 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The highdose quadrivalent influenza vaccine (QIVHD) has shown improved protection against influenza and its complications in older adults. We aimed to evaluate the costeffectiveness of QIVHD compared with QIVSD among Korean adults aged ≥ 65 years in reducing influenzarelated disease burden. We evaluated the 2016/2017 and 2017/2018 seasons and their average values using a static decision tree model. The difference in efficacy between standard-dose (SD) and high-dose (HD) was calculated based on the results of a clinical trial comparing Fluzone® High-Dose Vaccine and Fluzone® Vaccine in older adults. Incremental cost-effectiveness ratios (ICERs) were assessed from the healthcare system perspective. A discount rate of 4.5% was applied to life-year-gained (LYG) values and utilities. We performed deterministic and probabilistic sensitivity analyses to account for both epidemiological and economic sources of uncertainty. In the analysis of the 2017/2018 season, the QIV-HD strategy generated an excess of 0.00182 life-years (Lys)/person and 0.003953 quality-adjusted life-years (QALYs)/person compared with QIV-SD. The ICER was 6,467.56 United States Dollars (USD)/QALY. In the analysis from the 2016/2017 season, QIV-HD caused a surplus of 0.00117 Lys/person and 0.003272 QALYs/person compared with QIV-SD. ICER was 7,902.46 USD /QALY. From the average data of the 2016/2017 and 2017/2018 seasons, an excess of 0.00147 Lys/person and 0.003561 QALYs/person were generated using QIV-HD compared with QIV-SD, while the ICER was 7,190.44 USD /QALY. From the healthcare system perspective, QIV-HD was a more cost-effective vaccination option in reducing influenza-related disease burden and healthcare costs in Koreans aged ≥ 65 years compared with QIV-SD.
Introduction
Influenza is a contagious respiratory viral illness, causing 290,000–650,000 global deaths each season.Citation1 Older individuals (aged ≥ 65 years) are at a higher risk of influenza-associated hospitalization and death due to age-related immune dysfunction and a higher prevalence of underlying chronic medical conditions.Citation2 Influenza can cause a significant burden on the healthcare system. From a previous local study based on the Hospital-based Influenza Morbidity and Mortality Surveillance, the influenza-related socioeconomic cost of 2013–2014 seasonal influenza in the Korean adult population was estimated to be 125 million US Dollars (USD).Citation3
Influenza vaccination is an important preventive tool for all populations who are at risk of severe outcomes. This includes individuals with chronic medical conditions. It has been associated with a lower rate of cardiac events among people with heart disease, especially among those who have experienced a cardiac event in the past year.Citation4 It can also reduce exacerbation and subsequent hospitalization due to chronic lung diseases such as chronic obstructive pulmonary disease, asthma, and interstitial lung disease.Citation5 Especially, after the coronavirus disease 2019 pandemic, the Centers for disease control and prevention (CDC) recommends influenza vaccination to reduce the strain on healthcare systems responding to the pandemic.Citation6 Traditionally, influenza vaccines protect against three different seasonal influenza viruses (trivalent vaccines; TIV), influenza A (H3N2), pandemic A (H1N1), and one of the two influenza B subtype. However, quadrivalent vaccines (QIV), including both influenza B-subtypes, have become available in most countries.Citation7,Citation8
Korean adults aged ≥ 65 years can receive free influenza vaccination under the National Immunization Program (NIP). Until the 2019/2020 influenza season, the NIP provided standard-dose trivalent influenza vaccines (TIV-SD); however, from the 2020/2021 season, the NIP started to provide standard-dose quadrivalent influenza vaccines (QIV-SD).Citation9 Approximately 83% of the Korean population aged ≥ 65 years were vaccinated against influenza, while the average vaccination rate of people aged ≥ 65 years is approximately 42% in 32 Organization for Economic Cooperation and Development countries.Citation10 Based on data obtained from the Korean national vaccination registry, the number of influenza vaccine recipients has more than tripled in 20 years, from 3,200,000 people in the 1997/1998 season to 9,890,000 in the 2016/2017 season.Citation11 Despite high vaccine coverage, influenza-associated excess mortality in adults ≥ 65 years is substantially high in Korea.Citation12 In addition, the influenza-related disease burden, such as complications, hospitalization, and death, is still high in the older population, reflecting the low vaccine effectiveness in older adults and/or vaccine mismatching.Citation3
Recently, a high-dose (HD) influenza vaccine containing hemagglutinin antigen four times that of a standard-dose (SD) vaccine, was developed to provide improved protection among older adults in whom immune responses to SD vaccines can be suboptimal.Citation13 The further development of high-dose quadrivalent influenza vaccine (QIV-HD), containing an additional influenza B subtype lineage, represents an important step in the continued improvement of protection against influenza and its complications among older adults.Citation14 Considering the limited vaccine efficacy (VE) of conventional standard-dose influenza vaccines among older adults, the CDC preferentially recommends the use of highly immunogenic influenza vaccines for adults aged ≥ 65 years, including higher doses and adjuvanted influenza vaccines as of June 2022.Citation15
This study aimed to evaluate the cost-effectiveness of QIV-HD compared with QIV-SD in preventing influenza-related hospital visits which includes General Practitioner (GP) and Emergency Department (ED) visits, hospitalizations, and deaths among Korean adults aged ≥ 65 years.
