?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Neutralizing antibodies (NTAb) play a significant role in preventing and protecting against SARS-CoV-2 virus infection. Identifying NTAb is undoubtedly imperative in understanding the immunity toward COVID-19 better. However, it is interesting to note that the production of NTAb varies among individuals, especially among healthcare workers (HCWs), as they are exposed to the virus daily. Hence, we would like to investigate factors affecting the production of S-RBD IgG and NTAb among different categories of HCWs, particularly after receiving the third dose of the BNT162b2 mRNA COVID-19 Vaccine. A total of 361 HCWs from our hospital were prospectively enrolled and had their S-RBD IgG and NTAb titers measured. They were studied in relation to the degree of exposure to COVID-19, breakthrough infections, gender, age, race, household income, housing type, household number, and education levels. HCWs with the highest risk of exposure to COVID-19, breakthrough infections, and male gender displayed the highest median titers of both S-RBD IgG and NTAb, and the differences were statistically significant (p < .05). Age, race, household income, housing type, household number, and education levels were revealed to be insignificant. We concluded that the degree of exposure to COVID-19, breakthrough infections, and male gender are significant factors in NTAb production among HCWs.
Introduction
In December 2019, a cluster of cases of pneumonia with no known identifiable cause was reported by several health centers in Wuhan, Province of Hubei, China.Citation1 Interestingly, these patients exhibit similar signs and symptoms to those previously infected with SARS and MERS, particularly viral pneumonia with fever, chest discomfort, cough, and breathing difficulties with bilateral lung infiltration, which are apparent in severe cases.Citation1,Citation2 Wuhan Municipal Health Commission subsequently notified the public of a pneumonia outbreak of unknown cause and informed the World Health Organization (WHO) on the 31st of December 2019.Citation3 The infectious and deadly etiological agent was later identified as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS- CoV-2).Citation4 From the cases in Wuhan, it was later declared a global pandemic by the WHO on 11th March 2020. As of 17th June 2023, 766,440,796 cases with 6,932,591 deaths were reported. In Malaysia, 5,073,136 cases have been reported by the Ministry of Health, with 37,118 deaths.Citation5
Among the antibodies of interest, Neutralizing Antibodies (NTAb) toward SARS-CoV-2 play a significant role in the prevention of infection as well as pathogen elimination by inhibiting the interaction of the virus with the host cell receptors as well as by interacting with complement, natural killer cells, and phagocytes.Citation6 The most potent NTAb toward the virus targets the RBD and the heptad repeat two domains of the viral spike protein. This prevents viral attachment on the ACE-2 receptor on the host cell surface and subsequently prevents fusion between the viral and host cell membranes and, hence, entrance into the host cell.Citation6 S-RBD IgG antibody titers significantly correlate with neutralizing activity. They are associated with early virus control, highlighting the relevance of such antibodies as a reliable immunological correlate of protection (CoP) against infection.Citation7 Neutralizing anti-RBD antibodies are species-specific, but antibodies targeting non-RBD regions are also present, which confers no neutralizing activity.Citation8
Identifying NTAb is undoubtedly imperative in understanding the immunity toward COVID-19 better, yet it is interesting to note that the production of NTAb varies between individuals. Favreese et al.Citation9 identified varying levels of NTAb among different categories of individuals. In their study, they demonstrated that mean NTAb titers among previously infected and vaccinated COVID-19 patients had the highest level with mean NTAb of 888 AU/ml, followed by uninfected and vaccinated at 527 AU/ml (p < .0001). Among those infected, patients with moderate to severe symptoms had higher mean NTAb levels at 125 AU/ml than those with mild symptoms at 33.9 AU/ml (p < .0001). They concluded that vaccinated participants had significantly higher NTAb titers than COVID-19 patients.
With various studies producing different outcomes and conclusions, there is still a lot that can be explored in the field of NTAb in COVID-19. Demographics and regional aspects could also play a role. As per reviewing, most studies were conducted in the European and East Asian regions; hence, we would also like to explore the titers of NTAb among the Malaysian population, particularly among HCWs, which hopefully would benefit the country in combating the pandemic.
Materials and methods
Study design & patient selection
A cross-sectional study was conducted after approval from the Universiti Kebangsaan Malaysia Research Ethics Committee (JEP-2021-865). Initially, we received an overwhelming response from 602 eager volunteers to join the study. A questionnaire was given to each volunteer where details of dates of vaccinations, type of vaccine, demographics, and factors we wish to study, which included risk of exposure to COVID-19, were asked. Seeing that HCWs in our hospital work in different departments and with varying degrees of contact with COVID-19 patients, we were curious to find out whether NTAb titers would differ among varying levels of risk of exposure to COVID-19. Hence, from our questionnaire, we divided our HCWs into three groups, namely low, medium, and high-risk of exposure to COVID-19, based on the responses given by our participants. HCWs whose job scope does not involve contact with any COVID-19 patients or samples were categorized as low risk of exposure. HCWs involved with oral or nasal sampling of patients for COVID-19 or in handling COVID-19 samples (blood, saliva, urine, bodily fluids) were categorized as medium-risk. We consider HCWs with at least seven consecutive days of direct exposure actively engaging with COVID-19 patients at the ward as high-risk.
