ABSTRACT
Second-generation COVID-19 vaccines with improved immunogenicity (e.g., breadth, duration) and availability (e.g., lower costs, refrigerator stable) are needed to enhance global coverage. In this work, we formulated a clinical-stage SARS-CoV-2 receptor-binding domain (RBD) virus-like particle (VLP) vaccine candidate (IVX-411) with widely available adjuvants. Specifically, we assessed the in vitro storage stability and in vivo mouse immunogenicity of IVX-411 formulated with aluminum-salt adjuvants (Alhydrogel™, AH and Adjuphos™, AP), without or with the TLR-9 agonist CpG-1018™ (CpG), and compared these profiles to IVX-411 adjuvanted with an oil-in-water nano-emulsion (AddaVax™, AV). Although IVX-411 bound both AH and AP, lower binding strength of antigen to AP was observed by Langmuir binding isotherms. Interestingly, AH- and AP-adsorbed IVX-411 had similar storage stability profiles as measured by antigen-binding assays (competitive ELISAs), but the latter displayed higher pseudovirus neutralizing titers (pNT) in mice, at levels comparable to titers elicited by AV-adjuvanted IVX-411. CpG addition to alum (AP or AH) resulted in a marginal trend of improved pNTs in stressed samples only, yet did not impact the storage stability profiles of IVX-411. In contrast, previous work with AH-formulations of a monomeric RBD antigen showed greatly improved immunogenicity and decreased stability upon CpG addition to alum. At elevated temperatures (25, 37°C), IVX-411 formulated with AH or AP displayed decreased in vitro stability compared to AV-formulated IVX-411and this rank-ordering correlated with in vivo performance (mouse pNT values). This case study highlights the importance of characterizing antigen-adjuvant interactions to develop low cost, aluminum-salt adjuvanted recombinant subunit vaccine candidates.
Introduction
To date, the COVID-19 pandemic has resulted in >770 million confirmed cases and >6.9 million deaths worldwide.Citation1 Although first-generation COVID-19 vaccines are currently available based on mRNA-LNP, adenoviral-vector, and subunit protein vaccine platforms,Citation2,Citation3 there remains an urgent need for second-generation vaccines with improved immunogenicity to ensure efficacy against circulating variants of concernCitation4,Citation5 as well as greater global access. For example, mosaic nanoparticles with multimeric display of SARS-CoV-2 RBDs have been shown to induce a wide breadth of protection against many different SARS-like betacoronaviruses.Citation6,Citation7 Such subunit vaccine candidates are well suited for improving global access due to low cost-of-goods, high production capacity, and storage stability profiles compatible with refrigerated cold-chain infrastructure.Citation8–10
Subunit vaccines, however, generally require one or more immunostimulatory adjuvants to be effective. Several diverse adjuvant systems are available, which range from colloidal suspensions of aluminum-salts of varying chemical composition and morphology (generally referred to as alum), to oil-in-water emulsions, to specific immunostimulatory molecules (i.e., TLR agonists including oligonucleotides, phosphoryl lipid A, saponins) as well as combinations thereof.Citation11 Although the choice of adjuvant depends foremost on clinical performance, additional practical considerations including cost, availability of GMP materials, and effects on antigen compatibility must be considered.Citation11 This is especially true if the ultimate goal is to produce effective, lower-cost vaccines targeted for global use in low- and middle-income countries.
In this work, we focused on a subset of widely available adjuvants that are currently found in licensed vaccine products including aluminum-salts (alum), cytosine phosphoguanine (CpG) oligodeoxynucleotides and oil-in-water emulsions. These adjuvants differ in their composition, immunological effects, commercial availability, and cost. Alum adjuvants have been commonly used in vaccines for nearly a century, are inexpensive, readily available at large-scale, and have an excellent safety profile in a variety of infant, pediatric and adult vaccines.Citation12,Citation13 The CpG 1018™ oligonucleotide adjuvant (referred to herein as CpG) is a TLR9 agonist with demonstrated safety and efficacy in subunit vaccines including a licensed hepatitis B vaccine (HEPLISAV-B®) as well as when co-formulated with alum in a SARS-CoV-2 RBD-based vaccine.Citation14–18 The oil-in-water emulsion adjuvants MF59 and AS03 are currently licensed for use in seasonal and pandemic influenza vaccines (e.g., Fluad®, Focetria®, Pandemrix®, and Celura®). The major components of MF59 are squalene oil and the nonionic surfactants Polysorbate 80 and Span 85.Citation19,Citation20 There are considerable costs associated with access to and manufacturing of oil-in-water emulsion adjuvants to GMP standards at large scale.Citation21,Citation22 In addition, squalene is harvested from the livers of deep-sea sharks, which raises environmental, sustainability, and ecological concerns.Citation23 Since alum and CpG adjuvants do not have these same concerns, this work with a second-generation COVID-vaccine antigen focused on comparing formulations of alum (Alhydrogel™, AH and Adjuphos™ AP), with and without CpG, as bench-marked to an oil-in-water emulsion adjuvant, AddaVaxTM (AV), which is equivalent to MF59, but unavailable for human use.Citation24,Citation25
There are several different protein-based COVID-19 subunit vaccine candidates approved for use or under clinical development based on multimeric display of antigens on nanoparticles.Citation18,Citation26 Multivalent presentation of antigens has been successfully implemented previously in commercial vaccines using virus-like particles (VLP), including licensed vaccines against Hepatitis B Virus (HBV) and Human Papilloma Virus (HPV). These VLP-based vaccines consist of a viral surface protein (i.e., HBsAg and L1, for HBV and HPV, respectively), which self-assemble into VLPs that enable multimeric antigen display.Citation27,Citation28 In general, multivalent presentation of an antigen on the surface of a VLP generates higher cellular and humoral immune responses when compared to monomeric antigens.Citation29–36 Since VLPs are typically >10 fold larger by molecular weight when compared to monomeric proteins angiens, VLPs are more efficiently taken up by antigen presenting cells (APC), proteolytically processed, and presented by MHC class II. Furthermore, the repetitive conformational epitopes on the surface of VLPs promotes crosslinking of surface immunoglobulin B-cell receptors, which leads to a robust B-cell response.Citation37
One promising example of a COVID-19 vaccine antigen designed for multimeric antigen display on a nanoparticle is RBD-NPs, which are composed of a computationally designed nanoparticle (I53–50) engineered to display 60 copies of the Wuhan-1 SARS-CoV-2 receptor-binding domain (RBD).Citation33,Citation38 RBD-NPs elicit robust immune responses in mice and non-human primates (NHPs) when formulated with adjuvants such as AH, AH+CpG, AS37, a TLR7 agonist adsorbed to alum, and various oil-in-water emulsions. RBD-NPs formulated with the AS03 and AH+CpG adjuvant systems provided protective immunity based on induction of virus neutralizing antibody titers.Citation33,Citation39,Citation40 Furthermore, results from a Phase 1/2 and Phase 3 clinical trialsCitation41,Citation42 demonstrated an RBD-nanoparticle-based vaccine adjuvanted with AS03 (GP150) was highly immunogenic and well tolerated in humans and has now been approved for use in South Korea (SKYCovine® from SK Bioscience).
The major objective of this work was to assess the feasibility of developing a lower-cost, aluminum-salt adjuvanted formulation of a SARS-CoV-2 vaccine candidate composed of a two-component I53–50 nanoparticle displaying 60 copies of the SARS-CoV-2 Wuhan-1 RBD (termed IVX-411). Previous work has demonstrated that AV-adjuvanted IVX-411 induces robust neutralizing antibody responses in mice against both Wuhan-1 and variants of concern and protected Syrian hamsters in a SARS-CoV-2 challenge study.Citation31 In this work, we first performed physicochemical characterization and antigen-adjuvant interaction studies of IVX-411 formulated with aluminum-salt, CpG and oil-in-water emulsion adjuvants. We then developed stability-indicating antigen-binding assays to monitor the structural integrity of key epitopes of formulated IVX-411 in the presence of different adjuvants. Finally, we evaluated the storage stability and in vivo mouse immunogenicity profiles of the various IVX-411-adjuvant formulations. The results of this work are compared to previous work with similar formulations of monomeric RBD and are discussed more generally in the context of formulation development of low-cost vaccine candidates targeted for use in low- and middle-income countries (LMICs).
Materials and methods
Materials
IVX-411 antigen was provided frozen by Icosavax on dry ice at a protein concentration of 0.68 mg/mL in a formulation buffer (50 mM Tris, 150 mM NaCl, 0.1 M L-Arginine, 5% Sucrose, pH 8.0), thawed, aliquoted and stored at −80°C. CpG adjuvant was provided by Dynavax in a lyophilized powder form and was reconstituted in 20 mM Tris, 100 mM NaCl, pH 7.5 and stored at −20°C at a final concentration of 12–15 mg/mL. AH, AP, and AV adjuvants were purchased from InvivoGen (San Diego, CA). All other chemicals were of high purity and purchased from Sigma-EMD Millipore (Burlington, MA) except for sucrose, which was purchased from Pfanstiehl (Waukegan, IL).