Materials and methods
There is considerable seasonal variation in the subtype proportion of prevalent influenza viruses. In Korea, the proportion of influenza A (H1N1) was 0.5% in the 2016/2017 season and 7.0% in the 2017/2018 season, while that for the Influenza B subtype was 24.3% in 2011/2012–2016/2017 seasons, but increased to 55% in the 2017/2018 season.Citation16 The increase in influenza B was caused by a mismatch between vaccine composition and circulating subtype, resulting in a higher disease burden due to influenza B in Korea. The proportion of vaccines matching circulating influenza B subtype in the 2016/2017 and 2017/2018 seasons was 12.9% and 1.4% respectively. To reflect such seasonal variability in influenza characteristics, the model evaluated two influenza seasons, 2016/2017 and 2017/2018. In addition, we analyzed a virtual season composed of the average values of variables in the two seasons, with the exception of the proportion of each subtype (i.e., A and B), for which average values from 2010 to 2019 was used. This was to evaluate the overall benefit of the vaccines over a longer period. The 2016/2017 influenza season was from December 8, 2016, to June 2, 2017, and the 2017/2018 influenza season was from December 1, 2017, to May 25, 2018. GP and ED visits during these periods were included in the analysis. To reflect the late consequences of influenza complications, hospitalizations up to 4 weeks after the last day of each flu season were included. The symptomatic period was assumed to be 6 days.
Model design and structure
A static decision tree model in Microsoft Excel (Microsoft Corporation, Redmond, Washington; 2013) was used (). Decision trees are schematic representations of the question of interest and the possible consequences of each strategy.Citation17
Figure 1. Schematic description of the static decision tree model.
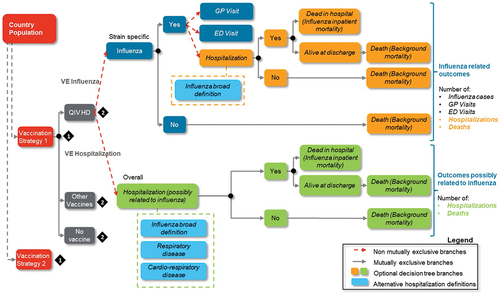
In a cohort cost-effectiveness analysis (CEA) model, the impact of the vaccination program can be assessed across either a short time horizon or a lifetime horizon.Citation18 The selection of the time horizon is contingent on the vaccine’s duration of effectiveness and its potential influence on long-term disease outcomes, including chronic sequelae and delayed disease manifestations attributed to the new vaccination program. Similarly, in a population CEA model, the time horizon employed to estimate cumulative costs and benefits should encompass a sufficiently extended period that captures the stabilization of changes in disease outcomes resulting from the new vaccination program.Citation19 In light of these considerations, the model estimated several key health outcomes at the population level for influenza: the number of influenza cases, general practitioner visits related to influenza, emergency department presentations related to influenza, influenza-related hospitalizations, deaths occurring during the influenza season, years of life lost (LYs) over a lifetime horizon, and quality-adjusted life years (QALYs) over a lifetime horizon.Citation20 The model foresees a discount rate exclusively for the health benefits. Influenza-related deaths were conditional on developing influenza, and individuals who survived from influenza were subject to background mortality. To avoid double counting we considered background mortality based on the general population life table. The model estimates the hypothetical number of influenza cases in individuals aged ≥ 65 years, using influenza attack rates and VE against influenza. The probability of contracting influenza directly depends on the vaccine received. The relative VE between HD and SD was sourced from the FIM12 clinical trial which compared Fluzone HD vaccine with Fluzone vaccine in elderly adults.Citation21
Epidemiological and health care resource utilization (HCRU) data were collected from the Health Insurance Review and Assessment Service-National Patient Sample during the 2016/2017 and 2017/2018 seasons when TIV-SD was the standard of care and administered under the NIP. Consequently, hospitalization data collected from a population partially vaccinated with TIV-SD was applied in the model. The relative VE of QIV-SD compared with TIV-SD was used to estimate the effectiveness of QIV-SD. Although QIV-SD was available with out-of-pocket expenses during that period, it was not considered in this analysis because of the low vaccination coverage of approximately 2%.
The model was conducted over a single average influenza season and had a life-time horizon for influenza-related costs and health outcomes. The clinical outcomes estimated by the model included influenza cases, GP consultations, ED visits, hospitalizations, and premature deaths due to influenza. The model considered hospitalizations possibly related to influenza. This approach leverages the results derived from the abovementioned FIM12 trial, which assessed the impact of using HD vs. SD on respiratory and cardiorespiratory hospitalization events without an influenza laboratory confirmation.Citation21 Three definitions of hospitalizations possibly related to influenza were considered based on the International Classification of Diseases 10th Revision (ICD-10) codes: (1) influenza and pneumonia, “broadly defined influenza” (hospitalizations for influenza and pneumonia), (2) respiratory events (hospitalizations possibly related to influenza due to respiratory illness, including confirmed influenza and pneumonia), and (3) cardiorespiratory events (hospitalizations possibly related to influenza due to cardiorespiratory illness, including respiratory events).
The model was designed to assess cost-effectiveness from a healthcare system perspective, including direct medical costs. We assumed that all the events of interest occurred within a single influenza season. A discount rate of 4.5% per annum was applied to life-year-gained (LYG) values and utilities according to the most recent Korean guidelines for economic evaluation from 2021. Costs were not discounted because they were based only on the healthcare use reported during the study period. All costs in the 2016/2017 season were reported in 2017 Korean Won (KRW), and those in the 2017/2018 season are reported in 2018 KRW. For reference purposes, all costs in this report are expressed in USD according to the average KRW-to-USD exchange rate from January 2022 to June 2022.