Other questions asked included breakthrough infections with COVID-19, education levels, household income, housing type, and amount of household individuals) and questions to select volunteers who fulfill the inclusion criteria and have no exclusion criteria. The criteria were as follows:
Inclusion criteria
Vaccinated HCWs
All HCWs currently working at UKMMC who completed three doses of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2 mRNA COVID-19 vaccine)
Age more than 18 y old.
Exclusion criteria
Participants with underlying human immunodeficiency virus (HIV), hepatitis B virus, or hepatitis C virus infection.
Any participant who is taking immunosuppressive therapy or medication. Immunosuppressive therapy or medication includes antineoplastic agents or Prednisone greater than 7.5 mg or equivalent.
Unvaccinated HCW.
Following the questionnaire, a total of 392 vaccinated HCWs of UKMMC who completed three doses of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2 mRNA COVID-19 vaccine) fulfilled the inclusion criteria and without any exclusion criteria were admitted prospectively into the study. To ensure volunteers were vaccinated, each volunteer’s vaccination certificate with vaccination dates and types was counter-checked by checking the Malaysian government mandatory issued COVID-19 tracking application “My Sejahtera” App on their smartphones. This app was also used to authenticate participants’ responses regarding previous breakthrough infections, including how they were diagnosed (rapid test kit or PCR). To avoid bias due to the well-known deteriorating levels of antibody titers following vaccination, we had to accept only volunteers with the exact duration of days following the third dose of vaccination. After further evaluation, we identified that 60–90 d following the third vaccination dose was the appropriate choice, as most volunteers were in this category. The interval between the second and third vaccination doses was between 200 and 220 d. Blood samples were taken during the third week of January 2022, where, during this period, the Omicron variant was predominant in Malaysia. However, only 361 volunteers were present for the sample collection. shows the consort diagram of the study. The study was conducted following the Declaration of Helsinki 1975. Informed consent was obtained from all subjects involved in the study.
Sample size calculation
The sample size calculation for this study was performed using the prevalence formulaCitation10 as presented below:
n = sample size
Z = Z statistic for a level of confidence (for a level of confidence of 99%, the Z value is 2.58)
P = expected prevalence or proportion (in a proportion of 1, if 20%, P = .02)
d = precision (in a proportion of 1, if 5%, d = 0.05)
Based on the seroprevalence studyCitation11 done in a non-COVID-19 hospital in Sarawak, Malaysia, the prevalence of seropositivity among asymptomatic HCWs was 4.5%. Z-value of 2.58 for a 99% level of confidence was used. However, because the prevalence was less than 10% and because of resource limitations, we reduced the accuracy to 0.03 and got 319 participants. After considering a 10% drop-out rate from the calculated sample size, the target sample size was 351. We managed to attain 361 participants, slightly more than the targeted 351 participants.
Laboratory analysis
Blood samples were collected at the assigned date for each participant. A maximum of 4 mL of venous blood was collected in a plain tube and centrifuged immediately. The serum was aliquoted and stored frozen at −80°C until analysis. SARS-CoV-2 S-RBD IgG (S-RBD IgG) and Neutralizing Antibodies (NTAb) of each patient were measured using the Mindray SARS-CoV-2 S-RBD IgG assay and Mindray SARS-CoV-2 neutralizing antibody assay (CL-900i Chemiluminescence Immunoassay System Analyzer, Shenzhen Mindray Bio-Medical Electronics Co., Ltd.). The principle and methods of measuring the two analytes are as follows:
SARS-CoV-2 S-RBD IgG
The CL-series SARS-CoV-2 S-RBD IgG assay is a two-step assay to quantitatively detect S-RBD IgG antibodies to SARS-CoV-2. In the first step, the sample, sample treatment solution, and paramagnetic microparticles coated with SARS-CoV-2 S-RBD antigens are added into a reaction vessel. After incubation, SARS-CoV-2 S-RBD IgG in the sample will bind RBD antigens coated on the microparticles. Afterward, microparticles are magnetically captured, while other unbound substances are removed by washing. In the second step, diluent solution and ALP-labeled anti-human IgG monoclonal antibody are added to the reaction vessel. After incubation, ALP-labeled anti-human IgG monoclonal antibody will form a sandwich structure with the S-RBD IgG. Afterward, microparticles are magnetically captured, while other unbound substances are removed by washing. Then, the substrate solution is added to the reaction vessel. The resulting chemiluminescent reaction is measured as relative light units (RLUs) by a photomultiplier in the instrument. The amount of S-RBD IgG in the sample is proportional to the relative light units (RLUs) generated during the reaction. The S-RBD IgG concentration can be determined via a calibration curve established on an encoded Master Calibration Curve and three-level product calibrators.