Methods
The analytical methods employed in this study, including physicochemical characterization techniques, i.e., SDS-PAGE, Fourier transform infrared (FTIR) and intrinsic fluorescence (IF) spectroscopy, differential scanning calorimetry (DSC), sedimentation velocity analytical ultracentrifugation (SV-AUC), and dynamic light scattering (DLS), as well as antigen binding in vitro potency assays (competitive ELISAs) and mouse immunogenicity studies including pseudovirus neutralization titers are described in detail in the Supplemental Methods. Experimental procedures for antigen-adjuvant interaction (i.e., Langmuir adsorption isotherms) and storage stability studies are also provided in the Supplemental Methods. Briefly, for biophysical measurements in solution, IVX-411 was diluted to 0.1–0.3 mg/mL in the formulation buffer. For antigen-adjuvant binding studies, IVX-411 was diluted to 0.05–0.2 mg/mL in formulation buffer. For storage stability and mouse immunogenicity studies, IVX-411 was diluted to 2 mcg/mL in the formulation buffer with adjuvant concentrations of (i) 1.5 mg/mL AH, (ii) 1.5 mg/mL AP, (iii) 1.5 mg/mL AH and 0.3 mg/mL CpG, (iv) 1.5 mg/mL AP and 0.3 mg/mL CpG, or (v) 1X final concentration of AV.
Results
Analytical characterization of IVX-411 antigen
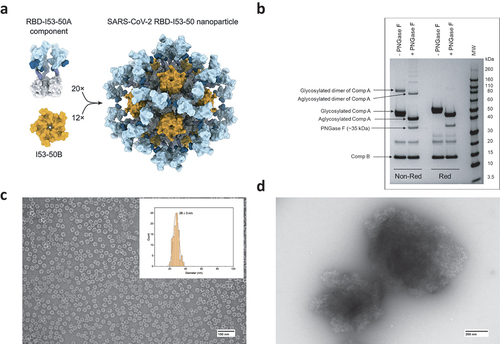
IVX-411 is a computationally designed, two-component VLP-based antigen consisting of the receptor-binding domain (RBD) of the spike protein from SARS-CoV-2 genetically fused to a homotrimeric complex (Component A) and a homopentameric complex (Component B). When Components A and B are mixed in solution, a VLP (IVX-411) spontaneously self assembles into a nanoparticle with multimeric display of RBD, as represented schematically in .Citation33 SDS-PAGE analysis () shows Components A and B migrate at the expected ~50 and ~15 kDa molecular weight (MW) values, respectively. Higher MW species (~90 kDa) were also observed in the non-reduced sample that were absent under reducing conditions, indicating the presence of disulfide-linked oligomers. After PNGase-F treatment, Component A shifted to a lower MW indicating the presence of N-linked glycans. An additional minor band at ~20–25 kDa could represent the core trimeric domain of Component A without RBD. The diameter of IVX-411 was 28 ± 3 nm as determined by negative staining Transmission Electron Microscopy (TEM) (). TEM analysis of IVX-411 formulated with AH showed the antigen adsorbed to the adjuvant surface (). Similar results were observed when IVX-411 was adsorbed to AP adjuvant (data not shown).
Figure 1. Analysis of IVX-411 VLP. (a) Schematic representation of the two-component IVX-411 VLP (also see text). (b) Representative reducing and non-reducing SDS-PAGE analysis of components A and B of IVX-411 following treatment with or without PNGase-F. (c) Representative negative stained TEM of IVX-411 in solution with particle size distribution indicated in the insert (n = 100 VLPs, mean particle size ± SD = 28 ± 3 nm). (d) TEM analysis of IVX-411 antigen adsorbed to the aluminum-salt adjuvant Alhydrogel. Samples were formulated in a buffer containing 50 mM Tris, 150 mM NaCl, 5% sucrose, 0.1 M L-arginine, pH 8.0. The cartoon display in panel (a) was reproduced from Walls et al.Citation33.
Overall secondary and tertiary structure analysis of proteins compromising the IVX-411 antigen was performed by Fourier transform infrared (FTIR) and intrinsic fluorescence (IF), respectively. The second-derivative FTIR spectra of the Amide I region (1700–1600 cm−1) indicate that the secondary structure content is a mixture of α-helix and β turn/sheet consistent with the expected structure of the components of IVX-411 (). IF analysis (excitation at 295 nm) showed an emission peak maximum of 332 nm, a result indicating the average tryptophan residue in both components (including RBD) is in a relatively apolar environment (). For particle size determination of IVX-411, sedimentation velocity analytical ultracentrifugation (SV-AUC) analysis revealed three different species with the major ~36S species (~77%) presumably intact, assembled nanoparticles (). Two low abundant species at approximately 9S and 53S were also observed, which were likely impurities and higher ordered species, respectively. An estimated MW of roughly 4.1 MDa was calculated for IVX-411 from the SV-AUC analysis, a result close to the predicted MW of 3.9 MDa based on the primary sequence.
Figure 2. Biophysical characterization of the IVX-411 VLP. (a) Second derivative FTIR spectra of the Amide I band, and (b) intrinsic Tryptophan emission spectra (excitation at 295 nm) of IVX-411 sample at 10°C. (c) Molecular size distribution of VLP species as measured by SV-AUC. (d, e) The overall conformational stability and aggregation behavior as a function of increasing temperature as measured by DSC (Tm = 47.5 ± 0.1) and DLS (Tm = 48.6 ± 0.7), respectively. Data shown are the mean of at least three independent measurements while the error bars represent one standard deviation. All experiments were performed in a 50 mM Tris, 150 mM NaCl, 5% sucrose, 0.1 M L-Arginine, pH 8.0 formulation buffer.
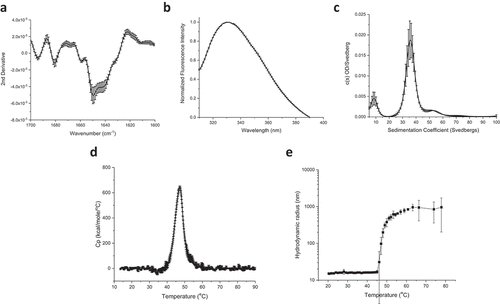
The thermal stability of IVX-411 was evaluated by differential scanning calorimetry (DSC) and dynamic light scattering (DLS). A single endothermic event was observed by DSC that initiated at approximately 42°C (onset temperature, Tonset) with a thermal melting temperature of ~48°C (). The aggregation profile of IVX-411 was measured by DLS as a function of temperature (). A steep increase in the hydrodynamic radius from ~14 nm to ~1000 nm, which is indicative of aggregation, was observed initiating at ~45°C. Taken together, the DSC and DLS results indicated structural alterations within the IVX-411 antigen initiated at ~42°C followed by aggregation at ~45°C.
IVX-411 antigen binds both AH and AP adjuvants, but with greater binding strength to AH
Prior to evaluating the binding of IVX-411 to AH and AP, the point of zero charge was measured by phase analysis light scattering and was ~5.8 (data not shown). When combined with AH (pI ~ 11) in a formulation buffer at pH 8, IVX-411 bound 100% to AH, a result not unexpected if binding occurred via electrostatic interactions. Surprisingly, IVX-411 also bound 100% to AP adjuvant (pI ~ 5) in the same formulation buffer (), suggesting IVX-411 bound to AP via other mechanisms than solely electrostatic. To better understand these observations, unassembled components A and B were evaluated for their ability to bind AH and AP under the same conditions as IVX-411. Both bound nearly 100% to AH, but only bound ~30% and 56%, respectively, to AP (). This result suggests that the binding of IVX-411 to each of the alum adjuvants (AH and AP) is mediated by both components of the mature, two-component VLP.
Figure 3. IVX-411 binds to both Alhydrogel (AH) and Adjuphos (AP) while the individual components bind completely to AH and partially to AP. (a) Representative reducing SDS-PAGE of binding of each component and the assembled VLP to AH and AP (U = unbound; B = bound). (b) The percentage of each component that is bound to either AH or AP was determined by gel densitometry. The data are the mean of three independent binding experiments with the error bars representing the standard deviation. (c, d) Increasing amounts of IVX-411 (0–600 mcg) were added to a fixed amount (50 mcg) of alum adjuvant (c) Alhydrogel, AH or (d) Adjuphos, AP and the amount of unbound antigen was determined by UV-Visible spectroscopy. The data from individual experiments were each fit using the Langmuir equation (red lines) with the red shading indicating the 95% CIs of the linear fits. (e) Statistical comparisons of the experimentally determined binding strength (KL) and binding capacity (Qmax) values of IVX-411 when bound to AP and AH by a two tailed student’s t-test. The KL and Qmax data are the mean of 3 or 5 binding isotherms for AH and AP, respectively, ± one standard deviation. (f) Addition of 1 M NaCl enhances the binding of IXV-411 to AH and AP and could no longer be fit to the Langmuir equation. The data are the mean of two independent experiments with the error bars representing the data range. All experiments were performed in a 50 mM Tris, 150 mM NaCl, 5% sucrose, 0.1 M L-Arginine, pH 8.0 formulation buffer.