The current model presents the incremental cost-effectiveness ratio (ICER). The ICER was expressed as the ratio of the difference in total cost between the QIV-HD and QIV-SD groups to the difference in health outcomes measured in QALYs. ICERs were analyzed in the context of a willingness-to-pay (WTP) threshold, defined as one unit of South Korea’s Gross Domestic Product per capita, in accordance with the World Health Organization (WHO) recommendations. As such, 44,159,631 KRW/QALY (35,751 USD/QALY, 1 USD = 1,235.2 KRW) from studies by Yun et al., corresponding to the year 2016 and translated to USD using the average exchange rate from January 2022 to June 2022, was applied for WTP threshold.Citation22–24
Model inputs
All model inputs are summarized in .
Table 1. Input data for population, efficacy, and utilities.
Population data
The target population in the model was defined as Korean citizens aged ≥ 65 years, stratified into 65–74 years and 75 years old or older (≥ 75 years) groups. The study population was further stratified into high- and low-risk groups. The high-risk group comprised people with comorbidities that increased the risk of influenza-related complications. The low-risk group consisted of relatively healthy seniors without comorbidities or those with lower rates of influenza-related complications. The proportion of the high-risk group was based on NHIS data for each equivalent influenza season. The number of general populations in each age group was extracted from the residential statistics published by the Korean Statistical Information Service (KOSIS) in December 2017 for the 2017/2018 season and December 2016 for the 2016/2017 season.Citation25 The life table was based on the KOSIS complete life table of the population aged ≥ 65 years in 2016–2018; the 2016/2017 season used 2017 data, and the 2017/2018 season used 2018 data.Citation26
Vaccine efficacy data
The model estimated the number of influenza cases on the basis of the vaccine coverage rates, influenza attack rate and the efficacy of each vaccine (Probability of being influenza case = vaccine coverage rate* influenza attack rate * (1- absolute vaccine efficacy)). Vaccine coverage rates were provided by Korea Disease Control and Prevention Agency (KDCA).Citation27 The influenza attack rate was extracted from published literature.Citation28 The relative VE of QIV-HD vs. QIV-SD in influenza-associated hospitalization was derived from a systematic review.Citation29 The relative VE for HD-QIV vs. SD-QIV in the prevention of influenza cases was assumed to be identical to that of HD-TIV vs. SD-TIV (i.e. 24.2%), which was obtained from the FIM12 trial.Citation21 The clinical efficacy data of TIV vaccines were used to calcultate the efficacy of QIV vaccines following immuno-bridging QHD00013 study; first, using VE data for each influenza subtype, proportion of influenza cases by strain and proportion of matched B strain cases, we calculated the VE for QIV-SD and QIV-HD, as below formulas.Citation30
By using VE of QIV-SD as calculated above and relative efficacy of QIV-HD compared to QIV-SD, we calculated VE of QIV-HD against influenza cases. The relative efficacy of preventing hospitalization due to cardio-respiratory hospitalization for QIV-HD compared to QIV-SD was 18.2% based on research of Lee et al.Citation29
Disease burden
Influenza-related GP visits, hospitalization rates, and ED visits were based on NHIS data analyses in the 2016/2017 and 2017/2018 seasons using ICD-10 codes J09 – J18 (Table S1). The codes for chronic medical conditions defining high-risk groups are presented in Table S2.Citation9 Hospitalizations for broadly defined influenza (ICD-10 code: J09–J18), respiratory events (ICD-10 code: J09–J18, J40–J45, J96), and cardiorespiratory events (ICD-10 codes: I20, I21, I24, I25, I50, I63, J09–J18, J40–J45, J96) were included in the model. Re-hospitalization within 30 days with the same diagnosis was considered to be the same as the previous hospitalization. The mean values of GP visits, ED visits, hospitalizations, and length of stay (LOS) data for each influenza season were applied ( and Table S3). The average LOS was 12.20 days in the 2016/2017 season and 11.88 days in the 2017/2018 season.
The case-fatality rate associated with influenza was calculated from the NHIS analysis data and KOSIS cause-of-death statistical data (Table S3). Deaths up to 30 days after hospitalization with a cardiorespiratory ICD-10 code were counted.
Cost data
In accordance with the 2021 Korean guidelines for economic evaluation, the model used a healthcare system perspective in base-case analysis, including direct medical costs of vaccination, vaccine administration costs, prescribed influenza medication costs, costs of influenza-related GP visits and ED visits, and hospitalization costs.Citation31 Vaccine acquisition costs (QIV-SD and QIV-HD) and were obtained from the Health Insurance Review and Assessment (HIRA) 2021 drug price tableCitation32 and vaccine administration costs from the Korea Disease Control Agency (KDCA) announcement.Citation33 Prescribed influenza medication was assumed to be oseltamivir (Tamiflu®), and its drug acquisition cost was sourced from the HIRA weighted average price table in 2021.Citation34 The testing kit cost was based on the HIRA service fee table.Citation35 The copayment rates of GP and ED visits were considered in the analysis according to the HIRA guidelines.Citation31 The costs of influenza-related GP visits, ED visits, and hospitalizations were based on NHIS data and fully reflected the consumer price index of each equivalent season.Citation36 The costs of influenza-related ED visits also considered emergency administration costs ( and Table S3).