SARS-CoV-2 neutralizing antibody
The CL-series SARS-CoV-2 neutralizing antibody assay is a competitive binding immunoenzymatic assay to quantitatively detect neutralizing antibodies to SARS-CoV-2. In the first step, the sample, sample treatment solution, and paramagnetic microparticles coated with SARS-CoV-2 antigens are added into a reaction cuvette. After incubation, the SARS-CoV-2 neutralizing antibody in the sample will bind antigens coated on the microparticles. ACE2-alkaline phosphatase (ALP) conjugate is added to the reaction cuvette in the second step. After incubation, the ACE2-ALP conjugate competes with neutralizing antibody in the sample for binding sites of SARS-CoV-2 antigens. Neutralizing antibodies or ACE2-ALP conjugates are bound to antigens on the microparticles, which are magnetically captured while other unbound substances are removed by washing. Then, the substrate solution is added to the reaction cuvette. The result of the chemiluminescent reaction is measured as relative light units (RLUs) by a photomultiplier built inside the system. The amount of neutralizing antibody in the sample is inversely proportional to the relative light units (RLUs) generated during the reaction. The neutralizing antibody concentration can be determined via a calibration curve established on an encoded Master Calibration Curve and three-level product calibrators.
Before sample analysis, method validation was conducted to ensure the assays were fit for use as per the manufacturer’s claims.
Statistical analysis
The categorical data were presented in frequencies (n) and percentages (%). Continuous data were presented in median and inter-quartile ranges as the assumption of the data was not normally distributed based on the Kolmogorov–Smirnov/Shapiro–Wilk test. The Mann–Whitney U-test analyzed the comparison between the median of two groups, and the Kruskal–Wallis test was used to analyze the median of three or more groups. P < .05 (95% confidence interval) was considered significant in all statistical analyses. All clinical and laboratory data were stored and analyzed using GraphPad Prism, Version 9.4.0.
Results
Baseline characteristics of HCWs and factors under study
A total of 361 HCWs were recruited prospectively into the study. The youngest participant was 24, while the eldest was 59, with a median age of 37. Female participants significantly outnumbered male participants, with 280 females (77.6%) and 81 males (22.4%). Many participants were Malays, constituting 327 participants (90.6%), with other participants being Chinese, Indian, and smaller indigenous tribes. The participants were then divided into three groups, mainly low, medium, and high risk of exposure to COVID-19, segregated based on their answers from the questionnaire. One hundred twelve participants were in the low-risk group (31%), 182 were in medium risk (50.4%), and 67 were in the high-risk group (18.6%). Breakthrough infections (infection with SARS-CoV-2 virus of a completely vaccinated individual) were also studied, where 49 participants (13.6%) developed breakthrough infections. In terms of education levels, 72 (19.9%) were certificate holders, 121 (33.5%) were diploma holders, and 168 (46.6%) were degree holders or higher. For monthly household income, participants were divided into three income brackets based on the Household Income & Basic Amenities Survey Report 2019 by the Malaysian Ministry of Economy for Kuala Lumpur,Citation12 namely low income (less than RM 9150) making up the majority of participants (250 participants − 69.3%), medium (RM9150-RM 16 639: 80 participants − 22.2%), and high income (≥ RM 16 640: 31 participants − 8.5%). In terms of household number, 104 participants (28.8%) had less than three individuals in the house, 170 participants (47.1%) between 3 and 5 individuals, and 87 participants (24.1%) had more than five individual households. Different housing types revealed that 200 participants (55.4%) lived in flats/apartments, 141 participants (39%) in terrace/townhouses, and 20 participants (5.6%) lived in bungalows. summarizes the baseline characteristics of the HCWs and the associated factors under study.
Table 1. Demographics & characteristics of participants.
Studied factors and association with S-RBD IgG and neutralizing antibody titers
shows the median titers of S-RBD IgG and NTAb of each factor under study and its associated significance. HCWs in the high risk of exposure to COVID-19 group showed higher median levels of S-RBD IgG (1796 AU/mL) and NTAb (731 AU/mL) titers compared to the low (S-RBD IgG: 1327 AU/mL, NTAb: 538 AU/mL) and medium groups (S-RBD IgG: 1501 AU/mL, NTAb: 664 AU/mL). These differences were significant with a p-value of (S-RBD IgG: 0.0372, NTAb: 0.0137). is a graphical representation of these titers.
Figure 2. S-RBD IgG & neutralizing antibody titers among HCWs with different risks of exposure to COVID-19.
