similar rate among both vaccine recipients (84.75% in the high-dose group; 85.63% in the low-dose group) and placebo (80.00%) recipients. The incidences of vaccine-related adverse events in the three groups were 71.88%, 66.25%, and 65.63%, respectively. All adverse reactions were mild or moderate in severity, and most resolved shortly after their onset. At least one solicited AE was recorded in 114 infants (71.25%) of the high dose of vaccine group, 106 infants (66.25%) of the low dose of vaccine group and 104 infants (65.00%) of the placebo group. At least one unsolicited AE was recorded in 86 infants (53.75%) of the high dose of vaccine group, 91 infants (56.88%) of the low dose of vaccine group and 92 infants (57.50%) of the placebo group. Upper respiratory tract infection (28.75% in the high-dose group, 35.00% in the low-dose group, and 32.50% in the placebo group) was the most common unsolicited AE reported in the groups ().
Table 3. Summary of adverse events (AEs).
During the 6-month postvaccination follow-up period, serious adverse events (SAEs) were reported in 2 of the 160 high-dose vaccine recipients (1.25%), 5 of 160 low doses of vaccine recipients (3.13%) and 2 of 160 placebo recipients (1.25%). During the entire study period, no fatal events were reported. None of the SAEs recorded were causally related to vaccination. No intussusception was reported ().
AEs of special interest are shown in . Fever (58.13% in the high-dose group, 51.88% in low-dose group, and 52.50% in placebo group) and diarrhea (23.13% in high-dose group, 25.63% in low-dose group, and 25.00% in placebo group) were the most common solicited AEs reported in the groups. There was no statistically significant difference among the three groups with respect to the incidence of fever, diarrhea, vomiting, anorexia, irritability/abnormal crying, or drowsiness. However, a higher frequency of allergy reported by participants in the low-dose group was observed than among participants in the high-dose group and in the placebo group (p = .04). All four allergic reactions occurred in the low-dose group.
Table 4. Summary of solicited adverse events (AEs).
Discussion
Rotavirus vaccines have been shown to be safe and effective in preventing deaths from rotavirus gastroenteritis infection.Citation12 In low-income and middle-income countries, including China, issues such as the cost, purchase, and supply of vaccine stocks have always been points of concern.Citation16 Therefore, cost-effective and safe rotavirus vaccines for the countries that need them the most remain an overwhelming need.Citation14 Recently, a novel hexavalent rotavirus vaccine (serotypes G1, G2, G3, G4, G8, and G9) candidate was developed by the Wuhan Institute of Biological Products Co., Ltd. (WIBP) in China and was evaluated for safety and preliminary immunogenicity in phase I trials.Citation14 We therefore aimed to further evaluate the immunogenicity and safety of the vaccine and to determine the dose for a planned phase 3 efficacy trial.
In this study, our findings showed that the hexavalent rotavirus vaccine was well tolerated at both high and low vaccine doses, without identifiable safety concerns. During the 28-d postvaccination follow-up period, AEs (solicited and unsolicited) occurred at a similar rate among both vaccine recipients (84.75% in high-dose group; 85.63% in low-dose group) and placebo (80.00%) recipients. The incidence of adverse reactions in different dose groups was similar, indicating that there were no dose-related aggravation concerns regarding safety. Moreover, most adverse reactions were mild and transient, and most resolved shortly after their onset. The results were similar to those of our study on a phase 1 clinical trialCitation14 as well as those of other studies on rotavirus vaccines.Citation17–25 Fever was the most frequently reported symptom. Nine serious adverse events were reported during the trial, and none of the SAEs recorded were causally related to vaccination. Correspondingly, no intussusception or death was reported. There were four cases of allergy in the low-dose group (one subject had systemic red miliary papules; one subject had cheek eczema; and one subject had systemic rash, which appeared 3 d after receipt of the vaccine, mostly on the chest, back, and elbows, and the rest appeared scattered and untreated, lasting for 7 d; and one subject had systemic water blisters), but the same allergy was not observed in the groups and did not increase with the time since inoculation. Nevertheless, we will still focus on this adverse event in further large-scale population research.