Utility data
The EuroQol 5 dimensions-3 level (EQ-5D-3 L) of the general population ≥ 65 years in Korea was obtained from the 2019 KDCA report.Citation37 The EQ-5D-3 L questionnaire consists of five questions on self-reported problems in five dimensions (mobility, self-care, usual activities, pain or discomfort, and depression or anxiety). There are three levels for each item (1 = no problem, 2 = moderate problem, 3 = severe problem). For individuals who experienced laboratory-confirmed influenza, a loss of utility value specific to clinically defined influenza disease was applied for the mean duration of the illness, assumed as six days. The utility loss due to influenza (0.35), hospitalization with complications (0.5), and hospitalization without complications (0.4) was based on a previous study.Citation38 For individuals who experienced influenza-related hospitalization, quality-adjusted life-years (QALY) loss due to hospitalization and LOS were applied (, Table S3).
Sensitivity analysis
We performed one-way deterministic sensitivity analysis (DSA) to identify the most impactful parameters. The analyses were performed by changing the key model inputs and widening the analysis perspective from the healthcare system to society. The value of each parameter was changed within the range of 20–25%. The results were presented as tornado diagrams. Hospitalization data regarding broadly defined influenza and respiratory events were presented in the sensitivity analysis. All ICER results are presented as the additional costs per QALY gained.
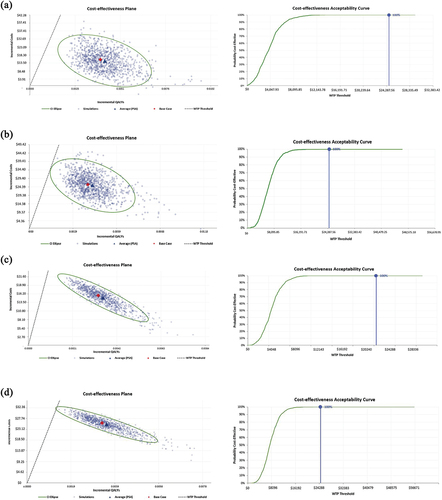
Probabilistic sensitivity analyses (PSA) were conducted to demonstrate the parameter uncertainty and robustness of the model. Sampling parameters from their respective distributions (beta, gamma, lognormal, and uniform) were included in PSA (). The results were presented as cost-effectiveness acceptability curves. One-way and probabilistic sensitivity analyses were performed for the 2016/2017 and 2017/2018 seasons and their averages.
Results
In the 2017/2018 season, a total average per-person cost for QIV-SD was 93.33 USD, while a total average per-person cost for QIV-HD was 118.97 USD. Total LYs per person for QIV-SD was 9.6954 and for QIV-HD was 9.6973. Total QALYs per person for QIV-SD was 8.7334 and for QIV-HD was 8.7374. QIV-HD would have prevented an additional 45,570 influenza cases, 17,258 GP visits, 1,040 ED visits, 33,198 cardiorespiratory event-related hospitalizations, and 1,755 deaths compared with QIV-SD (). The QIV-HD strategy generated an excess 0.00182 Life-years (LYs)/person and 0.003953 QALYs/person compared with QIV-SD, while spending additional 25.57 USD. ICER was 6,467.56 USD/QALY. Considering a WTP of 35,751 USD/QALY, these results support the cost-effectiveness of QIV-HD. By changing the definition of hospitalization to include broadly defined influenza-related admissions, QIV-HD averted 12,437 hospitalizations, generated an excess of 0.001211 QALYs/person compared with QIV-SD, and was a cost-effective strategy with an ICER of 27,21259 USD/QALY (Table S4).
Table 2. Base-case analysis results when hospitalization was defined as cardiorespiratory event-related.
In the 2016/2017 season, a total average per-person cost for QIV-SD was 91.22 USD, while a total average per-person cost for QIV-HD was 117.08 USD. Total LYs per person for QIV-SD was 9.7598 and for QIV-HD was 9.7609. Total QALYs per person for QIV-SD was 8.7934 and for QIV-HD was 8.7967. The QIV-HD strategy would have averted an additional 43,007 influenza cases, 6,446 GP visits, 357 ED visits, 30,369 cardiorespiratory event-related hospitalizations, and 1,061 deaths compared with QIV-SD. QIV-HD resulted in an excess of 0.00117 LYs/person and 0.003272 QALYs/person compared with QIV-SD, while spending 25.86 USD more. ICER was 7,902.46 USD/QALY, again proving the cost-effectiveness of QIV-HD compared with QIV-SD. By changing the definition of hospitalization to include broadly defined influenza-related admissions, QIV-HD averted 11,782 hospitalizations, generated excess 0.001206 QALYs/person, and was a cost-effective strategy with an ICER of 27,379.04 USD/QALY.
When estimated using the average data of the 2016/2017 and 2017/2018 seasons, a total average per-person cost for QIV-SD was 92.32 USD, while a total average per-person cost for QIV-HD was 117.92 USD. Total LYs per person for QIV-SD was 9.7269 and for QIV-HD was 9.7283. Total QALYs/person for QIV-SD was 8.7627 and for QIV-HD was 8.7662. An additional 43,804 influenza cases, 11,574 GP visits, 682 ED visits, 31,382 cardiorespiratory event-related hospitalizations, and 1,372 deaths were prevented using QIV-HD instead of QIV-SD. Excess 0.00147 LYs/person and 0.003561 QALYs/person were generated using QIV-HD, resulting in an ICER of 7,190.44 USD/QALY. This analysis also supports the cost-effectiveness of QIV-HD. By changing the definition of hospitalization to include broadly defined influenza-related admissions, QIV-HD averted 11,947 hospitalizations, generated an excess of 0.001190 QALYs/person compared with QIV-SD, and was a cost-effective strategy with an ICER of 27,536.86 USD/QALY.