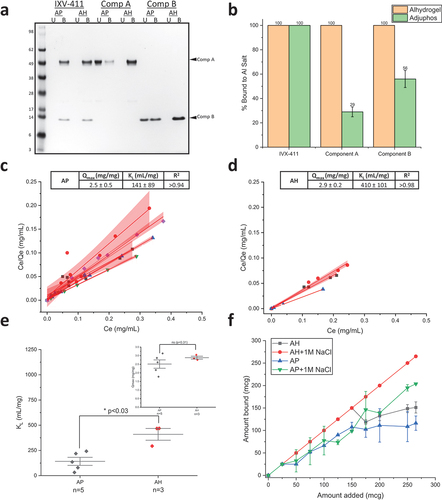
We determined the monolayer binding capacity and binding strength of IVX-411 to AH and AP via Langmuir binding isotherm studies (). The monolayer binding capacity (Qmax) values of IVX-411 to AH and AP were similar, 2.9 and 2.5 mg IVX-411/mg Al salt, respectively, which was statistically insignificant by a two tailed t-test (p > 0.3; inset). The mean binding strength (KL) values, however, differed by approximately three-fold for AH (~410 mL/g) compared to AP (~141 mL/g), indicating tighter antigen binding to AH, a result which was statistically significant by a two-tailed t-test (p < 0.03) (). When the NaCl concentration in the formulation buffer was increased to 1 M, more IVX-411 bound to AH and AP, and the binding data could no longer be fit to the Langmuir equation using the same experimental parameters at lower ionic strength (0.15 M) (). This latter result indicates that electrostatic interactions are not the primary driver in the binding of IVX-411 antigen to alum (AH or AP) adjuvants, and other molecular interactions are likely primarily responsible for antigen adsorption.
AH and CpG adjuvants conformationally destabilize IVX-411 while AP and the oil-in water emulsion have minimal effect
Having previously observed conformational destabilization of monomeric RBD when co-formulated with alum and/or CpG adjuvants,Citation43 one objective in this work was to determine if multimeric presentation of RBD in a VLP-based antigen had the same destabilizing effects. First, IVX-411 at the protein concentration used in the in vivo immunogenicity studies (2 mcg/mL) was formulated with the various adjuvants and antigen-adjuvant interactions were analyzed by SDS-PAGE (). As expected, IVX-411 was 100% bound to AH or AP adjuvants independent of the addition of CpG. When IVX-411 was co-formulated with Alum and CpG (5:1 Alum to CpG by weight), approximately 10% and 100% of the CpG was bound to AP and AH, respectively. In the oil-in-water emulsion (AV) formulation, the component A and B bands displayed an apparent slower migration in the gel, likely due to the sample’s higher viscosity ().
Figure 4. Effect of various adjuvants on the overall conformational stability of IVX-411. (a) Percent antigen bound vs unbound to adjuvant as determined by SDS-PAGE analysis with representative gel showing IVX-411 antigen samples formulated with various adjuvants (2 mcg/mL IVX-411 in presence of AH, AP, with or without CpG, or AV). The unbound (U) and the bound (B) fractions were separated by centrifugation followed by removal of alum-bound antigen (and CpG) using “strong” desorption conditions (see methods). (b, c) DSC thermograms comparing IVX-411 in solution with IVX-411 formulated with (b) AH or (c) AP alum-adjuvants, both with or without CpG. *The onset temperature value of antigen with AP alone could not be calculated due to baseline noise. (d) DSC thermograms comparing IVX-411 in solution to IVX-411 formulated with AV. As shown in the inset bar graphs, the effect of the adjuvants on the overall conformational stability of IVX-411 was summarized by calculating delta Tm and delta Tonset values (i.e., subtracting the Tm and Tonset values of the no adjuvant control from the Tm and Tonset values of formulated samples). Thermograms are a mean of duplicate scans and the data in the inserts of B-D are the mean of two measurements and the error bars represent the data range after accounting for error propagation. All DSC experiments were performed at 200 mcg/mL IVX-411 in a 50 mM Tris, 150 mM NaCl, 5% sucrose, 0.1 M L-Arginine, pH 8.0 formulation buffer.
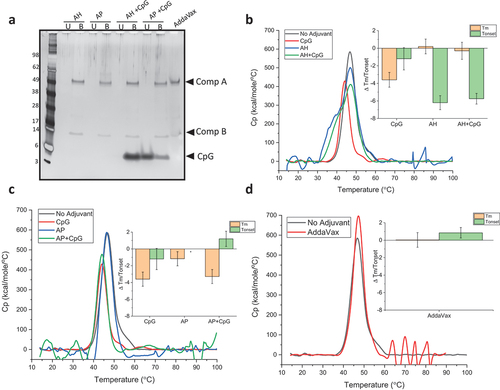
The conformational stability of IVX-411 formulated with the various adjuvants was then assessed by DSC. Compared to unadjuvanted IVX-411, the addition of CpG resulted in a ~ 4°C decrease in Tm, without affecting Tonset, values. In contrast, when IVX-411 was formulated with AH, the value of Tonset decreased by ~6°C with no change in Tm. When IVX-411 was formulated with AP, only a slight change in Tm value was observed (compared to the unadjuvanted control), but Tonset values could not be reliably determined due to baseline noise. When IVX-411 was co-formulated with CpG + AH or CpG +AP, none to small additional destabilizing effects of CpG were observed in the DSC thermograms, respectively (). Taken together, IVX-411 was 100% bound to both AH and AP, but displayed more structural destabilization when adsorbed to AH (compared to AP) as measured by DSC analysis. Although CpG alone destabilized the overall conformational stability of IVX-411, relatively small, additional destabilizing effects of CpG were observed in the presence of alum. Finally, when formulated in the AV emulsion, no notable changes in Tm or Tonset values of IVX-411 were observed compared to unadjuvanted control ().
Real-time, accelerated and stressed stability studies of adjuvanted IVX-411 formulations as measured by competitive ELISAs
To determine the relative stability of the various adjuvanted IVX-411 formulations (), we developed competitive binding ELISAs with three different ligands: (i) ACE2 receptor, a well-established ligand which binds to neutralizing epitopes on RBD,Citation44 (ii) mAb S309, a conformational-dependent, neutralizing mAb that binds adjacent to the ACE2 site,Citation45 and (iii) mAb CR3022, a conformational-dependent, non-neutralizing mAb that binds on the opposite side of the ACE2 receptor-binding surface.Citation46 Briefly, in this assay format, the ligands are preincubated with the formulated IVX-411 samples, centrifuged, and the free ligand concentration (not bound to antigen) is determined by measuring its binding to monomeric RBD adsorbed on the ELISA assay plate (see Supplemental methods and as described previously).Citation43 Representative binding curves of unadjuvanted IVX-411 and IVX-411 formulated with AH (with and without 37°C incubation for 2 weeks) with each of the three ligands are displayed in .
Figure 5. In vitro binding assays of IVX-411 in presence of various adjuvants before and after thermal stress. Representative competitive ELISA binding curves of (a) ACE2, (b) S309, and (c) CR3022 ligands to IVX-411 antigen in solution or formulated with either AH or AP. Representative ELISA binding curves of IVX-411 formulated with either AH or AP after incubation at 37°C for two weeks as measured by binding to (d) ACE2, (e) S309, and (f) CR3022. The IVX-411 antigen concentration was calculated from the OD450 values using a 4-point logistic fit of a reference sample. This reference sample was prepared using a frozen aliquot of IVX-411 that was freshly thawed and then mixed with each respective adjuvant at a known antigen concentration (see Supplemental methods). Summary of the relative in vitro binding results of formulated IVX-411 samples after incubation at 37°C for one and two weeks for each ligand (g) ACE2, (h) S309, and (i) CR3022. Data shown in g–i are the mean of two samples measured in duplicate (n = 4) with the error bars representing one standard deviation. *Indicates that no protein was detected. All samples contained 2 mcg/mL IVX-411 formulated with 1X AV, 1.5 mg/mL AH, 1.5 mg/mL AP, 1.5 mg/mL AH +0.3 mg/mL CpG, or 1.5 mg/mL AP +0.3 mg/mL CpG.
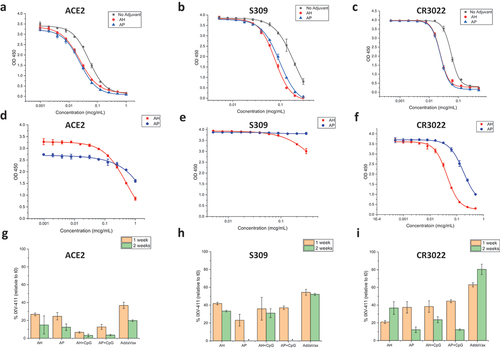
Table 1. Summary of the composition of the various adjuvanted IVX-411 formulations used in mouse immunogenicity studies. AH, aluminum hydroxide (Alhydrogel™); AP, aluminum phosphate (Adjuphos™); CpG, CpG-1018™ oligonucleotide, AV: AddaVax™ (oil-in-water emulsion).
To further establish the stability-indicating nature of this competitive ELISA assay, the various adjuvanted IVX-411 samples were incubated at 37°C for one to 2 weeks (). When compared to 4°C control samples, losses were observed in IVX-411’s ability to bind each of the three ligands after thermal stress. For example, after thermal stress, IVX-411 binding to the ACE2 receptor displayed a ~ 80–90% loss vs. binding to the CR3022 and S309 epitopes (60–80% loss). When comparing different formulations, the AV formulation of IVX-411 displayed a trend of the slowest degradation rate as measured by the binding to each of the three ligands.