The results of our study suggest that the hexavalent rotavirus vaccine is strongly immunogenic with a positive cumulative effect, with evidence that it might provide protection against rotavirus in infants. The IgA antibody responses observed in the infants who had received three vaccine doses of 1 × 105.5 FFU or 1 × 106.5 FFU were similar. Although it was not possible to establish a direct correlation with protectionCitation26–31 because serological correlates of protection were lacking, this vaccine can be expected to be effective on the basis of the excellent vaccine response found in the present study. The immunogenicity of rotavirus vaccines has been evaluated in numerous phase I, II, and III studies.Citation32–36 Rong-Cheng Li et al. showed that the anti-rotavirus IgA seroconversion rate was 74.7%,Citation34 whereas the study by Zhaojun Mo et al. showed that the anti-rotavirus IgA seroconversion rate was 89.4%. In our study, the anti-rotavirus IgA seroconversion rate of the vaccine groups (high-dose group and low-dose group) reached more than 70.00%, and the seroconversion rates ranged from 74.63% to 76.87% in the two vaccine groups. The PD3 GMCs of anti-rotavirus IgA among vaccine recipients were higher than those among placebo recipients; additionally, a significant difference was not observed between the low-dose group and the high-dose group. The 105.5 FFU dosage formulation was selected for further investigation of efficacy.
There are some limitations to this study. First, the coadministration of the study vaccine with other vaccines could have affected the assessment of the safety and immunogenicity of the study vaccine. Although the subjects were required to be immunized with other vaccines 2 weeks after oral administration of the study vaccine, overlap might still have occurred, so further study on simultaneous administration must be conducted. In addition, the relatively small number of subjects in this study could have resulted in some bias or uncertainty, and since the sample size was small, it was not possible to identify potentially increased risks of some rare but severe adverse reactions. However, further studies, including an efficacy trial of the vaccine currently underway in China, will assist in addressing these important questions.
In conclusion, the novel oral hexavalent rotavirus vaccine was safe and immunogenic in infants aged 6–12 weeks, supporting the advancement of the vaccine candidate to phase 3 efficacy trials.
Acknowledgments
We greatly appreciate all the researchers who were involved in this clinical trial and the participants and their parents or legal guardians who participated in this study.
Disclosure statement
Qing-Liang Li, Kai Duan, Wei Chen, Ge-Lin Xu, Biao Yang, Ben Dong, and Jiu-Wei Zhang are currently employees of the Wuhan Institute of Biological Products Co., Ltd. Xiao-Ming Yang is currently an employee of China National Biotec Group Co., Ltd., Beijing, China. The other authors declare that they have no conflicts of interest.
Additional information
Funding
References
- Kuri PR, Goswami P. Current update on rotavirus in-silico multiepitope vaccine design. ACS Omega. 2023;8(1):190–7. doi:10.1021/acsomega.2c07213.
- Britoh Mlan A, Burke RM, Koné H, Boni-Cisse C, N’Guessan R, Zaba F, Aka LN, N’Zue K, Adom SK, Kouadio SK, et al. Impact of rotavirus vaccine introduction in Abidjan, Côte d’Ivoire. Hum Vaccin Immunother. 2023;19(1):2156231. doi:10.1080/21645515.2022.2156231.
- Gundogdu Z, Yendur Sezer O. Changing parental attitudes towards rotavirus vaccine. Cureus. 2023;15:e35348. doi:10.7759/cureus.35348.
- Ndwandwe D, Runeyi S, Mathebula L, Wiysonge C. Rotavirus vaccine clinical trials: a cross-sectional analysis of clinical trials registries. Trials. 2022;23(1):945. doi:10.1186/s13063-022-06878-6.
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–41. doi:10.1016/S1473-3099(11)70253-5.
- Soares-Weiser K, Bergman H, Henschke N, Pitan F, Cunliffe N. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev. 2019. doi:10.1002/14651858.CD008521.pub5.
- Bergman H, Henschke N, Hungerford D, Pitan F, Ndwandwe D, Cunliffe N, Soares-Weiser K. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev. 2021;11:Cd008521. doi:10.1002/14651858.CD008521.pub6.
- Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1459–544. doi:10.1016/S0140-6736(16)31012-1.
- Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, Armah G, Bines JE, Brewer TG, Colombara DV, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018;172(10):958–65. doi:10.1001/jamapediatrics.2018.1960.
- Buchy P, Chen J, Zhang XH, Benninghoff B, Lee C, Bibera GL. A review of rotavirus vaccine use in Asia and the Pacific regions: challenges and future prospects. Expert Rev Vaccines. 2021;2021:1–16. doi:10.1080/14760584.2020.1853532.