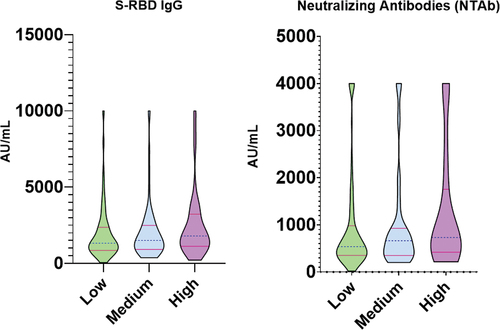
Table 2. S-RBD IgG & NTAb titers among HCWs based on different factors studied.
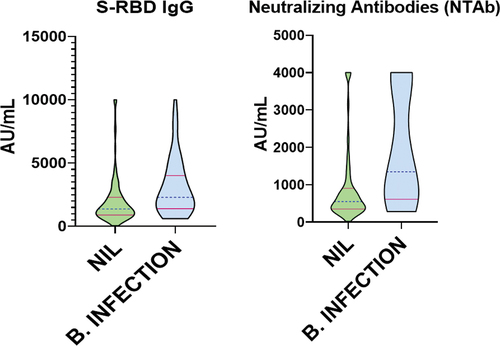
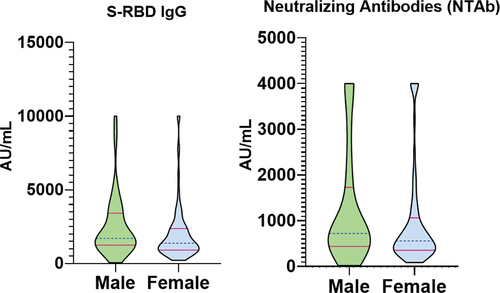
HCWs with breakthrough infections also recorded higher median levels of S-RBD IgG (2295 AU/mL) and NTAb (1346 AU/mL) titers compared with no breakthrough infections, S-RBD IgG (1377 AU/mL) and NTAb (548.1 AU/mL), . The differences were significant, with a p-value of < .0001 for both types of antibodies. Male HCWs recorded higher median titers of S-RBD IgG (1700 AU/mL) and NTAb (723.1 AU/mL) compared to female HCWS, with S-RBD IgG (1377 AU/mL) and NTAb (557.8 AU/mL), . These differences were also significant with the p-value of S-RBD IgG (p = .0046) and NTAb (p = .0119). Monthly household income in the medium category recorded higher median values of S-RBD IgG (1683 AU/mL) and NTAb (652.6 AU/mL); however, it was only borderline significant for the S-RBD IgG (p = .0447) and insignificant for NTAb (p = .0821). Other factors, including age, race, housing type, and education, revealed various median values of S-RBD IgG & NTAb levels but were all insignificant ().
Seeing that different levels of risk of exposure to COVID-19 bring about significant differences between the median titers of S-RBD IgG and NTAb, we decided to explore further and identify which pair is the most significant. shows a pairwise comparison done using Dunn’s test, which indicated that the low risk of exposure to COVID-19 group median antibody titers was observed to be significantly different from those of the high risk of exposure to COVID-19 group with S-RBD IgG (adjusted p = .0318) and NTAb (adjusted p = .0103). No other differences were statistically significant.
Table 3. Dunn’s multiple comparison test for different risks of exposure to COVID-19.
Discussion
This study evaluated S-RBD IgG and Neutralizing antibody (NTAb) titers among HCWs post-third dose of the BNT162b2 mRNA COVID-19 Vaccine. S-RBD IgG and NTAb production are believed to protect the human body; however, specific titers vary between various documented studies. Morales-Núñez et al.Citation13 evaluated the Pfizer-BioNTech (BNT162b2) efficacy among HCWs in a hospital in Mexico. They found that among 143 vaccinated HCWs without prior COVID-19 infection, only 7.7% did not have NTAb after the first dose, and 100% generated NTAb 21 d after the second dose. A 100% of HCWs with prior COVID-19 had NTAb after the first dose and increased their neutralization percentage in the second. This indicates that vaccinated individuals produce significant NTAb after the second-dose administration. It is well known that vaccination induces the production of NTAb to the SARS-CoV-2 virus, and different vaccines elicit different dynamics to the production of these antibodies. It is also well documented that antibody titers depreciate over time despite being vaccinated. In our study, we decided to evaluate the different characteristics of HCWs to understand whether other factors can also affect the production of NTAb.