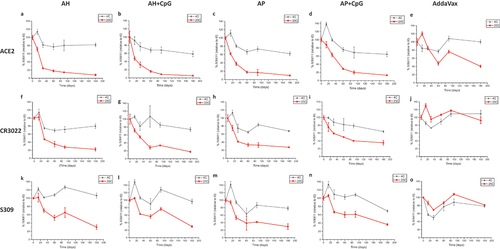
Real-time (2–8°C) and accelerated (25°C) storage stability studies that lasted up to 6 months were then set up for five IVX-411 adjuvanted formulations () and the relative stability profiles were monitored by competitive ELISA (). When compared to ACE2 receptor binding (), the CR3022 () and S309 () mAb binding profiles displayed less structural changes in the IVX-411 antigen during longer-term storage at 4 and 25°C, a result consistent with the short-term incubations at 37°C described above. For the alum containing formulations, IVX-411 retained approximately 70–80% of antigen binding relative to time zero after storage at 4°C up to 6 months as measured by all three competitive ELISAs. At 25°C, loss of antigen binding appeared multiphasic with a more rapid loss during the initial 30–60 days. Overall, the IVX-411 stability profiles at 25°C appeared similar across the alum formulations with ~20–40% of antigen binding remaining after 6 months. In contrast, the AV formulation of IVX-411 showed the best stability profile at 4°C and 25°C across the three competitive ELISAs (). For example, the AV-formulated IVX-411 maintained roughly 80–100% antigen binding (relative to time zero) with each ligand during storage at 4°C up to 6 months. At 25°C, approximately 40% of antigen binding was observed with the ACE2 receptor and approximately 80–90% with the CR3022 or S309 mAb. In summary, the oil-in-water emulsion-based AV formulation of IVX-411 was the most stable during storage at elevated temperatures, and there were no notable differences in the stability profiles of the alum containing formulations independent of co-formulation with CpG.
Figure 6. Real-time, accelerated and stressed stability studies of IVX-411 in presence of various adjuvants as measured by in vitro binding assays. Competitive ELISA results with formulated IVX-411 samples as measured by binding to (a–e) ACE2, (f–j) CR0322, and (k–o) S309. Samples were stored at 4° and 25°C and consisted of 2 mcg/mL IVX-411 formulated with either 1X AV, 1.5 mg/mL AH, 1.5 mg/mL AP, 1.5 mg/mL AH +0.3 mg/mL CpG, or 1.5 mg/mL AP +0.3 mg/mL CpG. Data shown are the mean of two samples measured in duplicate (n = 4) with the error bars representing one standard deviation.
Effect of IVX-411 dose and adjuvant formulation on neutralizing antibody titers in mice
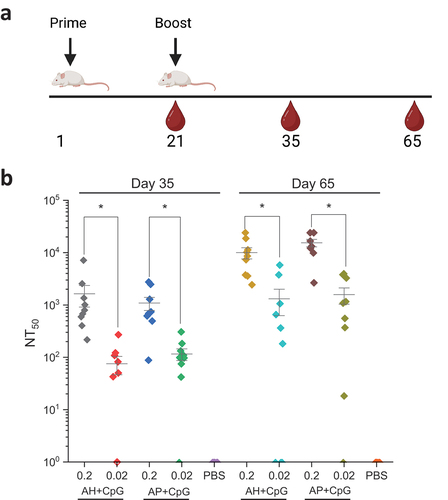
As an initial evaluation of mouse immunogenicity profiles of the adjuvanted IVX-411 formulations, we examined the dose effect of IVX-411 antigen on pseudovirus neutralizing antibody titers (pNT). We immunized mice with 0.2 or 0.02 mcg IVX-411 (total protein) adjuvanted with either 150 mcg AH or AP and co-formulated with 30 mcg CpG. Mice were primed on Day 1 with a booster dose administered on Day 21 (). On Day 21, prior to the booster dose, neutralizing titers at both doses in all formulations were below levels required to generate NT50 values (data not shown). On Day 35, after the booster dose, we observed NT50 values > 10 fold higher in the 0.2 mcg dose independent of the formulation (). This trend continued on Day 65, where the NT50 values increased ~10 fold in both formulations at both doses. No significant differences in NT50 values were observed between AH+CpG vs AP+CpG formulations on days 35 or 65 ().
Figure 7. SARS-CoV-2 pseudovirus neutralization activity in the sera of mice immunized with low and high doses of IVX-411 formulated with CpG 1018 and AH or AP. (a) Groups of mice (n = 9/group) were immunized on study day 0 with 0.2 or 0.02 mcg IVX-411 adjuvanted with either 150 mcg AH and 30 mcg CpG or 150 mcg AP and 30 mcg CpG. Mice were boosted with a second dose on Day 21. Serum samples were collected on days 35 and 65. (b) NT50 values from sera collected on days 35 and 65 using SARS-CoV-2 (D614G B.1, 20A) reporter virus particles. Asterisks indicates a statistically significant difference by Kruskal-Wallis analysis using Dunn’s multiple comparisons test (*p < .03). The diagram in (a) was created using BioRender.com.
In a second mouse immunogenicity study, various alum adjuvanted formulations of IVX-411 were compared head-to-head with the AV formulation. In addition, to correlate the results of the in vitro competitive ELISA results to in vivo performance, the IVX-411 formulations were stored at 4°C or 37°C for 2 weeks prior to immunization. The IVX-411 antigen (0.2 mcg dose) was adjuvanted with either AH or AP, both with and without CpG using the same prime-boost dosing regimen described above (). Similar to the dose-ranging results described above, NT50 values could not be determined at Day 21 (data not shown).
Figure 8. SARS-CoV-2 pseudovirus neutralization titers of mice immunized with IVX-411 in presence of various adjuvants with and without thermal treatment. (a) groups of mice (n = 8/group) were immunized with 0.2 mcg IVX-411 along with either 150 mcg AH, 150 mcg AP, 150 mcg AH + 30 mcg CpG, 150 mcg AP + 30 mcg CpG, or 1X AV. The vaccine formulations were either stored at (b) 4°C or (c) 37°C for 2 weeks prior to immunization and Day 35 neutralization titers were measured. (d) NT50 values derived using SARS-CoV-2 (D614G B.1, 20A) and sera from the AP and AV formulations at days 35 and 65 were compared after storage at 4°C and 37°C for 2 weeks. Mice were boosted on Day 21 and sera collected on days 35 and 65. Asterisks indicates a statistically significant difference by Kruskal-Wallis analysis using Dunn’s multiple comparisons test (*p < .03). The diagram in (a) was created using BioRender.com.
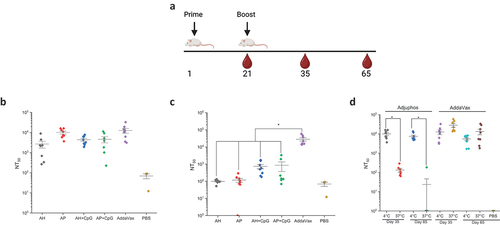
On Day 35, the highest neutralizing titers were observed in the IVX-411 groups formulated with either AV or AP (). Approximately 2–4 fold lower neutralization titers were observed when the AV group was compared to the other groups (AH, AH+CpG and AP+CpG). For the IVX-411 formulations stored at 37°C for 2 weeks, at least a 5-fold decreases in neutralization titers were observed in all formulations except for the AV-adjuvanted formulation, which displayed a trend of slightly higher titers relative to the 4°C samples (). When comparing the alum adjuvanted groups of IVX-411, with or without CpG, no differences in pNT values were observed with non-stressed samples. After thermal stress, although a ~ 6–7 fold higher mean neutralization titers were observed in the alum + CpG containing formulations (vs alum alone), these differences were statistically insignificant (p > 0.9, a result due to the two mice in each group that generated pNT >1000, which influenced the calculated the mean values).
We further compared the neutralizing titer responses of the two best performing IVX-411 formulations, adjuvanted with either AP or AV, at day 35 and day 65. The levels of neutralizing antibodies were sustained through day 65 in both formulations (). When incubated at 37°C for 2 weeks prior to immunization, there was, however, a ~500 fold decrease in the neutralization titers generated by the AP (versus AV) adjuvanted formulations (). For the IVX-411 adjuvanted formulations stored at 4°C, the AV or AP formulations displayed statistically similar, and the highest levels of, neutralizing titers in mice. The AV-formulated IVX-411, however, maintained the ability to induce a relatively high neutralizing antibody response after storage at 37°C for 2 weeks, whereas the capacity of the alum adjuvanted formulations to elicit neutralizing antibodies was greatly diminished. Finally, since the highest neutralization titers were observed in mice given AV-formulated IVX-411, we evaluated the ability of sera from this group to neutralize the Omicron pseudovirus. Only a negligible neutralizing response was observed in that NT50 values could not be determined (data not shown.) These results, taken together with the known effectiveness of the bivalent mRNA vaccines,Citation47 suggests that an effective boosting strategy using this multivalent VLP vaccine candidate could include RBD antigens from both the Wuhan-1 RBD and circulating variants.