- Rodriguez WJ, Kim HW, Brandt CD, Schwartz RH, Gardner MK, Jeffries B, Parrott RH, Ksalow RA, Smith JI, Kapikian AZ, et al. Longitudinal study of rotavirus infection and gastroenteritis in families served by a pediatric medical practice: clinical and epidemiologic observations. Pediatr Infect Dis J. 1987;6(2):170–6. doi:10.1097/00006454-198702000-00006.
- Kanungo S, Chatterjee P, Bavdekar A, Murhekar M, Babji S, Garg R, Samanta S, Nandy RK, Kawade A, Boopathi K, et al. Safety and immunogenicity of the rotavac and rotasiil rotavirus vaccines administered in an interchangeable dosing schedule among healthy Indian infants: a multicentre, open-label, randomised, controlled, phase 4, non-inferiority trial. Lancet Infect Dis. 2022;22(8):1191–9. doi:10.1016/S1473-3099(22)00161-X.
- Wu Z, Li Q, Liu Y, Lv H, Mo Z, Li F, Yu Q, Jin F, Chen W, Zhang Y, et al. Efficacy, safety and immunogenicity of hexavalent rotavirus vaccine in Chinese infants. Virol Sin. 2022;37(5):724–30. doi:10.1016/j.virs.2022.07.011.
- Wu ZW, Li QL, Zhou HS, Duan K, Gao Z, Zhang XJ, Jiang ZJ, Hao ZY, Jin F, Bai X, et al. Safety and immunogenicity of a novel oral hexavalent rotavirus vaccine: a phase I clinical trial. Hum Vaccines Immunother. 2021;17:2311–8. doi:10.1080/21645515.2020.1861874.
- China National Medical Products Administration. Guidelines for grading standards of adverse events in clinical trials of preventive vaccines. 2019 [accessed 2020 Nov 3]. https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20191231111901460.html.
- Ravish HS, Sudarshan MK, Madhusudana SN, Annadani RR, Narayana DH, Belludi AY, Anandaiah, G., Vijayashankar, V. Assessing safety and immunogenicity of post-exposure prophylaxis following interchangeability of rabies vaccines in humans. Hum Vaccines Immunother. 2014;10:1354–8. doi:10.4161/hv.28064.
- Groome MJ, Koen A, Fix A, Page N, Jose L, Madhi SA, McNeal M, Dally L, Cho I, Power M, et al. Safety and immunogenicity of a parenteral P2-VP8-P[8] subunit rotavirus vaccine in toddlers and infants in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2017;17(8):843–53. doi:10.1016/S1473-3099(17)30242-6.
- Chilengi R, Mwila-Kazimbaya K, Chirwa M, Sukwa N, Chipeta C, Velu RM, Katanekwa N, Babji S, Kang G, McNeal MM, et al. Immunogenicity and safety of two monovalent rotavirus vaccines, ROTAVAC(R) and ROTAVAC 5D(R) in Zambian infants. Vaccine. 2021;39:3633–40. doi:10.1016/j.vaccine.2021.04.060.
- Coldiron ME, Guindo O, Makarimi R, Soumana I, Matar Seck A, Garba S, Macher E, Isanaka S, Grais RF. Safety of a heat-stable rotavirus vaccine among children in Niger: data from a phase 3, randomized, double-blind, placebo-controlled trial. Vaccine. 2018;36:3674–80. doi:10.1016/j.vaccine.2018.05.023.
- Cohet C, Cheuvart B, Moerman L, Bi D, Caplanusi A, Kariyappa M, Lalwani S, Mitra M, Sapru A, Saha S, et al. A phase III randomized, open-label, non-inferiority clinical trial comparing liquid and lyophilized formulations of oral live attenuated human rotavirus vaccine (HRV) in Indian infants. Hum Vaccines Immunother. 2021;17(11):4646–53. doi:10.1080/21645515.2021.1960136.
- Kanchan V, Zaman K, Aziz AB, Zaman SF, Zaman F, Haque W, Khanam M, Karim MM, Kale S, Ali SK, et al. A randomized phase I/II study to evaluate safety and reactogenicity of a heat-stable rotavirus vaccine in healthy adults followed by evaluation of the safety, reactogenicity, and immunogenicity in infants. Hum Vaccines Immunother. 2020;16(3):693–702. doi:10.1080/21645515.2019.1664239.