Our results showed that HCWs in the high-risk group had the highest median titers of S-RBD IgG and NTAb titers compared to the other groups. These were significantly different, particularly from the titers in the low-risk group. Close contact and an increase in frequency of exposure to COVID-19 patients are some of the factors we believe to attribute to this finding. This aligns with previous studies that depict HCWs with close contact with COVID-19 patients would have a higher risk of getting infected.Citation14,Citation15 Interestingly, despite wearing high-grade personal protective equipment (PPE), titers were still high, indicating that significant exposure still occurs. We attribute this to a possibility of improper donning and doffing practices, hand hygiene practices, and contact precautions. Mumma et al.Citation16 conducted a study in a large tertiary care hospital where improper doffing practices were observed even among experienced HCWs in the presence of a trained observer. This brings into question the compliance of HCWs toward infection prevention control procedures. Furthermore, as demand for PPE skyrocketed during pandemic times, the quality of PPE provided could also be questioned. Hence, we postulate that these issues are potential hazards that may put HCWs at risk for self-contamination. The titers among the medium-risk group compared to the high- and low-risk group were insignificant. We believe that high and continuous exposure to the virus is needed to elicit a strong NTAb immune response. Furthermore, the virus spreads mainly via droplet transmission; hence, medium-risk HCWs handling samples are less likely to get transmitted, particularly if safety precautions are taken.
Since vaccination drives started worldwide, cases of breakthrough infections have been reported; hence, we also evaluated the antibody titers among our HCWs who had breakthrough infections (infection occurring after completion of at least two doses of BNT162b2 mRNA COVID-19 Vaccine) and found the titers to be significantly higher than those without breakthrough infections. We postulated a few factors to be considered from this finding. First, despite being completely vaccinated, HCWs were still infected, indicating that the immune response and hemodynamics of antibody production among individuals differ, leading to some being more vulnerable than others. Secondly, our HCWs were vaccinated with the BNT162b2 mRNA COVID-19 vaccine, an mRNA vaccine, while other vaccines with different targeted regions could have different outcomes. It also depicts that direct infection with COVID-19 induces a more robust response to NTAb production than vaccination-induced antibody production. This agrees with other previous studies, where Psaridi et al.,Citation17 in their study of 739 HCWs in Greece, reported that laboratory-confirmed SARS-CoV-2 infection was associated with sustained high NTAb immune responses after the second dose of mRNA BNT162b2 vaccine. Interestingly, it was also reported that high levels of S-RBD IgG and NTAb titers had been detected even after only one dose of mRNA vaccine among most vaccinated persons recovered from SARS-CoV-2 infection.Citation18 Furthermore, Bergwerk et al.Citation19 reported in their study that among 1497 fully vaccinated HCWs, 39 SARS-CoV-2 breakthrough infections were documented. NTAb titers among these patients during the peri-infection period were lower than those in matched uninfected controls. Higher peri-infection NTAb titers were associated with lower infectivity (higher CT values). They concluded that the occurrence of breakthrough infections was correlated with NTAb titers. To make things more interesting, Brehm et al.Citation20 reported a case of a 27-y-old immunocompetent female nurse working in a COVID-19 treating ward who developed reinfection of COVID-19 282 d after the first infection despite the detection of NTAb following the first infection. This raises a question as to how protective NTAb are toward SARS-CoV-2.
Next, we evaluated the gender aspect to see whether there were any differences in NTAb production. We found that male HCWs produced significantly higher levels of NTAb than females. Other studies have also shown a male preponderanceCitation21 where Robbiani et al.Citation22 found that among 149 COVID-19 convalescent participants, there was a significant difference in neutralizing activity between males and females where higher anti-RBD and -S IgG titers were seen in males. Klein et al.Citation23 also reported a stronger antibody response among males than females in their study involving 126 convalescent plasma donors. The exact reason males elicit stronger antibody responses is unknown; however, it could be related to an uncoordinated response between CD4+ T cell responses and serum antibody responses.Citation24 Some studies have even suggested that a higher thromboembolism risk in males than in females could be a contributing factor.Citation25 Interestingly, other viral infections and vaccinations, not limited to the influenza vaccine, display a stronger immune response in females than males,Citation26 while others show a male preponderance.Citation27 Despite this, it is postulated that differences in vaccine humoral responses may be attributed to a combination of sex hormone effects on immune cell signaling, X chromosome immune-related gene expression, and microRNA (miRNA) levels, as well as genetic polymorphismsCitation26 in genes encoding proteins such as interleukin-6 (IL-6)Citation28 and cytotoxic-T-lymphocyte-associated antigen 4 (CTLA-4).Citation29 Our findings are in line with these studies.
Another aspect we tried to look at was age. We anticipated that younger HCWs would produce higher titers of NTAb compared to older HCWs as the immune response was expected to be stronger; however, the results indicated that HCWs at the age of 41 and above produced higher levels of NTAb titers compared to those below 41. Walsh et al.,Citation30 in their study of 195 participants, found that the immune response to two doses of BNT162b2 is lower in people aged 65–85 y than in those aged 18–55 y. Levin et al.Citation31 reported similar findings. They concluded that humoral response was substantially decreased, especially among men, persons 65 y or older, and persons with immunosuppression 6 months after receiving the second dose of the BNT162b2 vaccine. Despite this, our results showed that the difference between the two age groups of the population was not statistically significant. This is probably attributed to our participants being 24–59 y of age, with a median age of 37; hence, immune responses could be similar based on previous studies.