Discussion
The major goal of this work was to assess the feasibility of developing a second-generation, low-cost COVID-19 vaccine candidate containing an aluminum-salt adjuvanted formulation of a two-component VLP displaying SARS-CoV-2 antigen. To this end, we tested aluminum-salt (i.e., AH, AP) adjuvanted formulations of IVX-411, and then compared their stability profiles and in vivo performance to IVX-411 formulated with newer adjuvant (i.e., alum+ CpG, oil-in-water emulsion, AV). In addition, results from the RBD-antigen displayed within a VLP (IVX-411) from this work can be compared to our recently reported results with a monomeric RBD antigen alone (RBD-J) formulated with the same alum adjuvants with and without CpG.Citation43,Citation48
Characterizing aluminum-salt formulations of IVX-411
Differences in antigen-aluminum adjuvant-binding strength can play a key role in immunopotentiation. If the adsorptive strength is too tight, it could potentially interfere with antigen processing and presentation to antigen presenting cells via MHC-II and thus impede a robust B-cell response. We observed a trend of reduced pNT values in mice immunized with IVX-411 adjuvanted with AH alone when compared to AP alone; however, this result was not significantly different (p >0.05). Interestingly, the IVX-411 antigen in the formulation buffer is completely bound to both AH and AP aluminum-salt adjuvants. When the binding interactions were quantified by Langmuir binding isotherm analysis, there was approximately 3-fold greater binding strength of IVX-411 to AH compared to AP. This result suggests increased binding strength of IVX-411 to AH may have resulted in reduced immunopotentiation. Hansen et al. observed similar results with decreased antibody responses of a vaccine antigen as a function of increasing adsorption strength to alum.Citation49 Similarly, increased immunogenicity was observed when Hepatitis B surface antigen (HBsAg) was less strongly bound to alum adjuvant.Citation50
In the formulation buffer at pH 8, the surface of the colloidal suspension of AH is positively charged while AP is negatively charged.Citation13 IVX-411 is composed of two components that have different isoelectric points, Comp A and Comp B with pI values of ~8.7 and ~6.7, respectively, as calculated from the primary sequence. Thus, it is possible that components A and B could be binding AP and AH, respectively, through preferential electrostatic interactions at pH 8. When we evaluated these individual components; however, we observed complete binding to AH. Binding of both individual components to AP, albeit incomplete, was also observed. Thus, it is unlikely that IVX-411 binds to AH and AP via a bipolar and strictly electrostatic mechanism as reported by Dagouassat et al.Citation51 for BBG2Na, which is another multicomponent vaccine (see below). On the other hand, it is possible that the increased avidity of IVX-411 through multivalent presentation of RBD could be responsible for binding to both AH and AP.
When the ionic strength of the formulation buffer was increased approximately six-fold (i.e., 0.15 to 1 M NaCl), not only did more IVX-411 antigen bound to AH or AP, but the binding results could no longer be fit to a Langmuir model. These observations further suggest that electrostatic interactions are not the primary forces responsible for IVX-411 binding to AH and AP. Higher NaCl concentrations could promote hydrophobic interactions between antigen-adjuvant by reducing solute solvation, increasing exposure of hydrophobic regions of the RBD-VLP, and thus increasing the amount of IVX-411 bound to the colloidal surface of the aluminum-salts (AH, AP) independent of the surface charge.Citation52,Citation53
We also observed a lowering of the conformational stability of IVX-411 when bound to AH, but not AP, as measured by DSC. This result is consistent with stronger interactions between IVX-411 and the surface of AH. Although varying degrees of conformational destabilization of different protein antigens after adsorption to aluminum-salt adjuvants have been widely reported,Citation43,Citation54–57 there are exceptions to this trend with other reports observing no notable structural destabilization after antigen adsorption.Citation58,Citation59 Taken together, the antigen-adjuvant binding data suggest hydrophobic interactions are the main driving force responsible for IVX-411 antigen adsorption to aluminum-salt adjuvants, although electrostatic interaction may also play a secondary role that could explain stronger IVX-411 antigen-alum adjuvant interactions with AH compared to AP.
These results, although intriguing, are not unprecedented and have been observed with other vaccine antigens. For example, the fusion protein BBG2Na comprising the central domain of RSV G glycoprotein, G2Na, and the albumin binding domain of Streptococcus protein G, BB, bound both AH and AP.Citation51 The mechanism of this binding, however, was primarily electrostatic via the acidic pI of BB (5.5) and the basic pI of G2Na (10.0) that was responsible for binding AH and AP, respectively.Citation51 Interestingly, robust in vivo immunogenicity and protection profiles in mice were observed with a subunit RSV fusion protein (BBG2Na) vaccine candidate upon RSV challenge independent of the type of aluminum-salt adjuvant used.Citation51 Another study found high binding (>90%) of recombinant botulinum toxin fragments to both AH and AP, although the results were solution pH and ionic strength dependent, which the authors proposed were a combination of electrostatic and ligand exchange interactions.Citation60 Finally, HPV 16 VLPs have been observed to bind both AH and AP adjuvants.Citation61
Stability profiles of alum adjuvanted formulations of IVX-411 (RBD-VLP) compared to other adjuvants and monomeric RBD antigen
The antigen-adjuvant interactions, storage stability profiles, and mouse immunogenicity results described above for alum formulations of IVX-411 were then compared with IVX-411 formulations containing either CpG, alum + CpG (i.e., AH+CpG, AP+CpG) or an oil-in-water emulsion (AV). In addition, we compared these results to our recently reported results examining the stability and immunogenicity of a monomeric RBD antigen (RBD-J) formulated with the same adjuvants.Citation43 Originally, we had also planned to evaluate the stability of IVX-411 in solution. . This analysis, however, was hindered due to adsorption of IVX-411 to the surface of the glass vial at the low protein doses examined in this work (data not shown). Primary container surface adsorption was not observed when IVX-411 was pre-adsorbed to alum, or formulated with AV adjuvants.
The molecular mechanisms by which adjuvants either stabilize, destabilize, or have no effect on the structural integrity of subunit vaccine antigens remain poorly understood.Citation54,Citation55 Several studies have examined the effects of thermal and freeze induced degradation of protein antigens on the surface of aluminum salts.Citation27,Citation55,Citation56,Citation62 In a recent study by our group, we observed that a monomeric RBD-J antigen adjuvanted with AH or AH+CpG (CpG ™ Adjuvant) displayed a dramatic decrease (8–27°C) in conformational stability as measured by DSC.Citation43 A roughly 12°C decrease in the Tm value was measured in the previous work when monomeric RBD (RBD-J) was adsorbed to AH, while no notable change in Tm values was observed in this work when multimeric RBD on a nanoparticle (IVX-411) was adsorbed to AH. Moreover, DSC results for IVX-411 upon the addition of CpG alone resulted in an approximately ~4°C decrease in Tm values, which was a notably less destabilization effect than was observed previously with monomeric RBD-J (~13°C decrease).Citation43 The RBD-J antigen is a stabilized form of RBD as a result of two amino acid substitutions, so the RBD portion of IVX-411 could be further optimized for greater stability.Citation29 These results are consistent with the RBD antigen being more stable when displayed on the surface of a VLP (IVX-411), compared to a monomeric protein (RBD-J), likely due to the nanoparticle component protecting the RBD antigen from direct destabilization via alum binding. Finally, no notable conformational destabilization of IVX-411 was observed in the AV formulation by DSC. This result indicates that the oil-in-water emulsion adjuvant (AV) was the least destabilizing for IVX-411 amongst the adjuvants analyzed in this study.
We also monitored the storage stability of the various IVX-411 adjuvanted formulations during real-time and accelerated temperature conditions. To this end, we developed an in vitro competitive ELISA to monitor the ability of IVX-411 to bind ACE2 receptor and the mAbs CR3022 and S309 as a function of time at three different temperatures with the objective to determine if the adjuvants affected the stability of IVX-411. One advantage of using competitive ELISA is its ability to monitor critical epitopes on RBD that are directly related to antigenicity of the vaccine candidate as opposed to measuring overall conformational changes by DSC. In addition, there is no need to desorb/extract the antigen from alum or the oil-in-water emulsion adjuvants, respectively, which allows for direct measurement of the antigen integrity in the presence of adjuvants.
Over a six-month timeframe during storage at 4° or 25°C, we observed similar IVX-411 stability profiles in the AH, AH+CpG, AP, or AP+CpG formulations. The AV adjuvanted formulation displayed superior stability profile over 6 months with 80–100% of CR3022 and S309 ligand-binding ability remaining after 6 months at 4°C and 25°C. At 37°C, similar losses in antibody binding were observed in all formulations with the AV formulation displaying the best relative stability profile. A similar degradation pattern was observed while studying monomeric RBD (RBD-J) formulated with AH at 4°C and 25°C over the course of 90 days,Citation43 but the loss of the ability to bind ACE2 was accelerated when CpG was co-formulated with AH. Conversely, IVX-411 adjuvanted with CpG had no effect on ACE2 binding as a function of time or temperature when co-formulated with alum adjuvants. Taken together, these data suggest that the stability profile of the formulated RBD antigen is stabilized by multimeric incorporation within a VLP (IVX-411) compared to the monomeric form (RBD-J).
Mouse immunogenicity profiles of aluminum-salt formulations of IVX-411 (RBD-VLP) compared to other adjuvants and monomeric RBD-antigen
Comparable pNT values were measured in mice immunized with AP and AV, while a trend of lower titers (albeit not significantly different, p > 0.05) wasobserved when IVX-411 was adjuvanted with AH, independent of co-formulation with CpG. These observations are in stark contrast to monomeric RBD-based subunit vaccine candidate (RBD-J), which had a higher neutralizing antibody response when AH was co-adjuvanted with CpG, yet was destabilized during storage.Citation43,Citation63 In the case of IVX-411, the addition of CpG to alum adjuvanted IVX-411 did not improve neutralization titers in mice when vaccinated with non-stressed samples (stored at 4°C). On the other hand, in mice administered stressed IVX-411 samples (37°C, 2 weeks), the same CpG + alum samples showed a trend toward improved neutralization titers (albeit statistically insignificant due high variability). Finally, alum formulations with and without CpG displayed similar IVX-411 stability profiles during real time and accelerated storage.