In the one-way sensitivity analysis of the 2016/2017 and 2017/2018 seasons and the average data for the two seasons, the parameters with the greatest impact on the results were the relative VE of QIV-HD vs. QIV-SD against influenza-associated hospitalization, the cost of QIV-HD, and the hospitalization rate ( and Figures S1–S3). In threshold analysis adjusting the relative VE of QIV-HD vs. QIV-SD against influenza-associated hospitalization, lowering the relative VE of QIV-HD vs. QIV-SD against influenza-associated hospitalization from 18.2% to 4.22–5.05% would make QIV-HD no longer cost-effective (4.22% in 2017/2018 season, 5.05% in 2016/2017 season, 4.63% in average, respectively). With the definition of cardiorespiratory hospitalizations, PSAs were conducted for the 2016/2017 and 2017/2018 seasons, while the cost of the QIV-HD vaccine was fixed at 50,000 KRW (40.48 USD) or 65,000 KRW (52.62 USD). The results showed that for a WTP threshold of 35,751 USD/QALY, the probability that QIV-HD is a cost-effective option for the Korean health system is 100% in all seasons and QIV-HD vaccine costs considered (). By changing the definition of hospitalizations to broadly defined influenza-related hospitalizations, the probability that QIV-HD is a cost-effective option for the Korean health system was 91% when the QIV-HD vaccine cost was 65,000 KRW (52.62 USD) in the 2016/2017 season. If the QIV-HD vaccine cost is 50,000 KRW (40.48 USD), the probability of QIV-HD being a cost-effective option for the Korean health system is 100% (Figure S4).
Figure 2. Deterministic sensitivity analysis results when hospitalization was defined as cardiorespiratory event-related for (a) 2017/2018 season, (b) 2016/2017 season, (c) average of the two seasons.
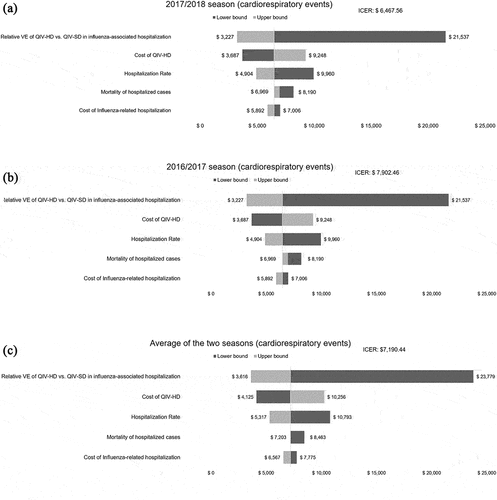
Figure 3. Probabilistic sensitivity analysis results when hospitalization was defined as cardiorespiratory event-related for (a) 2017/2018 season if the QIV-HD vaccine cost is 50,000 KRW (40.48 USD) or (b) 65,000 KRW (52.62 USD), (c) 2016/2017 season if the QIV-HD vaccine cost is 50,000 KRW or (d) 65,000 KRW. For each condition, two figures are presented: (1) cost-effectiveness plane and (2) cost-effectiveness acceptability curve.
Discussion
This is the first study to evaluate the cost-effectiveness of QIV-HD compared with QIV-SD in Korea. Our study showed that switching from QIV-SD to QIV-HD is a favorable influenza vaccination option for individuals aged ≥ 65 years. While the price of 65,000 KRW (52.62 USD) was set relatively high compared to other flu vaccine prices in Korea, when considering the threshold such as Korea’s 1 GDP (44,159,631KRW or35,751 USD), the ICER mentioned in the current paper appears to be favorable and falls within an acceptable range. One-way DSAs and PSAs showed that the results of our study were robust. Our results are consistent with those of previous economic evaluations conducted in different healthcare settings in other countries.Citation39–42
While older adults are at an increased risk of severe influenza-associated illness compared with younger persons, influenza vaccines are often less effective in this population. This tendency was found in annual VE reports by the CDC and Public Health England.Citation43,Citation44 According to a meta-analysis of six clinical trials, both seroprotection and seroconversion rates were significantly lower in older than in younger adults.Citation45 For the 2022/2023 influenza season, the CDC recommends adults aged ≥ 65 years to preferentially receive a higher-dose vaccine or adjuvanted vaccine.Citation46
Lower vaccine efficacy is not the only factor contributing to a higher disease burden in older adults. Predominant viral subtypes and degree of vaccine mismatch are also crucial. There was a remarkable difference in healthcare utilization and disease burden between the two seasons included in this study: QIV-HD was more cost-effective in the season with a higher disease burden. The preventive effect of vaccination against influenza itself, as well as against pneumonia, is important. However, in a previous study over three seasons (2014–2015, 2015–2016, 2016–2017), the SD influenza vaccine was effective in preventing pneumonia only during well-matched seasons, in contrast to the HD influenza vaccine, which showed efficacy irrespective of the circulating subtype and antigen match.Citation47,Citation48 Moreover, although the influenza season persists for more than six months, influenza vaccine immunity declines rapidly in adults aged ≥ 65 years.Citation49 Further studies are warranted to determine whether QIV-HD is effective in overcoming the problems of QIV-SD, such as low cross-reactive immunogenicity and the short duration of vaccine immunity in older adults.