As a multiracial country, we decided to also evaluate antibody titers among the different races, namely Malays, Chinese, Indians, and indigenous tribes of Malaysia. The results show that Malaysian Indians produce the highest titers of NTAb production compared to the other races. However, we could not come to a deduction as the amount of Malaysian Indian participants was very low at 1.4% compared to Malays at 90.6%, and the values were found to be statistically insignificant. Zhao et al.Citation32 reported that ACE2 expression is higher among Asians than African Americans and Caucasians. Smith et al.Citation33 reported various levels of antibody responses among Middle Eastern Qataris, Non-Middle Eastern Qataris, and South Asians admitted for COVID-19, and values differ interethnically based on the severity of the disease. They suggested that further studies, which include HLA allele sequencing, should be conducted. We postulated that race and ethnicity could affect NTAb production among our HCWs; a more homogenous and bigger sample size is required for further evaluation. Other factors also studied include household income, household number, housing type, and education level, which all had mixed results.
The method we used to measure both analytes was also based on immunoassay, specifically chemiluminescence immunoassay. Other laboratories are utilizing other methods, including the gold standard method of Plaque reduction neutralization test (PRNT). In PRNT, virus-Ab complexes are generated in vitro and added to virus-sensitive cells for culture and observation until a cytopathic effect (CPE) and plaque formation are observed. This allows measurement of the neutralizing effect of plaque formation on virus-susceptible cells.Citation34 Another method that is also being utilized is the Pseudovirus-based neutralization test (PBNT), where instead of using the actual virus, a pseudovirus is used instead, thus making it safer for HCWs to conduct the test in a Biosafety Level 2 laboratory. A pseudovirus is a synthetic virus that is as infectious and can simulate the whole process of virus-receptor binding and viral entry as the original virus but is unable to replicate.Citation35,Citation36 Other methods include enzyme-linked immunosorbent assay (ELISA), lateral immunochromatographic test a point of care NTAb detection method, quantum optical methods,Citation37 and covariant scanning.Citation38 It is recommended that further research be done to assess the correlation between all these methods and, if possible, to standardize NTAb assays or at least attain harmonization.
From the findings of this study, we learned that the high risk of exposure to COVID-19, male gender, and history of breakthrough infections were significant factors for S-RBD IgG & NTAb production by HCWs. This itself could have several implications and potential impacts on public health policies. The first is that vaccination policies should prioritize HCWs, particularly those whose work is in the high-risk bracket, as evident by the study. This is important to avoid debilitating infections and ensure the healthcare system functions optimally. Secondly, NTAb confers some form of protection from SARS-CoV-2, and in the study, males tend to produce higher titers; hence, further investigation could be done with regard to dosage, types, or variants of vaccine to understand the hemodynamics between males and females further and possibly improve antibody production among females. Third, breakthrough infections were found to be significant; hence, the duration and dosage of subsequent vaccine/booster doses should also be studied, given better NTAb titer production. Booster doses in the future could play a very imperative role, particularly among HCWs, due to the nature of their occupation. As S-RBD IgG confers strong neutralization potential, we believe both S-RBD IgG and NTAb should be measured among HCWs to assess protective immunity levels.
There are several limitations of this study. The first one is that the HCWs selected had their blood samples taken after 60–90 d following the booster shot; during that period, deterioration of antibody titers would have occurred compared to the first 14–30 d. However, this was unavoidable as we had to find a standard duration after the booster dose, as different durations can lead to bias in quantifying antibody titers. Secondly, our participants shared the same type of vaccine taken: the BNT162b2 mRNA COVID-19 Vaccine for all three doses. Other vaccines could induce different immune responses and have different levels of antibodies produced. Thirdly, we cannot rule out the possibility that male participants in the high-risk group who had breakthrough infections could have confounding levels of NTAb compared to other participants, as the three factors were significant. Another limitation of this study was that we focused on breakthrough infections; however, infections that occurred prior to vaccination or before completing the full vaccination doses were not addressed; hence, it could lead to confounding antibody levels as well. Next, the method we used to measure the NTAb was chemiluminescent immunoassay; other assays and methods could result in different levels of antibody titers and possibly even be able to identify strain-specific NTAb. This study was conducted at only one tertiary hospital; had more health centers been involved, the results could be variable. Overall, future studies can be performed on a bigger scale involving more health centers and other regions with different demographics, which could give us a better picture.
Conclusions
In conclusion, different degrees of exposure to COVID-19 among HCWs at the hospital would induce different levels of antibody production. COVID-19 breakthrough infections, as well as gender, also affect the production of NTAb among HCWs. Hence, more studies can be done to further evaluate the nature and hemodynamics of COVID-19 NTAb.
Acknowledgments
We want to acknowledge the contribution of phlebotomists, medical officers, and laboratory personnel for their assistance in making this study successful.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available because it contains sensitive information that could compromise the privacy of research participants.
Additional information
Funding
References
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–10. doi:10.1056/NEJMoa2001017.