The IVX-411 doses used in this mouse immunogenicity study were >1 order of magnitude lower (0.2 mcg total antigen which is ~40% RBD by weight) when compared to monomeric RBD-J formulations used in previous mice studies (5 mcg), a result which indicates IVX-411 induced a more potent immune response than monomeric RBD-antigens. Similar results have been reported with engineered RBD nanoparticles constructed using a SpyTagged RBD and I301 nanoparticles containing SpyCatcher.Citation29 Moreover, more robust neutralizing and cell-mediated responses were observed in an RBD/N subunit protein vaccine candidate adjuvanted with AH+CpG (ODN2395) when compared to other adjuvant combinations (i.e., MPLA/Quil A, AddaS03).Citation64
After thermal stress, the loss of IVX-411 binding to ACE2 receptor in the competitive ELISA assay correlated well with the reduction in pNT in mice with alum adjuvanted IVX-411 formulations. For thermally stressed AV-adjuvanted IVX-411 formulations, no reduction in neutralization titers in mice was observed, which correlated with the superior stability profiles at 4°C, 25°C, and 37°C observed in the competitive ELISA using ACE2, CR3022, and S309 ligands. The surfactants polysorbate 80 and sodium trioleate could be responsible for this enhanced stability in the AV formulation.Citation65,Citation66 By utilizing the stability-indicating methods identified in this work (i.e., competitive ELISA, SDS-PAGE and DSC), future work could include formulation optimization studies (e.g., buffer pH, stabilizing additives, antigen-adjuvant binding strength) to improve the stability profile of AP formulations of IVX-411. Moreover, the addition of stabilizing excipients could ensure bulk antigen stability during downstream processing (e.g., purification, ultrafiltration-diafiltration, large-scale bulk freezing) and drug product formulation processing (e.g., large-scale bulk thawing, mixing and compounding with adjuvant, aseptic filling).
Losses in an in vitro potency assay may or may not correlate with in vivo immunogenicity. For example, losses in D-antigenicity in an inactivated Sabin poliovirus vaccine subjected to thermal treatment did not correlate with reduced immunogenicity in rats.Citation67 Another example includes AH+CpG adjuvanted RBD-J vaccine candidate, which lost roughly 90% of the in vitro activity by ACE2 competitive ELISA, but retained the ability to generate robust pNT in mice (after two doses, but to a lesser extent after one dose).Citation43 The same study, however, demonstrated extensive denaturation of the RBD protein (pre-treatment with the reducing agent DTT) resulted in a complete loss of RBD ACE2 binding by competitive ELISA that correlated with the near complete abrogation of a neutralizing antibody response in mice.Citation43 Taken together, these results demonstrate that near complete loss of ACE2, CR3022, and S309 binding in the in vitro competitive ELISA assay can correlate well with the reduced ability to generate a robust in vivo neutralizing antibody response in mice.
Conclusions and future work
One major goal of this study was to characterize a low-cost alum adjuvanted formulation for IVX-411 and compare the results to other widely used adjuvants. Interestingly, although IVX-411 bound different aluminum-salt adjuvants (i.e., AP and AH) with similar antigen-adjuvant binding capacities, the AP-adjuvanted IVX-411 displayed superior neutralizing titers and weaker antigen-adjuvant binding strength. The AP-adjuvanted IVX-411 was compared to alum+CpG and oil-in-water emulsion (AddaVax, AV) formulations in terms of SARS-CoV-2 pseudovirus neutralizing antibody response in mice. The highest neutralization titers were generated in mice immunized with IVX-411 adjuvanted with either AP or AV. CpG addition to alum adjuvanted IVX-411 had no effect on pNT values when vaccinating with non-stressed samples but displayed a trend (yet statistically insignificant) of improved mean pNT values in mice immunized with stressed samples stored at 37°C for 2 weeks. This result differs from our recent report with monomeric RBD antigen (RBD-J) formulated with the same adjuvants, where AH+CpG adjuvanted RBD-J generated the highest pseudovirus neutralization titers, independent of thermal treatment.Citation43
We also compared these adjuvanted formulations of IVX-411 in terms of their relative stability profile as a function of time and temperature by developing competitive ELISAs that monitored antigen binding to the ACE2-receptor (or conformational mAb) in the presence of adjuvants. The AP and AV formulations of IVX-411 displayed similar storage stability profiles at 4°C as measured by competitive ELISAs. Interestingly, IVX-411 adsorption to alum or co-formulation with CpG did not affect storage stability, a result in contrast to our previous report with monomeric RBD which was destabilized by these adjuvants.Citation43 These results suggest the these adjuvanted formulations of the RBD are more stable when the RBD antigen is within in a multimeric display on the surface of a VLP compared to the monomeric form by itself. It will be of interest in future work to evaluate if other protein antigens display similar differences in their formulated stability profiles in the monomeric form vs. when expressed in a multimeric display on the surface of an I53–50-based VLP.
Finally, an important emphasis of future formulation optimization work with adjuvanted IVX-411 would include lowering costs to improve access for use in LMICs. Examples include lowering raw material and primary packaging costs (e.g., excipients, adjuvant, vials, stoppers), improving vaccine stability in the cold chain (e.g., vaccine vial monitor, VVM, designation),Citation9 and developing multidose (more doses per vial)Citation68,Citation69and combination vaccine (more vaccines per dose)Citation70 formulations.
Abbreviations
| AH: | = | Alhydrogel™ |
| AP: | = | Adjuphos |
| CpG: | = | CpG 1018™ Oligonucleotide Adjuvant |
| AV: | = | AddaVax™ |
| pNT: | = | pseudovirus neutralizing titers |
| TLR: | = | toll-like receptors |
| RBD: | = | receptor binding domain |
| GMP: | = | good manufacturing practices |
| mDa | = | megadalton |
| AS03: | = | Adjuvant System 03 |
| AS04: | = | Adjuvant System 04 |
| AS37: | = | Adjuvant System 37 |
| VLP: | = | Virus-Like Particle |
| IVX-411: | = | VLP vaccine containing RBD antigen using the I53–50 two component recombinant protein platform |
Supplementary Material
Download PDF (209.1 KB)Acknowledgments
We acknowledge the Bill and Melinda Gates Foundation for funding this work (# INV-021035 and INV-027417.) We thank the Global Health Discovery Collaboratory for supplying the recombinant ACE2-Fc receptor, CR3022 mAb, and the SARS-CoV-2 receptor-binding domain used as immunoassay reagents. We thank Dynavax Technologies for providing the CpG 1018™ adjuvant and Prem Thapa in the University of Kansas Microscopy and Analytical Imaging Core Lab for assistance with collecting and processing the TEM images. We also thank Scot Shepard, Ross Taylor, and Prathima Acharya at Icosavax, for thoughtful discussions and reviewing the manuscript.
Disclosure statement
CR and HL were employees and shareholders at Icosavax which was developing IVX-411 during this work.
Data availability statement
The datasets presented in this study are available in the KU ScholarWorks repository: https://doi.org/10.17161/1808.34795. The data are also available with the corresponding author.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2264594
Additional information
Funding
References
- WHO Coronavirus (COVID-19) dashboard. WHO, Geneva Switerland: 2023. https://covid19.who.int/.
- Kyriakidis NC, Lopez-Cortes A, Gonzalez EV, Grimaldos AB, Prado EO. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6(1):28. doi:10.1038/s41541-021-00292-w.
- Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–15. doi:10.1038/s41586-020-2798-3.
- Pulendran B, SA P., O’Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov. 2021;20(6):454–75. doi:10.1038/s41573-021-00163-y.
- Crommelin DJA, Anchordoquy TJ, Volkin DB, Jiskoot W, Mastrobattista E. Addressing the cold reality of mRNA vaccine stability. J Pharm Sci. 2021;110(3):997–1001. doi:10.1016/j.xphs.2020.12.006.
- Cohen AA, van Doremalen N, Greaney AJ, Andersen H, Sharma A, Starr TN, Keeffe JR, Fan C, Schulz JE, Gnanapragasam PNP, et al. Mosaic RBD nanoparticles protect against challenge by diverse sarbecoviruses in animal models. Science. 2022;377(6606):eabq0839. doi:10.1126/science.abq0839.
- Lee DB, Kim H, Jeong JH, Jang US, Jang Y, Roh S, Jeon H, Kim EJ, Han SY, Maeng JY, et al. Mosaic RBD nanoparticles induce intergenus cross-reactive antibodies and protect against SARS-CoV-2 challenge. Proc Natl Acad Sci USA. 2023;120(4):e2208425120. doi:10.1073/pnas.2208425120.
- Vu MN, Kelly HG, Kent SJ, Wheatley AK. Current and future nanoparticle vaccines for COVID-19. EBioMedicine. 2021;74:103699. doi:10.1016/j.ebiom.2021.103699.
- Kumru OS, Joshi SB, Smith DE, Middaugh CR, Prusik T, Volkin DB. Vaccine instability in the cold chain: mechanisms, analysis and formulation strategies. Biologicals. 2014;42(5):237–59. doi:10.1016/j.biologicals.2014.05.007.