This study has several strengths. First, we used the results of a large randomized trial as data sources.Citation21 The FIM12 trial included approximately 32,000 older adults over two seasons and reported exploratory endpoints of interest. A second strength of the study is that the use of meta-analysis data with endpoints and high-quality evidence across a range of seasons and settings. In addition, this study compared the up-to-date vaccination strategy in Korea according to the recent inclusion of QIV in the NIP as of the 2020/2021 season. Our study considered two seasons and their average to reflect variability in the seasonal influenza epidemic. Finally, this study included a broader range of health outcomes related to cardiorespiratory events, respiratory events, and influenza.
There are also some limitations to this study. First, because the relative VE data were derived from the FIM12 trial (i.e., using TIV-SD vs. TIV-HD, not QIV-SD vs. QIV-HD), it was difficult to estimate the true relative VE against influenza-related health outcomes. However, it is noteworthy that the rVE of HD TIV compared to SD TIV for HD QIV was reasonably inferred through reference to an immunobridging study and its recognition for European market authorization. Using local epidemiology data and HCRU data, an attempt has been made to apply the most up-to-date, appropriate data for accurate evaluation in the Korean healthcare setting. Second, the model in this study did not consider adverse effects of the influenza vaccine. However, a previous study regarding the safety of QIV and TIV reported no significant difference in the adverse event rates of the two vaccines.Citation50 Previous economic evaluation studies have also not considered adverse events.Citation51,Citation52 Third, utility inputs were derived from indirectly collected data, including those from non-influenza-patient-based studies. To address this issue, sensitivity analyses were conducted to complement these limitations, which showed robustness across varying utility inputs. Finally, the study did not consider the impact of vaccines on transmission dynamics, which could underestimate the benefits of vaccination. Nevertheless, this is in line with the WHO recommendations to use static decision trees in economic evaluations of infectious diseases to evaluate the cost and health outcomes of an intervention over a short period.Citation53
In conclusion, QIV-HD was a cost-effective measure for reducing the disease burden related to influenza in individuals aged ≥ 65 years compared with QIV-SD. Our findings offer important insights to public health policymakers and healthcare providers.
Author contributions
Conceptualization: EN, HS, JML, JYS
Methodology: EN, EK, LC, JYS
Investigation: EN, HS, HH, JGY, JYN, HJC, WJK, EK, LC, JML, JYS
Validation: EN, EK, LC, JYS
Writing – original draft: EN, JYS
Writing – review & editing: all authors
All authors attest they meet the ICMJE criteria for authorship.
Supplemental Material
Download MS Power Point (577.9 KB)Disclosure statement
HJC and JYS have participated in the advisory board meeting of Sequirus. JML is an employee of Sanofi and may hold shares and/or stock options in the company.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2266233
Additional information
Funding
References
- Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–11. doi:10.1016/S0140-6736(17)33293-2.
- Dao CN, Kamimoto L, Nowell M, Reingold A, Gershman K, Meek J, Arnold K, Farley M, Ryan P, Lynfield R, et al. Adult hospitalizations for laboratory-positive influenza during the 2005-2006 through 2007-2008 seasons in the United States. J. Infect Dis. 2010; 202:881–88. doi:10.1086/655904.
- Choi WS, Cowling BJ, Noh JY, Song JY, Wie SH, Lee JS, Seo YB, Lee J, Jeong HW, Kim YK, et al. Disease burden of 2013-2014 seasonal influenza in adults in Korea. PLoS One. 2017;12:e0172012. doi:10.1371/journal.pone.0172012.
- Behrouzi B, Bhatt DL, Cannon CP, Vardeny O, Lee DS, Solomon SD, Udell JA. Association of influenza vaccination with cardiovascular risk: a meta-analysis. JAMA Netw. Open. 2022;5:e228873. doi:10.1001/jamanetworkopen.2022.8873.
- Center of Disease Control and Prevention. Flu vaccination. Updated 2022 Sep 6. [Accessed Mar 27, 2023]. https://www.cdc.gov/flu/highrisk/heartdisease.htm.
- Center of Disease Control and Prevention. Flu vaccine recommendation in COVID-19 outbreak. Updated 2021 Apr 15. [Accessed Mar 27, 2023]. https://www.cdc.gov/vaccines/pandemic-guidance/index.html.
- World Health Organization. Types of seasonal flu vaccines. Updated 2010 May 13. [Accessed Mar 27, 2023]. https://www.euro.who.int/en/health-topics/communicable-diseases/influenza/vaccination/types-of-seasonal-influenza-vaccine.
- Choi WS, Choi JH, Kwon KT, Seo K, Kim MA, Lee SO, Hong YJ, Lee J-S, Song JY, Bang JH, et al. Revised adult immunization guideline recommended by the Korean society of infectious diseases, 2014. Infect Chemother. 2015;47(1):68–79. doi:10.3947/ic.2015.47.1.68.
- Choi EJ, Park JH, Chun BC. Cost effectiveness of trivalent and quadrivalent influenza vaccines in 50- to 64-year-old adults in Korea. Vaccine. 2020;38(32):5002–08. doi:10.1016/j.vaccine.2020.05.065.
- Organisation for Economic Co-operation and Development. OECD health statistics 2019. [Accessed Mar 27, 2023]. https://www.oecd-ilibrary.org/sites/2700bb99-en/index.html?itemId=/content/component/2700bb99-en.
- Yun JW, Noh JY, Song JY, Chun C, Kim Y, Cheong HJ. The Korean influenza national immunization program: history and present status. Infect Chemother. 2017;49(4):247–54. doi:10.3947/ic.2017.49.4.247.
- Park M, Wu P, Goldstein E, Kim WJ, Cowling BJ. Influenza-associated excess mortality in South Korea. Am. J. Prev. Med. 2016;50(4):e111–e9. doi:10.1016/j.amepre.2015.09.028.