- Gralinski LE, Menachery VD. Return of the coronavirus: 2019-nCoV. Viruses. 2020;12(2):135. doi:10.3390/v12020135.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–42. doi:10.1001/jama.2020.2648.
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–44. doi:10.1038/s41564-020-0695-z.
- KKMNOW. Department of statistics Malaysia, Ministry of health Malaysia. COVID -19. The latest data on the pandemic in Malaysia. [accessed 2023 June 17]. https://data.moh.gov.my/covid.
- Iwasaki A, Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nat Rev Immunol. 2020;20(6):339–41. doi:10.1038/s41577-020-0321-6.
- Wu J, Liang B, Chen C, Wang H, Fang Y, Shen S, Yang X, Wang B, Chen L, Chen Q, et al. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19. Nat Commun. 2021;12(1):1813. doi:10.1038/s41467-021-22034-1.
- Gorse GJ, Donovan MM, Patel GB. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus-associated illnesses. J Med Virol. 2020;92(5):512–7. doi:10.1002/jmv.25715.
- Favresse J, Gillot C, Di Chiaro L, Eucher C, Elsen M, Van Eeckhoudt S, David C, Morimont L, Dogné JM, Douxfils J. Neutralizing antibodies in COVID-19 patients and vaccine recipients after two doses of BNT162b2. Viruses. 2021;13(7):1364. doi:10.3390/v13071364.
- Daniel WW, Cross CL. Biostatistics: a foundation for analysis in the health sciences. Hoboken (NJ): Wiley; 2018.
- Ling HS, Pang IX, Fong AYY, Ong TK, Khiew NZ, Cham Y, Chen LS. Covid-19 antibody surveillance among healthcare workers in a non-covid designated cardiology centre. 2020. doi:10.22541/au.158955330.06881415.
- Statistics on Household Income & Basic Amenities. Ministry of economy, department of statis Malaysia, household income & basic amenities survey report 2019. [accessed 2022 Jan 3]. https://www.dosm.gov.my/portal-main/release-content/household-income-&-basic-amenities-survey-report-2019.
- Morales-Núñez JJ, Muñoz-Valle JF, Meza-López C, Wang LF, Machado Sulbarán AC, Torres-Hernández PC, Bedolla-Barajas M, De la O-Gómez B, Balcázar-Félix P, Hernández-Bello J. Neutralizing antibodies titers and side effects in response to BNT162b2 vaccine in healthcare workers with and without prior SARS-CoV-2 infection. Vaccines. 2021;9(7):742. doi:10.3390/vaccines9070742.
- Kumar SS, Kumar A, Kirtana J, Singh AK, Shankar SH, Khan MA, Srivastava AK, Kaur R, Wig N. Risk factors and outcome among COVID-19 exposed and quarantined healthcare workers: a study on the status of existing practices of standard precautions. J Family Med Prim Care. 2020;9(10):5355–9. doi:10.4103/jfmpc.jfmpc_1579_20.
- Das R, Sengupta B, Goswami B, Mog C. Assessment of exposure risk among healthcare workers exposed to confirmed cases of COVID-19 working in non-COVID zones of a teaching hospital in Northeast India: a cross-sectional study. J Family Med Prim Care. 2022;11(8):4483–7. doi:10.4103/jfmpc.jfmpc_50_22.
- Mumma JM, Durso FT, Ferguson AN, Gipson CL, Casanova L, Erukunuakpor K, Kraft CS, Walsh VL, Zimring C, DuBose J, Centers for Disease Control and Prevention Epicenters Program, Division of Healthcare Quality Promotion, et al. Human factors risk analyses of a doffing protocol for ebola-level personal protective equipment: mapping errors to contamination. Clin Infect Dis. 2018;66(6):950–8. doi:10.1093/cid/cix957.
- Psaridi L, Maltezou HC, Simonidou S, Lialliou I, Athanasopoulou D, Haila Z, Kyrimi A, Giannopoulou I, Giannousa S, Pseimada M, et al. Neutralizing antibody responses in healthcare personnel after three doses of mRNA BNT162b2 vaccine and association with baseline characteristics and past SARS-CoV-2 infection. Vaccine. 2022;40(40):5752–6. doi:10.1016/j.vaccine.2022.08.031.
- Demonbreun AR, Sancilio A, Velez MP, Ryan DT, Saber R, Vaught LA, Reiser NL, Hsieh RR, D’Aquila RT, Mustanski B, et al. Comparison of IgG and neutralizing antibody responses after one or two doses of COVID-19 mRNA vaccine in previously infected and uninfected individuals. EClinicalMedicine. 2021;38:101018. doi:10.1016/j.eclinm.2021.101018.
- Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, Mandelboim M, Gal Levin E, Rubin C, Indenbaum V, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–84. doi:10.1056/nejmoa2109072.