- Rosa SS, Prazeres DMF, Azevedo AM, Marques MPC. mRNA vaccines manufacturing: challenges and bottlenecks. Vaccine. 2021;39(16):2190–200. doi:10.1016/j.vaccine.2021.03.038.
- Shi S, Zhu H, Xia X, Liang Z, Ma X, Sun B. Vaccine adjuvants: understanding the structure and mechanism of adjuvanticity. Vaccine. 2019;37:3167–78. doi:10.1016/j.vaccine.2019.04.055.
- Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004;82(5):497–505. doi:10.1111/j.0818-9641.2004.01286.x.
- HogenEsch H, O’Hagan DT, Fox CB. Optimizing the utilization of aluminum adjuvants in vaccines: you might just get what you want. NPJ Vaccines. 2018;3(1):51. doi:10.1038/s41541-018-0089-x.
- Campbell JD. Development of the CpG adjuvant 1018: a case study. Methods Mol Biol. 2017;1494:15–27.
- Janssen JM, Jackson S, Heyward WL, Janssen RS. Immunogenicity of an investigational hepatitis B vaccine with a toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared with a licensed hepatitis B vaccine in subpopulations of healthy adults 18-70 years of age. Vaccine. 2015;33:3614–8. doi:10.1016/j.vaccine.2015.05.070.
- Pollet J, Strych U, Chen WH, Versteeg L, Keegan B, Zhan B, Wei J, Liu Z, Lee J, Kundu R, et al. Receptor-binding domain recombinant protein on alum-CpG induces broad protection against SARS-CoV-2 variants of concern. Vaccine. 2022;40(26):3655–63. doi:10.1016/j.vaccine.2022.05.007.
- Kuo TY, Lin MY, Coffman RL, Campbell JD, Traquina P, Lin YJ, Liu LTC, Cheng J, Wu Y-C, Wu C-C, et al. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci Rep. 2020;10(1):20085. doi:10.1038/s41598-020-77077-z.
- Kleanthous H, Silverman JM, Makar KW, Yoon IK, Jackson N, Vaughn DW. Scientific rationale for developing potent RBD-based vaccines targeting COVID-19. NPJ Vaccines. 2021;6(1):128. doi:10.1038/s41541-021-00393-6.
- El Sahly H. MF59™ as a vaccine adjuvant: a review of safety and immunogenicity. Expert Rev Vaccines. 2010;9(10):1135–41. doi:10.1586/erv.10.111.
- O’Hagan DT, Ott GS, Nest GV, Rappuoli R, Giudice GD. The history of MF59((R)) adjuvant: a phoenix that arose from the ashes. Expert Rev Vaccines. 2013;12:13–30. doi:10.1586/erv.12.140.
- Wilkins AL, Kazmin D, Napolitani G, Clutterbuck EA, Pulendran B, Siegrist CA, Pollard AJ. AS03- and MF59-adjuvanted influenza vaccines in children. Front Immunol. 2017;8:1760. doi:10.3389/fimmu.2017.01760.
- Tregoning JS, Russell RF, Kinnear E. Adjuvanted influenza vaccines. Hum Vaccin Immunother. 2018;14(3):550–64. doi:10.1080/21645515.2017.1415684.
- Popa O, Babeanu NE, Popa I, Nita S, Dinu-Parvu CE. Methods for obtaining and determination of squalene from natural sources. Biomed Res Int. 2015;2015:1–16. doi:10.1155/2015/367202.
- Calabro S, Tritto E, Pezzotti A, Taccone M, Muzzi A, Bertholet S, De Gregorio E, O’Hagan DT, Baudner B, Seubert A, et al. The adjuvant effect of MF59 is due to the oil-in-water emulsion formulation, none of the individual components induce a comparable adjuvant effect. Vaccine. 2013;31(33):3363–9. doi:10.1016/j.vaccine.2013.05.007.
- Kim EH, Woodruff MC, Grigoryan L, Maier B, Lee SH, Mandal P, Cortese M, Natrajan MS, Ravindran R, Ma H, et al. Squalene emulsion-based vaccine adjuvants stimulate CD8 T cell, but not antibody responses, through a RIPK3-dependent pathway. Elife. 2020;9. doi:10.7554/eLife.52687.
- Yadav T, Kumar S, Mishra G, Saxena SK. Tracking the COVID-19 vaccines: the global landscape. Hum Vaccin Immunother. 2023;19(1):2191577. doi:10.1080/21645515.2023.2191577.
- Jain NK, Sahni N, Kumru OS, Joshi SB, Volkin DB, Russell Middaugh C. Formulation and stabilization of recombinant protein based virus-like particle vaccines. Adv Drug Deliv Rev. 2015;93:42–55. doi:10.1016/j.addr.2014.10.023.
- Markowitz LE, Schiller JT. Human papillomavirus vaccines. J Infect Dis. 2021;224:S367–S78. doi:10.1093/infdis/jiaa621.
- Dalvie NC, Rodriguez-Aponte SA, Hartwell BL, Tostanoski LH, Biedermann AM, Crowell LE, Kaur K, Kumru OS, Carter L, Yu J, et al. Engineered SARS-CoV-2 receptor binding domain improves manufacturability in yeast and immunogenicity in mice. Proc Natl Acad Sci USA. 2021;118(38):118. doi:10.1073/pnas.2106845118.
- Dalvie NC, Tostanoski LH, Rodriguez-Aponte SA, Kaur K, Bajoria S, Kumru OS, Martinot AJ, Chandrashekar A, McMahan K, Mercado NB, et al. SARS-CoV-2 receptor binding domain displayed on HBsAg virus–like particles elicits protective immunity in macaques. Sci Adv. 2022;8:eabl6015. doi:10.1126/sciadv.abl6015.
- McKechnie JL, Fiala B, Wolf C, Ellis D, Holtzman D, Feldhaus A. Virus-like particle displaying SARS-CoV-2 receptor binding domain elicits neutralizing antibodies and is protective in a challenge model. bioRxiv. 2022;518404.
- Khan F, Porter M, Schwenk R, DeBot M, Saudan P, Dutta S, Tetteh KKA. Head-to-head comparison of soluble vs. Qβ VLP circumsporozoite protein vaccines reveals selective enhancement of NANP repeat responses. PLos One. 2015;10:e0142035. doi:10.1371/journal.pone.0142035.
- Walls AC, Fiala B, Schafer A, Wrenn S, Pham MN, Murphy M, Tse LV, Shehata L, O’Connor MA, Chen C, et al. Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2. Cell. 2020;183:1367–82 e17. doi:10.1016/j.cell.2020.10.043.
- Bruun TUJ, Andersson AC, Draper SJ, Howarth M. Engineering a rugged nanoscaffold to enhance plug-and-display vaccination. Acs Nano. 2018;12:8855–66. doi:10.1021/acsnano.8b02805.
- Tan TK, Rijal P, Rahikainen R, Keeble AH, Schimanski L, Hussain S, Harvey R, Hayes JWP, Edwards JC, McLean RK, et al. A COVID-19 vaccine candidate using SpyCatcher multimerization of the SARS-CoV-2 spike protein receptor-binding domain induces potent neutralising antibody responses. Nat Commun. 2021;12(1):542. doi:10.1038/s41467-020-20654-7.
- Lacasta A, Kim HC, Kepl E, Gachogo R, Chege N, Ojuok R, Muriuki C, Mwalimu S, Touboul G, Stiber A, et al. Design and immunological evaluation of two-component protein nanoparticle vaccines for east coast fever. Front Immunol. 2022;13:1015840. doi:10.3389/fimmu.2022.1015840.
- Kushnir N, Streatfield SJ, Yusibov V. Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine. 2012;31(1):58–83. doi:10.1016/j.vaccine.2012.10.083.
- Bale JB, Gonen S, Liu Y, Sheffler W, Ellis D, Thomas C, Cascio D, Yeates TO, Gonen T, King NP, et al. Accurate design of megadalton-scale two-component icosahedral protein complexes. Science. 2016;353(6297):389–94. doi:10.1126/science.aaf8818.
- Grigoryan L, Lee A, Walls AC, Lai L, Franco B, Arunachalam PS, Feng Y, Luo W, Vanderheiden A, Floyd K, et al. Adjuvanting a subunit SARS-CoV-2 vaccine with clinically relevant adjuvants induces durable protection in mice. NPJ Vaccines. 2022;7(1):55. doi:10.1038/s41541-022-00472-2.
- Arunachalam PS, Walls AC, Golden N, Atyeo C, Fischinger S, Li C, Aye P, Navarro MJ, Lai L, Edara VV, et al. Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature. 2021;594(7862):253–8. doi:10.1038/s41586-021-03530-2.
- Song JY, Choi WS, Heo JY, Lee JS, Jung DS, Kim SW, Park K-H, Eom JS, Jeong SJ, Lee J, et al. Safety and immunogenicity of a SARS-CoV-2 recombinant protein nanoparticle vaccine (GBP510) adjuvanted with AS03: a randomised, placebo-controlled, observer-blinded phase 1/2 trial. EClinicalMedicine. 2022;51:101569. doi:10.1016/j.eclinm.2022.101569.
- Song JY, Choi WS, Heo JY, Kim EJ, Lee JS, Jung DS, Kim SW, Park KH, Eom JS, Jeong SJ, Lee J. Immunogenicity and safety of SARS-CoV-2 recombinant protein nanoparticle vaccine GBP510 adjuvanted with AS03: randomised, active-controlled, observer-blinded, phase 3 trial. medRxiv. 2023;23284895.