- Kissling E, Rondy M. Early 2016/17 vaccine effectiveness estimates against influenza A (H3N2): i-MOVE multicentre case control studies at primary care and hospital levels in Europe. Eurosurveillance. 2017;22:30464. doi:10.2807/1560-7917.ES.2017.22.7.30464.
- Hollingsworth R, Palmu A, Pepin S, Dupuy M, Shrestha A, Jokinen J, Syrjänen R, Nealon J, Samson S, De Bruijn I, et al. Effectiveness of the quadrivalent high-dose influenza vaccine for prevention of cardiovascular and respiratory events in people aged 65 years and above: rationale and design of a real-world pragmatic randomized clinical trial. Am. Heart J. 2021;237:54–61. doi:10.1016/j.ahj.2021.03.007.
- Center of Disease Control and Prevention. Preference for specific flu vaccines for seniors. Updated 2022 June 30. [Accessed Mar 26, 2023]. https://www.cdc.gov/media/releases/2022/s0630-seniors-flu.html.
- Cheong HJ. Cost-effectiveness analysis of extension of influenza immunization program. 2020.
- United States Department of Veterans Affairs. Decision analysis: decision trees, simulation models, sensitivity analyses. Updated 2021 Mar 19. [Accessed Mar 27, 2023]. https://www.herc.research.va.gov/include/page.asp?id=decision-analysis.
- Wilkinson T, Sculpher MJ, Claxton K, Revill P, Briggs A, Cairns JA, Teerawattananon Y, Asfaw E, Lopert R, Culyer AJ, et al. The international decision support initiative reference case for economic evaluation: an aid to thought. Value Health. 2016;19(8):921–28. doi:10.1016/j.jval.2016.04.015.
- Mauskopf J, Standaert B, Connolly MP, Culyer AJ, Garrison LP, Hutubessy R, Jit M, Pitman R, Revill P, Severens JL, et al. Economic analysis of vaccination programs: an ISPOR good practices for outcomes research task force report. Value Health. 2018;21(10):1133–49. doi:10.1016/j.jval.2018.08.005.
- Redondo E, Drago G, Lopez-Belmonte JL, Guillen JM, Bricout H, Alvarez FP, Callejo D, Gil de Miguel Á. Cost-utility analysis of influenza vaccination in a population aged 65 years or older in Spain with a high-dose vaccine versus an adjuvanted vaccine. Vaccine. 2021;39(36):5138–45. doi:10.1016/j.vaccine.2021.07.048.
- DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, Pollak R, Christoff J, Earl J, Landolfi V, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N. Engl. J. Med. 2014;371(7):635–45. doi:10.1056/NEJMoa1315727.
- Ahn. Development of reasonable Korean evaluation criteria for osteoporosis. [Accessed Mar 27, 2023]. https://www.neca.re.kr/lay1/program/S1T11C145/report/view.do?seq=20. [ Korean].
- Yun. Cost-effetiveness of influenza vaccine strategies for the elderly in South Korea. PLoS One. 2019 Jan 25;14(1):e0209643. doi:10.1371/journal.pone.0209643.
- Korean Statistics Information Service. e national indicators - exchange rates. [Accessed Mar 27, 2023]. https://www.index.go.kr/potal/main/EachDtlPageDetail.do?idx_cd=1068123.
- Korean Statistics Information Service. Residence statistics. [Accessed Apr 25, 2022]. https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1B04006&conn_path=I2.
- Korean Statistics Information Service. Complete life table. [Accessed Apr 25, 2022]. https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1B42&conn_path=I2.
- Korean Disease Control and Prevention Agency. Vaccine coverage rates - provided by KDCA. [Accessed Apr 25, 2022]. https://www.open.go.kr/com/main/mainView.do?mainBgGubun=search.
- Somes MP, Turner RM, Dwyer LJ, Newall AT. Estimating the annual attack rate of seasonal influenza among unvaccinated individuals: a systematic review and meta-analysis. Vaccine. 2018;36(23):3199–207. doi:10.1016/j.vaccine.2018.04.063.
- Lee JKH, Lam GKL, Shin T, Kim J, Krishnan A, Greenberg DP, Chit A. Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: a systematic review and meta-analysis. Expert. Rev. Vaccines. 2018;17:435–43. doi:10.1080/14760584.2018.1471989.
- Safety and immunogenicity of high-dose quadrivalent influenza vaccine in participants ≥65 years in the US. [Accessed Jun 12, 2023]. https://classic.clinicaltrials.gov/ct2/show/NCT03282240.
- Health Insurance Review and Assessment Service. Guidelines for the economic evaluation of drugs. Updated 2021 Mar 15. [Accessed Mar 27, 2023]. https://www.hira.or.kr/ebooksc/ebook_630/ebook_630_202103150917443810.pdf.
- Health Insurance Review and Assessment Service. Drug reimbursement price ceiling table. [Accessed Mar 27, 2023]. https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA030014050000.
- Korean Disease Control and Prevention Agency. Vaccination costs in accordance with the regulations on entrustment of vaccination services (KDCA 2021–98). [Accessed Sep 15, 2023]. https://www.kdca.go.kr/filepath/boardSyview.es?bid=0017&list_no=712672&seq=1.
- Health Insurance Review and Assessment Service. Weighted average drug cost table. [Accessed Mar 27, 2023]. https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA030017000000.
- Health Insurance Review and Assessment Service. Fee-for-service price 2021. [Accessed Oct 19, 2022]. https://www.hira.or.kr/npay/index.do.