- Brehm TT, Pfefferle S, von Possel R, Kobbe R, Nörz D, Schmiedel S, Grundhoff A, Olearo F, Emmerich P, Robitaille A, et al. SARS-CoV-2 reinfection in a healthcare worker despite the presence of detectable neutralizing antibodies. Viruses. 2021;13(4):661. doi:10.3390/v13040661.
- Markmann AJ, Giallourou N, Bhowmik DR, Hou YJ, Lerner A, Martinez DR, Premkumar L, Root H, van Duin D, Napravnik S, et al. Erratum for markmann et al., “Sex disparities and neutralizing-antibody durability to SARS-CoV-2 infection in convalescent individuals”. mSphere. 2021;6:e00275–21. doi:10.1128/mSphere.00736-21.
- Robbiani DF, Gaebler C, Muecksch F, Lorenzi JCC, Wang Z, Cho A, Agudelo M, Barnes CO, Gazumyan A, Finkin S, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–42. doi:10.1038/s41586-020-2456-9.
- Klein SL, Pekosz A, Park HS, Ursin RL, Shapiro JR, Benner SE, Littlefield K, Kumar S, Naik HM, Betenbaugh MJ, et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest. 2020;130(11):6141–50. doi:10.1172/JCI142004.
- Yu KK, Fischinger S, Smith MT, Atyeo C, Cizmeci D, Wolf CR, Layton ED, Logue JK, Aguilar MS, Shuey K, et al. Comorbid illnesses are associated with altered adaptive immune responses to SARS-CoV-2. JCI Insight. 2021;6(6):e146242. doi:10.1172/jci.insight.146242.
- Pivonello R, Auriemma RS, Pivonello C, Isidori AM, Corona G, Colao A, Millar RP. Sex disparities in COVID-19 severity and outcome: are men weaker or women stronger? Neuroendocrinology. 2021;111(11):1066–85. doi:10.1159/000513346.
- Klein SL. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. BioEssays. 2012;34(12):1050–9. doi:10.1002/bies.201200099.
- Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26(29–30):3551–5. doi:10.1016/j.vaccine.2008.04.054.
- Cussigh A, Falleti E, Fabris C, Bitetto D, Cmet S, Fontanini E, Bignulin S, Fornasiere E, Fumolo E, Minisini R, et al. Interleukin 6 promoter polymorphisms influence the outcome of chronic hepatitis C. Immunogenetics. 2011;63(1):33–41. doi:10.1007/s00251-010-0491-7.
- Schott E, Witt H, Hinrichsen H, Neumann K, Weich V, Bergk A, Halangk J, Müller T, Tinjala S, Puhl G, et al. Gender-dependent association of CTLA4 polymorphisms with resolution of hepatitis C virus infection. J Hepatol. 2007;46(3):372–80. doi:10.1016/j.jhep.2006.09.011.
- Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, et al. Safety and immunogenicity of two RNA-Based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–50. doi:10.1056/NEJMoa2027906.
- Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, Doolman R, Asraf K, Mendelson E, Ziv A, et al. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi:10.1056/NEJMoa2114583.
- Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;202(5):756–9. doi:10.1164/rccm.202001-0179LE.
- Smith M, Abdesselem HB, Mullins M, Tan TM, Nel AJM, Al-Nesf MAY, Bensmail I, Majbour NK, Vaikath NN, Naik A, et al. Age, disease severity and ethnicity influence humoral responses in a multi-ethnic COVID-19 cohort. Viruses. 2021;13(5):786. doi:10.3390/v13050786.
- Chen C, Liang J, Hu H, Li X, Wang L, Wang Z. Research progress in methods for detecting neutralizing antibodies against SARS-CoV-2. Anal Biochem. 2023;673:115199. doi:10.1016/j.ab.2023.115199.
- Li Q, Liu Q, Huang W, Li X, Wang Y. Current status on the development of pseudoviruses for enveloped viruses. Rev Med Virol. 2018;28(1):e1963. doi:10.1002/rmv.1963.
- Nie J, Li Q, Wu J, Zhao C, Hao H, Liu H, Zhang L, Nie L, Qin H, Wang M, et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerging Microbes Infect. 2020;9(1):680–6. doi:10.1080/22221751.2020.1743767.
- Rajil N, Esmaeili S, Neuman BW, Nessler R, Wu HJ, Yi Z, Brick RW, Sokolov AV, Hemmer PR, Scully MO. Quantum optical immunoassay: upconversion nanoparticle-based neutralizing assay for COVID-19. Sci Rep. 2022;12(1):1263. doi:10.1038/s41598-021-03978-2.
- Heggestad JT, Britton RJ, Kinnamon DS, Wall SA, Joh DY, Hucknall AM, Olson LB, Anderson JG, Mazur A, Wolfe CR, et al. Rapid test to assess the escape of SARS-CoV-2 variants of concern. Sci Adv. 2021;7(49):eabl7682. doi:10.1126/sciadv.abl7682.