- Bajoria S, Kaur K, Kumru OS, Van Slyke G, Doering J, Novak H, Rodriguez Aponte SA, Dalvie NC, Naranjo CA, Johnston RS, et al. Antigen-adjuvant interactions, stability, and immunogenicity profiles of a SARS-CoV-2 receptor-binding domain (RBD) antigen formulated with aluminum salt and CpG adjuvants. Hum Vaccin Immunother. 2022;18(5):2079346. doi:10.1080/21645515.2022.2079346.
- Yang J, Petitjean SJL, Koehler M, Zhang Q, Dumitru AC, Chen W, Derclaye S, Vincent SP, Soumillion P, Alsteens D, et al. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat Commun. 2020;11(1):4541. doi:10.1038/s41467-020-18319-6.
- Pinto D, Park YJ, Beltramello M, Walls AC, Tortorici MA, Bianchi S, Jaconi S, Culap K, Zatta F, De Marco A, et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583(7815):290–5. doi:10.1038/s41586-020-2349-y.
- Wrobel AG, Benton DJ, Hussain S, Harvey R, Martin SR, Roustan C, Rosenthal PB, Skehel JJ, Gamblin SJ. Antibody-mediated disruption of the SARS-CoV-2 spike glycoprotein. Nat Commun. 2020;11(1):5337. doi:10.1038/s41467-020-19146-5.
- Arbel R, Peretz A, Sergienko R, Friger M, Beckenstein T, Duskin-Bitan H, Yaron S, Hammerman A, Bilenko N, Netzer D, et al. Effectiveness of a bivalent mRNA vaccine booster dose to prevent severe COVID-19 outcomes: a retrospective cohort study. Lancet Infect Dis. 2023;23(8):914–21. doi:10.1016/S1473-3099(23)00122-6.
- Bajoria S, Kumru OS, Doering J, Berman K, Slyke GV, Prigodich A, Rodriguez-Aponte SA, Kleanthous H, Love JC, Mantis NJ, et al. Nanoalum formulations containing aluminum hydroxide and CpG 1018(TM) adjuvants: the effect on stability and immunogenicity of a recombinant SARS-CoV-2 RBD antigen. Vaccines (Basel). 2023;11:11. doi:10.3390/vaccines11061030.
- Hansen B, Sokolovska A, HogenEsch H, Hem SL. Relationship between the strength of antigen adsorption to an aluminum-containing adjuvant and the immune response. Vaccine. 2007;25(36):6618–24. doi:10.1016/j.vaccine.2007.06.049.
- Hansen B, Belfast M, Soung G, Song L, Egan PM, Capen R, HogenEsch H, Mancinelli R, Hem SL. Effect of the strength of adsorption of hepatitis B surface antigen to aluminum hydroxide adjuvant on the immune response. Vaccine. 2009;27(6):888–92. doi:10.1016/j.vaccine.2008.11.078.
- Dagouassat N, Robillard V, Haeuw JF, Plotnicky-Gilquin H, Power UF, Corvaia N, Nguyen T, Bonnefoy J-Y, Beck A. A novel bipolar mode of attachment to aluminium-containing adjuvants by BBG2Na, a recombinant subunit hRSV vaccine. Vaccine. 2001;19:4143–52. doi:10.1016/S0264-410X(01)00168-2.
- Porath J, Sundberg L, Fornstedt N, Olsson I. Salting-out in amphiphilic gels as a new approach to hydrophobia adsorption. Nature. 1973;245(5426):465–6. doi:10.1038/245465a0.
- Rabe M, Verdes D, Seeger S. Understanding protein adsorption phenomena at solid surfaces. Adv Colloid Interface Sci. 2011;162(1–2):87–106. doi:10.1016/j.cis.2010.12.007.
- Clapp T, Siebert P, Chen D, Jones Braun L. Vaccines with aluminum-containing adjuvants: optimizing vaccine efficacy and thermal stability. J Pharm Sci. 2011;100(2):388–401. doi:10.1002/jps.22284.
- Jones LS, Peek LJ, Power J, Markham A, Yazzie B, Middaugh CR. Effects of adsorption to aluminum salt adjuvants on the structure and stability of model protein antigens. J Biol Chem. 2005;280(14):13406–14. doi:10.1074/jbc.M500687200.
- Peek LJ, Martin TT, Elk Nation C, Pegram SA, Middaugh CR. Effects of stabilizers on the destabilization of proteins upon adsorption to aluminum salt adjuvants. J Pharm Sci. 2007;96(3):547–57. doi:10.1002/jps.20762.
- Iyer V, Hu L, Liyanage MR, Esfandiary R, Reinisch C, Meinke A, Maisonneuve J, Volkin DB, Joshi SB, Middaugh CR, et al. Preformulation characterization of an aluminum salt-adjuvanted trivalent recombinant protein-based vaccine candidate against streptococcus pneumoniae. J Pharm Sci. 2012;101(9):3078–90. doi:10.1002/jps.23175.
- Jerajani K, Wan Y, Hickey JM, Kumru OS, Sharma N, Pullagurla SR, Ogun O, Mapari S, Whitaker N, Brendle S, et al. Analytical and preformulation characterization studies of human papillomavirus virus-like particles to enable quadrivalent Multi-dose vaccine formulation development. J Pharm Sci. 2022;111(11):2983–97. doi:10.1016/j.xphs.2022.07.019.
- Agarwal S, Hickey JM, McAdams D, White JA, Sitrin R, Khandke L, Cryz S, Joshi SB, Volkin DB. Effect of aluminum adjuvant and preservatives on structural integrity and physicochemical stability profiles of three recombinant subunit rotavirus vaccine antigens. J Pharm Sci. 2020;109(1):476–87. doi:10.1016/j.xphs.2019.10.004.
- DePaz RA, Henderson I, Advant SJ. Formulation of botulinum neurotoxin heavy chain fragments for vaccine development: mechanisms of adsorption to an aluminum-containing adjuvant. Vaccine. 2005;23(31):4029–35. doi:10.1016/j.vaccine.2005.03.028.
- Caulfield MJ, Shi L, Wang S, Wang B, Tobery TW, Mach H, Ahl PL, Cannon JL, Cook JC, Heinrichs JH, et al. Effect of alternative aluminum adjuvants on the absorption and immunogenicity of HPV16 L1 VLPs in mice. Hum Vaccin. 2007;3(4):139–45. doi:10.4161/hv.3.4.4309.
- Kurzatkowski W, Kartoglu U, Staniszewska M, Gorska P, Krause A, Wysocki MJ. Structural damages in adsorbed vaccines affected by freezing. Biologicals. 2013;41:71–6. doi:10.1016/j.biologicals.2011.10.011.
- Nanishi E, Borriello F, O’Meara TR, McGrath ME, Saito Y, Haupt RE, Seo H-S, van Haren SD, Cavazzoni CB, Brook B, et al. An aluminum hydroxide: CpG adjuvant enhances protection elicited by a SARS-CoV-2 receptor binding domain vaccine in aged mice. Sci Transl Med. 2022;14(629):eabj5305. doi:10.1126/scitranslmed.abj5305.
- Brzuska G, Zimna M, Baranska K, Szewczyk B, Strakova P, Ruzek D, Krol E. The influence of adjuvant type on the immunogenicity of RBD/N cocktail antigens as a vaccine candidate against SARS-CoV-2 virus. Microbiol Spectr. 2023;11:e0256422. doi:10.1128/spectrum.02564-22.
- Kerwin BA. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways. J Pharm Sci. 2008;97(8):2924–35. doi:10.1002/jps.21190.
- Shi L, Sanyal G, Ni A, Luo Z, Doshna S, Wang B, Graham TL, Wang N, Volkin DB. Stabilization of human papillomavirus virus-like particles by non-ionic surfactants. J Pharm Sci. 2005;94(7):1538–51. doi:10.1002/jps.20377.
- Murakami K, Fujii Y, Someya Y. Effects of the thermal denaturation of Sabin-derived inactivated polio vaccines on the D-antigenicity and the immunogenicity in rats. Vaccine. 2020;38(17):3295–9. doi:10.1016/j.vaccine.2020.03.027.
- Sharma N, Jerajani K, Wan Y, Kumru OS, Pullagurla SR, Ogun O, Mapari S, Brendle S, Christensen ND, Batwal S, et al. Multi-dose formulation development for a quadrivalent human papillomavirus virus-like particle-based vaccine: part II- Real-time and accelerated stability studies. J Pharm Sci. 2023;112(2):458–70. doi:10.1016/j.xphs.2022.11.021.
- Jerajani K, Wan Y, Kumru OS, Pullagurla SR, Kumar P, Sharma N, Ogun O, Mapari S, Brendle S, Christensen ND, et al. Multi-dose formulation development for a quadrivalent human papillomavirus virus-like particle-based vaccine: part I - Screening of preservative combinations. J Pharm Sci. 2023;112(2):446–57. doi:10.1016/j.xphs.2022.09.001.
- Kumar P, Bird C, Holland D, Joshi SB, Volkin DB. Current and next-generation formulation strategies for inactivated polio vaccines to lower costs, increase coverage, and facilitate polio eradication. Hum Vaccin Immunother. 2022;18:2154100. doi:10.1080/21645515.2022.2154100.