- Korean Statistics Information Service. Consumer price index. [Accessed Apr 25, 2022]. http://kostat.go.kr/incomeNcpi/cpi/index.action.
- Korean Disease Control and Prevention Agency. National health statistics. [Accessed Mar 27, 2023]. https://knhanes.kdca.go.kr/knhanes/sub04/sub04_04_01.do.
- Yun JW, Choi MJ, Shin GS, Lim JO, Noh JY, Kim YK, Song JY, Kim WJ, Choi S-E, Cheong HJ, et al. Cost-effectiveness of influenza vaccine strategies for the elderly in South Korea. PLoS One. 2019;14:e0209643. doi:10.1371/journal.pone.0209643.
- Becker DL, Chit A, DiazGranados CA, Maschio M, Yau E, Drummond M. High-dose inactivated influenza vaccine is associated with cost savings and better outcomes compared to standard-dose inactivated influenza vaccine in Canadian seniors. Hum. Vaccines Immunother. 2016;12:3036–42. doi:10.1080/21645515.2016.1215395.
- Colrat F, Thommes E, Largeron N, Alvarez FP. Economic evaluation of high-dose inactivated influenza vaccine in adults aged ≥65 years: a systematic literature review. Vaccine. 2021;39(Suppl 1):A42–a50. doi:10.1016/j.vaccine.2020.12.036.
- van Aalst R, Russo EM, Neupane N, Mahmud SM, Mor V, Wilschut J, Chit A, Postma M, Young-Xu Y. Economic assessment of a high-dose versus a standard-dose influenza vaccine in the US veteran population: estimating the impact on hospitalization cost for cardio-respiratory disease. Vaccine. 2019;37:4499–503. doi:10.1016/j.vaccine.2019.06.066.
- Chit A, Roiz J, Briquet B, Greenberg DP. Expected cost effectiveness of high-dose trivalent influenza vaccine in US seniors. Vaccine. 2015;33:734–41. doi:10.1016/j.vaccine.2014.10.079.
- Center of Disease Control and Prevention. Seasonal influenza vaccine effectiveness. Updated 2020 Dec 22. [Accessed Oct 6, 2022]. https://www.cdc.gov/flu/vaccines-work/2019–2020.html.
- Public Health England. Influenza vaccine effectiveness: seasonal estimates. Updated 2018 Jul 18. [Accessed Oct 6, 2022]. https://www.gov.uk/government/publications/influenza-vaccine-effectiveness-seasonal-estimates.
- Meng Z, Zhang J, Shi J, Zhao W, Huang X, Cheng L, Yang X. Immunogenicity of influenza vaccine in elderly people: a systematic review and meta-analysis of randomized controlled trials, and its association with real-world effectiveness. Hum. Vaccines Immunother. 2020;16:2680–89. doi:10.1080/21645515.2020.1747375.
- Grohskopf LA, Blanton LH, Ferdinands JM, Chung JR, Broder KR, Talbot HK, Morgan RL, Fry AM. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2022-23 influenza season. MMWR Recomm. Rep. 2022;71:1–28. doi:10.15585/mmwr.rr7101a1.
- Song JY, Noh JY, Lee JS, Wie SH, Kim YK, Lee J, Jeong HW, Kim SW, Lee SH, Park K-H, et al. Effectiveness of influenza and pneumococcal polysaccharide vaccines against influenza-related outcomes including pneumonia and acute exacerbation of cardiopulmonary diseases: analysis by dominant viral subtype and vaccine matching. PLoS One. 2018;13:e0207918. doi:10.1371/journal.pone.0207918.
- Lee JKH, Lam GKL, Shin T, Samson SI, Greenberg DP, Chit A. Efficacy and effectiveness of high-dose influenza vaccine in older adults by circulating strain and antigenic match: an updated systematic review and meta-analysis. Vaccine. 2021;39(Suppl 1):A24–a35. doi:10.1016/j.vaccine.2020.09.004.
- Song JY, Cheong HJ, Hwang IS, Choi WS, Jo YM, Park DW, Cho GJ, Hwang TG, Kim WJ. Long-term immunogenicity of influenza vaccine among the elderly: risk factors for poor immune response and persistence. Vaccine. 2010;28:3929–35. doi:10.1016/j.vaccine.2010.03.067.
- Chang LJ, Meng Y, Janosczyk H, Landolfi V, Talbot HK. Safety and immunogenicity of high-dose quadrivalent influenza vaccine in adults ≥65 years of age: a phase 3 randomized clinical trial. Vaccine. 2019;37:5825–34. doi:10.1016/j.vaccine.2019.08.016.
- Bianculli PM, Bellier L, Mangado IO, Pérez CG, Mieres G, Lazarov L, Petitjean A, Dibarboure H, Lopez JG. Switching from trivalent to quadrivalent inactivated influenza vaccines in uruguay: a cost-effectiveness analysis. Hum. Vaccines Immunother. 2022;18(5):2050653. doi:10.1080/21645515.2022.2050653.
- Clements KM, Meier G, McGarry LJ, Pruttivarasin N, Misurski DA. Cost-effectiveness analysis of universal influenza vaccination with quadrivalent inactivated vaccine in the United States. Hum. Vaccines Immunother. 2014;10(5):1171–80. doi:10.4161/hv.28221.
- Walker DG, Hutubessy R, Beutels P. WHO guide for standardisation of economic evaluations of immunization programmes. Vaccine. 2010;28(11):2356–59. doi:10.1016/j.vaccine.2009.06.035.