- Witte D, Handley A, Jere KC, Bogandovic-Sakran N, Mpakiza A, Turner A, Pavlic D, Boniface K, Mandolo J, Ong DS, et al. Neonatal rotavirus vaccine (RV3-BB) immunogenicity and safety in a neonatal and infant administration schedule in Malawi: a randomised, double-blind, four-arm parallel group dose-ranging study. Lancet Infect Dis. 2022;22(5):668–78. doi:10.1016/S1473-3099(21)00473-4.
- Bines JE, Danchin M, Jackson P, Handley A, Watts E, Lee KJ, West A, Cowley D, Chen M-Y, Barnes GL, et al. Safety and immunogenicity of RV3-BB human neonatal rotavirus vaccine administered at birth or in infancy: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2015;15(12):1389–97. doi:10.1016/S1473-3099(15)00227-3.
- Bines JE, At Thobari J, Satria CD, Handley A, Watts E, Cowley D, Nirwati H, Ackland J, Standish J, Justice F, et al. Human neonatal rotavirus vaccine (RV3-BB) to target rotavirus from birth. New Engl J Med. 2018;378(8):719–30. doi:10.1056/NEJMoa1706804.
- At Thobari J, Damayanti W, Haposan JH, Nirwati H, Iskandar K, Samad, Fahmi J, Sari RM, Bachtiar NS, Watts E, et al. Safety and immunogenicity of human neonatal RV3 rotavirus vaccine (bio farma) in adults, children, and neonates in Indonesia: phase I trial. Vaccine. 2021;39:4651–8. doi:10.1016/j.vaccine.2021.06.071.
- Angel J, Franco MA, Greenberg HB. Rotavirus immune responses and correlates of protection. Curr Opin Virol. 2012;2(4):419–25. doi:10.1016/j.coviro.2012.05.003.
- Offit PA. Correlates of protection against rotavirus infection and disease. Novartis Found Symp. 2001;238:106–13. discussion 14-24.
- Plotkin SA. Recent updates on correlates of vaccine-induced protection. Front Immunol. 2022;13:1081107. doi:10.3389/fimmu.2022.1081107.
- Pollock L, Bennett A, Jere KC, Mandolo J, Dube Q, Bar-Zeev N, Heyderman RS, Cunliffe NA, Iturriza-Gomara M. Plasma rotavirus-specific IgA and risk of rotavirus vaccine failure in infants in Malawi. Clin Infect Dis. 2022;75:41–6. doi:10.1093/cid/ciab895.
- Holmgren J, Parashar UD, Plotkin S, Louis J, Ng SP, Desauziers E, Picot V, Saadatian-Elahi M. Correlates of protection for enteric vaccines. Vaccine. 2017;35:3355–63. doi:10.1016/j.vaccine.2017.05.005.
- Clarke E, Desselberger U. Correlates of protection against human rotavirus disease and the factors influencing protection in low-income settings. Mucosal Immunol. 2015;8(1):1–17. doi:10.1038/mi.2014.114.
- Mo Z, Ma X, Luo P, Mo Y, Kaplan SS, Shou Q, Zheng M, Hille DA, Arnold BA, Liao X, et al. Immunogenicity of pentavalent rotavirus vaccine in Chinese infants. Vaccine. 2019;37(13):1836–43. doi:10.1016/j.vaccine.2019.02.018.
- Li RC, Li YP, Mo ZJ, Luo D, Huang T, Kong JL, Wang L-H, Song N-S, Liu A, Zhang H, et al. Reactogenicity and safety of a liquid human rotavirus vaccine (RIX4414) in healthy adults, children and infants in China: randomized, double-blind, placebo-controlled phase I studies. Hum Vaccines Immunother. 2013;9(8):1638–42. doi:10.4161/hv.25076.
- Li RC, Huang T, Li Y, Wang LH, Tao J, Fu B, Si G, Nong Y, Mo Z, Liao X, et al. Immunogenicity and reactogenicity of the human rotavirus vaccine, RIX4414 oral suspension, when co-administered with routine childhood vaccines in Chinese infants. Hum Vaccines Immunother. 2016;12(3):785–93. doi:10.1080/21645515.2015.1085143.
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. New Engl J Med. 2006;354:23–33. doi:10.1056/NEJMoa052664.
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. New Engl J Med. 2006;354(1):11–22. doi:10.1056/NEJMoa052434.